Abstract
Primate Cognition is the study of cognitive processes, which represent internal mental processes involved in discriminations, decisions, and behaviors of humans and other primate species. Cognitive control involves executive and regulatory processes that allocate attention, manipulate and evaluate available information (and, when necessary, seek additional information), remember past experiences to plan future behaviors, and deal with distraction and impulsivity when they are threats to goal achievement. Areas of research that relate to cognitive control as it is assessed across species include executive attention, episodic memory, prospective memory, metacognition and self-control. Executive attention refers to the ability to control what sensory stimuli one attends to and how one regulates responses to those stimuli, especially in cases of conflict. Episodic memory refers to memory for personally experienced, autobiographical events. Prospective memory refers to the formation and implementation of future-intended actions, such as remembering what needs to be done later. Metacognition consists of control and monitoring processes that allow individuals to assess what information they have and what information they still need, and then if necessary to seek information. Self-control is a regulatory process whereby individuals forego more immediate or easier to obtain rewards for more delayed or harder to obtain rewards that are objectively more valuable. The behavioral complexity shown by nonhuman primates when given tests to assess these capacities indicates psychological continuities with human cognitive control capacities. However, more research is needed to clarify the proper interpretation of these behaviors with regard to possible cognitive constructs that may underlie such behaviors.
Introduction
Cognitive control refers to a number of regulatory or executive processes within the mind that allocate and focus attention, manipulate and evaluate information (including initiating searches for new information when it is needed), remember past events so as to anticipate, plan and remember future responses, and deal with distraction and impulsivity when they are threats to goal achievement by exhibiting self-control. For humans, these are hallmark features of our cognitive system and critical developmental milestones1–2. Cognitive control is not a single ability. Rather, the term refers to a suite of constructs that reflect multiple capacities, including (at least in humans) degrees of self-knowledge, episodic and prospective memories, and the representation of goals that remain to be obtained in the future if behaviour can be coordinated to accomplish that in the present.
Cognitive control is perhaps best exemplified in the executive control processes that allow for focal and directed attention, or in the metacognitive capacity in which the environment and cognitive representations are monitored so as to generate feelings of confidence or uncertainty and then controlled to guide information-seeking behaviors when needed3–4. Another critical aspect of cognitive control concerns managing time-based memories, and how those memories impact present and future choices. The key issue is whether and how organisms use memories of past experiences or anticipate future conditions when they structure their present behavior. To do so most effectively, they must allocate resources and engage cognitive control processes to incorporate temporally-removed sources of information. And, finally, cognitive control can manifest in choice behaviour in which one weighs the value of more immediate, but objectively lower-valued outcomes with more delayed but higher valued ones. These intertemporal choices, when they reflect self-control, require controlled behavioral responding (e.g., self-distraction) if delayed rewards are to be earned. Of course, these are not the only processes that constitute a full cognitive control system, but they are processes well-studied in nonhuman primates in an effort to provide a comparative assessment of cognitive control.
Identifying behavioral competencies that reflect cognitive control requires recognizing the contrast between instances of deliberate and effortful responding on the one hand and the large number of habitual and automatic actions on the other [4]. Highly practiced responses can become very efficient, stereotyped and inflexible, stimulus-driven routines that do not require willful, executive processing. Indeed, the best evidence for cognitive control is often found in an organism's capacity to inhibit these strongly associative, automatic responses in favor of adaptive and flexible (but characteristically slower and more effortful) behavioral outcomes. Note that animal behavior tends to be silent on the question of whether this processing is conscious; rather, the goal is to infer where any particular behavioral competency falls on the stimulus-control/cognitive-control continuum, with the burden of proof being to show that responding is neither the manifestation of randomness nor automaticity.
In comparative research, efforts to establish executive attentional control have led to new and sometimes controversial ideas about the role of language, experience, and species-specific factors in how well cognitive processes can be controlled and allocated. In comparative research, it also has been an ongoing debate as to whether being able to anticipate the future may offer greater adaptive advantages than being able to remember the past5, or whether these two aspects of “mental time travel” are intricately related6–8. In any case, there are increasingly compelling data that nonhuman primates (and other animals) can use past experiences to anticipate future ones. It is important here to acknowledge and emphasize that the documentation of the more controversial and difficult to study aspects of so-called “mental time travel” (e.g., autonoetic experiences9–11) is not necessary to illustrate cognitive control processes at work when animals use past experiences or anticipate future ones as they behave and respond to their environments. Such questions about subjective experiences and whether nonhuman primates and other animals experience themselves as the actors of past and future events is an interesting and important area of inquiry, but it is not central to the cognitive control processes that are the focus of this article. The same is true for research into animal metacognition, in which one can focus on objective claims of the degree to which animals monitor their sensory and memory experiences and control their search for information to guide efficient responding without having to make strong claims that such control and monitoring must reflect self-awareness or specific conscious states that are believed to accompany human metacognitive processes12–14. This last point also holds for comparative research into self-control, where objective data on choices for smaller, sooner rewards or larger, later ones does not have to reflect strong claims for strategic responding, although some paradigms used with animals suggest that such claims are justified. In all of these areas, nonhuman animal performances suggest psychological continuities with humans, but more research is needed, and ongoing controversies and debates exist. Finally, we would note that this article focuses discussion on nonhuman primates as a consequence of its assigned subject area. This does not mean that other, non-primate species are not equally valuable as participants in a truly comparative psychology, and with regard to studying cognitive control specifically. They certainly are, and we provide some additional readings that can direct the reader to some of that important work.
Executive Attention
The act of paying attention is among the strongest expressions of cognitive control, as when searching systematically for a hidden image, listening intently for a sound amid noise, or struggling to remain alert when bored. This experience of controlled or executive attention15–17 is made noteworthy by the contrast with bottom-up, data-driven attention that is involuntarily elicited by unexpected movement, sudden sounds, or strongly habitual responding. Attention processes in nonhuman animals have been examined in hundreds of studies (e.g., reviews by 18–20). Most of these animal-attention studies were conducted with birds, but much of what is known about the neural circuitry of (visual, in particular) selective attention was gleaned from studies with monkeys (e.g., reviews in 21–23). However, relatively few of the studies of primate attention were designed to determine where on the stimulus-control/cognitive-control continuum the animals’ attention falls for the tasks being studied. Because performance on attention-demanding activities is determined by the interaction of top-down (i.e., knowledge-driven) and bottom-up (i.e., stimulus-driven) influences, the control of attention in nonhuman primates is best revealed using tasks that place these sources of constraint in direct competition24.
Consider for example the classic Stroop color-word task25 and its variations26, which require executive attention to overcome strong, associative response tendencies to incongruent stimulus cues. For example, a language-experienced chimpanzee (Pan troglodytes) named Lana was tested on a task requiring her to select a “B” on a computer screen if a blue stimulus was presented, but a “Y” if a yellow image was displayed on the computer screen27. On some trials, the blue or yellow stimuli were visuographic symbols (“lexigrams”28) that Lana had learned, including the specific symbols she had previously learned to mean “blue” and “yellow” to label colors. Thus, the lexigram that meant “blue” could be presented either in blue (congruous) color or yellow (incongruous) color. In any case, the meaning of the stimulus was irrelevant, as the rule was to label the stimulus color. Nevertheless, Lana showed Stroop-like interference from this irrelevant, but highly salient associative cue—as has been reported in thousands of Stroop-task studies with humans29
Washburn30–31 reported similar interference effects for a numerical-Stroop task administered to rhesus monkeys (Macaca mulatta) and undergraduate volunteers. This task required rapid judgment of which of two arrays of stimuli contained the most items. On baseline trials, the two arrays contained letters (e.g., A A A A vs. B B B where the set of A stimuli is more numerous). On other trials, the arrays contained Arabic numerals in congruous (e.g., 4 4 vs. 2 where the greater number of numerals also consisted of the higher value numeral type) or incongruous (e.g., 5 5 5 vs. 1 1 1 1 where the greater number of numerals consisted of the lower value numeral type) configurations. Compared to performance on baseline and congruous trials, human participants took significantly longer to judge the larger array when it consisted of the smaller Arabic numerals. Similarly, the monkeys, which had learned Arabic-numeral values through previous training32, showed longer response times and more errors on these incongruous versus baseline or congruous trial types. Moreover, the magnitude of interference was a function of the strength-of-association between the Arabic numerals and response, such that larger symbolic distances produced greater Stroop-like interference. These effects were amplified in the macaques compared to the humans, suggesting that whereas both species had the capacity for executive control of attention (i.e., they performed the task at levels greater than chance), the monkeys were less able than human adults of ignoring irrelevant stimulus-response associative cues.
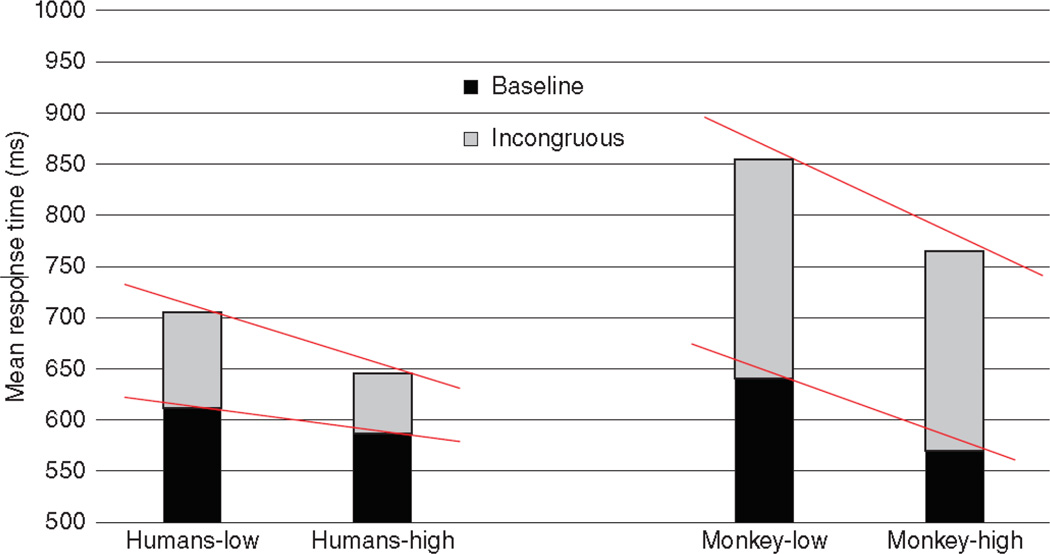
Washburn31 replicated this pattern of results and manipulated the relative demands on the cognitive control of attention, for example by varying the ratio of incongruous-to-congruous trials, and by incentivizing fast and accurate responding on incongruous trials. As shown in Figure 1, the monkeys were particularly susceptible to Stroop-like interference, and appeared to be less able than humans to attenuate control by irrelevant, incongruous stimuli on performance, even when motivation was high. This highlighted a strong species contrast with regard to executive attention.
Figure 1.
Mean response time for humans and monkeys under low-incentive and high-incentive conditions. The red bars show the Stroop-like interference (longer response times on incongruous than baseline trials). The diagonal lines highlight the finding that, although incentive improved performance overall, it reduced Stroop interference for humans but not for monkeys. From “The Stroop effect at 80: The competition between stimulus control and cognitive control,” by DA Washburn, Journal of the Experimental Analysis of Behavior, 105: 3–13. Copyright 2016 by Wiley. Reprinted with permission.
Stroop-like effects have also been reported for nonhuman primates by other researchers33–34; however, this is not the only paradigm that reveals species similarities and differences in controlled attention. Visual search tasks require location of a specific target stimulus within a search array—as, for instance, if you search the previous sentence for any appearance of the letter “z”. By manipulating the set-size of the search array and the similarity of the target to the non-target images, researchers can examine the degree to which attention scanning is controlled in a top-down, endogenous, voluntary way or in a bottom-up, exogenous, automatic way. For example, locating a stimulus within similar-looking foils typically requires controlled searching; thus, it takes longer to locate a target like “Z” buried in 30 letters than in 10 letters, if the letters are X, E, K and the like. In contrast, the set-size slope (the change in response time as a function of set size) is near zero if the non-target foils are O, C, Q and similar letters that are visually dissimilar to Z. That is, the target stimulus seems to “pop out” of the array, such that it takes no longer to find the target stimulus as the number of non-targets increases. Color, size, and movement are other cues that elicit attention automatically.
