Abstract
Objective
To determine the normal ranges of vital signs, including blood pressure (BP), mean arterial pressure (MAP), heart rate (HR) and shock index (SI) (HR/systolic BP), in the immediate postpartum period to inform the development of robust obstetric early warning scores.
Study Design
We conducted a secondary analysis of a prospective observational cohort study evaluating vital signs collected within one hour following delivery in women with estimated blood loss (EBL) <500ml (316 women) delivering at a UK tertiary centre over a one-year period. Simple and multiple linear regression were used to explore associations of demographic and obstetric factors with SI.
Results
Median (90% reference range) was 120 (100–145) for systolic BP, 75 (58–90) for diastolic BP, 90 (73–108) for MAP, 81 (61–102) for HR, and 0.66 (0.52–0.89) for SI. Third stage Syntometrine® administration was associated with a 0.03 decrease in SI (p = 0.035) and epidural use with a 0.05 increase (p = 0.003). No other demographic or obstetric factors were associated with a change in shock index in this cohort.
Conclusion
This is the first study to determine normal ranges of maternal BP, MAP, HR and SI within one hour of birth, a time of considerable haemodynamic adjustment, with minimal effect of demographic and obstetric factors demonstrated. The lower 90% reference point for systolic BP and upper 90% reference point for HR correspond to triggers used to recognise shock in obstetric practice, as do the upper 90% reference points for systolic and diastolic BP for obstetric hypertensive triggers. The SI upper limit of 0.89 in well postpartum women supports current literature suggesting a threshold of 0.9 as indicating increased risk of adverse outcomes.
Introduction
The UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity Report of 2009–2012 (Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE)) highlighted that failures by healthcare professionals to immediately recognise and act on signs of life-threatening conditions, including haemorrhage, severe pre-eclampsia and sepsis, may have contributed to potentially avoidable direct maternal deaths [1]. The report emphasised the importance of routine measurement of vital signs and recommended the use of modified early obstetric warning score (MEOWS) charts in all pregnant and postpartum women to aid more timely recognition of compromise [1]. According to the 2014 systematic review of causes of maternal mortality by the World Health Organisation, obstetric haemorrhage, hypertensive disorders and sepsis contribute to approximately 50% of maternal deaths worldwide. All are associated with changes in vital signs, including blood pressure (BP) and heart rate (HR) [2]. Obstetric haemorrhage is the leading cause of death (27.1% of all deaths, 95% CI 19.9 to 36.2%) [2] with the majority occurring during labour, delivery and the immediate postpartum period [3]. Failure to recognise deterioration contributes to many of these avoidable deaths [4].
Changes in conventional vital signs into the abnormal range are late markers of compromise [5–6]. Relying solely on changes in BP and HR as individual parameters may delay vital interventions in women with postpartum haemorrhage (PPH), defined as estimated blood loss (EBL) of 500ml or more, and may contribute to avoidable mortality and morbidity.[6] Shock Index (SI), the ratio of HR to systolic BP, has been proposed as an alternative measure of early compromise and compares favourably to conventional vital signs in predicting risk of adverse clinical outcomes in women with PPH [7]. Despite this, the normal ranges of SI and conventional vital signs in the immediate postpartum period (i.e. within one hour of delivery) have not yet been adequately defined.
The aim of this study was to determine the normal range of maternal SI, in addition to BP, mean arterial pressure (MAP) and HR within an hour of birth in women with normal blood loss, in order to enable subsequent exploration of its use as an early warning system to identify the deteriorating woman.
