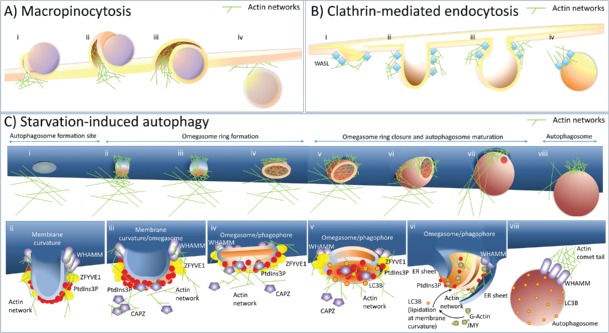
Figure 1.
A model explaining the role of actin in membrane modulation and shaping of autophagosomes. (A) Actin networks are involved in the plasma membrane protrusion(s) during macropinocytosis. (i) To form a macropinosome, actin networks force the plasma membrane to engulf extracellular cargo, for instance, as a cup-shaped extension of the cell membrane. (ii) These plasma membrane extrusions may fold back on the plasma membrane and form a cave-like invagination. (iii) A membrane fusion/fission event separates the lumen of the macropinosome from the extracellular space. (iv) After cargo engulfment, the newly formed macropinosome is moved further into the cytoplasm. (B) During clathrin-mediated endocytosis, actin polarization/depolarization events promote clathrin-coated vesicle formation. (i) Clathrin-mediated endocytosis is initiated at PtdIns(4,5)P2-rich sites in the plasma membrane enriched also for subunits of clathrin coat proteins (not shown) which bind the actin nucleation factor, WASL/N-WASP. (ii) WASL initiates actin nucleation, which causes plasma membrane deformation into a bulb. (iii) Further actin polymerization and branching promote elongation and contraction of the neck required for vesicle closure. (iv) After vesicle scission, the newly formed endosome is moved further into the cytoplasm by a comet-tail mechanism, which involves actin filaments. (C) During starvation-induced autophagy, actin networks shape autophagosomes from inside and outside the phagophore. (i) Autophagosome formation starts at ER membrane curvature sites rich in ATG14. At this stage actin is not detected at the ER sites specific to autophagosome formation. (ii) ATG14 recruits the PIK3C3/VPS34 kinase complex (not shown) required for PtdIns3P generation. PtdIns3P is then recognized by effectors, such as ZFYVE1. The actin nucleation-promoting factor, WHAMM, is also recruited to ER sites specific to autophagosome formation, via its N-terminal segment. Actin nucleation stabilizes and promotes membrane curvature, which can stimulate further PtdIns3P and ZFYVE1 recruitment. (iii) High levels of PtdIns3P remove from the actin filaments a negative regulator of actin polymerization and branching, the actin-capping protein, CAPZ. (iv) This removal of CAPZ stimulates actin polymerization and may cause flattening of the structure and omegasome formation with a highly-curved membrane existing at the limiting edge that is saturated with ZFYVE1. (v) PtdIns3P produced by the membrane-bound PIK3C3/VPS34 kinase complex further enhances curvature of the double-membrane structure facilitating phagophore formation. Simultaneously, the edge exhibiting local lipid-packing defects serves as a platform for the recruitment of WIPI2 (not shown), followed by recruitment of the ATG12–ATG5-ATG16L1 complex (not shown) and LC3/GABARAP lipidation. (vi) LC3 recruits another nucleation-promoting factor, JMY, and this attracts more actin and expands the actin network to allow phagophore extension. The growing phagophore is also surrounded by 2 sheets of ER, which hypothetically might be used by the actin network to create the autophagosome shape, as well as being used as a force required to stabilize a highly-curved limiting edge required for phagophore expansion. (vii) Further formation of actin networks affects autophagosome shape and size preceded by phagophore closure and omegasome dissociation. (viii) When an autophagosome is formed, WHAMM drives it into the cytoplasm using an actin comet-tail mechanism.