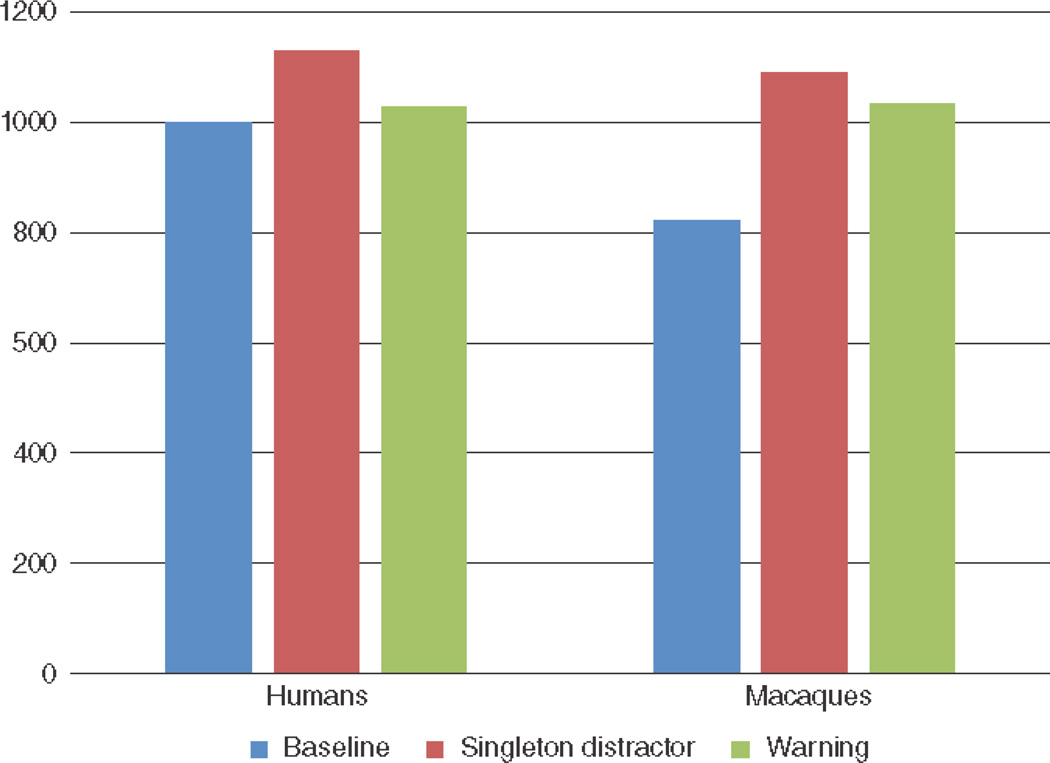
Research with monkeys trained to perform visual search tasks has been useful for distinguishing the neural systems associated with controlled versus data-driven attention scanning35–38. Behavioral research with nonhuman primates using the visual-search paradigm has also been informative, indicating the spatial or object-based information used to guide attention (e.g., 39–40), the role of inhibitory control41, and the consequences of competition between bottom-up and top-down search cues 42–44. For example, how is search performance affected when there is a singleton, pop-out distractor stimulus in the array? Salient distractors compromise search efficiency in humans, but cognitive control can be used to ameliorate attention capture45, although this capacity declines as executive attention declines46. Similarly, chimpanzees43 and rhesus monkeys24 showed less efficient visual search when a pop-out non-target stimulus was in the display (Figure 2). Although human adults were able to make use of warning cues to attenuate this effect, the monkeys did seem to be able to make use of such pre-trial information to over-ride attention capture via cognitive control. A similar interference effect of irrelevant but salient faces on target detection performance by monkeys has also been reported47.
Figure 2.
Mean response time on a visual search task for undergraduate participants and rhesus monkeys, averaged across set sizes (5- to 30-letter arrays). For both species, response times were significantly longer when a non-target stimulus appeared in a unique color (singleton distractor). Humans but not the monkeys performed significantly faster if this pop-out distractor was preceded by warning information informing the participant of exactly what stimuli would appear in the search array. Data are from Washburn and Taglialatela24.
A similar conclusion can be drawn from other paradigms that have been used to study attention control by nonhuman animals. Nonhuman primates show evidence of cognitive control of attention, even in the face of strong, conflicting stimulus cues. Thus, monkeys and apes manifest patterns of executive attention on tests of dimensional sorting and set-switching (e.g., 48), multiple-object tracking49, working memory50, and practically every other paradigm used to study attention control in human adults and children. When these tasks permit comparison of the relative potency of environmental constraints, experiential constraints, and executive constraints on attention, the general finding is that nonhuman primates seem more susceptible than humans to the stimulus-control of attention, and less effective than humans (healthy adults at least) in biasing attention using executive control51–52.
Episodic Memory
Human episodic memory – encompassing the ability to recall myriad features (“what,” “where,” “when,” “who,” etc.) of specific, personally witnessed events – is often described as a recently evolved, late-developing memory system9. Definitions of human episodic memory have emphasized the re-experiencing of personal events, self-knowing reflection, linguistic skill, and representation of subjective time9,53. Episodic memory is proposed to support thinking, communication, planning, and projections of an imagined self into different times and situations (“mental time travel”). It is posited to be a central factor in the emergence and subsequent evolution of both long-range planning abilities54 and language55 in the human lineage. The richness, complexity, and flexibility of human episodic memory has led some scientists to doubt the existence of closely similar processes in nonhumans and to regard human memory as unique and apart from all other forms6, 56 even in the face of evidence of episodic-like memory in birds and rodents. However, if we focus exclusively on recently evolved functions in humans, or if we limit comparative research to the work so far done on birds and rodents11, 57, this may come at the expense of recognizing foundational memory structures that support surprisingly detailed, flexible recall in both humans and great apes58–59 and possibly other nonhuman primates60. For example, rhesus monkeys re-created simple shapes on a computer monitor, providing evidence of recall memory as well as recognition memory, and also demonstrating that recall memory was less accurate than recognition memory in these tests61. In addition, it has been demonstrated that monkeys engage in active cognitive control when remembering visual stimuli in a memory test62. When concurrent cognitive demands were presented during a delay period in which a sample also had to be remembered, performance was disrupted on the matching to sample task. This occurred to a much greater degree when the to-be-remembered stimuli were familiar than when they were novel. This suggested that maintaining familiar images in memory during concurrent task performance taxed limited cognitive resources whereas maintaining unfamiliar images did not, a pattern indicating a dissociation of active from passive memory processes where active memory was under greater cognitive control.
As Fivush63 noted, the goal of human developmental studies is not merely to pinpoint the moment at which a child is capable of full-blown episodic or autobiographical memory, but to study how the underlying abilities change in their organization and permit new functions over time. Similar logic applies to the goals of comparative and evolutionary studies. Living primate species and individuals are remarkably diverse in the social and ecological problems they face, and they offer rich possibilities for comparative investigations of memory. Each species is the product of a long evolutionary history, and memory capabilities develop during ontogeny in ways that tend to provide a biological advantage to the particular individual in its specific situation. At the same time, primates share basic developmental and cognitive mechanisms for solving problems, and the emergence of episodic memory and its elements in primates can be viewed as part of an evolved life-history strategy that includes an extended lifespan, a prolonged period of immaturity, developmental flexibility and openness to experience64–65, and the construction of a detailed internal model of the structure and changes of the environment66–67.
Until recently, studies of nonhuman primate memory rarely have tested how well organisms can recall and transmit detailed information about events that occurred hours earlier, from situations that are at a great distance from, and completely out of view of, the original learning situation. Such recall of spatially and temporally distant events has been described as an essential and possibly human-specific feature of episodic memory9. Wild primates possess mechanisms for mapping and monitoring the structure and changes of their home range, i.e. for finding food, avoiding dangers, discovering pathways, and keeping track of social partners over a large area. Relevant to the question of recall, not all key features are visible from a single vantage point. A growing body of research indicates that apes68–69, monkeys70–73, and lemurs74 can orient their travel toward profitable resources that are dozens or hundreds of meters away and completely out of view. The ability to aim travel toward profitable resources from different starting points improves an animals’ foraging efficiency and can be expected to provide fitness benefits75.
A continuing research task for studies of memory in the ethological tradition76–77 is thus to study the features and processes that guide travel in the animals’ evolutionarily relevant environment. Such studies potentially can offer fresh perspectives on the “contents” of episodic memory. That is, the richness of memory and representational systems that have evolved in primates cannot be captured by a simple breakdown of episodes into “what,” “where,” and “when” components. To take an example from the realm of social behavior, the most prominent feature in a young primate’s world is its primary attachment figure, typically its mother, and at later points in life an animal may be strongly influenced by the direction of travel of its social group. A chimpanzee or a gelada baboon that encounters another adult group member after being apart for many days, or possibly months, needs to recognize that specific individual78 and to show behavior that is appropriate to the stage of dyad formation reached previously79. Some primate research has studied memory for “who” was involved in a specific event80. Future research potentially will explore other basic features of events that make them prominent in memory for a given ape, monkey, or lemur. Candidate features include certain classes of individuals (adult males, adult females, etc.), specific individuals (with highly differentiated, individual-specific relations), the traveling social group (composition varies sharply by species and populations); classes of structure (arboreal substrates for manual grasping and travel, objects, edges, verticals, trees, water holes, open spaces); predators; novelty and movement of objects; and natural time periods.
Studies conducted in small forests and naturalistic enclosures show that apes are capable of a form of free recall of events. Findings show that apes use this information flexibly and adaptively in behavior58, 81. In humans, this often would be called episodic memory. One approach has been to study chimpanzees who are symbol-competent. These apes provide a method for investigating the detailed nature of the information they possess and convey about their environment. Such apes can potentially "comment on" environmental features and events82–83. They can be tested outside the spatiotemporal contexts in which they encountered the original events. Symbol-competent chimpanzees at the Language Research Center can use lexigrams to provide information about objects and resources in the environment, and they do this after long delay periods, and after complicated encoding events have occurred. Three examples will be provided.
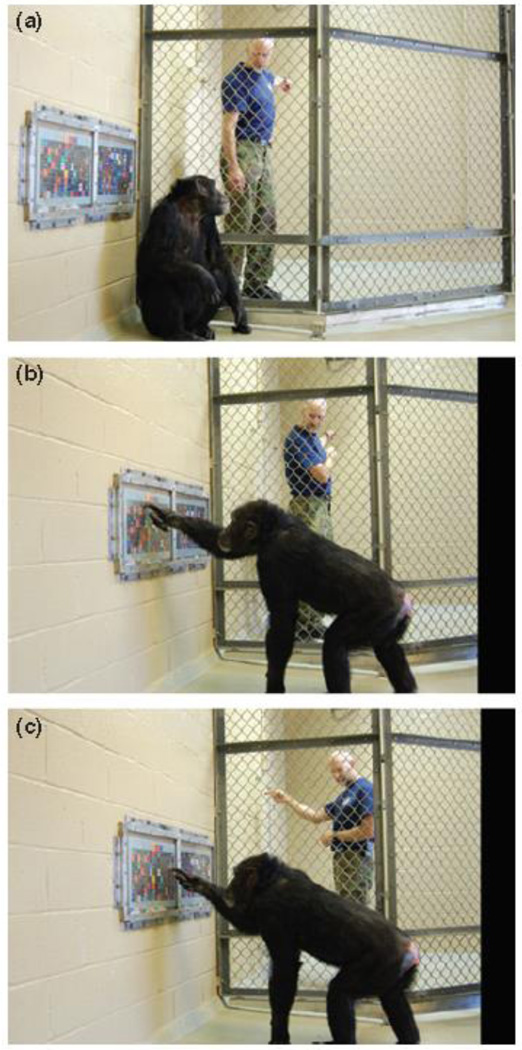
First, we have obtained evidence of unprompted recall and reporting of object types after extended delays. In our basic paradigm, a chimpanzee (in this example, Panzee) watched as an experimenter hid an object in the woods outside her outdoor enclosure. Panzee was not allowed to leave her enclosure or navigate to the location. The type and location of the object varied across trials. After an imposed delay of up to 16 h, Panzee could interact indoors with a person, who did not know that an object had been hidden, let alone its type or location. A keyboard in the indoor cage displayed 256 lexigrams. From Trial 1, Panzee used vocalization and gesture to catch the person's attention and then touched the lexigram corresponding to the type of object hidden, pointed outdoors, went outdoors (if followed), and continued to vocalize and point manually toward the object through the wire of the enclosure until the person found the object84 (Figure 3). The chimpanzee, rather than the experimenters, determined the exact time of reporting, and she reported items after delays ranging from several minutes to more than 90 hours. She selected lexigrams from a large set, not simply from a small set of alternatives as in traditional primate matching-to-sample tasks. She selected the lexigrams indoors, without an immediate view of the area in which the object was hidden. Finally, she did not simply touch lexigrams; she pointed toward the outdoor area and persisted in the interaction until the person found the object outdoors. To our knowledge, this is the strongest evidence to date of recall memory capabilities in a nonverbal animal58,85–86. We have replicated these findings in more than 14 experiments with Panzee including variations in type of item hidden, the number of items hidden, the modality of cue giving (direct view versus video representation), the modality of recruitment (direct pointing versus reporting on a video representation), and the outcome of recruitment (whether or not the item was removed and given to Panzee). We have also replicated the basic findings in an additional chimpanzee84. Aspects of these performances that are particularly relevant to the concept of “cognitive control” include the self-initiation of object reporting after long delays and the flexible control of upcoming action plans in working memory – the prospective dimensions of behavior87. Some have suggested that the performance of Panzee and other apes in these tests is reflective of mental time travel, and may even be the closest evidence we have from non-humans animals of autonoetic metacognition88 (see later section on Uncertainty Monitoring and Metacognition).
Figure 3.
After touching the lexigram on her keyboard (visible in background) corresponding to the type of object hidden, Panzee points toward the location of the object. Photograph by Charles R. Menzel.