Materials and Methods
The case records of all women chosen for the control arm within a prospective weighted-sample cohort study were examined i.e. women with blood loss at delivery within normal limits. The purpose of the original study was to determine risk factors for PPH. Women delivering at a UK maternity unit with an EBL of less than 500mls were chosen as a one-in-twelve random sample (for comparison against women with PPH delivering over a one-year period) and the data used in this analysis. At this unit, blood loss at caesarean section was calculated through weighing of swabs and drapes and 10% of documented data on blood loss values underwent rigorous validation involving an independent expert obstetrician. It is possible that a minority of women with blood losses above the normal threshold have been included in the cohort (unlikely to be overt haemorrhages). As these women were identified as having ‘normal’ blood losses and were therefore treated as clinically ‘normal’, our results remain valid and valuable. The original study was approved by the South East multi-centre research ethics committee [8]. Individual informed consent was not required for this observational study, as data collection did not directly involve contact with women. All women with normal blood loss following delivery (including, for example, those with multi-fetal pregnancies or caesarean delivery) were included, to provide a representative sample of women. The first BP and HR values recorded in clinical practice within the hour following delivery were used in the analysis. Method of measurement varied according to clinician preference but automated devices with digital displays were routinely available. In women who underwent caesarean section or instrumental delivery, the durations of the first, second and third stages may have been iatrogenically altered. Third stage policy was to offer uterotonic agents for active third stage prophylaxis as then recommended by the National Institute for Health and Care Excellence (NICE) but physiological third stage was also practiced [9].
Data analysis was conducted using Stata version 11.2 (StataCorp, College Station, Texas). Median, lower and upper quartiles and 90% reference ranges were calculated for BP, HR, MAP and SI.
Using simple and multiple linear regression analysis, the association with SI of nineteen predefined demographic and obstetric factors was investigated, based on prior examination of the literature and sufficient data on the variable: age, parity, ethnicity, weight, height, body mass index, multi-fetal pregnancy, duration of first stage, second stage and third stage of labour, mode of delivery (vaginal or caesarean section), Syntometrine® administration for the third stage, oxytocin administration for the third stage, epidural anaesthetic, spinal anaesthetic, temperature during labour, anaemia, gestational hypertension, pre-eclampsia. For variables with strong non-normal distributions (duration of first, second and third stage of labour and EBL) medians and IQR were calculated.
Results
384 control women with an EBL <500ml were identified. There were 68 exclusions due to missing vital signs documentation (n = 24) and delivery-observation time greater than one hour (n = 44). 316 women were included in statistical analysis, allowing estimation of the 95th centile to within 0.17 of a standard deviation [10]. Participant characteristics are shown in Table 1.
Table 1. Participant Characteristics.
