Abstract
In allergic asthma, inhalation of airborne allergens such as house dust mite (HDM) effectively activates both innate and adaptive immunity in the lung mucosa. To determine the role of the eicosanoid PGI2 and its receptor IP during allergic airway sensitization, HDM responses in mice lacking a functional IP receptor (IP−/−) were compared to wild type (WT) mice. Surprisingly, IP−/− mice had increased numbers of pulmonary CD3−NK1.1+Ly49b+ NK cells producing IFN-γ that was inversely associated with the number of type 2 innate lymphoid cells (ILC2s) expressing IL-33Rα and IL-13 compared to WT animals. This phenomenon was associated with elevated CX3CL1 levels in the airways of IP−/− mice and treatment with a neutralizing antibody to CX3CL1 reduced IFN-γ production by the lung NK cells. Remarkably, IP−/− mice were less responsive to HDM challenge than WT counterparts since intranasal instillation of the allergen induced markedly reduced levels of airway eosinophils, CD4+ lymphocyte infiltration and mucus production, as well as depressed levels of CCL2 chemokine and Th2 cytokines. NK cells were responsible for such attenuated responses since depletion of NK1.1+ cells in IP−/− mice restored both the HDM-induced lung inflammation and ILC2 numbers, while transfer of CD3−NK1.1+ NK cells into the airways of WT hosts suppressed the inflammatory response. Collectively, these data demonstrate a hitherto unknown role for PGI2 in regulating the number and properties of NK cells resident in lung tissue and reveal a role for NK cells in limiting lung tissue ILC2s and preventing allergic inflammatory responses to inhaled HDM allergen.
Keywords: Rodent, Natural Killer Cells, Th1/Th2 Cells, Lipid Mediators, Allergy, Inflammation, Transgenic/Knockout Mice, Lung and Mucosa
INTRODUCTION
Asthma is largely associated with atopy, typified by an IgE response to known allergens. Exacerbations of this disease are coincident with Th2-mediated airway inflammation characterized by the infiltration of eosinophils, goblet cell hyperplasia and airway remodeling resulting in airway hyperreactivity and luminal narrowing. In highly populated areas of North and South America, almost 85% of asthmatics are allergic to house dust mite (HDM) (1, 2). It seems likely that an improved understanding of the cellular and molecular events that underpin bronchial sensitization to allergens will prove prerequisite for the development of novel intervention strategies. The interaction of HDM allergens with innate cells in the respiratory tract is a critical event that precedes adaptive immunity. In this context, HDM entering the mouse airway has been shown to interact with formyl peptide receptors on eosinophils (3), protease activated receptor-2 on epithelial cells (4), TLR4 on stromal cells (5) and Dectin-2 on dendritic cells (DC) (6) and alveolar macrophages (7).
Natural Killer (NK) cells are effector cells of innate immunity classically recognized for their ability to identify and kill tumors and virally-infected cells (8). Such reactivity is tightly regulated by an expansive system of activating (e.g. NKp46 and NKG2D) and inhibiting (e.g. NKG2A and NKG2B) receptors expressed on the surface of NK cells (9, 10). Recent advances in the understanding of NK cell interaction with other innate and adaptive immune cell populations has generated interest in the immunoregulatory role of NK cells beyond their capacity as ‘killers’. NK cells residing in the lung mucosal tissue have been described previously, however, their relationship to conventional circulating NK cells remains unclear (11–13). Previous reports have investigated the effect of NK cells on allergic lung inflammation with contradictory findings, with some instances inhibiting allergic lung inflammation (14, 15) and in others promoting eosinophilic inflammation (16, 17). Importantly, NK cells are responsive to a range of endogenously produced eicosanoids which include Resolvin-E1 (18), Lipoxin-A4 (19), PGD2 (20), PGE2 (21) and leukotrienes (22). Eicosanoids generated in the lung likely regulate the inflammatory response and provide a means to integrate the opposing needs of maintaining the normal barrier function of both endothelial and epithelial surfaces yet facilitate immune responses to airborne pathogens and allergens. The early events elicited by the interaction of allergen with the innate immune system include the biosynthesis of cysteinyl leukotrienes and prostaglandins (23–25). PGI2 (also known as prostacyclin) is a metabolite of arachidonic acid and forms a prominent component of the COX-2 response (reviewed in (26)). This mediator plays a critical role in pain perception and inflammation and is a potent vasodilator and inhibitor of platelet aggregation (27).
To resolve the contribution of PGI2 and its IP receptor during sensitization of the airways to the common airborne allergen HDM, lung mucosal immune responses were examined in the IP−/− mice and compared to WT counterparts. In mice lacking IP, the size of the NK cell pool in the lung tissue was increased two-fold and these cells produced high levels of IFN-γ. Remarkably, IP−/− mice were less responsive to HDM allergen sensitization than WT counterparts since intranasal instillation of the allergen induced markedly reduced levels of eosinophils, CD4+ lymphocyte infiltration and mucus production in the airways. The reduced allergic inflammation was associated with a decrease in the number of ILC2s in the lungs of HDM challenged IP−/− mice. NK cells appeared linked to the reduced ILC2s numbers since depletion of NK cells in IP−/− mice restored ILC2 numbers. These findings reveal a previously unknown capacity for PGI2 in regulating pNK cells and reveal a role for NK cells in preventing airway sensitization to inhaled allergen and subsequent development of HDM-induced allergic lung inflammation.
MATERIALS AND METHODS
Mice
C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) and IP−/− mice (10–16 week old) were used throughout this study. IP−/− mice were originally developed and kindly provided by Dr. Garret A. FitzGerald’s Laboratory, Institute for Translational Medicine and Therapeutics, Philadelphia, PA. All mice were bred under pathogen-free conditions in a barrier facility. Experimental animals were maintained in micro-isolator cages and treated in accordance with National Institutes of Health guidelines and the American Association of Laboratory Animal Care regulations. Animal experiments were approved by the University of Montana, Institutional Animal Care and Use Committee (IACUC) according to National Institute of Health guidelines.