A second memory function studied in symbol-competent apes involves multiple comparisons in simulated foraging tasks. We examined the information chimpanzees retain from trial-unique events, varying factors that are included in models from optimal foraging theory. We hypothesized that when multiple food items were hidden, they would be recovered in the order of their profitability (energy/handling time). The assessment of “profitability” involved multiple dimensions of quantity, expected recovery time, and expected processing time89. We sequentially presented two chimpanzees, Panzee and Sherman, with ten transparent bags of almonds per trial, one at a time. The bags were then hidden, each varying in quantity (kilocalories) and shell presence/absence (high/low processing time). After delays of 15 minutes to 23 hours, subjects interacted with an uninformed person and directed them to the hidden items. There was a strong negative correlation between recovery order and profitability (kcal/handling time) of each bag for both subjects, across trials and even within many of the individual trials. The order in which bags were recovered was related to both quantity and shell presence/absence (Figure 4). In effect, Panzee and Sherman retained multiple dimensions of the hidden foods – several “what” variables and ten unique “where” locations per trial – combined these into a single measure of preference, and recovered them based on that preference. New aspects of these performances relevant to “cognitive control” include the comparison and ordering of multiple items in memory (in trial-unique experimental problems), and foregoing near items when larger or better items were available elsewhere – the self-controlled dimension of foraging.
Figure 4.
Panzee’s recovery order of 10 bags of food by memory on a sample trial. Diamond indicates subject’s position in tower; the line emanating from this point traces through hidden items in the order Panzee directed an uninformed person to them. Size of circle denotes almond quantity, open circles almonds without shells, closed circles almonds with shells. Hatches on the perimeter represent 1-meter increments. On this trial, all 10 hidden items were recovered and there were no unsuccessful searches. Reprinted from Animal Behaviour, 84, Sayers K, Menzel CR, “Memory and foraging theory: chimpanzee utilization of optimality heuristics in the rank-order recovery of hidden foods”, 795–803, 2012, with permission from Elsevier.
A third example from chimpanzee research concerns communication of information about resources that are displaced in space and time and completely out of view. The ability to respond accurately to probes and queries in real-time, face-to-face interactions arguably requires flexible use of memory. We have found that a chimpanzee can convey the types of items hidden in distant, invisible, trial-unique locations, in response to a human’s directional pointing query90. While Panzee watched from her outdoor enclosure, an experimenter hid two different objects in two different locations in the woods. The types of objects and specific locations varied across trials. After a delay of about 20 minutes, her caregiver interacted with her inside the building. The caregiver stood next to Panzee’s indoor cage and pointed toward each location, one after the other, (see Figure 5). The caregiver knew the locations of the objects but not their type. From the indoor cage, neither Panzee nor her caregiver could see the outdoor area or any structure that had been visible to Panzee when the objects were hidden. The caregiver correctly deduced from Panzee’s keyboard use the type of object in both locations on 17 of 20 trials; the chance probability of naming both objects correctly on any given trial was 1 in 600. Thus, Panzee reliably reported from inside a windowless room which type of object, from a set of 25 possible types, lay in each of two directions outside the building, in response to human manual pointing queries. In further tests, 3 objects per trial were shown to Panzee, both inside and outside the building. Panzee’s performance remained accurate (28 of 30 objects correctly identified). The findings suggest that Panzee could recall specific with an arm extended and said “what is that way?” information about objects in particular locations in invisible areas, that she could read her caregiver’s directional pointing, and that she could discriminate between the multiple spatial contexts of “right here” versus “out there.” She displayed flexibility in her use of memory. That is, she did not simply recall the item at the top of her priority queue, or the one that was most recently seen.
Figure 5.
Caregiver (J) with Panzee (P) indoors. a) J points manually to the west and says “what’s out there?” Unknown to J, but known to P, peanuts lie beyond the cinder block wall of the building in the direction in which J is pointing. b) P responds to J’s query by touching the PEANUT lexigram on her keyboard. c) J now points to the south. M&M candies lie beyond the wall in that direction. P responds by touching the M&M lexigram on her keyboard. Photographs by Charles R. Menzel from 90.
To summarize our lab findings on chimpanzees, examples of cognitive control include voluntary recruitments of caregivers, the bypassing of nearby visible items when better items are available elsewhere, and answering queries about hidden objects. Thus, an aspect of chimpanzee memory crucial to its proper functionality is its flexibility in weighting the episodic features of events. The weight attached to any given feature (e.g. quantity) is not fixed but depends on other aspects of the situation. The ape does not simply form an action plan at time-1 and stick to it; episodic features are re-weighted according to prevailing circumstances at time-2. The environment and task can change, and the ape uses past and current cues to solve current tasks efficiently. Flexibility in using the priority queue appears to be considerable, which suggests a measure of control in the use of memory. Further investigations along these lines should be productive in understanding the cognitive control of behavior.
Prospective Memory
Prospective memory (PM) involves forming intentions, retaining those intentions during a delay period, and properly executing the intended responses when the correct time is reached or correct event is experienced91. As such, it is clear example of cognitive control through these sub-processes. Prospective memory is clearly established in human behavior92–95, and countless examples in everyday life highlight these processes. For example, one might set an intention to purchase a food item on the way home from work, and then one must maintain that intention until driving by the store and remembering to stop for the correct item on the way home. Control is needed to switch between the present intentions that cannot be acted upon immediately and then responding appropriately when the circumstances are right. Cognitive control certainly manifests in the monitoring that is needed to be responsive to environmental cues one sets to invoke the prospective memory (in this example, seeing the store). In formal tests of PM, two additional critical features are that execution of the intended action cannot occur immediately, and that continuous rehearsal must be prevented to the greatest extent possible96 so that prospection is engaged rather than ongoing working memory.
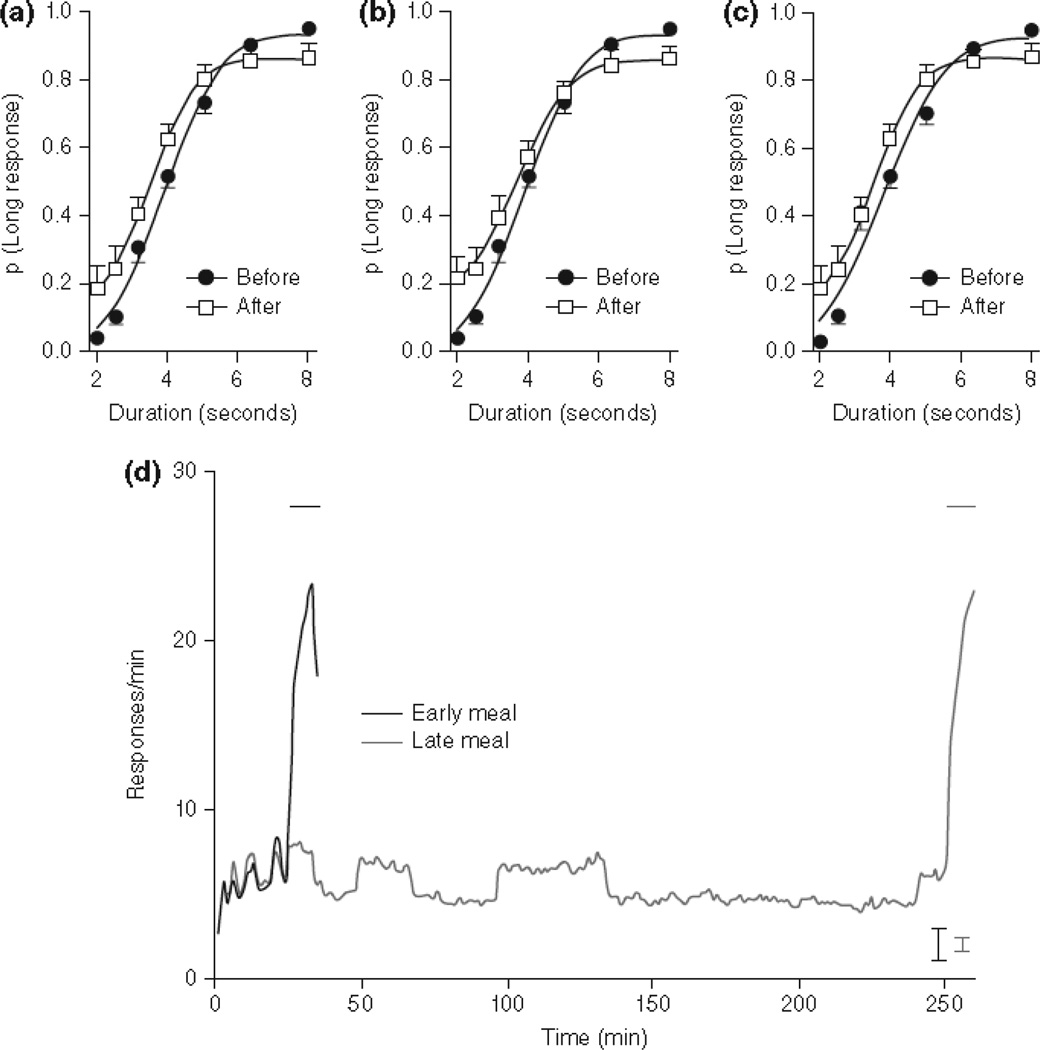
In the comparative literature, relatively little research has directly assessed human PM-like abilities in animals. A few animal studies have focused on the ability to remember information for future use (sometimes called prospective coding97), but often such studies do not demonstrate all of the essential characteristics of human PM. Other studies with birds98–100 and primates101–102 have focused on future-oriented planning, often with successful results. Some of the best research using PM tasks that approximate those used with humans comes from studies with rats103–104 (Figure 6), but there is also growing evidence of human-like PM in nonhuman primates. For example, we tested the same chimpanzee, Panzee, who had years of experience in recall and reporting tests58,85,89 to document her prospective memory105. When a trial began, Panzee was given a choice between two tokens that represented two food types. The tokens were blank on one side and had a lexigram icon printed on the other. What she chose was given to her immediately, and the unchosen item was saved for later if she remembered to ask for it by bringing a token indoors to request that item. Panzee then went outdoors to enjoy eating her chosen item, and this was where eight such tokens were spread throughout her enclosure. If she remembered later to search for, locate and bring in the correct token, she could get the other food item. So, in essence, the task was one of choosing what she most wanted now, but then having to remember later to get the token she would need when she returned if she wanted the other item. Panzee was quite good at doing just that. In fact, other experiments have shown that chimpanzees sometimes match the PM skills of young children106.
Figure 6.
Testing rats in a prospective memory test. Anticipation of early (A) and late (B) meals severely disrupted performance in an ongoing task (an auditory discrimination) after the event, relative to excellent performance at an earlier time point. (C) When event and time were dissociated (using data from 25–34 min, with and without the event), performance was severely disrupted by the event. (D) Rats anticipated the arrival of the meal, as shown by the increase in food-trough responses when the event provided information that the meal could be obtained soon; the meal could be obtained early or late (beginning at 35 or 260 min, respectively), which was randomly determined on each day. Horizontal lines indicate the last 10-min before the meal when the event was presented. From “Event-based prospective memory in the rat” by Wilson AG, Pizzo MJ, Crystal JD, Current Biology, 23, 1089–1093. Copyright 2013 by Elsevier Press. Reprinted with permission.
And, when one approximates even more closely the PM tasks given to adult humans, chimpanzees appear to show the control necessary to encode, retain, and implement intended future actions. In another study107, we reported that chimpanzees could disengage from a concurrent task (sorting images of items/foods) when they needed to take a specific image that was presented as part of the sorting task, but had also been prompted earlier as the PM cue, to obtain a preferred item from a second experimenter. A primary question that was proposed in that study was whether performance on the ongoing task might vary as a function of whether there was a PM requirement or not. Having to remember to perform the delayed PM response (taking the specific token to the second experimenter in the back of the enclosure rather than sort it) influenced the accuracy of chimpanzees’ ongoing matching task performance. With humans, it is sometimes reported that the degree of monitoring resources individuals devote to remembering to perform a delayed intention can affect ongoing task performance, an indication that cognitive control is at work when one uses PM91,95,108. This is important because it suggests that working memory load and prospective memory may have a similar relationship in nonhuman primates, and more specifically that the cognitive control needed to encode and remember future responses comes with some cost to present task performance. Previously, research with rats had shown that a disruption in ongoing task performance occurred when PM was engaged during that ongoing task103–104, and the work with chimpanzees confirmed this as well. This is a critical point, and one demonstrated in other excellent research that placed nonhuman primates in situations with increased cognitive demands and found that control and monitoring effects emerged as a function of conflict and cognitive load109. As a whole, prospective memory studies with nonhuman primates (and other animals) show that animals sometimes demonstrate evidence of critical components of prospective memory, and that such components are under controlled cognitive processing. And, as is evidenced in humans, such control is often fallible, and can lead to missed opportunities to put prospective intentions into play. This is another striking similarity between nonhuman primates and humans.
