Abstract
Importance and Objective
Clinical trials testing treatments for Alzheimer’s disease (AD) are increasingly focused on cognitively normal individuals in the preclinical phase of the disease. To optimize observing a treatment effect, such trials need to enroll cognitively normal individuals likely to show cognitive decline over the duration of the trial. The goal of the current study was to identify which group of cognitively normal individuals showed the greatest cognitive decline over time based on their cerebrospinal fluid (CSF) biomarker profile.
Design, Setting, and Participants
Cognitively normal participants (n=222, mean follow-up 11 years, follow-up range=0–18 years, mean baseline age=57 years, range=22–85 years) were classified into one of four hypothetical preclinical AD groups using baseline CSF levels of amyloid-beta and tau, or amyloid-beta and phosphorylated tau (p-tau): Stage 0 (high abeta/low tau), Stage 1 (low abeta/low tau), Stage 2 (low abeta/high tau), and suspected non-AD pathology, SNAP (high abeta/high tau).
Main Outcome Measure
An a-priori cognitive composite score based on four tests previously shown to predict progression from normal cognition to symptom onset of mild cognitive impairment or dementia: Paired Associates immediate recall, Logical Memory delayed recall, Boston Naming, and Digit-Symbol Substitution. Linear mixed effects models were used to compare the cognitive composite scores across the four groups over time, adjusting for baseline age, gender, education and their interactions with time.
Results
Individuals in Stage 2 (low abeta and high tau (or p-tau)) showed lower baseline cognitive scores and greater decline in the cognitive composite score relative to the other three groups (all p<=0.001). Subjects in Stage 0, 1, and SNAP did not differ from one another in cognitive performance at baseline or over time (11 years) and showed practice-related improvement in performance. APOE-ε4 genotype was not associated with baseline or rate of change in the cognitive score.
Conclusions and Relevance
These results suggest that in order to optimize observing a treatment effect, clinical trials enrolling cognitively normal individuals should selectively recruit participants with abnormal levels of both amyloid and tau (i.e., Stage 2), as this group would be expected to show the greatest cognitive decline over time if untreated.
Current evidence suggests that the neuropathological processes associated with Alzheimer’s disease (AD) begin a decade or more before the emergence of obvious cognitive impairment.1 This preclinical phase of the disease is currently the focus of clinical trials, as it is hypothesized that disease-modifying therapies are likely to be most successful when administered before the initial symptomatic phase, known as Mild Cognitive Impairment (MCI), in which there is substantial synaptic and neuronal damage2–4. The primary goal of the current study was to examine which cognitively normal individuals with evidence of AD pathology are most likely to demonstrate cognitive decline over time. This information would have important implications for determining subject selection criteria for clinical trials, since the rate of cognitive change over time must be sufficient to permit seeing a drug effect, if one is present.
We tested whether individuals with differing biomarker profiles show different cognitive trajectories over time, as would be predicted by the hypothetical staging model of preclinical AD laid out by the Preclinical AD Workgroup sponsored by the National Institute on Aging and Alzheimer’s Association (NIA/AA).1 This model proposes that the preclinical phase of AD can be subdivided into three successive stages. Stage 1 is characterized by amyloid pathology, but the absence of tau-related neurodegeneration. During Stage 2, both amyloid pathology and tau-related neurodegeneration are evident. Finally, during Stage 3, subtle cognitive decline becomes detectable in addition to amyloid and tau pathology. Individuals with normal measures of both amyloid and neurodegeneration are classified as Stage 0. Additionally, it has been proposed that individuals with evidence of neurodegeneration but normal levels of amyloid might be classified as having suspected non-Alzheimer pathology (SNAP).5
The subjects in this study were part of a longitudinal cohort of individuals with normal cognition when first assessed. We used baseline cerebrospinal fluid (CSF) measures of amyloid (abeta1–42), total tau, and phosphorylated tau (p-tau) to classify individuals into the hypothetical stages of preclinical AD and SNAP. These CSF biomarkers are particularly useful in addressing the goals of the study because they directly reflect the levels of abnormal brain proteins associated with the AD pathology, i.e., plaques and tangles.6, 7.