Intra-nasal administration of house dust mite allergen
To elicit allergic lung inflammation, mice were sensitized to HDM on day 0 by the intra-nasal administration of 100μg of HDM allergen (Dermatophagoides pteronyssinus, Greer Laboratories, Lenoir, NC) in 30μl of PBS and then on days 7 and 14 by intranasal treatment with 50μg of HDM (30μl total volume). HDM allergen preparations used throughout this study contained minimal levels of LPS. Control groups comprised mice receiving 30μl of PBS on days 0, 7 and 14. To determine the level of mucosal inflammation, BALF and lung tissue were harvested 48h after the last challenge on day 16.
Determining the level of pulmonary inflammation
Bronchoalveolar lavage was performed to collect bronchoalveolar lavage fluid (BALF) for analysis. Eosinophil peroxidase (EPO) levels in the lavage cells were determined by colorimetric analysis. Cell differential percentages were determined by light microscopic evaluation of Hema3-stained cytospin preparations and expressed as absolute cell numbers. Lung tissue was dispersed by collagenase (Type IV; Sigma-Aldrich, St Louis, MO), and lung mononuclear cells (LMC) were isolated by Percoll (Sigma-Aldrich) density gradient for functional analysis.
CX3CL1 immunochemical detection and neutralization in vivo
Paraffin-embedded lung tissues were deparaffinized and treated with 0.3% H2O2, followed by blocking non-specific binding and the sections were then incubated with 5 μg/ml goat anti-mouse CX3CL1 polyclonal Ab (R&D Systems, Minneapolis, MN) at 4°C overnight. After washing, they were incubated with horse anti-goat IgG peroxidase (Vector Labs, Burlingame, CA) for 1hr at room temperature. Peroxidase binding sites were amplified with avidin-biotin complex reagent (ABC reagent, Vector Labs) for 1hr, and peroxidase activity was visualized using diaminobenzidine. Sections were counterstained with hematoxylin and visualized using Nikon Eclipse 800 light microscope equipped with Olympus DP71 camera and cellSens software.
In certain experiments, CX3CL1 activity was inhibited by the administration of a neutralizing mAb to CX3CL1 (rat IgG2A, R&D Systems) or control IgG2A isotope mAb by oropharyngeal instillation (25 μg in 30μl PBS over a 6 day period on days 0 and 3) into the airways of naïve mice prior to isolating lung tissues for generating LMC and enumeration of pNK cell numbers (on day 6).
Measurement of cytokines, chemokines and eicosanoids
To examine cytokine production in vitro, dispersed LMC or spleen cells were stimulated with immobilized anti-CD3, (2 μg/ml, 2C11, ATCC), anti-NKp46 (10μg/ml, 29A1.4 Biolegend, San Diego, CA) or anti-NK1.1 antibody (PK136, 20μg/ml) in the absence or presence of 10ng/ml mouse IL-2 (R&D Systems). After culture for 24h, supernatants were harvested and cytokines (IL-4, IL-5, IL-13, TNF-α, IFN-γ, IL-12, IL-18 and IL-15) measured by ELISA (R&D Systems).
BALF chemokine or cytokine levels were determined using ELISA for measurement of CCL2, CX3CL1 (Duoset, R&D Systems) and IL-13 (Quantikine, R&D Systems), or using the sensitive Mouse V-Plex Pro-Inflammatory Panel 1 assay and Meso Quickplex 120 reader (MesoScale Discovery, MD) for measurement of other cytokines (IL-4, IL-5, TNF-α, IFN-γ and IL-12p70). Prostaglandin and cysteinyl leukotriene levels in the BALF were measured using prostaglandin screening and cysteinyl leukotriene ELISA kits (Cayman Chemical, Ann Arbor, MI).
Histological determination of peribronchial inflammation and goblet cell hyperplasia
Lung tissue was fixed in 4% paraformaldehyde and embedded in paraffin using a Leica ASP 300 tissue processor (Leica, Bannockburn, IL). Microtome sections were cut at 5-μm thickness and stained with hematoxylin and eosin (H&E) using a Shandon Varistain 24–4 (Thermo Fisher Scientific). Alternatively, sections were stained using periodic acid-Schiff (PAS) reagent. The level of peribronchial inflammation (H&E stained) or mucus production (PAS stained) was analyzed by microscopy and the transmitted light images were collected on a Nikon Eclipse 800 microscope equipped with an Olympus DP 71 camera and cellSens software (Version 1.9).
In vivo depletion of NK1.1+ NK cells
In order to determine the in vivo effects of NK cells on allergic lung inflammation, NK1.1+ cells were depleted using anti-NK1.1 antibody. The anti-NK1.1 MoAb (PK136 obtained from the American Type Culture Collection (ATCC), Manassas, VA) was purified by protein-A affinity chromatography (28). Briefly, C57BL/6 WT and IP−/− mice were injected i.p with 250 μg anti-NK1.1 antibody or control IgG (mouse IgG2a) 24h prior to the start of HDM challenge and then twice weekly for a period of 2 weeks (on days -1, 3, 6, 10 and 13). Mice were challenged with PBS (control) or HDM allergen on days 0, 7 and 14 with the allergic inflammatory response characterized on day 16. In certain experiments, naïve IP−/− or WT mice were similarly treated with the anti-NK1.1 or control mAb, twice weekly over a period of 2 weeks. After treatment the number of NK cells remaining in lungs and spleen was determined by enumerating CD3−NK1.1+NKp46+ cells.