Of course, the results from studies of nonhuman primate prospective memory (and episodic memory, see previous section) do not necessarily indicate that these animals have the same experiences that humans have when they remember things that happened to them or remember to do things later. They may not, or the experiences may be quantitatively much less personal with regard to the qualia of mental time travel8–14. Thus, nonhuman animals may not share the same degree or experience of mental time travel that humans experience, but they appear to experience the same demands on their cognitive control processes when they remember past events and anticipate future needs as do humans, and it is these shared aspects of memory systems across species that should attract future research efforts110. It will be more difficult to understand whether cognitive control processes such as those that allow for searches of past memories or for structuring future responses require also the conscious experiences that humans report, but even if they do not, there is no question that animals can show behavior guided by the past and focused on the future.
Uncertainty Monitoring and Metacognition
Extensive research explores humans’ capacity for metacognition—by which they oversee and regulate their ongoing cognitive processes adaptively3,111–112. The theoretical core of this research is that some minds—human minds, at least—have a cognitive executive that monitors cognition, sensing difficulty and potential failure, and controls cognition recruiting alternative or compensatory strategies.
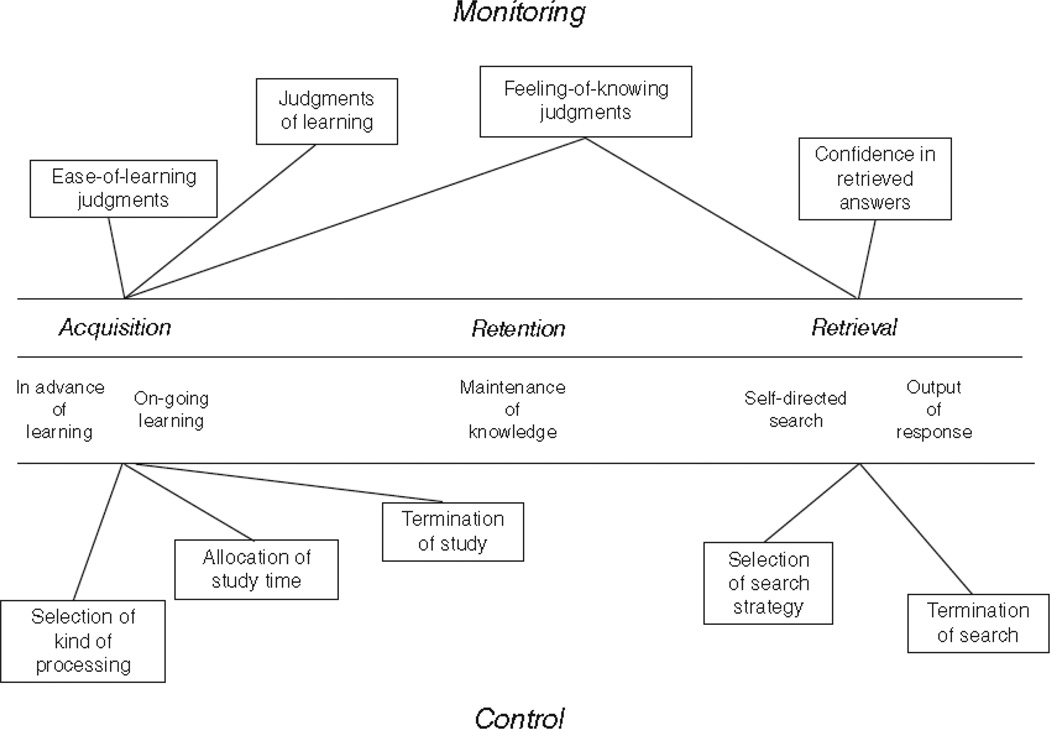
Nelson and Narens113 provided a useful theoretical perspective on metacognition (Figure 7). They distinguished cognitive processes occurring on a first-order object level from those occurring on a second-order, meta level. The meta level would monitor first-order processes to gauge their efficacy and control those processes to steer them adaptively toward task solution or problem resolution. Other theorists have proposed different kinds of metacognitive experiences88. These include anoetic metacognition, which occurs when one evaluates an external stimulus, noetic metacognition, which involve evaluations about representations of internal representations of things no longer present and thus no longer available to the sensory systems of the organism, and autonoetic metacognition, which are judgments of representations that are specifically self-referential, and can include source judgments, remember/know judgments, and agency judgments. These and other frameworks for metacognition share the idea that the capacity for metacognition reveals important things about a cognitive system, including its hierarchical structure, its recursive self-awareness, and its control systems. It is one of humans’ most sophisticated cognitive capacities—indeed, it might be humanly unique. But if it is not, then demonstrating a metacognitive capacity in animals could reveal important things about their cognitive systems, too.
Figure 7.
A theoretical framework for research on metacognition, showing examples of process-monitoring capacities above and process-control capacities below. From “Metamemory: A theoretical framework and new findings,” by T. O. Nelson and L. Narens, The Psychology of Learning and Motivation, 26, p. 129. Copyright 1990 by Academic Press. Reprinted with permission.
Thus, the important theoretical question arose of whether animals share facets of a metacognitive capacity with humans. Smith and his colleagues inaugurated this research area114–118. Obviously, animals will not declare their confidence or uncertainty. Therefore, researchers had to construct purely behavioral paradigms by which animal might express and cope with their doubts through behavioral responses. In these paradigms, one key element was to intermix trials known to be easy/manageable for the animal with trials known to be difficult/dubious. The second key element was to provide animals with an additional uncertainty response (UR) by which they might decline to complete any trials of their choosing. The idea was that animals would use the UR selectively for the dubious class of trials.
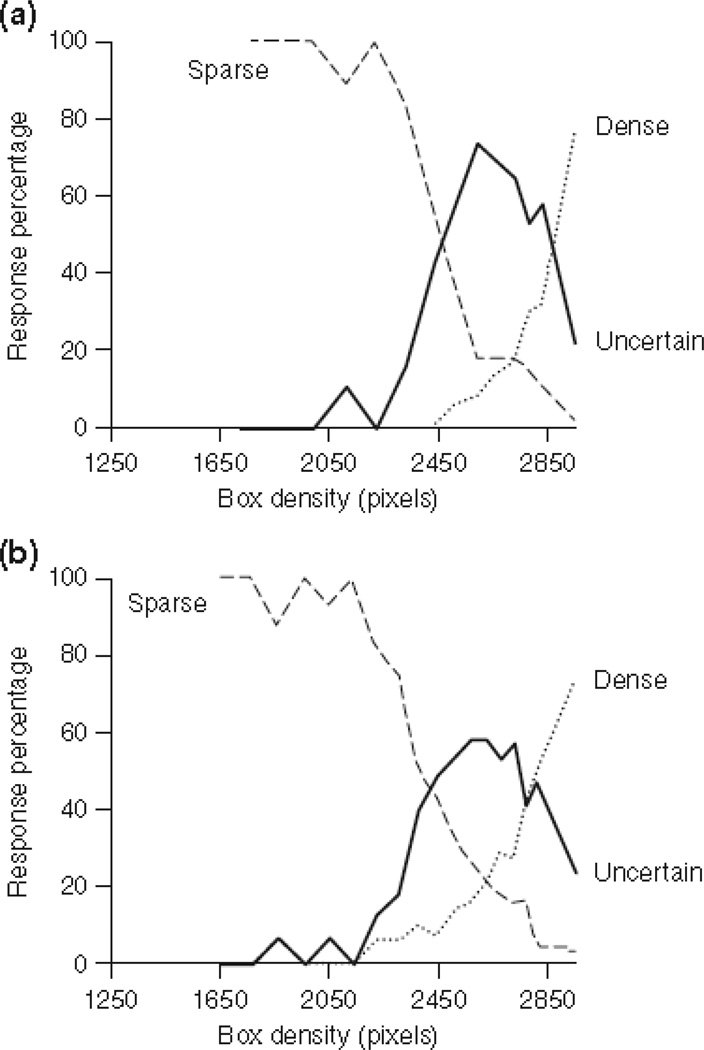
These early studies were highly successful but possibly flawed. For example, Figure 8A shows the result when macaques were brought to their discrimination threshold in a Sparse-Dense perceptual-discrimination task115. They responded Sparse and Dense when they thought they could. But, most important, they made URs for the trials that were presented at density levels near threshold, where they demonstrably could not tell Sparse from Dense. These results were identical to those produced by humans who reported making URs when they were metacognitively uncertain (Figure 8B). Perhaps macaques, too, were monitoring their confidence and controlling their responding adaptively. However, the task yoked difficulty/uncertainty to particular stimulus levels. Some have argued that this introduces an associative element. Animals received fewer rewards/more error timeouts for threshold stimulus levels. They might have felt averse to those stimuli, avoided responding to them directly, and made URs to cope with aversion, not to manage metacognitive uncertainty. This metacognitive-associative debate dominated the first decade of research in this area119–131, casting doubt over whether animals do have monitoring and control systems within cognition. This theoretical debate is nearing resolution. Many researchers have now actively explored animal metacognition, creating one of comparative psychology’s influential literatures. Primates have shown diverse, seemingly metacognitive performances in many tasks132–148, and in some cases the metacognitive interpretation of those performances is clearly more plausible and parsimonious. In some cases, the behaviour of animals highlights a link between areas of cognitive control we are outlining, as with the suggestion that Panzee’s recall memory task (see above section on Episodic Memory) also may illustrate one of the strongest instances of autonoetic metacognition in nonhuman animals88.
Figure 8.
A. Performance by a monkey in a Sparse-Dense discrimination. The horizontal axis indicates the density of the trial. The Dense response was correct for 2,950-pixel trials—these trials are represented by the rightmost data point for each curve. All trials with fewer pixels deserved the Sparse response. The solid line represents the percentage of trials receiving the uncertainty response at each trial level. The percentages of trials ending with the Sparse response (dashed line) or Dense response (dotted line) are also shown. B. The performance of humans in the Sparse-Dense discrimination, depicted in the same way. To equate discrimination performance across subjects, the data were normalized to place each subject's discrimination crossover at a pixel density of about 2,700. From “The comparative psychology of uncertainty monitoring and metacognition,” by J. D. Smith, W. E. Shields, and D. A. Washburn, Behavioral and Brain Sciences, 26, p. 322. Copyright 2003 by the Cambridge University Press. Reprinted with permission.
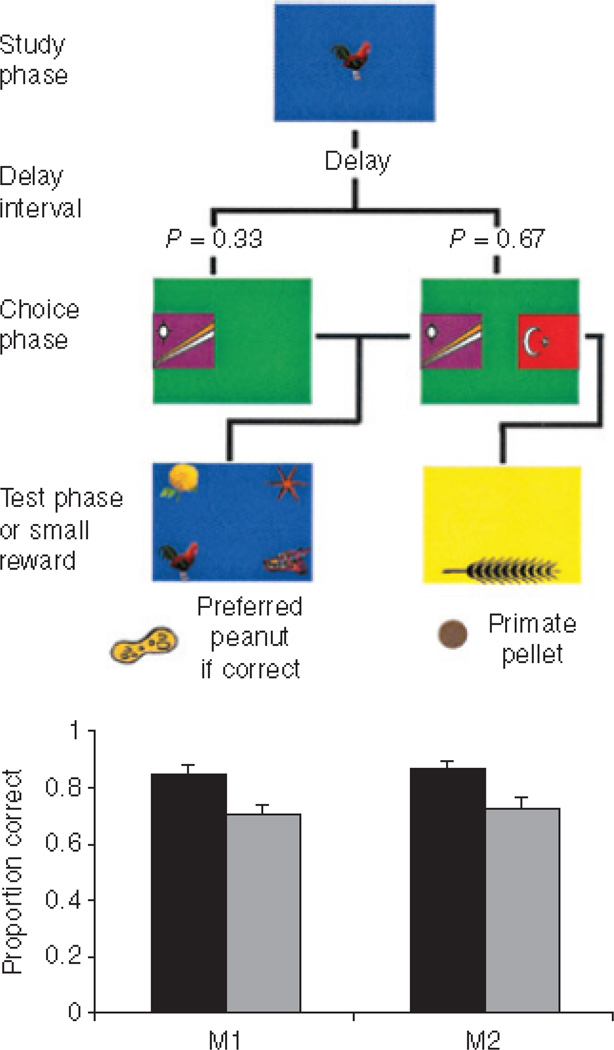
We can only consider a few other examples from among many. In one influential case, Hampton139 asked whether macaques could monitor their recent memory (a form of metamemory). Monkeys performed a delayed matching-to-sample trial, and, following the delay, they could choose either to continue to the memory test or to bail from the trial using a UR. Hampton showed that animals made more URs after longer forgetting intervals, perhaps because they had forgotten more of what they had seen (Figure 9). Strikingly, even at long delays, macaques performed very well when they made the behavioral choice to continue with the trial. This result is perhaps only consonant with the idea that they knew they still remembered the sample item and so could safely take the test. Emphasizing this point, Basile et al.120 conducted multiple studies testing possible low-level explanations for macaques’ memory monitoring. They found no evidence to support the associative hypotheses of behavioral cue association, rote response learning, expectancy violation, response competition, generalized search strategy, or postural mediation. Instead, they consistently found evidence for the metacognitive hypothesis.
Figure 9.