Values are given as number (percentage), mean (SD) or median [IQR].
| Characteristics | Participants (n = 316) |
|---|---|
| Mean (SD) age at delivery, years | 30.7 (5.6) |
| Parity at trial entry n (%) | |
| 0 | 163 (51.6) |
| 1 | 85 (26.9) |
| 2 | 42 (13.3) |
| 3+ | 26 (8.2) |
| Ethnicity n (%) | |
| White | 156 (49.9) |
| Black | 89 (28.2) |
| Asian | 39 (12.3) |
| Other | 32 (10.1) |
| Mean (SD) Body Mass Index, kg/m2 | 24.5 (5.2) |
| Multi-fetal pregnancy n (%) | 6 (1.9) |
| Median [IQR] duration of 1st Stage, minutes | 270 [140, 475] |
| Median [IQR] duration of 2nd Stage, minutes | 30 [10, 90] |
| Median [IQR] duration of 3rd Stage, minutes | 8 [5,14] |
| Mode of delivery n (%) | |
| Vaginal delivery (including instrumental) | 278 (88.0) |
| Caesarean delivery | 38 (12.0) |
| Syntometrine® administration in the 3rd stage n (%) | 206 (65.2) |
| Oxytocin administration in the 3rd stage n (%) | 66 (20.9) |
| Physiological management of 3rd stage n (%) | 44 (13.9) |
| Epidural anaesthetic n (%) | 69 (21.8) |
| Spinal anaesthetic n (%) | 33 (10.4) |
| Mean (SD) temperature during labour,°C | 36.7 (0.49) |
| Anaemia (Hb <10.5 g/ml in pregnancy) n (%) | 43 (13.6) |
| Gestational hypertension n (%) | 8 (2.5) |
| Pre-eclampsia n (%) | 4 (1.3) |
| Median [IQR] blood loss, ml | 300 [200, 400] |
| Median [IQR] time from delivery to SI reading, minutes | 8 [5, 13] |
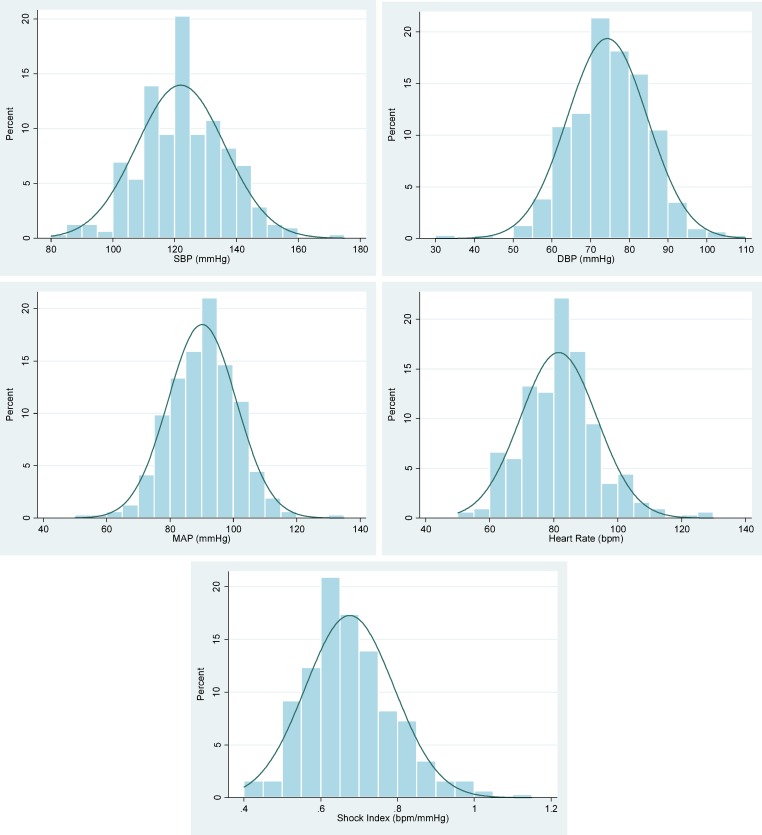
Median, lower and upper quartile and 90% reference ranges for systolic BP, diastolic BP, MAP, HR and SI are shown in Table 2. The 90% reference range of SI is 0.52–0.89. Histograms for systolic BP, diastolic BP, MAP, HR and SI, including superimposed normal distribution curves, are shown in Fig 1. The similarity between the histograms and the normal distribution curve confirms that tests based on the normal distribution are appropriate.
Table 2. Median, lower and upper quartile and 90% reference ranges for systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate and shock index.
| Median | Lower quartile | Upper quartile | 90% Reference Range | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 120 | 111 | 131 | 100–145 |
| Diastolic BP (mmHg) | 75 | 68 | 80 | 58–90 |
| Mean arterial pressure (mmHg) | 90 | 83 | 97 | 73–108 |
| Heart rate (bpm) | 81 | 74 | 88 | 61–102 |
| Shock index | 0.66 | 0.60 | 0.74 | 0.52–0.89 |
Significant associations (using simple then multiple linear regression methods) were noted between SI and third stage Syntometrine® and epidural use (Table 3). SI decreased by 0.03 with third stage use of Syntometrine®, predominantly due to HR decrease rather than systolic BP rise, equating to median SI decreasing from 0.66 to 0.63. SI increased by 0.05 with epidural use, due to HR increase rather than BP decrease, equating to median SI increase from 0.66 to 0.71. No other factors retained significance in the multiple regression analysis. In this cohort of normal women, length of labour was relatively short (compared to duration for an unselected population of labouring women) and this may have been due to exclusion of prolonged labour in women who went on to have postpartum haemorrhage. The median [IQR] time from delivery to SI reading was 8 [5, 13]. The mean SI was 0.63 prior to delivery of placenta, 0.68 at the same time as delivery of placenta, and 0.68 following delivery of placenta. An observed difference in SI of 0.05 is clinically unimportant.
Table 3. Association between shock index and demographic and obstetric factors.