To our knowledge, only one prior study has examined the combined effects of CSF measures of amyloid and tau on cognitive change among individuals who were cognitively normal at baseline.8 Vos et al. (2013) reported a greater decline on the Mini-Mental State Exam (MMSE) over an average of 3.9 years among individuals with evidence of both amyloid and tau pathology (Stage 2) compared to individuals classified as Stages 0, 1, and SNAP. The only other two studies to investigate the combined effects of amyloid and neuronal injury on cognitive change used imaging-based biomarkers, such as MRI, which do not provide a direct measure of tau-related neurofibrillary tangle pathology. These studies reported similar results as Vos et al. (2013), but the mean follow-up period was limited to 2–4 years.9, 10
The availability of CSF at baseline, when the subjects were cognitively normal, the extensive cognitive testing, and the unusually long duration of follow-up (mean=11 years) allowed us to examine several questions of particular relevance to clinical trials in preclinical AD. First, the present study used a cognitive composite score covering multiple domains of cognition as the outcome, allowing us to determine whether prior findings regarding the MMSE generalized to a broader range of cognitive domains and to tests likely to be more sensitive to subtle cognitive change. Second, most prior studies that have examined rates of cognitive change among cognitively normal individuals have been of short duration (mean follow-up 1–4 years),8–10 have not included measures of tau pathology,11–14 or did not examine possible interactions between amyloid and tau on the rate of change in cognition.15–20 Third, it remains unclear whether the major genetic risk factor for AD, the apolipoprotein (APOE) ε4 genotype,21 modulates the associations between amyloid, tau, p-tau and cognitive change. This may be highly relevant for the selection of subjects in clinical trials, since subgroups with differing rates of decline would make it more challenging to identify drug effects.
Methods
Study Design
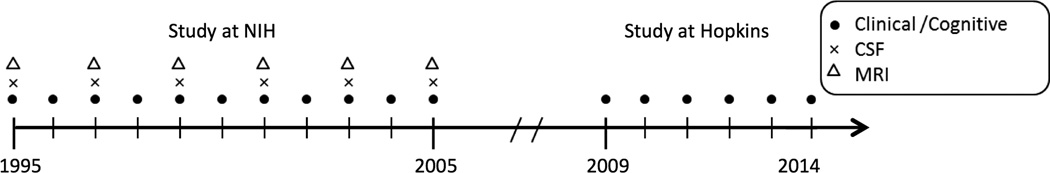
The parent study from which these data are derived is known as the BIOCARD study, which was initiated at the National Institutes of Health (NIH) in 1995. By design, approximately 75% of the participants had a first degree relative with dementia of the Alzheimer type. The study was stopped in 2005 for administrative reasons and re-established at Johns Hopkins in 2009. While at the NIH, subjects were administered a comprehensive neuropsychological battery annually. MRI scans, cerebrospinal fluid, and blood specimens were obtained approximately every two years. See Figure 1 for a schematic representation of the study design.
Figure 1. Timeline showing the design of the BIOCARD study.
Legend: Types of data collected each year for the BIOCARD study between 1995 and 2014.
Selection of Participants
Recruitment was conducted by the staff of the Geriatric Psychiatry branch of the intramural program of the National Institute of Mental Health. At baseline, all participants completed a comprehensive evaluation at the NIH, consisting of a physical and neurological examination, an electrocardiogram, standard laboratory studies, and neuropsychological testing. Individuals were excluded from participation if they were cognitively impaired, or had significant medical problems such as severe cerebrovascular disease, epilepsy or alcohol or drug abuse. See eMethods-1 for details regarding the selection of participants.
A total of 349 individuals were initially enrolled in the study, after providing written informed consent. The analyses presented here are based on 222 participants of the 335 participants who provided baseline CSF (see eMethods-2 for reasons for exclusion of subjects from analyses).
Clinical and Cognitive Assessment of Participants
A cognitive and clinical assessment and a consensus diagnosis were completed annually at the NIH and at Johns Hopkins (see Albert et al., 2014 for further details).22 Each participant included in our analyses received a consensus diagnosis by the staff of the Johns Hopkins BIOCARD Clinical Core. Each case was handled in a similar manner: (1) clinical data pertaining to the medical, neurologic and psychiatric status of the subject were examined, (2) reports of changes in cognition by the subject and by collateral sources were reviewed, and (3) decline in cognitive performance, based on review of longitudinal testing from multiple domains, was established. We followed the diagnostic recommendations incorporated in the NIA/AA working group reports for the diagnosis of MCI23 and dementia due to AD.24 See eMethods-3 for additional details. The clinical diagnoses were blinded to CSF assessments.