Purification of splenic NK cells and transfer to the airways
NK cells were purified by magnetic cell sorting (MagCellect, R&D Systems) of spleen cells prepared from WT or IP−/− mice. Sorted cells were selected on the basis of being CD3−NK1.1+ and purity was checked by measuring the proportion of CD3−NK1.1+ cells (87–92% over three experiments). Purified splenic NK cells (5×105) were suspended in RPMI (lacking FBS) and instilled directly into the airways of WT mice by the oropharyngeal route in a 30μl volume 24h after the start of HDM allergen sensitization period as detailed above. Purified NK cells were pretreated with 10ng/ml of IL-2 prior to transfer into hosts to maintain cell viability and function. Sham control mice received an equivalent suspension media alone by the oropharyngeal route. Mice were subsequently challenged with 50μg of HDM allergen on days 7 and 14 and the airway inflammatory characterized on day 16. Examination of the eosinophilic infiltration into the airways was determined by cell differential counts and measuring cell-associated EPO activity.
Flow Cytometry
Cells (LMC, BALF or splenic cells) were FcγR blocked using 2.4G2 antibody (ATCC) and stained with combinations of the following mouse conjugated mAb (all purchased from BioLegend): allophycocyanin (APC) or FITC anti-CD3, APC/Cy7 anti-CD4, PE anti-CD8a, APC-Cy7 anti-CD19, APC or PE anti-CD49b DX5 (pan-NK cells) or Ly49a, FITC or APC anti-NK1.1 (PK136), PE or APC anti-CD335 (NKp46), PE or APC CD27, PE anti-CD94 (NKG2), FITC or PE anti-NKG2D (CD314), APC or PE anti-CD11c, PE or APC/Cy7 anti-I-A/I-E, APC/Cy7 anti-Ly6G, APC or APC/Cy7 anti-Ly6C, APC/Cy7 anti-Ly-6G/Ly6C (Gr1), PE, FITC or Brilliant Violet 421 anti-CD11b, APC or PE anti-F4/80. In addition, PE anti-Siglec-F (BD Biosciences, to stain eosinophils), Alexa Fluor® 647 anti-Dectin-2 (AbD Serotec), and PE or APC anti-CX3CR1 (R&D Systems) mAb were used. For intracellular staining, FITC anti-granzyme B, APC anti-granzyme A, and APC anti-IFN-γ, (all antibodies from BioLegend) were utilized and cells stained intracellularly as previously (29). Flow cytometric acquisition was performed on a FACSAria II (BD Biosciences, San Jose, CA) by 4-color analysis using FACSDiVa software and FlowJo, and a minimum of 50,000 live, single-cell events collected. Lung ILC2s were lineage negative (Lin−) cells that stained with Thy1.2/CD90.2 (AlexaFluor 405), CD45 (CLA) (APC/Cy7), and IL-33Rα/ST2 (APC) mAb (all from Biolegend). Lin− cell determination was attributed to cells that were CD3−, CD4−, CD8α−, CD5−, CD11c−, CD19−, B220−, TCRβ−, γδ-TCR−, Gr1−, NK1.1−, TER-119− (all FITC mAb from BioLegend) and Siglec-F− (using PE mAb, BD Biosciences). Additionally, eFluor® 660 anti-IL-13 (Affymetrix eBioscience, San Diego, CA) was utilized for intracellular staining. Flow cytometric acquisition was performed on a FACSAria II (BD Biosciences) by 6-color analysis using FACSDiva software or Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA).
Statistical analyses
Data were analyzed using GraphPad Prism 5.0 (GraphPad, La Jolla, CA). Results involving two variables were analyzed by two-way ANOVA with a Bonferroni post test. Data comparing two groups were analyzed using an unpaired t test. Figures show combined data from multiple studies or independent repeats (two or more). Data shown are mean ± SEM. A p value < 0.05 was considered statistically significant.
RESULTS
The lungs of mice lacking the PGI2 receptor, IP−/−, contain increased numbers of NK cells that express elevated levels of NKp46
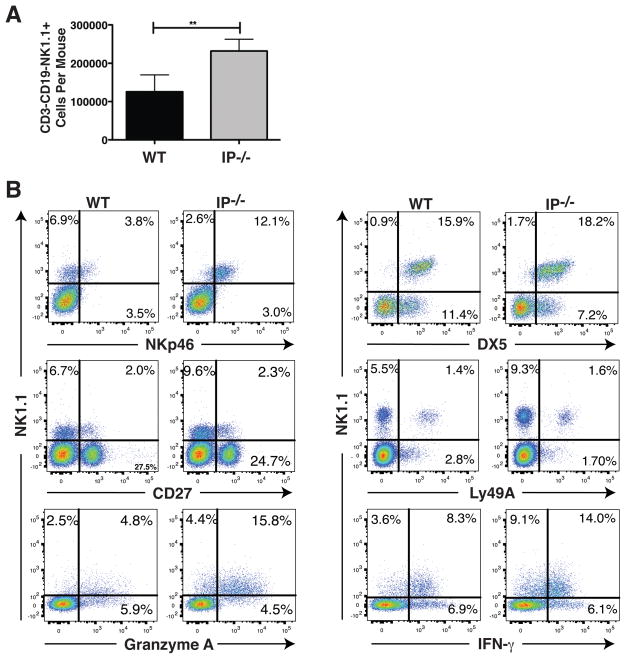
Pulmonary NK cells (pNK) were characterized in IP−/− mice following dispersion of lung tissue using collagenase and the resulting LMC examined by multicolor flow cytometry. The NK1.1 alloantigen (a member of the Mkrp1c gene family) was used as a specific marker for NK cells (30). CD3−CD19−NK1.1+ pNK cells from IP−/− mice were enumerated and compared with C57BL/6 WT counterparts. Unexpectedly, a 2-fold increase in the number of pNK cells was found in the lungs of IP−/− compared to WT mice (2.3×105 vs 1.2×105 NK cells per mouse respectively, Fig. 1A), although the total number of leukocytes present in the lungs of these mice was similar. Given the elevated numbers of IP−/− pNK cells it was important to establish their relationship to circulating NK cells. To this end, the expression of known NK differentiation markers by CD3−CD19−NK1.1+ cells was evaluated. IP−/− pNK cells expressed over 4-fold elevated levels of NKp46 (CD335 or