The metamemory task of Hampton139. The top panel outlines the procedure. Each colored panel represents what monkeys saw on a touch-sensitive computer monitor at a given stage in a trial. At the start of each trial, monkeys studied a randomly selected image. A delay period followed over which monkeys often forgot the studied image. In two-thirds of trials, animals chose between taking a memory test (Right, left-hand stimulus) and declining the test (Right, right-hand stimulus). In one-third of trials, monkeys were forced to take the test (Left). Better accuracy on chosen than on forced tests indicates that monkeys know when they remember and decline tests when they have forgotten, if given the option. The bottom panel shows the results. Dark bars represent accuracy on tests the monkeys chose to take. Light bars represent performance on trials where the animals were not given the choice of declining tests. Monkeys were more accurate on tests they chose to take than those they were forced to take. From “Rhesus monkeys know when they remember,” by RR Hampton, Proceedings of the National Academy of Sciences, 98: 5359–5362. Copyright 2001 by HighWire Press. Reprinted with permission.
Smith et al.50 explored the level that URs may occupy within the cognitive system. They added a concurrent working-memory load to macaques’ ongoing uncertainty-monitoring performance. They found a striking interaction. The memory load left the primary perceptual responses in the task—like Sparse and Dense—untouched in frequency and distribution along the task continuum—consonant with the idea that these responses do not expend or depend on working-memory resources. In contrast, they found that the memory load sharply reduced the frequency and range of URs—consonant with the idea that URs do depend on working-memory resources.
These results complement research in which humans performed memory tasks while reporting metacognitive states150. Here, too, memory loads strongly affected metacognitive judgments, sharply decreasing tip-of-the-tongue experiences. Schwartz149 concluded that working memory and metamemory use similar processes, a conclusion possibly extended to macaques50. It is an intuitive theoretical possibility that metacognitive monitoring/control systems would occupy a higher level within the overall cognitive system. This is fully consistent with Nelson and Narens’114 original theoretical formulation of the object and meta levels. But it is very important theoretically that animals may share with humans at least some aspects of the capacity for cognitive monitoring and control. In short, these and many other studies have established clearly that macaques and ape species are showing at least the beginnings of the capacities that let them monitor cognition and control it adaptively.
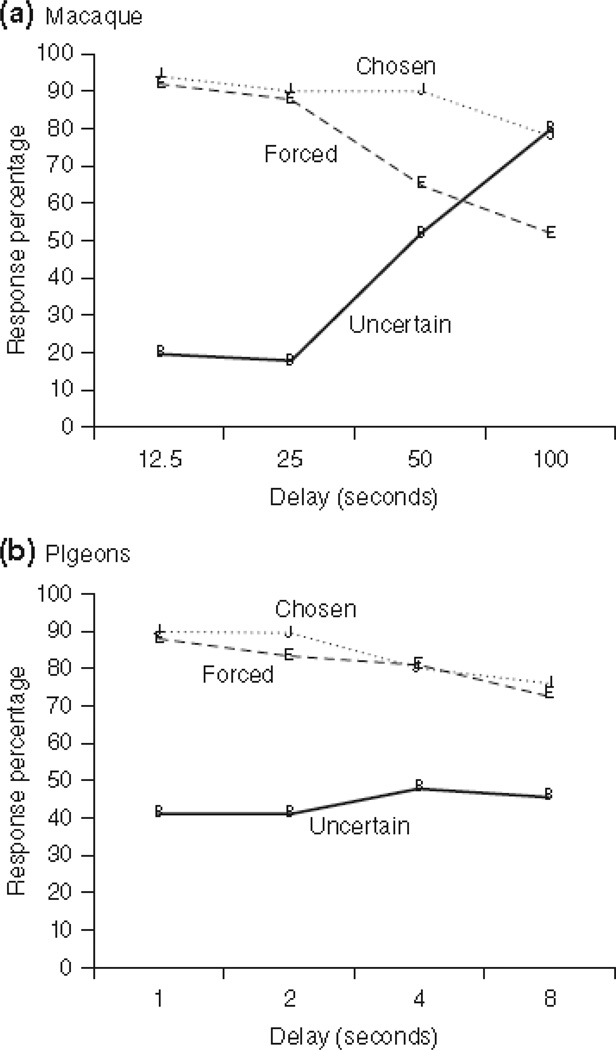
Metacognitive “failures” by other species reinforce this conclusion. For example, pigeons’ metamemory has been asssessed150. The pattern of results for macaques/pigeons is contrasted in Figure 10. Pigeons’ URs hardly increase at longer forgetting intervals, as they should if pigeons appreciate that they gradually forget the sample information. Nor do pigeons still perform well at long forgetting intervals when they choose to take the memory test, as they should if they know when they remember the sample. They barely show any components of macaques’ metamemory capacity. Other research151 confirmed this empirical conclusion through multiple experiments, although pigeon studies continue with varying outcomes152. Pigeons are adept associative learners. If there were low-level cues in these tasks to support low-level learning, pigeons would likely use them. Therefore, these results highlight the psychological sophistication of the metacognitive performance pattern when it occurs, and possibly suggest the distinctive psychological organization of macaques’ cognitive-control systems. These control systems may not have evolved equally robustly in all vertebrate lines.
Figure 10.
A. Memory performance by a macaque in the delayed matching-to-sample task of Hampton (2001). The horizontal axis indicates the length of the retention interval before matching could occur. The percentage of trials that received the uncertainty response is shown (solid line). The percentages correct of memory tests completed are also shown, on occasions when the memory test was mandatory (dashed line) or optional and voluntarily selected by the macaque (dotted line). B. Memory performance by a pigeon in the delayed matching-to-sample task of Inman and Shettleworth (1999). From “Animal metacognition: A tale of two comparative psychologies,” by J. D. Smith, J. J. Couchman, & M. J. Beran, Journal of Comparative Psychology, 128, p. 115. Copyright 2014 by the American Psychological Association. Reprinted with permission.
In fact, one can consider a cognitive-control example directly. For example, pigeons have been given the chance to make information-seeking responses in a matching-to-sample task153. The researchers asked simply whether pigeons would realize that they needed to make a response to reveal an occluded sample before trying to choose the shape that matched that sample. In several experiments, pigeons did not robustly make this response that let them see or study the sample. They just kept trying to match the sample that they had not seen and that therefore they could not possibly match. Beran and Smith134 gave the Roberts et al. test to macaques. In a sharp comparative contrast, macaques understood essentially immediately that you have to reveal the sample if you hope to match it successfully. They easily brought to bear a cognitive-control process to optimize performance in this task. But of course one need not attribute fully conscious, self-imbued control to macaques even given this successful information-seeking performance, or given other successes by great apes when presented with information-seeking tasks133, 135–137, 143. Such species differences that broadly suggest better metacognitive capacities in primates than in non-primates underscore the cognitive complexity and the distinctive psychology of the macaques’ and apes’ cognitive-control performances.
Self-Control and Delay of Gratification
Self-control is defined as foregoing an immediate reward in the interest of a higher-valued, but delayed reward154, and it is essential to a wide-variety of future-oriented behaviors and outcomes, including planning behavior, physical health and well-being, and social success155–156. Self-control is most impressively documented within humans, with examples including years of savings for retirement, extreme weight loss, and successful attempts to overcome drug and alcohol addictions. Early developmental work by Mischel and colleagues underscored the importance of establishing the ontogenetic trajectory of the full-fledged self-control exhibited by many human adults through simple behavioral measures157–159. In addition to the long-term developmental work on self-control, comparative research with nonhuman primates sheds light on the conditions under which self-control emerges in our closest-living relatives, and how it relates to the impressive inhibitory control expressed within our own species. These inhibitory skills can be viewed as foundational to many other cognitive control processes, as they underlie the slowed, voluntarily controlled allocation of attention, the controlled accessing of memories, and the monitoring and control that occur in information-seeking behaviors when uncertainty is experienced. As such, self-control supports each of the previous capacities highlighted in this article, and is therefore an important capacity to understand developmentally and comparatively.
Self-control often is tested using an intertemporal choice task, also known as temporal discounting or delay choice tasks, in which participants choose between an immediately-available, but less desirable option (5 food rewards now) and a higher-valued, but delayed option (10 food rewards in 60 s). A variety of nonhuman primate species have been tested using the delay choice task, with considerable variability in performance within and across species, with animals waiting on the order of several seconds to several minutes for higher-valued but delayed options160–164. An important component of the delay choice task concerns the nature of the choice options presented to animals. Historically, comparative tasks presented arbitrary stimuli (e.g., light keys, levers) to animal subjects, which represented the smaller-sooner and larger-later options under comparison165. More recently, food items have been used as choice options in which primates are faced with a smaller food set immediately available for consumption versus a larger food set that can be obtained after some delay160–161, 166–167. In this scenario, selection of the larger food set represents the self-controlled response for the better, but delayed reward. However, this choice of the larger-later reward also may be reflective of a prepotent, impulsive response to point to an overall larger food amount (see 168 for a full discussion).
One of the dominant paradigms in comparative self-control research is the reverse reward task. It was first reported that chimpanzees failed to learn a reverse-reward contingency, which required individuals to point to a smaller food set in order to obtain a larger, more desirable food set169. The failures of the reverse-reward task have since then been replicated in other primates, including capuchin monkeys170, Japanese macaques171, and squirrel monkeys172. However, chimpanzees and capuchin monkeys have demonstrated more promising performance when food sets were substituted with arbitrary items (Arabic numerals or tokens) that removed the prepotent, quantitative features of the stimuli170, 173. Ongoing research is focused on what conditions might allow animals to overcome these prepotent response tendencies toward the goal of more optimal responding in reversed-reward contingencies.
Of particular focus in the developmental literature are the behavioral and mental processes underlying the ability to “bridge a delay interval” between when one chooses to try to wait for the better reward and when it is received156–159. For example, choosing to place money in a retirement savings not only requires the initial choice to do so, but also the continual act of refraining from removing that money until retirement age. Originally adapted for developmental work, a seminal delay of gratification paradigm presented children with a desirable treat (marshmallow) that would double in number after some time period if the child refrained from eating the first treat156,159. Importantly, this task requires sustained inhibition during the delay to maximize rewards beyond the initial choice of the larger-later reward, and variables such as attention to the reward and reward visibility typically reduced performance of young children in this task156–159, 174–175.
Adapted from the developmental literature176 delay maintenance tasks present participants with a reward that grows in number or size with the passage of time until the participant takes the reward (e.g., the growing savings account). Great apes have been at the center of much of the comparative work and typically outperform other species of primates and non-primates, with convincing self-control demonstrated by bonobos177, orangutans178–179, and chimpanzees178,180–181. For example, chimpanzees have waited for an upwards of 11 minutes in some experiments to accumulate the total number of rewards available180, a performance level comparable to that of the human child. Unlike with children, however, attention to and visibility of the reward set does not decrease performance by chimpanzees180. In addition, great apes have been known to use self-distraction in accumulation situations requiring sustained self-control (i.e., by playing with toys and enrichment181; see Figure 11), a strong continuity to the best strategies children learn to employ. Chimpanzees perform well in a variety of accumulation scenarios, including in the absence of experimenters (i.e., automated delivery of rewards180), in the presence of social partners performing the same self-control task183, and when coordinated activity with a conspecific is required to facilitate accumulation of rewards183. Monkey species generally are out-performed by their great ape relatives on the accumulation task, with wide-spread individual differences within and between monkeys species, and with variables such as reinforcer quality, reward visibility, and experimental history impacting performance160, 172, 184–186. For example, accumulation tests with ascending reward sizes (i.e., where each newly added item was the best item yet seen) increased performance by capuchin monkeys and squirrel monkeys173 and the presence of a high-valued reward embedded late in the accumulation set increased performance by rhesus monkeys184.
Figure 11.
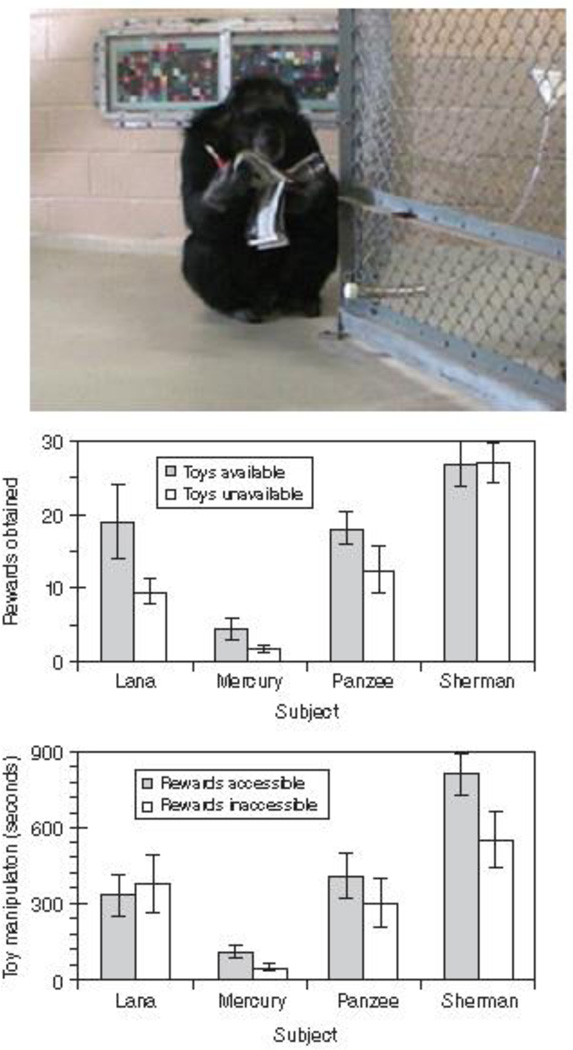
The top panel shows chimpanzee Sherman with items he could use for self-distraction during an accumulation self-control test. An automated dispenser delivered food items, one-at-a-time, into the tube that projected into his enclosure. Food items would continue to accumulate as long as Sherman did not take the tube and eat the items. The middle panel shows that three of four chimpanzees obtained more food items when they had toys to act as distractions than when they did not. However, this result is the not the critical one to showing self-distraction, because it may have been that simply having toys made chimpanzees play with the more, and thus wait longer. The bottom panel shows the crucial result. Three chimpanzees showed a statistically greater level of item manipulation when rewards were accessible (i.e., they had to self-impose the continued delay of eating those items) than when rewards were inaccessible and delay was imposed by the experimenters. This meant that having toys available was not the key factor in whether chimpanzees interacted with those toys. The key factor was whether the chimpanzees had to maintain self-control in the face of temptation or did not. These data are reported in Evans and Beran (2007)181.