The main outcome variable was an a-priori derived global cognitive composite score based on four individual measures that were identified previously to be the best combination of cognitive predictors of time to progress from normal cognition to clinical symptom onset.22 These measures were the: (1) Logical Memory Delayed Recall (Story A) score of the Wechsler Memory Scale – Revised (WMS-R); (2) Verbal Paired Associates – Immediate subtest of the WMS-R; (3) Digit Symbol Test from the Wechsler Adult Intelligence Scale – Revised; (4) Boston Naming Test. These measures were administered annually at the NIH and are part of the annual neuropsychological battery at Johns Hopkins. To calculate the cognitive composite score, the individual measures were transformed to z-scores and then averaged, with the requirement that at least two of the four scores were present at a given time point. eFigure-1 shows a histogram of baseline scores.
CSF Assessments
The CSF specimens were analyzed using the same protocol employed in the Alzheimer’s Disease Neuroimaging Initiative. This protocol employed the xMAP-based AlzBio3 kit [Innogenetics] run on the Bioplex 200 system. Each subject had all samples (run in triplicate) analyzed on the same plate (see eMethods-4 and eFigure-2 for details regarding the CSF assay and baseline biomarker frequency distributions; additional details have been published elsewhere25).
APOE Genotyping and Coding
APOE genotype was established in all but one of the cohort participants (n=348). Genotypes were determined by restriction endonuclease digestion of polymerase chain reaction amplified genomic DNA (performed by Athena Diagnostics, Worcester, MA). APOE ε4 carrier status was coded by an indicator variable, with ε4 carriers coded as 1 if they had at least one ε4 allele and non-carriers coded as 0. Analyses that included APOE carrier status excluded individuals with the ε2/ε4 genotype because the ε4 allele increases AD dementia risk,21 whereas the ε2 allele decreases AD dementia risk.26
Statistical Methods
Based on the observation that about one-third of cognitively normal older adults have AD pathology in their brains, as indicated by amyloid imaging27–29 and neuropathologic studies30–32, biomarker abnormality was defined as having CSF abeta1–42 levels in the lower one-third of the distribution of participants (<374.5 pg/mL), or having tau (>74.9 pg/mL) or p-tau (>39.4 pg/mL) levels in the upper one-third of the distribution. The resulting proportion of individuals in the hypothetical preclinical AD groups (i.e., Stages 0, 1, and 2) was comparable to that reported in the literature.5 The pattern of results was similar when using a median split (data not shown) or quintile split (supplementary eTable-1, eTable-2) to classify individuals into groups, suggesting robustness to cut-point variations.
The data were analyzed using general linear mixed regression models, including linear effects of time, to test if the rate of change in cognition differed across the groups. Two main analyses were performed, one using CSF abeta1–42 and tau to classify individuals into the four groups, the other using CSF abeta1–42 and p-tau for classification. Group status was coded using binary predictors (0 or 1) for each group. The following predictors were included in both models, treating Stage 0 as the implicit baseline: baseline age, gender, years of education, time, Stage 1 indicator, Stage 2 indicator, SNAP indicator, and the interaction (cross-product) of each predictor with time. In these models, the stage indicator×time interaction terms test if the rate of change in the cognitive composite score differs between Stage 0 and the other stages. The outcome variable in all analyses was the cognitive composite score (including baseline and all available follow-up scores, as defined above). Models were specified with a random intercept and slope.
To examine the role of APOE-ε4 genotype on cognitive change, both models were re-run, including the indicator for APOE-ε4, and the APOE-ε4 genotype×time interaction term. Additionally, to test if the cognitive trajectories within a given stage differ by APOE-ε4 genotype, four mixed-effects models were run, one for each group, with the following predictors: baseline age, gender, education, APOE-ε4 indicator, time, baseline age×time interaction, and APOE-ε4 indicator×time interaction. (The gender×time and education×time interactions were not included because they were not significant in any previous analysis).
Differences in baseline characteristics of participants in Stage 0 compared to the other three groups were assessed using two-tailed t-tests or chi-square tests, with a significance level of p<.05, uncorrected for multiple comparisons. All data analyses used R, version 3.2.1.