natural cytotoxicity triggering receptor-1) compared to WT NK cells (Fig. 1B). In marked contrast to the elevated pNK cell numbers, the number of CD3−CD19−NK1.1+ NK cells present in spleen, bone marrow, axillary and mesenteric lymph nodes, and Peyer’s Patches of IP−/− mice was not significantly different from that observed in WT mice. However, NKp46 expression by NK cells in IP−/− mice in all such tissue sites examined was elevated and comparable to pNK cells (Supplemental Fig. S1). The IP−/− pNK cells were indistinguishable from circulating NK cells since >95% of CD3−CD19−NK1.1+ pNK cells were DX5+ (the α2 integrin CD49b), a late NK maturation marker (Fig. 1B). Subsets of pNK cells in IP−/− mice expressed CD27 and Ly49A (approx. 19.9% and 14.7%, respectively) but no appreciable difference to that observed in WT mice was evident. Intracellular staining of the pulmonary cells revealed that the majority (>95%) of the IP−/− pNK cells expressed granzyme A (and granzyme B, data not shown). Innate lymphoid cells have also been reported to express NKp46 in some instances but these cells typically co-express CD127 (31). CD127 was universally absent from the CD3−CD19-NK1.1+ pNK cells in both IP−/− and WT mice (data not shown). Collectively, these results demonstrate that IP−/− mice have elevated numbers of NK cells expressing NKp46, DX5, granzymes A and B, and revealed that the pNK cells are indistinguishable from mature NK cells.
FIGURE 1.
IP−/− mice exhibit increased numbers of pulmonary NK cells that express elevated levels of NKp46. Lung tissue from C57BL/6 WT and IP−/− mice (6 per group) was dispersed using collagenase to prepare lung mononuclear cells (LMC) and the characteristics of CD3−CD19−NK1.1+ NK cells was determined by 4-color flow cytometry. (A) After gating on CD3−CD19− cells, the total number of NK1.1+ NK cells was assessed, and (B) the level of NKp46, DX5, CD27 and Ly49A expression as well as intra-cellular Granzyme A and IFN-γ expression by the NK1.1+ NK cells was determined. Data are mean ± SEM and expressed as total number of lung CD3−CD19−NK1.1+ NK cells per mouse (n = 6), **p < 0.01. Results are representative of 3 independent experiments.
Increased production of IFN-γ by pNK cells in IP−/− mice
Although largely recognized by their ability to mediate cytolytic activity, NK cells are also an important source of pro-inflammatory cytokines that include IFN-γ and TNF-α. Given the elevated numbers of NK1.1+ NK cells present in IP−/− lungs, we next examined the levels of IFN-γ and TNF-α produced by these pNK cells. Intracellular staining of IFN-γ revealed that the majority of pNK in WT and IP−/− mice expressed this cytokine (Fig. 1B). Since NK1.1 and NKp46 are “activating” receptors, an effective way to determine cytokine production by NK cells was to determine LMC response to immobilized anti-NK1.1 or anti-NKp46 antibodies. Cytokine release from LMC was determined using antibody coated plates in the absence or presence of exogenous IL-2 as detailed previously (32). A significant elevation in IFN-γ production in response to anti-NKp46 or anti-NK1.1 stimulation (in the presence of IL-2) was observed in LMC from IP−/− mice (2-fold or more) compared with WT counterparts (Fig. 2). These responses are consistent with a 2-fold increase in the number of NK1.1+ cells present in the lungs and elevated NKp46 expression by NK cells. Interestingly, a slight elevation in IFN-γ production by LMC from IP−/− mice was also observed in the presence of exogenous IL-2 alone or spontaneously, possibly due to elevated myeloid cells in these mice (Fig. 2). In both IP−/− and WT LMC the amount of production of TNF-α by pNK was negligible and <40pg/ml. Since NK cell are responsive to the cytokines IL-12, IL-18 and IL-15, we examined whether any of these cytokines were elevated in the airways or lung tissue of IP−/− mice. Although, none of these cytokines were detected in the BALF of naïve IP−/− mice (data not shown) their contribution to the increased numbers of pNK cells cannot be excluded.
FIGURE 2.
Increased IFN-γ production by pulmonary NK cells in IP−/− mice. The production of IFN-γ by NK cells was determined by stimulating LMC, generated by collagenase dispersion of lung tissue, from naïve WT or IP−/− mice (4 per group) with either immobilized anti-NK1.1 or anti-NKp46 antibody in the presence or absence of IL-2. After culture for 24h, IFN-γ production was determined using ELISA (in triplicates). Data represent mean ± SEM of 3 independent experiments, ***p < 0.001.