Change in mean shock index associated with a 1-unit change in each predictor is shown.
| Change in mean SI | Confidence Interval | P-value | |
|---|---|---|---|
| Simple Linear Regression Analysis | |||
| Age (in decades) | -0.01 | -0.03, 0.01 | 0.332 |
| Parity | |||
| 1 | 0.001 | -0.029, 0.032 | 0.938 |
| 2 | -0.013 | -0.053, 0.026 | 0.507 |
| 3+ | -0.032 | -0.080, 0.016 | 0.195 |
| Ethnicity | |||
| Black | 0.017 | -0.014, 0.047 | 0.281 |
| Asian | 0.030 | -0.011, 0.071 | 0.148 |
| Other | 0.001 | -0.043, 0.045 | 0.968 |
| Weight (kg) | 0.000 | -0.001, 0.001 | 0.903 |
| Height (cm) | -0.002 | -0.004, 0.000 | 0.052 |
| BMI | 0.001 | -0.001, 0.004 | 0.380 |
| Multiple Pregnancy | -0.093 | -0.186, 0.0001 | 0.050 |
| Duration of 1st Stage | 0.003 | -0.002, 0.003 | 0.816 |
| Duration of 2nd Stage | -0.001 | -0.009, 0.007 | 0.818 |
| Duration of 3rd Stage | -0.001 | -0.014, 0.012 | 0.890 |
| Mode of Delivery (caesarean vs vaginal) | 0.011 | -0.028, 0.051 | 0.572 |
| Syntometrine® administration in the 3rd stage | -0.029 | -0.055, -0.002 | 0.035 |
| Oxytocin administration in 3rd stage | 0.031 | 0.000, 0.062 | 0.053 |
| Epidural anaesthetic | 0.046 | 0.0159, 0.770 | 0.003 |
| Spinal anaesthetic | -0.160 | -0.579, 0.026 | 0.451 |
| Temperature during labour | -0.004 | -0.031, 0.022 | 0.741 |
| Anaemia (last recorded in pregnancy) | -0.003 | -0.143, 0.009 | 0.619 |
| Gestational hypertension | 0.011 | -0.710, 0.920 | 0.800 |
| Pre-eclampsia | -0.058 | -0.172, 0.056 | 0.319 |
| Multivariable Regression Analysis | |||
| Epidural anaesthetic | -0.030 | -0.057, -0.002 | 0.036 |
| Syntometrine® administration in 3rd stage | 0.047 | 0.016, 0.079 | 0.003 |
Discussion
This study is original, defining the normal range of BP, HR, MAP and SI within the first hour after birth. Previous studies evaluating postpartum haemodynamic changes have proposed normal ranges for vital signs. However, all have used time points beyond the first hour [11–14], despite recognition in the World Health Organization Post Natal Guidelines of the need for appropriate surveillance to start within the first hour after birth [15]. This study defines the normal ranges for maternal BP, HR, and MAP in the first hour postpartum, all of which are used routinely to assess the haemodynamic status of women following birth. The lower 90% reference range for systolic BP (100mHg) corresponds with the amber lower threshold of systolic BP (100 mmHg) and the upper 90% reference range for HR (102 bpm) corresponds with the amber upper threshold of HR (100 bpm) for the recognition of shock on the currently recommended MEOWS chart [16]. The upper 90% reference ranges for systolic and diastolic BP (145 mmHg and 90 mmHg, respectively) correspond well with the early warning chart amber hypertensive triggers (150 mmHg and 90 mmHg) [16]. It is important to define a normal range as pregnant and recently pregnant women may decompensate relatively late following haemorrhage and sepsis compared to other adults.
The strength of this study is that it addresses the need for evidence-based vital sign reference ranges to guide the monitoring of postpartum women worldwide and subsequent timely intervention. The multi-ethnic population studied is a further strength of the study increasing the generalisability of the findings.
It is recognised that the values may not be applicable beyond the first hour and that results may be influenced by the particular setting (i.e. maternity unit in a high-income country). Considering the dramatic haemodynamic changes during pregnancy and postpartum, more precise prospective studies are ongoing to define the normal ranges of BP, HR, MAP and SI at different gestations, intrapartum and in the later postpartum period, and in low-resource settings.