Self-control in primates has been measured in other paradigms as well, including exchange tasks in which primates must trade an inedible token or food item currently in their possession for a different, potentially more desirable option187–191. For example, capuchin monkeys learned to exchange food items for qualitatively or quantitatively better rewards, although quantitative exchanges proved more difficult for the monkeys189. Similarly, chimpanzees readily inhibited the consumption of low- or medium-valued food items within their possession in exchange for qualitatively or quantitatively better food rewards, including visible and non-visible exchange sets, with quantitative exchanges more difficult for some individuals187. The exchange task offers an intuitive measure of self-control in which animals can monitor forth-coming rewards and experience the immediate positive impact of the exchange. Another intuitive task involved a rotating food tray that held two food rewards of different value or quantity. Monkeys allowed less-preferred food items to pass by so that they could obtain a more delayed, but better food item192. Another clever design demonstrated that capuchin monkeys would forego eating an immediately available but lower-valued food item (celery sticks and pretzel rods) to instead obtain an out-of-reach, higher-valued food reward (peanut-butter) using the less-preferred food as a tool193.
Self-control research among primates is broadening in scope beyond existence-based proof of continuity in inhibitory control among human and nonhuman primates to a more in-depth view of the variables impacting differences within and across species. Self-control studies among primates are beginning to link inhibitory performance in one paradigm to related or unrelated performance by the same individuals in separate self-control tasks194. Further, establishing the degree to which self-control is related to other future-oriented cognition, including planning and metacognition, will allow for a broad comparative assessment of cognitive control, its precursors, and its expression in our closest-living relatives, the primates.
Conclusion
In each of the areas we have outlined, cognitive control is evident in the behaviour of nonhuman primates, even though in many cases the degree to which nonhuman primates match the self-control, or metacognitive capacities, or memory and attentional processes of humans may differ. Continuing to assess these differences and similarities in the capacity for such cognitive processes will allow researchers to highlight both the uniqueness of our species, but also its connectedness to the rest of the animal kingdom, and particularly our primate relatives.
Whether a nonhuman primate must control its visual attention, directing it toward one stimulus but not another, or recall specific aspects of a past event to structure its current retrieval and recruitment behaviors, or anticipate future reward opportunities and then encode intentions and monitor when to implement them, or search its memory or assess its perceptual acuity and decide whether to respond, to bail, or to seek more information, cognitive control is at work. As, in each of these cases, inhibitory demands that underlie a self-control capacity are at work and must be employed. As such, these behaviors, although often discussed separately and assessed as solitary constructs, are intricately related. They offer an explicit contrast to stimulus-control of behavior. Cognitive control is required to prevent or transcend stimulus-associative responding. Future research efforts need to be more sensitive to the interplay of these control processes no matter the central focus on one particular construct from the group. It will be interesting and informative to see just how tightly the relation is between, for example, self-control and metacognition, or between prospective memory and attentional control.
In addition, the comparative approaches to these topics need to remain integral and connected to other approaches in psychological science. Newer technologies afford the hope that we can look at the functioning brain in awake, non-invasively studied nonhuman primates who willingly engage in these kinds of cognitive tasks. A developmental-comparative component also offers great value, as researchers attempt to link ontogeny to phylogeny with regard to the emergence of cognitive control. Such efforts will highlight not only the capacities for such control as they emerged in evolution of the primates, but also as they grow and develop in human children. Ultimately, developmental, cognitive, comparative, and neuroscientific approaches complement each other in critical ways, particularly for phenomena such as those outlined in this article, that are considered hallmarks of the human experience, and among the most fascinating subjects in cognitive science. The continued effort to study such processes requires a continued commitment to maintaining, protecting, and supporting nonhuman primate research that is non-invasive, behavioral, and perhaps most importantly, enjoyable to the primates who participate in this research. Thirty years ago, many of these topics had not yet even been discussed in regards to nonhuman animal cognition (which, itself, was only beginning to take hold as a dominant research area). To protect what scientists can learn 30 years from now requires a continued financial and scientific commitment to this kind of research, and to the goal of using cognitive primatology to learn more about all aspects of human cognition as well.
Footnotes
Preparation of this article and support for the research of these authors on this topic was provided by the National Institutes of Health (grants HD38051, HD056352, HD060563, HD061455, MH58855, and 1F32HD061177), the National Science Foundation (grants BCS 0956993, BCS 0924811, BCS 0634662, and SBR-9729485) the L.S.B. Leakey Foundation, the Wenner-Gren Foundation, and the College of Arts and Sciences at Georgia State University. The contents of this article do not necessarily represent the official views of these funding agencies.
All authors declare that they have no conflicts of interest in preparing this article.
Related WIREs Articles
| DOI | Article title |
|---|---|
| COGSCI228 | Animal Cognition |
| COGSCI303 | Mental Time Travel/Prospective Thinking |
| COGSCI312 | Cognitive Control/EF |
Contributor Information
Michael J. Beran, Georgia State University
Charles R. Menzel, Georgia State University
Audrey E. Parrish, Georgia State University
Bonnie M. Perdue, Agnes Scott College
Ken Sayers, Georgia State University.
J. David Smith, Georgia State University.
David A. Washburn, Georgia State University
References
- 1.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posner MI, Snyder CRR. Attention and cognitive control. In: Solso R, editor. Information processing and cognition: The Loyola Symposium. Hillsdale, NJ: Lawrence Erlbaum; 1975. pp. 55–85. [Google Scholar]
- 3.Nelson TO, editor. Metacognition: Core readings. Needham Heights, MA: Allyn & Bacon; 1992. [Google Scholar]
- 4.Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- 5.Klein SB, Robertson TE, Delton AW. Facing the future: Memory as an evolved system for planning future acts. Memory and Cognition. 2010;38:13–22. doi: 10.3758/MC.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suddendorf T, Busby J. Mental time travel in animals? Trends in Cognitive Sciences. 2003;7:391–396. doi: 10.1016/s1364-6613(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths D, Dickinson A, Clayton N. Episodic memory: What can animals remember about their past? Trends in Cognitive Sciences. 1999;3:74–80. doi: 10.1016/s1364-6613(98)01272-8. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WA. Mental time travel: Animals anticipate the future. Current Biology. 2007;17:418–420. doi: 10.1016/j.cub.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace H, Metcalfe J, editors. The missing link in cognition: Evolution of self-knowing consciousness. New York: Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- 10.Zentall TR. Mental time travel in animals: A challenging question. Behavioural Processes. 2006;72:173–183. doi: 10.1016/j.beproc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Crystal JD. Remembering the past and planning for the future in rats. Behavioural Processes. 2013;93:39–49. doi: 10.1016/j.beproc.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beran MJ, Perdue BM, Futch SE, Smith JD, Evans TA, Parrish AE. Go when you know: Chimpanzees’ confidence movements reflect their responses in a computerized memory task. Cognition. 2015;142:236–246. doi: 10.1016/j.cognition.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JD, Couchman JJ, Beran MJ. The highs and lows of theoretical interpretation in animal-metacognition research. Philosophical Transactions of the Royal Society. 2012;367:1297–1309. doi: 10.1098/rstb.2011.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JD, Couchman JJ, Beran MJ. Animal metacognition: A tale of two comparative psychologies. Journal of Comparative Psychology. 2014;128:117–131. doi: 10.1037/a0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of Learning and Motivation. 2004;44:145–200. [Google Scholar]
- 16.Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- 17.Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- 18.Blough DS. Reaction-time explorations of visual perception, attention, and decision in pigeons. In: Zentall TR, Wasserman EA, editors. The Oxford handbook of comparative cognition. New York: Oxford University Press; 2012. pp. 83–99. [Google Scholar]
- 19.Teng Y, Vyazovska OV, Wasserman EA. Selective attention and pigeons’ multiple necessary cues discrimination learning. Behavioural Processes. 2015;112:6171. doi: 10.1016/j.beproc.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Zentall TR. Selective and divided attention in animals. Behavioural Processes. 2005;69:1–15. doi: 10.1016/j.beproc.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 22.Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: An ambiguity resolution theory of visual selective attention. Cognitive Psychology. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- 23.Posner MI, editor. Cognitive neuroscience of attention. Guilford Press; 2011. [Google Scholar]
- 24.Washburn DA, Taglialatela LA. The competition for attention in humans and other animals. In: Zentall TR, Wasserman EA, editors. The Oxford handbook of comparative cognition. New York: Oxford University Press; 2012. pp. 110–116. [Google Scholar]
- 25.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643. [Google Scholar]
- 26.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 27.Beran MJ, Washburn DA, Rumbaugh DM. A stroop-like effect in color-naming of color-word lexigrams by a chimpanzee (Pan troglodyte) The Journal of General Psychology. 2007;134:217–228. doi: 10.3200/GENP.134.2.217-228. [DOI] [PubMed] [Google Scholar]
- 28.Rumbaugh DM, editor. Language learning by a chimpanzee: The Lana project. New York: Academic Press; 1977. [Google Scholar]
- 29.MacLeod CM. The Stroop task: The "gold standard" of attentional measures. Journal of Experimental Psychology: General. 1992;121:12–14. [Google Scholar]
- 30.Washburn DA. Stroop-like effects for monkeys and humans: Processing speed or strength of association? Psychological Science. 1994;5:375–379. doi: 10.1111/j.1467-9280.1994.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 31.Washburn DA. The Stroop effect at 80: The competition between stimulus control and cognitive control. Journal of the Experimental Analysis of Behavior. 2016;105:3–13. doi: 10.1002/jeab.194. [DOI] [PubMed] [Google Scholar]
- 32.Washburn DA, Rumbaugh DM. Ordinal judgments of numerical symbols by macaques (Macaca mulatta) Psychological Science. 1991;2:190–193. doi: 10.1111/j.1467-9280.1991.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 33.Allritz M, Call J, Borkenau P. How chimpanzees (Pan troglodytes) perform in a modified emotional Stroop task. Animal Cognition. 2015:1–15. doi: 10.1007/s10071-015-0944-3. [DOI] [PubMed] [Google Scholar]
- 34.Tomonaga M. Relative numerosity discrimination by chimpanzees (Pan troglodytes): Evidence for approximate numerical representations. Animal Cognition. 2008;11:43–57. doi: 10.1007/s10071-007-0089-0. [DOI] [PubMed] [Google Scholar]
- 35.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 36.Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: How top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- 38.Treue S. Neural correlates of attention in primate visual cortex. Trends in Neurosciences. 2001;24:295–300. doi: 10.1016/s0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- 39.Lanyon LJ, Denham SL. A model of active visual search with object based attention guiding scan paths. Neural Networks. 2004;17:873–897. doi: 10.1016/j.neunet.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Ushitani T, Imura T, Tomonaga M. Object-based attention in chimpanzees (Pan troglodytes) Vision Research. 2010;50:577–584. doi: 10.1016/j.visres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Fagot J, Bonté E, Hopkins WD. Age-dependant behavioral strategies in a visual search task in baboons (Papio papio) and their relation to inhibitory control. Journal of Comparative Psychology. 2013;127:194–201. doi: 10.1037/a0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee B, McPeek RM. The effects of distractors and spatial precues on covert visual search in macaque. Vision Research. 2013;76:43–49. doi: 10.1016/j.visres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomonaga M. Attentional capture in the chimpanzee (Pan troglodytes): Effects of a singleton distractor on visual search performances. Current Psychology Letters: Behaviour, Brain & Cognition. 2000;8:97–105. [Google Scholar]
- 44.Tomonaga M. Precuing the target location in visual searching by a chimpanzee (Pan troglodytes): Effects of precue validity. Japanese Psychological Research. 1997;39:200–211. [Google Scholar]