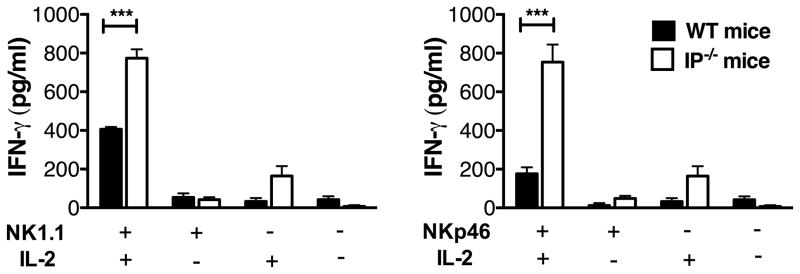
Elevated CX3CL1 levels in the airways of IP−/− mice regulate cytokine production by pNK cells
CX3CL1/fractalkine and its receptor CX3CR1 promotes the chemotaxis of macrophages (33) and cytolytic lymphoid cells including NK cells, CD8+ cells and γδ T cells (34). CX3CL1 is unusual insofar as it exists as a membrane-associated form that is proteolytically released from the cells. Given the elevated numbers of pNK cells in IP−/− mice, we examined whether the chemokine CX3CL1 was present in the airways of these mice. Interestingly, two-fold higher levels of CX3CL1 were found in the BALF of naïve IP−/− mice compared to WT animals (Fig. 3A). Since expression of this chemokine is reported to be predominantly by epithelial and endothelial cells (35), we investigated its expression in lung tissue of both WT and IP−/− mice by immunohistochemistry. The majority of CX3CL1 expression in the lungs of IP−/− mice was found to be associated with the airway epithelium, with lower levels of expression observed in the lung tissue of WT mice (Supplemental Fig. S2). To confirm that NK cells in both IP−/− and WT mice potentially respond to this chemokine, the expression of CX3CR1 receptor by NK cells was examined. Flow cytometric analysis revealed that CD3−NK1.1+ NK cells in both naïve IP−/− and WT mice expressed CX3CR1 (Fig. 3B). To examine whether CX3CL1 was responsible for influencing cytokine production by pNK cells or promoting the homeostatic recruitment of these cells to the lungs of IP−/− mice, a neutralizing antibody to CX3CL1 or control IgG2A isotope antibody (25μg in 30μl PBS over a 6 day period on days 0 and 3) was administered by oropharyngeal instillation into the airways of naïve IP−/− or WT mice. Lung tissues were harvested (on day 6) and cytokine production or the frequency of CD3−CD19−NK1.1+ NK cells in LMC was determined. Interestingly, in vivo administration of anti-CX3CL1 mAb to naïve WT or IP−/− mice resulted in a significant reduction in the production of IFN-γ by pNK from IP−/− mice (but not WT) in response to IL-15 + IL-18 or NKp46 stimulation (Fig. 3C). In contrast, the number of CD3−CD19−NK1.1+ NK cells present in the lungs of both WT or IP−/− mice was unchanged after treatment with anti-CX3CL1 antibody (Fig. 3D). These findings suggest that CX3CL1 plays a role in the maintenance of cytokine production by pNK cells rather than the recruitment of NK cells to the lungs.
FIGURE 3.
Elevated CX3CL1 levels in the airways of IP−/− mice regulate cytokine production by pNK cells. CX3CL1 chemokine levels in BALF from naïve WT and IP−/− mice (6 per group) were determined in conjunction with CX3CR1 receptor expression by NK1.1+ NK cells. (A) CX3CL1 levels present in the BALF from naïve IP−/− or WT mice measured by ELISA. Data are mean ± SEM (n = 6), **p < 0.01. (B) Receptor CX3CR1 expression by purified splenic CD3−NK1.1+ NK cells from WT or IP−/− mice determined by flow cytometry. The contribution of CX3CL1 in influencing cytokine production by pNK cells in IP−/− or WT mice was examined in vivo using a neutralizing antibody to CX3CL1. Anti-CX3CL or isotope control antibody was administered by oropharyngeal instillation (25μg in 30μl PBS over a 6 day period on days 0 and 3) into the airways of naïve IP−/− or WT mice (6 per group). Lung tissue was collected and dispersed in collagenase to prepare LMC. (C) IFN-γ production by pNK cells was determined using ELISA (in triplicates) following stimulation of LMC with either IL-15 + IL-18 or anti-NKp46 antibody for 24h. Data are mean ± SEM, *p < 0.05. (D) The effect of anti-CX3CL1 treatment on the frequency of pulmonary CD3−CD19−NK1.1+ NK cells was determined by flow cytometry. Results are representative of 3 independent experiments.
HDM-induced allergic lung inflammation and Th2 cytokine production are attenuated in IP−/− mice
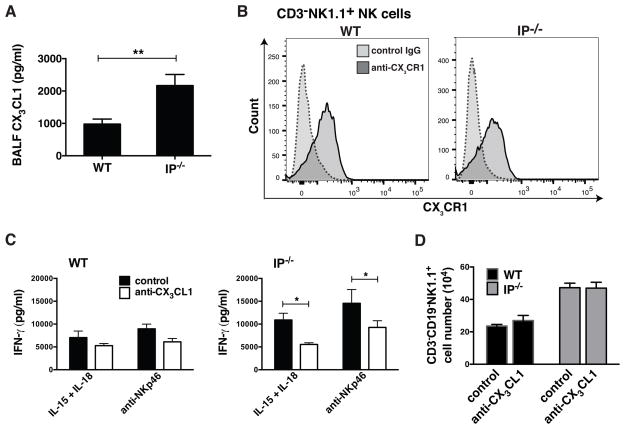
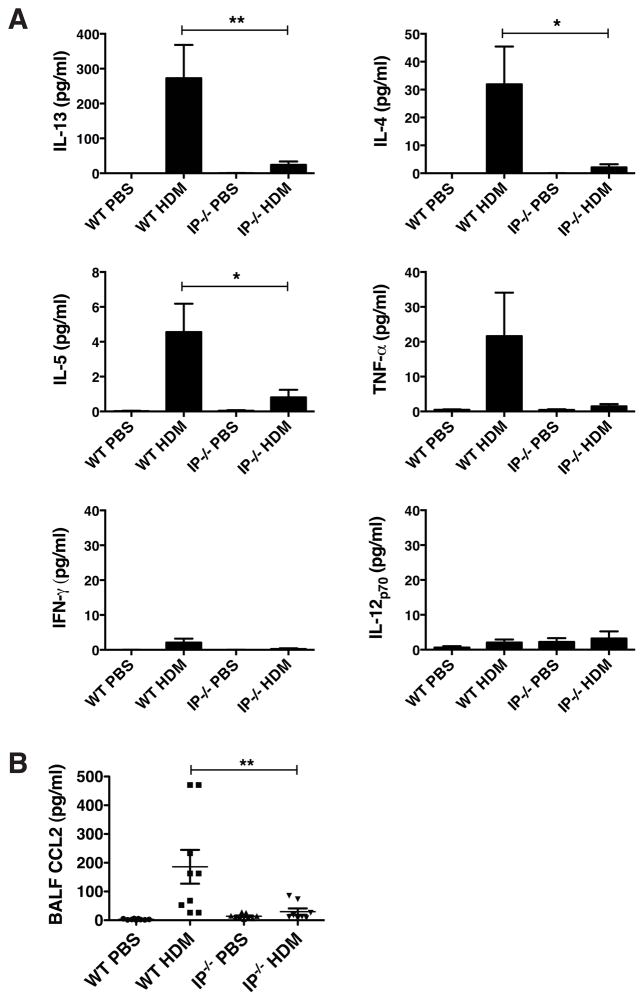