As a secondary analysis of a prospective observational study, the accuracy of vital sign values recorded depended on the BP devices used and the healthcare provider measuring and recording the vital signs. Fig 1 highlights the issue of digit preference, with a striking proportion of values recorded as systolic BP of 120mmHg and HR of 80bpm (standard values), potentially confounding the results. Future work should collect vital signs prospectively in women immediately postpartum, using automated devices that have been validated for use in pregnancy, to minimise user error and improve accuracy.
Fig 1. Histograms of distribution of BP, MAP, HR and SI values (with superimposed normal distribution curves).
SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure, HR: heart rate, SI: shock index.
Clinical assessment of postpartum haemorrhage includes accurate estimation of blood loss and timely measurement of vital signs. It is well recognised that, despite being the most commonly used technique, visual estimation of blood loss frequently underestimates [17]. As a consequence, appropriate use of vital signs is critical, as reflected in The UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity Report of 2009–2012 (Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE)). SI was first proposed as an early marker of haemodynamic instability in non-obstetric shock in 1967 [18]. It has been studied extensively in non-specific shock [19–21], trauma [22–28], and sepsis [29] as an earlier identifier of circulatory shock than conventional vital signs. In a non-obstetric population the normal range has been defined and validated as 0.5 to 0.7 [19, 21, 30–31], with a SI >0.9 indicating increased risk of mortality and morbidity [27]. In pregnancy, studies have suggested an upper limit of normal of 0.7 [5], 0.81 [32], and 0.85 [33], based on prediction of ruptured ectopic pregnancy, and 0.9 [7], based on prediction of ICU admission in women with PPH. Only one study has attempted to define SI normal range, in a retrospective case-control analysis of women experiencing PPH [34]. In their control group of only 50 women with normal blood loss, mean SI was 0.74 with a range of 0.4–1.1, ten minutes following birth. Although the methodology was unclear, a normal range of 0.7–0.9 for an obstetric population was proposed from these data. This previous work is supported by the upper limit of normal of 0.9 in the current cohort (0.52–0.89). Both are higher than the upper limit of normal of 0.7 in a non-obstetric population. The difference can be explained by the haemodynamic changes of pregnancy and delivery, namely an increase in resting HR, which is often further increased during the immediate postpartum period owing to pain and exertion.
Pregnancy involves significant haemodynamic changes [35]. As the placenta is delivered, auto-transfusion results in cardiac output increasing to a maximal 80% above pre-pregnancy values [35]. It is in this dynamic intrapartum and immediate postpartum period that haemorrhage and sepsis is most prevalent, compensatory mechanisms can mask hypovolaemia and subtle changes in vital signs measurement may alert healthcare providers prior to severe compromise, thereby limiting short- and longer-term maternal morbidity.
The UK Confidential Enquiry report into Maternal Death repeatedly asserts the importance of vital signs measurement and recommends early warning charts for all antenatal and postnatal admissions despite lack of evidence of clinical and cost-effectiveness [1]. These charts are held as a beacon of good practice internationally and have been widely emulated in other countries, including USA [36] and South Africa [37]. However, a standardised obstetric-specific chart does not exist; there is no agreed combination of vital signs and trigger thresholds for vital signs have not been adequately determined [1]. One example of a early warning chart (from the Confidential Enquiry in 2007) has been adopted by hospitals in the UK and elsewhere, but was only validated in 2012, demonstrating adequate predictive value, but suggesting further refinements were required [16]. There has been very little research since about the vital signs thresholds that specifically indicate decompensation in an obstetric population, a particular concern considering the haemodynamic changes of pregnancy and postpartum. Our findings support current early warning thresholds in the immediate postpartum period.