Results
Baseline characteristics for the BIOCARD cohort and for subjects in the analyses are shown in Table 1. Table 2 shows baseline characteristics separately for the four groups (Stage 0, 1, 2, and SNAP). The groups did not differ in education, gender, or MMSE at baseline. However, compared to Stage 0, individuals in Stage 2 were older, were more likely to be APOE-ε4 carriers, had lower baseline cognitive composite scores, and were more likely to progress to MCI or AD-dementia (Table 2, eTable-2). Individuals in Stage 0 had more follow-up cognitive testing than the other groups.
Table 1.
| Participant Characteristics at Baseline | Cohort as a whole |
Subjects in analyses |
|---|---|---|
| Variable | (N = 349) | (N=222) |
| Age, mean number of years (SD) | 57.3 (10.4) | 56.9 (10.1) |
| Age, range (min, max) | 20.0 – 85.8 | 22.1 – 85.8 |
| Follow-up time, mean number of years (SD) | 10.9 (4.6) | 11.0 (4.1) |
| Follow-up time, range (years) | 0 – 18.7 | 0 – 18.3 |
| Gender, females (%) | 57.6% | 59.9% |
| Ethnicity, Caucasians (%) | 97.1% | 97.3% |
| ApoE ε4 carriers (%) | 33.6% | 32.9% |
| MMSE, mean score (SD) | 29.5 (0.9) | 29.5 (0.8) |
| Education, mean years (SD) | 17.0 (2.4) | 17.2 (2.3) |
| Education, range (years) | 12 – 20 | 12 – 20 |
| Paired Associates Immediate (SD) | 20.2 (3.4) | 20.1 (3.4) |
| Logical Memory Delayed (SD) | 12.3 (4.0) | 14.8 (4.1) |
| Boston Naming, % Correct (SD) | 96.0 (5.3) | 95.9 (5.3) |
| Digit Symbol Substitution (SD) | 52.2 (11.7) | 55.0 (12.6) |
| Cognitive Composite, mean (SD) | −0.10 (0.6) | −0.05 (0.6) |
Table 2.
Baseline characteristics of participants in each of the four preclinical AD groups
| Using baseline CSF abeta1–42 and total tau to define group membership | ||||
|---|---|---|---|---|
| Baseline Participant Characteristics | Stage 0 | Stage1 | Stage 2 | SNAP |
| Variable | (N=102) | (N=46) | (N=28) | (n=46) |
| Age, mean number of years (SD) | 54.8 (10.3) | 56.8 (8.1) | 63.6 (9.9)** | 57.6 (9.9) |
| Follow-up time, mean years (SD) | 12.0 (3.5) | 10.0 (3.6)** | 8.6 (5.2)** | 11.2 (4.3) |
| Gender, females (%) | 60.8% | 58.7% | 57.1% | 60.9% |
| Ethnicity, Caucasians (%) | 96.1% | 97.8% | 100.0%* | 97.8% |
| ApoE ε4 carriers (%) | 23.5% | 34.8% | 50.0%* | 41.3* |
| MMSE, mean score (SD) | 29.6 (0.8) | 29.4 (0.9) | 29.6 (0.8) | 29.5 (0.8) |
| Education, mean years (SD) | 17.2 (2.4) | 16.9 (2.4) | 17.1 (2.1) | 17.2 (2.2) |
| Paired Associates Immediate (SD) | 20.3 (2.9) | 20.0 (3.4) | 20.3 (2.8) | 20.7 (2.9) |
| Logical Memory Delayed (SD) | 13.5 (3.8) | 13.0 (3.9) | 10.8 (4.5)* | 12.8 (4.1) |
| Boston Naming, % Correct (SD) | 96.1 (5.9) | 96.4 (5.7) | 94.8 (6.0) | 95.1 (6.5) |
| Digit Symbol Substitution (SD) | 54.5 (11.6) | 52.2 (12.5) | 47.9 (13.0)* | 53.4 (11.9) |
| Cognitive Composite, mean (SD) | 0.03 (0.6) | −0.06 (0.7) | −0.32 (0.6)* | −0.05 (0.6) |
| CSF abeta1–42 in pg/mL (SD) | 447.7 (51.7) | 315.3 (41.2)** | 250.8 (71.8)** | 476.9 (57.2) |
| CSF p-tau181 in pg/mL (SD) | 30.7 (8.7) | 26.6 (8.3) | 60.5 (22.7)** | 42.5 (13.5)** |
| CSF total tau in pg/mL (SD) | 56.8 (11.1) | 45.4 (13.3) | 109.6 (31.7)** | 95.8 (30.0)** |
| Using baseline CSF abeta1–42 and p-tau181 to define group membership | ||||
| Variable | (N=102) | (N=46) | (N=28) | (n=46) |
| Age, mean number of years (SD) | 56.0 (9.9) | 57.1 (8.5) | 63.1 (9.6)** | 55.1 (11.1) |