Given the increase in pNK cell numbers in IP−/− mice, it was important to resolve whether these cells influenced the development of adaptive immunity in the lung. NK cells have been shown to be capable of augmenting or suppressing the development of allergic lung inflammation (15, 17, 36). We thus evaluated whether the presence of increased numbers of pNK cells evident in the IP−/− mice impacts lung mucosal immune responses in these animals. To this end, acute allergic sensitization and challenge was elicited by repeated intra-nasal administration of HDM allergen over a 2-week period. The level of HDM-induced pulmonary inflammation in IP−/− mice was compared with WT counterparts. HDM inhalation evoked a pronounced lung inflammation in C57BL/6 WT mice, characterized by the influx of eosinophils and lymphocytes into the airways and an increase in cell-associated eosinophil peroxidase (EPO) levels in the BALF (Fig. 4A). In marked contrast, the number of eosinophils and lymphocytes present in BALF of HDM-challenged IP−/− mice was markedly reduced compared with WT mice (by approx. 50%, Fig. 4A). IP−/− and WT mice that were repeatedly exposed to intra-nasal PBS (control) did not exhibit any evidence of eosinophil infiltration into the airway. Lung histological analysis (using H&E staining and PAS staining) revealed that HDM-exposed WT mice developed a pronounced peribronchial and perivascular inflammation and an increase in mucus production or goblet cell hyperplasia compared to control animals exposed to PBS (Fig. 4B). Both the peribronchial inflammation and mucus secretion were markedly reduced in HDM-challenged IP−/− mice (Fig. 4B), with limited evidence for eosinophil infiltration. The infiltration of eosinophils into the airway was further demonstrated by flow cytometry where the number of Gr1−CD11b+Siglec-F+ eosinophils present in the BALF of HDM-treated IP−/− mice was clearly reduced compared to WT counterparts (Fig. 4C). To determine if the reduced inflammatory response was associated with decreased numbers of antigen-presenting cells present in the lungs, we enumerated the number of monocyte-derived DC (CD11c+MHC−IIbrightCD11b+) and epithelial-associated DC (CD11c+MHC−IIbrightCD103+) in the LMC. Following the onset of HDM-induced allergic inflammation, the number of CD11c+MHC−IIbright DC in WT mice was markedly increased (0.64% in PBS controls vs 4.52% in HDM-challenged) and these were predominantly CD11b+ monocyte-derived DC rather than CD103+ conventional DC (Fig. 4D). In marked contrast, the number of CD11c+MHC−IIbrightCD11b+ DC in IP−/− mice was not significantly increased. Coincident with the onset of allergic inflammation in HDM-challenged WT mice, was the presence of high levels of IL-13 in the BALF and increased concentration of IL-4, IL-5 and TNF-α (Fig. 5A). The production of all three cytokines was markedly depressed in BALF from IP−/− mice treated with HDM allergen. However, levels of IFN-γ and IL-12 in the BALF of HDM-exposed IP−/− or WT mice were undetectable (Fig. 5A), suggesting that the unresponsiveness in IP−/− mice was not a consequence of immune deviation of the HDM response toward a Th1 phenotype. CCL2 has been implicated in the recruitment of monocyte-derived DC during allergic inflammation (37) and is essential for Th2 polarization (38). Coincident with the onset of allergic inflammation in HDM-challenged WT mice, high levels of CCL2 were produced in the airways of these animals. However, consistent with the depressed Th2 cytokine levels, there was a marked reduction in CCL2 production in the BALF of HDM-exposed IP−/− compared to WT mice (Fig. 5B). To confirm that the observed effects in IP−/− mice did not arise as a consequence of altered eicosanoid biosynthesis, the levels of prostaglandins and cysteinyl leukotrienes present in BALF of HDM-challenged or control IP−/− mice was compared to WT littermates. However, no significant differences in de novo levels of eicosanoids were observed in the airways of WT and IP−/− mice, although production in both strains tended to increase after allergen inhalation (Supplemental Fig. S3). The early induction of allergic airway inflammation has been reported to involve HDM interaction with alveolar macrophages via a Dectin-2 dependent mechanism (7). Given that the airways of IP−/− animals had elevated levels of CX3CL1, we examined whether the reduced responsiveness of IP−/− mice to allergen sensitization was due to changes in phenotype and early allergen responses of CX3CR1+ myeloid cells present in the lung tissue and airways. Flow cytometric analysis revealed that naïve IP−/− mice had augmented numbers of CD11c+CX3CR1+ cells in the BALF that exhibited markedly increased levels of MHC-IIint expression but reduced levels of Dectin-2 compared to WT counterparts (Supplemental Fig. S4).