Demographic and iatrogenic influences impacting on the haemodynamic physiology of pregnancy will influence vital signs. In this setting (a UK maternity unit), uterotonics are routinely administered as the baby is delivered to prevent PPH. In low-resource settings, active management of the third stage is used less frequently, due to limited availability of uterotonic medication. Syntometrine®, a combination of ergometrine and oxytocin, is the most commonly used preparation in high-income countries but is usually contraindicated in those with hypertension, owing to the hypertensive effects of ergometrine [38]. Oxytocin alone is often used in those with Syntometrine® contraindications and also has important side-effects, including hypotension and tachycardia [39]. In women receiving either epidural or spinal analgesia or anaesthesia, blood pressure can be reduced due to sympathetic block. These data show that Oxytocin alone had no significant effect on SI. The observed decrease in SI with Syntometrine® use was largely due to a decrease in HR, rather than the anticipated increase in SBP. Epidural use was associated with an SI increase, again, largely due to changes in HR, rather than the expected hypotensive effects of epidural use [40]. Although statistically significant, syntometrine use and epidural use did not move the median SI outside of the normal range and are likely of minimal clinical importance. Spinal anaesthetic use was not associated with a change in SI, likely due to the timing of vital signs used for analysis; the vasodilatory effects of spinal anaesthesia are most marked at administration prior to delivery, rather than at the time of vital sign measurement postpartum.
Study participants were defined by having normal outcome blood loss and there was a low incidence of hypertension and anaemia. We have previously shown that these variables have little influence on SI in postpartum haemorrhage. In women with postpartum haemorrhage, confounding factors including spinal and epidural use, and Syntometrine for the management of the third stage, have previously been shown to have negligible effects on SI [7]. Considering the minimal effect of demographic and obstetric factors on SI in women with postpartum haemorrhage and women with normal blood loss, the normal range of SI does not need to be altered according to the presence or absence of these factors, despite their prevalence differing between settings e.g. well-resourced and low-resourced settings.
A robust evidence-base underpinning the recommended and widely used MEOWS chart does not exist. Studies are required to establish the impact, risks and benefits and cost-effectiveness of MEOWS charts. SI has previously been shown to be a consistently strong predictor of a range of adverse clinical outcomes in women with PPH and has the advantage of being automatable [7]. This work defines the normal range of maternal SI in the first hour after birth. Future work should focus on prospective evaluation of SI in uncomplicated pregnancy, obstetric haemorrhage and sepsis. Studies should also consider the impact of gestational age and stage of labour on SI and assess the added value of incorporating SI into MEOWS charts.
In conclusion, this study is the first to determine the normal range of SI of 0.52–0.89 in the first hour postpartum as well as the normal range of BP, HR and MAP. These findings may have implications for guiding intervention immediately postpartum.
Acknowledgments
Thanks to those who worked on the original study including Jane Sandall, Rachel Tribe, Graham Tydeman, Mark Waterstone.
Data Availability
This data is ethically restricted. At the time of gaining consent for the primary study, which this secondary analysis is based upon, consent did not include explicit approval of data sharing of this nature. The South East Multicentre Research Ethics Committee (07/H1102/79) approved the study. We are therefore unable to allow our minimal dataset to be freely available online. We are able to share anonymised data with researchers who contact us directly, at annette.l.briley@kcl.ac.uk.
Funding Statement
Funded by Bill and Melinda Gates Foundation (OPP1086183), MQAWLAR, HLN, AHS, The National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London, LCC, AB, PTS, CLAHRC South London (NIHR), PTS, Tommy's Charity (registered charity no 1060508), PTS, AB. LCC is funded by a NIHR Research Professorship, (RP-2014-05-019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk J. Saving lives, improving mothers’ care—lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12.: MBRRACE-UK, Oxford: National Perinatal Epidemiology Unit, University of Oxford 2014. 2014.
- 2.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2(6): e323–e33. 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 3.Campbell OM, Graham WJ. Strategies for reducing maternal mortality: getting on with what works. The lancet. 2006; 368(9543): 1284–99. [DOI] [PubMed] [Google Scholar]
- 4.Walvekar V, Virkud A, Majumder R. (2012). Management of Postpartum Haemorrhage in Low Resource Settings. In: Arulkumaran S, Karoshi M, Keith L, Lalonde A, B-Lynch C, editors. A Comprehensive Textbook of Postpartum Haemorrhage 2nd Edition p. 539–44.