| Follow-up time, mean years (SD) | 12.4 (3.9) | 10.2 (3.9)** | 8.3 (4.7)** | 10.2 (3.1)** |
| Gender, females (%) | 58.8% | 54.3% | 64.3% | 65.2% |
| Ethnicity, Caucasians (%) | 96.1% | 97.8% | 100.0%* | 97.8% |
| ApoE ε4 carriers (%) | 25.5% | 34.8% | 50.0%* | 37.0% |
| MMSE, mean score (SD) | 29.5 (0.8) | 29.5 (0.9) | 29.6 (0.7) | 29.7 (0.7) |
| Education, mean years (SD) | 17.3 (2.4) | 17.1 (2.4) | 16.8 (2.2) | 17.0 (2.2) |
| Paired Associates Immediate (SD) | 20.4 (2.9) | 20.0 (3.5) | 20.3 (2.5) | 20.5 (2.9) |
| Logical Memory Delayed (SD) | 13.3 (3.9) | 13.0 (4.0) | 10.8 (4.3)* | 13.3 (4.0) |
| Boston Naming, % Correct (SD) | 95.9 (6.3) | 96.0 (6.2) | 95.5 (5.3) | 95.5 (5.7) |
| Digit Symbol Substitution (SD) | 54.1 (11.9) | 53.1 (12.9) | 46.5 (11.8)* | 54.4 (11.3) |
| Cognitive Composite, mean (SD) | 0.00 (0.6) | −0.06 (0.7) | −0.32 (0.6)* | 0.00 (0.6) |
| CSF abeta1–42 in pg/mL (SD) | 454.2 (57.2) | 308.1 (51.8)** | 262.7 (69.7)** | 462.5 (50.0) |
| CSF p-tau181 in pg/mL (SD) | 28.6 (7.2) | 25.6 (6.9) | 62.2 (20.7)** | 47.1 (9.8)** |
| CSF total tau in pg/mL (SD) | 61.9 (20.3) | 47.1 (16.7) | 106.9 (34.5)** | 84.7 (30.9)** |
Significant differences between Stages 1, 2, and SNAP relative to Stage 0 are indicated by asterisks: * p < 0.05; ** p < 0.005.
The results from the mixed effects models comparing the cognitive trajectories of individuals in Stage 0 to the other groups are shown in Table 3. The results were nearly identical whether CSF abeta1–42 and tau or CSF abeta1–42 and p-tau were used to define group membership. In both models, there was a main effect of time (reflecting practice-related improvement in cognitive performance over time), a main effect of age, and an age×time interaction (signifying lower cognitive performance and less improvement in performance over time with increasing age). Higher education was associated with better cognitive performance, but did not alter the rate of cognitive change over time.
Table 3.
Results of linear mixed effects models
| Groups defined by CSF abeta and p-tau |
Groups defined by CSF abeta and total tau |
|||||
|---|---|---|---|---|---|---|
| Model Predictors | Estimate | SE | p-value | Estimate | SE | p-value |
| Time | 0.0905 | 0.0318 | 0.0050 | 0.0915 | 0.0324 | 0.0053 |
| Baseline age | −0.0158 | 0.0043 | 0.0003 | −0.0157 | 0.0043 | 0.0003 |
| Gender (male) | −0.2590 | 0.0779 | 0.0010 | −0.2508 | 0.0777 | 0.0015 |
| Education | 0.0466 | 0.0164 | 0.0051 | 0.0489 | 0.0164 | 0.0032 |
| Stage 1 | −0.0579 | 0.0977 | 0.5539 | −0.0426 | 0.0987 | 0.6665 |
| Stage 2 | −0.2613 | 0.1211 | 0.0320 | −0.2288 | 0.1214 | 0.0609 |
| SNAP | −0.0755 | 0.0999 | 0.4511 | 0.0068 | 0.0980 | 0.9444 |
| Baseline age × time | −0.0017 | 0.0004 | <0.0001 | −0.0018 | 0.0004 | <0.0001 |
| Gender × time | −0.0029 | 0.0068 | 0.6657 | −0.0019 | 0.0068 | 0.7778 |
| Education × time | 0.0011 | 0.0014 | 0.4465 | 0.0014 | 0.0015 | 0.3536 |
| Stage 1 × time | 0.0009 | 0.0085 | 0.9179 | −0.0048 | 0.0088 | 0.5825 |
| Stage 2 × time | −0.0526 | 0.0116 | <0.0001 | −0.0494 | 0.0116 | <0.0001 |
| SNAP × time | 0.0028 | 0.0087 | 0.7508 | −0.0090 | 0.0084 | 0.2844 |
Note: Stage 0 was used as the implicit baseline in these models. Thus, estimates for Stage 1, 2, and SNAP and their interactions with time reflect differences relative to Stage 0.