FIGURE 4.
HDM-induced allergic lung inflammation and mucus production is attenuated in IP−/− mice. WT or IP−/− mice (6 per group) were subjected to acute allergen sensitization and challenge by repeated intra-nasal administration of HDM allergen or PBS (control) on days 0, 7 and 14. BALF and lung tissue were harvested (on day 16) to determine the level of allergic inflammation. (A) The inflammatory cells present in the BALF was determined by cell differential counts and expressed as absolute cell numbers (per mouse) of lymphocytes (Lym), macrophages (Mac), eosinophils (Eos), and neutrophils (Neu). Eosinophil infiltration was also determined by measuring cell-associated eosinophil peroxidase (EPO) by colorimetric analysis. Date are mean ± SEM (n = 6), *p< 0.05 and **p<0.01. (B) Peribronchial and perivascular inflammation (H&E) and mucus production (PAS staining) was determined using histological analysis (20x). (C) The number of Gr1−CD11b+Siglec-F+ eosinophils present in BALF was determined using 4-color flow cytometry. (D) The presence of monocyte-derived DC in LMC (prepared by collagenase dispersion of lung tissue) from WT and IP−/− mice following HDM challenge was examined by 4-color flow cytometry. After gating on CD11c+MHC-IIbright cells, the expression of αE integrin (CD103) and CD11b was determined. Data are representative of 3 or 4 independent experiments.
FIGURE 5.
The attenuated allergic inflammation in IP−/− mice is associated with a reduction in Th2 cytokine and CCL2 chemokine production. WT or IP−/− mice (8–10 per group) were subjected to acute allergen challenge by repeated intra-nasal administration of HDM allergen or PBS (control) on days 0, 7 and 14. (A) Cytokine production in the BALF was assessed using ELISA or V-Plex assay, and (B) CCL2 chemokine levels were determined using ELISA. Results represent mean ± SEM of 3 independent experiments, *p < 0.05 and **p < 0.01.
Depletion of NK cells restores allergic lung inflammation in IP−/− mice
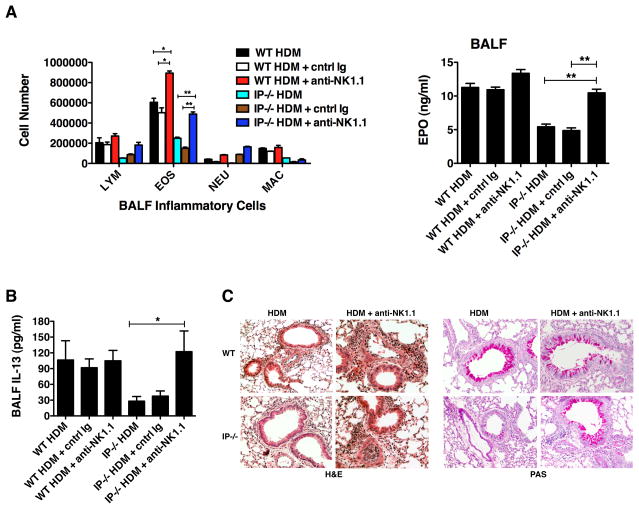
Since naïve IP−/− mice had elevated numbers of CD3−CD19−NK1.1+ NK cells in the lungs compared to WT animals, we examined the effect of depleting NK cells on the allergic inflammatory response. To deplete NK cells in vivo, IP−/− and WT mice were treated twice weekly with either the anti-NK1.1 antibody, PK136 (250 μg), or control IgG during the acute allergen challenge period. Treatment with PK136 resulted in >98% depletion of NK1.1+ cells when evaluated at day 16 (data not shown). Interestingly, depletion of NK1.1+ cells in HDM-exposed IP−/− mice restored the airway eosinophilia and cell-associated EPO levels to an amount similar to that observed in WT mice and this effect was not evident in mice receiving control IgG (Fig. 6A). The restoration of eosinophilic inflammation in IP−/− mice by depletion of NK1.1+ cells, strongly implies that NK cells are responsible for preventing the development of allergic lung inflammation in response to HDM. Treatment of WT mice with anti-NK1.1 also resulted in a slight increase in the number of eosinophils, possibly due the presence of fewer NK cells in these mice. As observed previously, production of the Th2 cytokine IL-13 in the airways of mice after allergen challenge was clearly reduced in IP−/− mice. However, treatment of HDM-exposed IP−/− mice with anti-NK1.1 antibody restored airway IL-13 production to a level similar to that found in WT mice (Fig. 6B). Moreover, lung histological analysis revealed that HDM-exposed IP−/− mice had reduced peribronchial inflammation and mucus production compared to allergen-challenged WT mice. Consistently, depletion of NK1.1 cells markedly increased the inflammatory pockets and mucus production in the lungs of HDM-challenged IP−/− mice (Fig. 6C). Dectin-2 expression by myeloid cells in the lungs of IP−/− mice was not restored to normal levels following NK cell depletion (data not shown) even though lung inflammatory responses were restored.
FIGURE 6.
Depletion of NK1.1+ cells in IP−/− mice restores allergic lung inflammation. To deplete NK1.1+ cells in vivo, WT and IP−/− mice (6 per group) were injected with anti-NK1.1 antibody (250 μg i.p.) or control IgG twice weekly over a period of 2 weeks during the acute allergen sensitization and challenge period. Following intranasal administration of HDM or control PBS (on days 0, 7 and 14), BALF and lung tissue were harvested (on day 16) to examine the level of allergic airway inflammation and IL-13 production. (A) Cell differential counts in the BALF were determined and expressed as absolute numbers (per mouse) of lymphocytes (Lym), eosinophils (Eos), neutrophils (Neu) and macrophages (Mac). Eosinophil infiltration was also assessed by measuring cell-associated eosinophil peroxidase (EPO) using colorimetric analysis. Results are mean ± SEM (n = 6), *p < 0.05 and **p < 0.01. (B) IL-13 levels in BALF were measured by ELISA. Results are mean ± SEM (n = 6), *p < 0.05. (C) Peribronchial inflammation (H&E staining) and mucus production (PAS staining) was determined by histological analysis of lung tissue (20x). Data are representative of 3 or 4 independent experiments.