- 5.Birkhahn RH, Gaeta TJ, Van Deusen SK, Tloczkowski J. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003; 189(5): 1293–6. [DOI] [PubMed] [Google Scholar]
- 6.Hick JL, Rodgerson JD, Heegaard WG, Sterner S. Vital signs fail to correlate with hemoperitoneum from ruptured ectopic pregnancy. Am J Emerg Med. 2001; 19(6): 488–91. 10.1053/ajem.2001.27133 [DOI] [PubMed] [Google Scholar]
- 7.Nathan HL, El Ayadi AM, Hezelgrave NL, Seed P, Butrick E, Miller S, et al. Shock index: an effective predictor of outcome in postpartum haemorrhage?: BJOG 2015; 122(2): 268–75. 10.1111/1471-0528.13206 [DOI] [PubMed] [Google Scholar]
- 8.Briley A, Seed P, Tydeman G, Ballard H, Waterstone M, Sandall J, et al. Reporting errors, incidence and risk factors for postpartum haemorrhage and progression to severe PPH: a prospective observational study. BJOG 2014; 121: 876–88. 10.1111/1471-0528.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guildelines NC. Intrapartum Care: Care of healthy women and their babies during childbirth. NICE clinical guideline 55 2007. [Google Scholar]
- 10.Altman DG, Chitty LS. Charts of fetal size: 1. Methodology. BJOG. 1994; 101(1): 29–34. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Boys RJ, Hunter S, Dunlop W. Maternal hemodynamics after normal delivery and delivery complicated by postpartum hemorrhage. Obstet Gynecol. 1989; 74(2): 234–9. [PubMed] [Google Scholar]
- 12.Robson S, Dunlop W, Hunter S. Haemodynamic changes during the early puerperium. BMJ. 1987; 294(6579): 1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990; 76(6): 1061–9. [PubMed] [Google Scholar]
- 14.Grindheim G, Estensen ME, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012; 30(2): 342–50. 10.1097/HJH.0b013e32834f0b1c [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation. WHO recommendations on Postnatal care of the mother and newborn. 2013. [PubMed]
- 16.Singh S, McGlennan A, England A, Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS)*. Anaesthesia. 2012; 67(1): 12–8. 10.1111/j.1365-2044.2011.06896.x [DOI] [PubMed] [Google Scholar]
- 17.Schorn MN. Measurement of blood loss: review of the literature. Journal of Midwifery Womens Health. 2010; 55(1): 20–7. [DOI] [PubMed] [Google Scholar]
- 18.Allgower M BC. Shock Index. Dtsch Med Wochenschr. 1967; 92(43): 1947–50. 10.1055/s-0028-1106070 [DOI] [PubMed] [Google Scholar]
- 19.Rady MY, Nightingale P, Little RA, Edwards JD. Shock index: a re-evaluation in acute circulatory failure. Resuscitation. 1992; 23(3): 227–34. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y-c, Liu J-h, Fang ZA, Shan G-l, Xu J, Qi Z-w, et al. Modified shock index and mortality rate of emergency patients. World J Emerg Med. 2012; 3(2): 114–7. 10.5847/wjem.j.1920-8642.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rady MY, Smithline HA, Blake H, Nowak R, Rivers E. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Annals emerg med. 1994; 24(4): 685–90. [DOI] [PubMed] [Google Scholar]
- 22.Paladino L, Subramanian RA, Nabors S, Sinert R. The utility of shock index in differentiating major from minor injury. Eur J Emerg Med. 2011; 18(2): 94–8. 10.1097/MEJ.0b013e32833f212b [DOI] [PubMed] [Google Scholar]
- 23.Cancio LC, Wade CE, West SA, Holcomb JB. Prediction of mortality and of the need for massive transfusion in casualties arriving at combat support hospitals in Iraq. J Trauma. 2008; 64(2 Suppl): S51–5. 10.1097/TA.0b013e3181608c21 [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Reisner AT, McKenna TM, Gribok A, Reifman J. Diagnosis of hemorrhage in a prehospital trauma population using linear and nonlinear multiparameter analysis of vital signs. Conf Proc IEEE Eng Med Biol Soc. 2007: 3748–51. 10.1109/IEMBS.2007.4353147 [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara A, Kimura A, Kato H, Mizushima Y, Matsuoka T, Takeda M, et al. Hemodynamic reactions in patients with hemorrhagic shock from blunt trauma after initial fluid therapy. J Trauma. 2010; 69(5): 1161–8. 10.1097/TA.0b013e3181d27c94 [DOI] [PubMed] [Google Scholar]