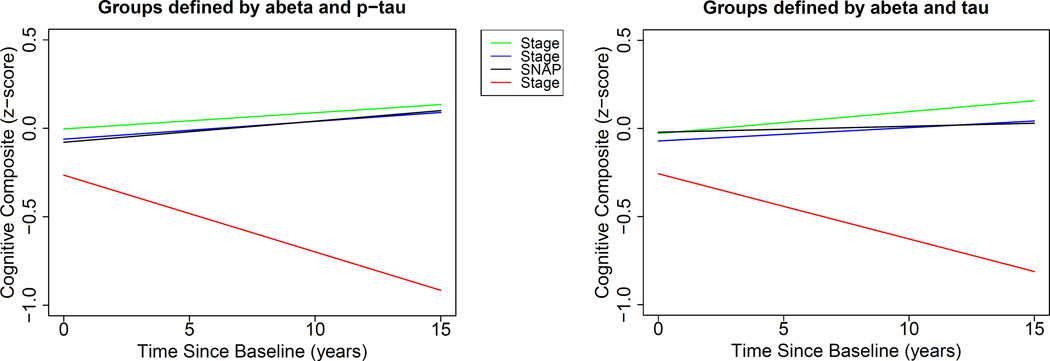
Importantly, the main effects of Stage 1 and SNAP were not significant, nor were the interactions between Stage 1×time and SNAP×time. This suggests that there was no difference in either the mean or the rate of change in the cognitive composite score over time between the Stage 0 group and the Stage 1 and SNAP groups. In contrast, the Stage 2×time interactions were highly significant, indicating a more negative rate of change in cognition for the Stage 2 group compared to the Stage 0 group (p<0.0001). Mean cognitive performance was also lower in the Stage 2 group compared to the Stage 0 group. These results are presented graphically in Figure 2. Post-hoc mixed effects models directly comparing Stage 1 and SNAP to Stage 2 revealed a more negative rate of change in cognition for individuals in Stage 2 compared to Stage 1 (estimate=-0.074, SE=0.018, p=0.0002 using p-tau and estimate=-0.068, SE=0.019, p=0.0001 using tau) and compared to SNAP (estimate=-0.074, SE=0.018, p=0.0002 for p-tau; and estimate=-0.06, SE=0.017, p=0.001 using tau). Results were similar using the individual cognitive measures as outcomes.
Figure 2. Estimates of longitudinal cognitive change for the four hypothetical preclinical AD groups.
Legend: Estimates from linear mixed effects models predicting longitudinal cognitive composite scores over time among individuals classified into the four preclinical AD groups (Stage 0, 1, 2, and SNAP) using baseline CSF abeta1–42 and p-tau (left) or abeta1–42 and total tau (right) for classification. The estimates are adjusted for baseline age, gender, education, and their interactions with time. Stage 2 showed a greater decline and lower baseline scores than the other groups, which did not differ from one another (see Table 3).
The analyses were repeated including APOE-ε4 and the APOE-ε4×time interaction term, but the results were unchanged and effects involving APOE-ε4 were non-significant (all p>0.4). Likewise, separate models for individuals in each stage showed no differences in the cognitive trajectories between APOE-ε4 carriers and non-carriers.