- 45.Lamy D, Tsal Y, Egeth HE. Does a salient distractor capture attention early in processing? Psychonomic Bulletin & Review. 2003;10:621–629. doi: 10.3758/bf03196524. [DOI] [PubMed] [Google Scholar]
- 46.Madden DJ, Parks EL, Davis SW, Diaz MT, Potter GG, Chou Y, Cabeza R. Age mediation of frontoparietal activation during visual feature search. Neuroimage. 2014;102:262–274. doi: 10.1016/j.neuroimage.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landman R, Sharma J, Sur M, Desimone R. Effect of distracting faces on visual selective attention in the monkey. Proceedings of the National Academy of Sciences. 2014;111:18037–18042. doi: 10.1073/pnas.1420167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AC. Comparison of cognitive function in human and nonhuman primates. Cognitive Brain Research. 1996;3:319–327. doi: 10.1016/0926-6410(96)00017-1. [DOI] [PubMed] [Google Scholar]
- 49.Matsuno T, Tomonaga M. Effects of target connectivity on multiple object tracking by chimpanzees (Pan troglodytes) The Japanese Journal of Psychonomic Science. 2008;27:123–124. [Google Scholar]
- 50.Smith JD, Coutinho MC, Church BA, Beran MJ. Executive-attentional uncertainty responses by rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: General. 2013;142:458–475. doi: 10.1037/a0029601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einhäuser W, Kruse W, Hoffmann K, König P. Differences of monkey and human overt attention under natural conditions. Vision Research. 2006;46:1194–1209. doi: 10.1016/j.visres.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko T, Tomonaga M. Differential reliance of chimpanzees and humans on automatic and deliberate control of motor actions. Cognition. 2014;131:355–366. doi: 10.1016/j.cognition.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Wheeler MA. Episodic memory and autonoetic awareness. In: Tulving E, Craik FIM, editors. Oxford handbook of memory. New York: Oxford University Press; 2000. pp. 597–608. [Google Scholar]
- 54.Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- 55.Corballis MC. Mirror neurons and the evolution of language. Brain and Language. 2010;112:25–35. doi: 10.1016/j.bandl.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 57.Clayton NS, Bussey TJ, Emery NJ, Dickinson A. Prometheus to Proust: The case for behavioural criteria for ‘mental time travel’. Trends in Cognitive Sciences. 2003;7:436–437. doi: 10.1016/j.tics.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Menzel CR. Progress in the study of chimpanzee recall and episodic memory. In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self-reflective consciousness. Oxford: Oxford University Press; 2005. pp. 188–224. [Google Scholar]
- 59.Martin-Ordas G, Berntsen D, Call J. Memory for distant past events in chimpanzees and orangutans. Current Biology. 2013;23:1438–1441. doi: 10.1016/j.cub.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Hampton RR, Schwartz BL. Episodic memory in nonhumans: what, and where, is when? Current Opinion in Neurobiology. 2004;14:192–197. doi: 10.1016/j.conb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Basile BM, Hampton RR. Monkeys recall and reproduce simple shapes from memory. Current Biology. 2011;21:774–778. doi: 10.1016/j.cub.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basile BM, Hampton RR. Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition. 2013;126:391–396. doi: 10.1016/j.cognition.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fivush R. The development of autobiographical memory. Annual Review of Psychology. 2011;62:559–582. doi: 10.1146/annurev.psych.121208.131702. [DOI] [PubMed] [Google Scholar]
- 64.Mason WA. Ontogeny of social behavior. In: Marler P, Vandenbergh JG, editors. Handbook of behavioral neurobiology, Volume 3, social behavior and communication. New York: Plenum Press; 1979. pp. 1–28. [Google Scholar]
- 65.Rumbaugh DM, Washburn DA. Intelligence of apes and other rational beings. New Haven: Yale University Press; 20003. [Google Scholar]
- 66.Mason WA. Environmental models and mental modes: Representational processes in the great apes and man. American Psychologist. 1976;31:284–294. [Google Scholar]
- 67.Menzel EW., Jr . Cognitive mapping in chimpanzees. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale: Lawrence Erlbaum Associates; 1978. pp. 375–422. [Google Scholar]
- 68.Ban SD, Boesch C, Janmaat KR. Taï chimpanzees anticipate revisiting high-valued fruit trees from further distances. Animal Cognition. 2014;17:1353–1364. doi: 10.1007/s10071-014-0771-y. [DOI] [PubMed] [Google Scholar]
- 69.Janmaat KRL, Polansky L, Ban SD, Boesch C. Wild chimpanzees plan their breakfast time, type, and location. Proceedings of the National Academy of Sciences. 2014;111:16343–16348. doi: 10.1073/pnas.1407524111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunningham E, Janson C. Integrating information about location and value of resources by white-faced saki monkeys (Pithecia pithecia) Animal Cognition. 2007;10:293–304. doi: 10.1007/s10071-007-0077-4. [DOI] [PubMed] [Google Scholar]
- 71.Garber PA. Role of spatial memory in primate foraging patterns: Saguinus mystax and Saguinus fuscicollis. American Journal of Primatology. 1989;19:203–216. doi: 10.1002/ajp.1350190403. [DOI] [PubMed] [Google Scholar]
- 72.Janson CH, Byrne R. What wild primates know about resources: opening up the black box. Animal Cognition. 2007;10:357–367. doi: 10.1007/s10071-007-0080-9. [DOI] [PubMed] [Google Scholar]
- 73.Noser R, Byrne RW. Travel routes and planning of visits to out-of-sight resources in wild chacma baboons, Papio ursinus. Animal Behaviour. 2007;73:257–266. [Google Scholar]
- 74.Lührs ML, Dammhahn M, Kappeler PM, Fichtel C. Spatial memory in the grey mouse lemur (Microcebus murinus) Animal Cognition. 2009;12:599–609. doi: 10.1007/s10071-009-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menzel CR. Solving ecological problems. In: Mitani JC, Silk JB, Palombit RA, Call J, Kappeler PM, editors. The evolution of primate societies. Chicago: University of Chicago Press; 2012. pp. 609–627. [Google Scholar]
- 76.Gould JL. The biology of learning. Annual Review of Psychology. 1986;37:163–192. doi: 10.1146/annurev.ps.37.020186.001115. [DOI] [PubMed] [Google Scholar]
- 77.Shettleworth SJ. Cognition, evolution, and behavior. New York: Oxford University Press; 2009. [Google Scholar]
- 78.Myowa-Yamakoshi M, Yamaguchi MK, Tomonaga M, Tanaka M, Matsuzawa T. Development of face recognition in infant chimpanzees (Pan troglodytes) Cognitive Development. 2005;20:49–63. [Google Scholar]
- 79.Kummer H. Rules of dyad and group formation among captive gelada baboons (Theropithecus gelada) In: Kondo S, Kawai M, Ehara A, Kawamura S, editors. Proceedings from the symposia of the 5th Congress of the International Primatological Society. Tokyo: Japan Science Press; 1975. pp. 129–159. [Google Scholar]
- 80.Schwartz BL, Meissner CA, Hoffman M, Evans S, Frazier LD. Event memory and misinformation effects in a gorilla (Gorilla gorilla gorilla) Animal Cognition. 2004;7:93–100. doi: 10.1007/s10071-003-0194-7. [DOI] [PubMed] [Google Scholar]
- 81.Menzel CR, Savage-Rumbaugh ES, Menzel EW. Bonobo (Pan paniscus) spatial memory and communication in a 20-hectare forest. International Journal of Primatology. 2002;23:601–619. [Google Scholar]
- 82.Savage-Rumbaugh ES, Murphy J, Sevcik RA, Brakke KE, Williams SL, Rumbaugh DM, Bates E. Language comprehension in ape and child. Monographs of the Society for Research in Child Development. 1993:58. (3-4, Serial No. 233) [PubMed] [Google Scholar]
- 83.Brakke KE, Savage-Rumbaugh ES. The development of language skills in Pan—II. Production. Language & Communication. 1996;16:361–380. [Google Scholar]
- 84.Roberts AI, Vick SJ, Roberts SGB, Menzel CR. Chimpanzees modify intentional gestures to coordinate a search for hidden food. Nature Communications. 2014;5 doi: 10.1038/ncomms4088. article 3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menzel CR. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology. 1999;113:426–434. doi: 10.1037/0735-7036.113.4.426. [DOI] [PubMed] [Google Scholar]
- 86.Menzel CR. Spatial cognition and memory in symbol-using chimpanzees. In: Mitchell R, Dolins F, editors. Spatial cognition, spatial perception: Mapping the self and space. New York: Cambridge University Press; 2010. pp. 539–564. [Google Scholar]
- 87.Putney RT. Willful apes revisited: The concept of prospective control. In: Washburn DA, editor. Primate perspectives on behavior and cognition. Washington: American Psychological Association; 2007. pp. 207–219. [Google Scholar]
- 88.Metcalfe J, Son L. Anoetic, noetic, and autonoetic metacognition. In: Beran MJ, Brandl JL, Perner J, Proust J, editors. Foundation of metacognition. Oxford, UK: Oxford University Press; 2012. pp. 289–301. [Google Scholar]
- 89.Sayers K, Menzel CR. Memory and foraging theory: chimpanzee utilization of optimality heuristics in the rank-order recovery of hidden foods. Animal Behaviour. 2012;84:795–803. doi: 10.1016/j.anbehav.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menzel CR, Menzel EW., Jr . Enquiries concerning chimpanzee understanding. In: de Waal FBM, Ferrari PF, editors. The primate mind. Cambridge: Harvard University Press; 2012. pp. 265–287. [Google Scholar]
- 91.Einstein GO, McDaniel MA. Prospective memory: Multiple retrieval processes. Current Directions in Psychological Science. 2005;14:286–290. [Google Scholar]
- 92.Hicks JL, Marsh RL, Cook GI. Task interference in time-based, event-based, and dual intention prospective memory conditions. Journal of Memory and Language. 2005;53:430–444. [Google Scholar]
- 93.Kliegel M, Mackinlay R, Jager T. Complex prospective memory: Development across the lifespan and the role of task interruption. Developmental Psychology. 2008;44:612–617. doi: 10.1037/0012-1649.44.2.612. [DOI] [PubMed] [Google Scholar]
- 94.Marsh RJ, Hancock TW, Hicks JL. The demands of an ongoing activity influence the success of event-based prospective memory. Psychonomic Bulletin and Review. 2002;9:604–610. doi: 10.3758/bf03196319. [DOI] [PubMed] [Google Scholar]
- 95.Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- 96.McDaniel MA, Einstein GO. Los Angeles: Sage Publications; 2007. [Google Scholar]
- 97.Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in the radial maze. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:453–469. [PubMed] [Google Scholar]
- 98.Correia SPC, Dickinson A, Clayton NS. Western scrub-jays anticipate future needs independently of their current motivational state. Current Biology. 2007;17:856–861. doi: 10.1016/j.cub.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 99.Feeney MC, Roberts WA, Sherry DF. Black-capped chickadees (Poecile atricapillus) anticipate future outcomes of foraging choices. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:30–40. doi: 10.1037/a0019908. [DOI] [PubMed] [Google Scholar]
- 100.Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- 101.Mulcahy NJ, Call J. Apes save tools for future use. Science. 2006;312:1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- 102.Volter CJ, Call J. Younger apes and human children plan their moves in a maze task. Cognition. 2014;130:186–203. doi: 10.1016/j.cognition.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Wilson AG, Crystal JD. Prospective memory in the rat. Animal Cognition. 2012;15:349–358. doi: 10.1007/s10071-011-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson AG, Pizzo MJ, Crystal JD. Event-based prospective memory in the rat. Current Biology. 2013;23:1089–1093. doi: 10.1016/j.cub.2013.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beran MJ, Perdue BM, Bramlett JL, Menzel CR, Evans TA. Prospective memory in a language-trained chimpanzee (Pan troglodytes) Learning and Motivation. 2012;43:192–199. doi: 10.1016/j.lmot.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perdue BM, Beran MJ, Williamson RA, Gonsiorowski A, Evans TA. Prospective memory in children and chimpanzees. Animal Cognition. 2014;17:287–295. doi: 10.1007/s10071-013-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans TA, Perdue BM, Beran MJ. The relationship between event-based prospective memory and ongoing task performance in chimpanzees (Pan troglodytes) PLoS ONE. 2014;9:e112015. doi: 10.1371/journal.pone.0112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guynn MJ. A two-process model of strategic monitoring in event-based prospective memory: Activation/retrieval mode and checking. International Journal of Psychology. 2003;38:245–256. [Google Scholar]
- 109.Templer VL, Hampton RR. Episodic memory in nonhuman animals. Current Biology. 2013;23:R801–R806. doi: 10.1016/j.cub.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tu H, Hampton RR. Control of working memory in rhesus monkeys (Macaca mulatta) Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:467–476. doi: 10.1037/xan0000030. [DOI] [PubMed] [Google Scholar]
- 111.Dunlosky J, Metcalfe J. Metacognition. Los Angeles: Sage; 2009. [Google Scholar]
- 112.Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- 113.Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. Psychology of Learning and Motivation. 1990;26:125–141. [Google Scholar]
- 114.Shields WE, Smith JD, Guttmannova K, Washburn DA. Confidence judgments by humans and rhesus monkeys. Journal of General Psychology. 2005;132:165–186. [PMC free article] [PubMed] [Google Scholar]
- 115.Smith JD, Shields WE, Schull J, Washburn DA. The uncertain response in humans and animals. Cognition. 1997;62:75–97. doi: 10.1016/s0010-0277(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 116.Smith JD, Shields WE, Washburn DA. The comparative psychology of uncertainty monitoring and metacognition. The Behavioral and Brain Sciences. 2003;26:317–373. doi: 10.1017/s0140525x03000086. [DOI] [PubMed] [Google Scholar]