- 26.Vandromme MJ, Griffin RL, Kerby JD, McGwin G Jr., Rue LW 3rd, Weinberg JA. Identifying risk for massive transfusion in the relatively normotensive patient: utility of the prehospital shock index. J Trauma. 2011; 70(2): 384–8. 10.1097/TA.0b013e3182095a0a [DOI] [PubMed] [Google Scholar]
- 27.Cannon CM, Braxton CC, Kling-Smith M, Mahnken JD, Carlton E, Moncure M. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma and Acute Care Surg. 2009; 67(6): 1426–30. [DOI] [PubMed] [Google Scholar]
- 28.Baron BJ, Sinert R, Zehtabchi S, Stavile KL, Scalea TM. Diagnostic utility of sublingual PCO2 for detecting hemorrhage in penetrating trauma patients. J Trauma Acute Care Surg. 2004; 57(1): 69–74. [DOI] [PubMed] [Google Scholar]
- 29.Asaari H. Value of shock index in prognosticating the short term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012; 67(4): 407. [PubMed] [Google Scholar]
- 30.Rady MY, Rivers EP, Martin GB, Smithline H, Appelton T, Nowak RM. Continuous central venous oximetry and shock index in the emergency department: use in the evaluation of clinical shock. Am J Emerg Med. 1992; 10(6): 538–41. [DOI] [PubMed] [Google Scholar]
- 31.Pacagnella RC, Souza JP, Durocher J, Perel P, Blum J, Winikoff B, et al. A systematic review of the relationship between blood loss and clinical signs. PLoS One. 2013; 8(3): e57594 10.1371/journal.pone.0057594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaramillo S, Barnhart K, Takacs P. Use of the shock index to predict ruptured ectopic pregnancies. Int J Gynecol Obstet. 2011; 112(1): 68. [DOI] [PubMed] [Google Scholar]
- 33.Birkhahn RH, Gaeta TJ, Bei R, Bove JJ. Shock index in the first trimester of pregnancy and its relationship to ruptured ectopic pregnancy. Acad emerg med. 2002; 9(2): 115–9. [DOI] [PubMed] [Google Scholar]
- 34.Le Bas A, Chandraharan E, Addei A, Arulkumaran S. Use of the “obstetric shock index” as an adjunct in identifying significant blood loss in patients with massive postpartum hemorrhage. Int J Gynecol Obstet. 2013; 124: 253–5. [DOI] [PubMed] [Google Scholar]
- 35.Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol. 2005; 98(2): 179–89. 10.1016/j.ijcard.2003.10.028 [DOI] [PubMed] [Google Scholar]
- 36.Mhyre JM, D'Oria R, Hameed AB, Lappen JR, Holley SL, Hunter SK, et al. The maternal early warning criteria: a proposal from the national partnership for maternal safety. J Obstet Gynecol Neonatal Nurs. 2014; 43(6): 771–9. 10.1111/1552-6909.12504 [DOI] [PubMed] [Google Scholar]
- 37.Schoon M, editor Proposed colour banded early warning observation charts for South Africa: review. Obstetrics and Gynaecology Forum; 2012: Sabinet Online.
- 38.Dyer R, Van Dyk D, Dresner A. The use of uterotonic drugs during caesarean section. Int J obstet anesthes. 2010; 19(3): 313–9. [DOI] [PubMed] [Google Scholar]
- 39.Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anesthesiol. 2011; 24(3): 255–61. [DOI] [PubMed] [Google Scholar]
- 40.Holte K, Foss NB, Svensén C, Lund C, Madsen JL, Kehlet H. Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology. 2004; 100(2): 281–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This data is ethically restricted. At the time of gaining consent for the primary study, which this secondary analysis is based upon, consent did not include explicit approval of data sharing of this nature. The South East Multicentre Research Ethics Committee (07/H1102/79) approved the study. We are therefore unable to allow our minimal dataset to be freely available online. We are able to share anonymised data with researchers who contact us directly, at annette.l.briley@kcl.ac.uk.