Discussion
This study compared the cognitive trajectories of individuals with different CSF AD-biomarker profiles and normal cognition at baseline within the framework of four hypothetical groupings related to preclinical AD.1, 5 There was no difference in baseline cognitive performance or the rate of change in cognitive performance over an average of 11 years among individuals in Stage 1 (low levels of abeta) or SNAP (high levels of tau/p-tau) compared to those in Stage 0 (normal levels of both abeta and tau/p-tau). By comparison, individuals in Stage 2 (both low levels of abeta and high levels of tau/p-tau) showed lower cognitive performance at baseline and a more negative rate of change in cognition than the other three groups. Taken together, these results suggest that abnormal levels of both amyloid and tau are necessary for observing a marked decline in cognition among cognitively normal individuals.
These findings have important implications for the design of clinical trials aimed at individuals in the preclinical phase of AD. Our results suggest that in order to optimize observing a treatment effect, clinical trials enrolling cognitively normal individuals should selectively recruit participants with abnormal levels of both amyloid and tau (i.e., Stage 2), as this group would be expected to show the greatest cognitive decline over time if untreated. If participants are selected solely on the basis of their amyloid status (such as in the A4 study33), then the ability to observe a significant treatment effect on cognition might be greatly diminished because a large proportion of untreated participants (those with abnormal amyloid but normal tau levels) would not be expected to show meaningful cognitive decline over the relatively short time frame of a clinical trial. Our findings also suggest that while APOE-ε4 carriers may be more likely to be further along the AD trajectory and therefore have an earlier age of onset,12 the cognitive trajectories do not differ by ε4 carrier status after accounting for CSF amyloid and tau/p-tau levels. Though we do not have data regarding the effectiveness of anti-amyloid drugs in reducing cognitive decline, our results suggest that to the extent that amyloid and tau pathology arise independently and cognitive decline simply depends on their co-occurrence,34–36 anti-amyloid therapies may be effective in individuals with concurrent amyloid and tau pathology, and in those with amyloid pathology only who may subsequently develop tau pathology. However, if amyloid accumulation initiates a downstream cascade of tau-related neurodegeneration that becomes increasingly independent of amyloid itself,37 then anti-amyloid agents may only be effective if administered prior to the onset of the neurodegenerative process.
The present results are consistent with prior short-term longitudinal studies reporting a disproportionately greater rate of cognitive decline for individuals classified as Stage 2 compared to Stages 0, 1, and SNAP using CSF biomarkers8 or neuroimaging-based biomarkers9, 10. The study expands on prior findings in several ways. First, our cognitive outcome measure is clinically validated in the sense that it is based on neuropsychological tests previously shown to predict progression from normal cognition to MCI or dementia due to AD.22 Both the baseline score and the rate of change in the measures that compose our composite score are associated with the time to onset of clinical symptoms, suggesting that these types of measures are useful for tracking AD progression in clinical trials. Second, our results demonstrate that the pattern of short-term cognitive trajectories observed previously remains stable over the course of a decade. Third, we found that although APOE-ε4 carriers were over-represented among individuals classified as Stage 2, APOE-ε4 genotype did not modify the rate of change in cognition. Taken together, these two findings suggest that the APOE-ε4 allele does not significantly alter the rate of AD progression, but is associated with an earlier age of onset of AD.38, 39 Fourth, higher education was associated with better cognitive performance, after accounting for baseline CSF levels, but did not modify the rate of change in cognition. This supports the view that education reduces the impact of AD neuropathology on cognition, but does not alter the rate of disease progression.40, 41 Lastly, the current results point toward the utility of CSF biomarkers in identifying individuals at risk for cognitive decline at a significantly younger age (mean baseline age for Stage 2 = 63 years) than what has been reported by previous studies, which focused on individuals in their 70s at baseline.
Our study has several limitations. The participants are well educated, primarily Caucasian, primarily middle-aged at baseline, and the majority had a family history of dementia, so the results may not generalize to the population at large or to older cohorts. Additionally, the sample size may have been too small to detect differences by APOE-ε4 genotype. Future studies are necessary to determine if similar findings would be obtained using imaging-based biomarkers of amyloid and tau.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146, and T32-AG027668). The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Guy McKhann, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D’Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O’Brien, Abby Spangler); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Matt Toepfner, Lauren Parlett, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Qing Cai, Daisy Lu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs John Cernansky, David Holtzman, David Knopman, Walter Kukull, and John McArdle, and Drs Neil Buckholtz, John Hsiao, Laurie Ryan, and Jovier Evans, who provide oversight on behalf of the National Institute on Aging and the National Institute of Mental Health (NIMH), respectively. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Dr. Soldan had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures:
Dr. Soldan reports no disclosures.