- 117.Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty states and reinforcement signals in the comparative study of metacognition. Journal of Experimental Psychology: General. 2006;135:282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- 118.Smith JD, Shields WE, Allendoerfer KR, Washburn DA. Memory monitoring by animals and humans. Journal of Experimental Psychology: General. 1998;127:227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- 119.Basile BM, Schroeder GR, Brown EK, Templer VL, Hampton RR. Evaluation of seven hypotheses for metamemory performance in rhesus monkeys. Journal of Experimental Psychology: General. 2015;144:85–102. doi: 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Basile BM, Hampton RR. Metacognition as discrimination: Commentary on Smith et al. (2014) Journal of Comparative Psychology. 2014;128:135–137. doi: 10.1037/a0034412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carruthers P. Meta-cognition in animals: A skeptical look. Mind and Language. 2008;23:58–89. [Google Scholar]
- 122.Crystal JD. Where is the skepticism in animal metacognition? Journal of Comparative Psychology. 2014;128:152–154. doi: 10.1037/a0034427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Crystal JD, Foote AL. Metacognition in animals. Comparative Cognition and Behavior Reviews. 2009;4:1–16. doi: 10.3819/ccbr.2009.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hampton RR. Multiple demonstrations of metacognition in nonhumans: Converging evidence or multiple mechanisms? Comparative Cognition and Behavior Reviews. 2009;4:17–28. doi: 10.3819/ccbr.2009.40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jozefowiez J, Staddon JER, Cerutti DT. Metacognition in animals: How do we know that they know? Comparative Cognition and Behavior Reviews. 2009;4:29–39. [Google Scholar]
- 126.Kepecs A, Mainen ZF. A computational framework for the study of confidence in humans and animals. Philosophical Transactions of the Royal Society, B. 2012;367:1322–1337. doi: 10.1098/rstb.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kornell N. Metacognition in humans and animals. Current Directions in Psychological Science. 2009;18:11–15. [Google Scholar]
- 128.Kornell N. Where is the "meta" in animal metacognition? Journal of Comparative Psychology. 2014;128:143–149. doi: 10.1037/a0033444. [DOI] [PubMed] [Google Scholar]
- 129.Le Pelley ME. Metacognitive monkeys or associative animals? Simple reinforcement learning explains uncertainty in nonhuman animals. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2012;38:686–708. doi: 10.1037/a0026478. [DOI] [PubMed] [Google Scholar]
- 130.Smith JD. The study of animal metacognition. Trends in Cognitive Sciences. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 131.Smith JD, Beran MJ, Couchman JJ, Coutinho MVC, Boomer J. Animal metacognition: Problems and prospects. Comparative Cognition and Behavior Reviews. 2008;4:33–46. [Google Scholar]
- 132.Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beran MJ, Perdue BM, Futch SE, Smith JD, Evans TA, Parrish AE. Go when you know: Chimpanzees’ confidence movements reflect their responses in a computerized memory task. Cognition. 2015;142:236–246. doi: 10.1016/j.cognition.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beran MJ, Smith JD, Perdue BM. Language-trained chimpanzees name what they have seen, but look first at what they have not seen. Psychological Science. 2013;24:660–666. doi: 10.1177/0956797612458936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Call J. Do apes know that they could be wrong? Animal Cognition. 2010;13:689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- 137.Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- 138.Foote AL, Crystal JD. “Play it again”: A new method for testing metacognition in animals. Animal Cognition. 2012;15:187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hampton RR, Hampstead BM. Spontaneous behavior of a rhesus monkey (Macaca mulatta) during memory tests suggests memory awareness. Behavioural Processes. 2006;72:184–189. doi: 10.1016/j.beproc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- 142.Kornell N, Son L, Terrace H. Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 143.Marsh HL, MacDonald SE. Orangutans (Pongo abelii) “play the odds”: Information-seeking strategies in relation to cost, risk, and benefit. Journal of Comparative Psychology. 2012;126:263–278. doi: 10.1037/a0025906. [DOI] [PubMed] [Google Scholar]
- 144.Morgan G, Kornell N, Kornblum T, Terrace HS. Retrospective and prospective metacognitive judgments in rhesus macaques (Macaca mulatta) Animal Cognition. 2014;17:249–257. doi: 10.1007/s10071-013-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith JD, Redford JS, Beran MJ, Washburn DA. Rhesus monkeys (Macaca mulatta) adaptively monitor uncertainty while multi-tasking. Animal Cognition. 2010;13:93–101. doi: 10.1007/s10071-009-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suda-King C, Bania AE, Stromberg EE, Subiaul F. Gorillas’ use of the escape response in object choice memory tests. Animal Cognition. 2013;16:65–84. doi: 10.1007/s10071-012-0551-5. [DOI] [PubMed] [Google Scholar]
- 147.Templer VL, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Animal Cognition. 2012;15:409–419. doi: 10.1007/s10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zakrzewski AC, Perdue BM, Beran MJ, Church BA, Smith JD. Cashing out: The decisional flexibility of uncertainty responses in rhesus macaques (Macaca mulatta) and humans (Homo sapiens) Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40:490–501. doi: 10.1037/xan0000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwartz BL. Working memory load differentially affects tip-of-the-tongue states and feeling-of-knowing judgment. Memory & Cognition. 2008;36:9–19. doi: 10.3758/mc.36.1.9. [DOI] [PubMed] [Google Scholar]
- 150.Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: a test with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:389–395. [Google Scholar]
- 151.Sutton JE, Shettleworth SJ. Memory without awareness: pigeons do not show metamemory in delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- 152.Adams A, Santi A. Pigeons exhibit higher accuracy for chosen memory tests than for forced memory tests in duration matching-to-sample. Learning and Behavior. 2011;39:1–11. doi: 10.1007/s13420-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 153.Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E. Do pigeons (Columba livia) study for a test? Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- 154.Rachlin H, Green L. Commitment, choice, and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baumeister RF, Vohs KD, editors. Handbook of self-regulation: research theory and applications. New York: Guilford Press; 2004. p. 592. [Google Scholar]
- 156.Mischel W. Processes in delay of gratification. In: Berkowitz L, editor. Advances in Experimental Social Psychology. New York: Academic Press; 1974. pp. 249–292. [Google Scholar]
- 157.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 158.Mischel W, Ebbesen EB. Attention in delay of gratification. Journal of Personality and Social Psychology. 1970;16:329–337. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- 159.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 160.Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 161.Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 162.Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biology Letters. 2005;1:223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stevens JR, Mühlhoff N. Intertemporal choice in lemurs. Behavioural Processes. 2012;89:121–127. doi: 10.1016/j.beproc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 164.Tobin H, Logue AW, Chelonis JJ, Ackerman KT, May JG. Self-control in the monkey Macaca fascicularis. Animal Learning & Behavior. 1996;24:168–174. [Google Scholar]
- 165.Ainslie GW. Impulse control in pigeons. Journal of the Experimental Analysis of Behavior. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Current Biology. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 167.Stevens JR, Rosati AG, Ross KR, Hauser MD. Will travel for food: Spatial discounting in two new world monkeys. Current Biology. 2005;15:1855–1860. doi: 10.1016/j.cub.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paglieri F, Focaroli V, Bramlett J, Tierno V, McIntyre JM, Addessi E, Evans TA, Beran MJ. The hybrid delay task: Can capuchin monkeys (Cebus apella) sustain a delay after an initial choice to do so? Behavioural Processes. 2013;94:45–54. doi: 10.1016/j.beproc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boysen ST, Berntson GG. Responses to quantity: perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- 170.Addessi E, Rossi S. Tokens improve capuchin performance in the reverse–reward contingency task. Proceedings of the Royal Society of London B: Biological Sciences. 2010;278:849–854. doi: 10.1098/rspb.2010.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Silberberg A, Fujita K. Pointing at smaller food amounts in an analogue of Boysen and Berntson's (1995) procedure. Journal of the Experimental Analysis of Behavior. 1996;66:143–147. doi: 10.1901/jeab.1996.66-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anderson JR, Awazu S, Fujita K. Can squirrel monkeys (Saimiri sciureus) learn self-control? A study using food array selection tests and reverse-reward contingency. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:87–97. doi: 10.1037//0097-7403.26.1.87. [DOI] [PubMed] [Google Scholar]
- 173.Boysen ST, Mukobi KL, Berntson GG. Overcoming response bias using symbolic representations of number by chimpanzees (Pan troglodytes) Animal Learning and Behavior. 1999;27:229–235. [Google Scholar]
- 174.Mischel W. Objective and subjective rules for delay of gratification. In: d’Y-dewalle G, Lens W, editors. Cognition in Human Learning and Motivation. Hillsdale, NJ: Erlbaum; 1981. pp. 33–58. [Google Scholar]
- 175.Mischel W, Baker N. Cognitive appraisals and transformations in delay behavior. Journal of Personality and Social Psychology. 1975;31:254–261. [Google Scholar]
- 176.Toner IJ, Smith RA. Age and overt verbalization in delay-maintenance behavior in children. Journal of Experimental Child Psychology. 1977;24:123–128. [Google Scholar]
- 177.Stevens JR, Rosati AG, Heilbronner SR, Mühlhoff N. Waiting for grapes: Expectancy and delayed gratification in bonobos. International Journal of Comparative Psychology. 2011;24:99–111. [Google Scholar]
- 178.Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) The Journal of General Psychology. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- 179.Parrish AE, Perdue BM, Stromberg EE, Bania AE, Evans TA, Beran MJ. Delay of gratification by orangutans (Pongo pygmaeus) in the accumulation task. Journal of Comparative Psychology. 2014;128:209–214. doi: 10.1037/a0035660. [DOI] [PubMed] [Google Scholar]
- 180.Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): The effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behavioural Processes. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 181.Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biology Letters. 2007;3:599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Evans TA, Perdue BM, Parrish AE, Menzel EC, Brosnan SF, Beran MJ. How is chimpanzee self-control influenced by social setting? Scientifica. 2012:654094. doi: 10.6064/2012/654094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Parrish AE, Perdue BM, Evans TA, Beran MJ. Chimpanzees (Pan troglodytes) transfer tokens repeatedly with a partner to accumulate rewards in a self-control task. Animal Cognition. 2013;16:627–636. doi: 10.1007/s10071-013-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Evans TA, Beran MJ. Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta) The Journal of General Psychology. 2007;134:199–216. doi: 10.3200/GENP.134.2.199-216. [DOI] [PubMed] [Google Scholar]
- 185.Evans TA, Beran MJ, Paglieri F, Addessi E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): When quantity is salient, symbolic stimuli do not improve performance. Animal Cognition. 2012;15:539–548. doi: 10.1007/s10071-012-0482-1. [DOI] [PubMed] [Google Scholar]
- 186.Pelé M, Micheletta J, Uhlrich P, Thierry B, Dufour V. Delay maintenance in Tonkean macaques (Macaca tonkeana) and brown capuchin monkeys (Cebus apella) International Journal of Primatology. 2011;32:149–166. [Google Scholar]
- 187.Beran MJ, Rossettie MS, Parrish AE. Trading up: Chimpanzees (Pan troglodytes) show self-control through their exchange behavior. Animal Cognition. 2016;19:109–121. doi: 10.1007/s10071-015-0916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bourjade M, Thierry B, Call J, Dufour V. Are monkeys able to plan for future exchange? Animal Cognition. 2012;15:783–795. doi: 10.1007/s10071-012-0502-1. [DOI] [PubMed] [Google Scholar]
- 189.Drapier M, Chauvin C, Dufour V, Uhlrich P, Thierry B. Food-exchange with humans in brown capuchin monkeys. Primates. 2005;46:241–248. doi: 10.1007/s10329-005-0132-1. [DOI] [PubMed] [Google Scholar]
- 190.Dufour V, Pelé M, Sterck EH, Thierry B. Chimpanzee (Pan troglodytes) anticipation of food return: Coping with waiting time in an exchange task. Journal of Comparative Psychology. 2007;121:145–155. doi: 10.1037/0735-7036.121.2.145. [DOI] [PubMed] [Google Scholar]
- 191.Pelé M, Dufour V, Micheletta J, Thierry B. Long-tailed macaques display unexpected waiting abilities in exchange tasks. Animal Cognition. 2010;13:263–271. doi: 10.1007/s10071-009-0264-6. [DOI] [PubMed] [Google Scholar]
- 192.Bramlett JL, Perdue BM, Evans TA, Beran MJ. Capuchin monkeys (Cebus apella) let lesser rewards pass them by to get better rewards. Animal Cognition. 2012;15:963–969. doi: 10.1007/s10071-012-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Evans TA, Westergaard GC. Self-control and tool use in tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- 194.Addessi E, Paglieri F, Beran MJ, Evans TA, Macchitella L, De Petrillo F, Focaroli V. Delay choice versus delay maintenance: Different measures of delayed gratification in capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2013;127:392–398. doi: 10.1037/a0031869. [DOI] [PMC free article] [PubMed] [Google Scholar]