Dr. Pettigrew reports no disclosures.
Ms. Cai reports no disclosures.
Dr. Wang reports no disclosures.
Dr. Moghekar reports no disclosures.
Dr. O’Brien reports no disclosures.
Dr. Albert is an advisor to Eli Lilly.
Author Contributions:
Dr. Soldan took part in the design and conceptualization of the study, supervised the analysis of the data, participated in the interpretation of the analyses, drafted and revised the manuscript and takes overall responsibility for the data and the manuscript.
Dr. Pettigrew took part in the design and conceptualization of the study, participated in the interpretation of the data, and critically reviewed the manuscript.
Ms. Cai performed the statistical analyses, participated in the interpretation of the data, and critically reviewed the manuscript.
Dr. Wang supervised the statistical analyses, the interpretation of the data and critically reviewed the manuscript.
Dr. Moghekar supervised the analysis of the cerebrospinal fluid and critically reviewed the manuscript.
Dr. O’Brien supervised the analysis of the cerebrospinal fluid and critically reviewed the manuscript.
Dr. Albert took part in the design and conceptualization of the study, obtained funding, participated in the interpretation of the analyses, and critically reviewed the manuscript.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desikan RS, Cabral HJ, Hess CP, et al. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain : a journal of neurology. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grothe MJ, Heinsen H, Amaro E, Jr, Grinberg LT, Teipel SJ. Cognitive Correlates of Basal Forebrain Atrophy and Associated Cortical Hypometabolism in Mild Cognitive Impairment. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagani M, De Carli F, Morbelli S, et al. Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer's disease from healthy controls. A European Alzheimer's Disease Consortium (EADC) study. NeuroImage Clinical. 2015;7:34–42. doi: 10.1016/j.nicl.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtzman DM. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiology of aging. 2011;32(Suppl 1):S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of neurology. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 8.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. The Lancet Neurology. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA neurology. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement. 2013;9:687–698. e681. doi: 10.1016/j.jalz.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doraiswamy PM, Sperling RA, Johnson K, et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Molecular psychiatry. 2014;19:1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA neurology. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain : a journal of neurology. 2015;138:761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roe CM, Fagan AM, Grant EA, Holtzman DM, Morris JC. CSF biomarkers of Alzheimer disease: "noncognitive" outcomes. Neurology. 2013;81:2028–2031. doi: 10.1212/01.wnl.0000436940.78152.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Millard SP, Peskind ER, et al. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA neurology. 2014;71:742–751. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glodzik L, de Santi S, Tsui WH, et al. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of aging. 2011;32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aschenbrenner AJ, Balota DA, Fagan AM, Duchek JM, Benzinger TL, Morris JC. Alzheimer Disease Cerebrospinal Fluid Biomarkers Moderate Baseline Differences and Predict Longitudinal Change in Attentional Control and Episodic Memory Composites in the Adult Children Study. Journal of the International Neuropsychological Society : JINS. 2015;21:573–583. doi: 10.1017/S1355617715000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Insel PS, Mattsson N, Mackin RS, et al. Biomarkers and cognitive endpoints to optimize trials in Alzheimer's disease. Annals of clinical and translational neurology. 2015;2:534–547. doi: 10.1002/acn3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 22.Albert M, Soldan A, Gottesman R, et al. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Current Alzheimer research. 2014;11:773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghekar A, Goh J, Li M, Albert M, O'Brien RJ. Cerebrospinal fluid Abeta and tau level fluctuation in an older clinical cohort. Archives of neurology. 2012;69:246–250. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of neurology. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 31.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in "normal" aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. Journal of neuropathology and experimental neurology. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. Journal of neuropathology and experimental neurology. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 33.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Science translational medicine. 2014;6:228fs213. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81:1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Annals of neurology. 2013;73:472–480. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Archives of neurology. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 38.Resnick SM, Bilgel M, Moghekar A, et al. Changes in Abeta biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiology of aging. 2015;36:2333–2339. doi: 10.1016/j.neurobiolaging.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: The Cache County Study. Archives of general psychiatry. 2004;61:518–524. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- 40.Roe CM, Fagan AM, Grant EA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Archives of neurology. 2011;68:1145–1151. doi: 10.1001/archneurol.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soldan A, Pettigrew C, Li S, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer's disease. Neurobiology of aging. 2013;34:2827–2834. doi: 10.1016/j.neurobiolaging.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.