Adoptive transfer of NK cells into the airways suppresses the development of allergic lung inflammation
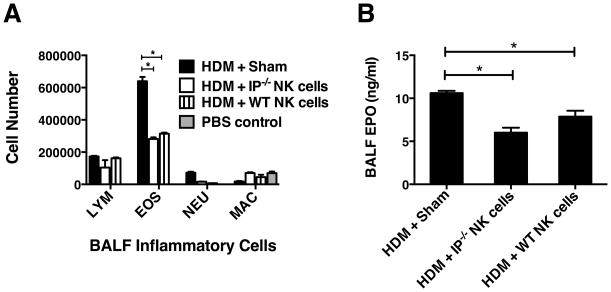
The depletion of NK cells using the anti-NK1.1 mAb (PK136) strongly implied that NK1.1+ cells in the IP−/− mice are responsible for preventing the development of HDM-induced allergic airway inflammation. However, a limitation encountered when treating mice with the PK136 antibody is that both invariant NK-T cells and NK cells are depleted. To resolve that the anti-inflammatory effects were mediated by NK cells, we examined whether purified IP−/− and WT splenic NK cells (5×105) instilled directly into the airways of WT mice via the oropharyngeal route (24h after the start of allergen sensitization period) were able to inhibit HDM-induced lung eosinophilic inflammation. Remarkably, adoptive transfer of either IP−/− or WT NK cells into the lungs of WT hosts significantly reduced the level of HDM-induced eosinophilic inflammation (Fig. 7A) and cell-associated EPO levels (Fig. 7B) compared to sham group (no cell transfer). Interestingly, on a “per cell” comparison both WT and IP−/− NK cells were equally effective at suppressing allergic pulmonary inflammation, suggesting that the major contributing factor to the attenuated responses evident in IP−/− mice likely arose from the preponderance of NK cells in their lungs.
FIGURE 7.
Adoptive transfer of NK cells suppresses allergic lung inflammation. NK cells were purified from spleens of IP−/− and WT mice (using MagCellect protocol) and the NK cells or PBS alone (Sham group) were adoptively transferred into the airways of WT mice (6 per group) by oropharyngeal instillation (5×105 cells per mouse). Following acute allergic sensitization and challenge with intranasal HDM or control PBS (on days 0, 7 and 14), the level of allergic lung inflammation was determined by examining the inflammatory cells present in the BALF. (A) Cell differential counts in the BALF were determined and expressed as absolute numbers (per mouse) of lymphocytes (Lym), eosinophils (Eos), neutrophils (Neu) and macrophages (Mac). (B) Eosinophil infiltration was assessed by measuring cell-associated eosinophil peroxidase (EPO) using colorimetric analysis. Results represent mean ± SEM (n = 6) of 3 independent experiments, *p < 0.05.
The elevated number of pNK cells in IP−/− mice is associated with depressed lung ILC2 numbers
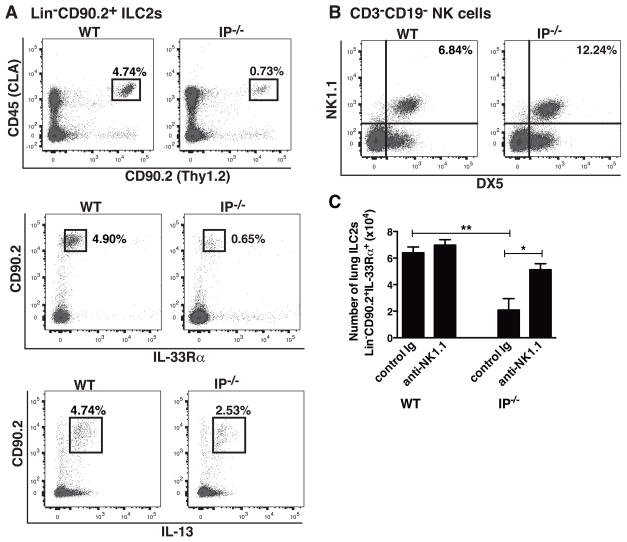
Although the elevated numbers of pNK cells in IP−/− mice appeared responsible for reduced HDM-induced inflammatory responses, how they mediate immune suppression was unclear. To examine if a defect in pulmonary ILC2 function was responsible for the attenuated HDM responses, we characterized the number and properties of ILCs present in the lungs of IP−/− mice by enumerating Lin− cells that co-expressed CD45 (CLA) and CD90.2 using flow cytometry. The ILC2 phenotype was confirmed by intracellular staining for IL-13 and surface expression of IL-33Rα/ST2, as detailed previously (39). This approach revealed that lung tissues from IP−/− mice had markedly reduced numbers of Lin−CD45+CD90.2+ ILC2s expressing IL-33Rα (4.90% in WT vs 0.65% in IP−/− mice ) and IL-13 (4.74% in WT vs 2.53% in IP−/− mice) compared to WT mice (Fig 8A). As evidenced by the previous observations, the reduced number of ILC2s in IP−/− mice was coincident with an increased total number of pNK cells (6.84% in WT vs 12.24% in IP−/−, Fig. 8B). To examine the possibility that NK1.1+ NK cells were directly linked to and/or responsible for the reduced numbers of ILC2s, NK cells were depleted by administration of anti-NK1.1 antibody to naïve WT and IP−/− mice. The effect of treating mice with anti-NK1.1 mAb or control Ig (twice weekly over a period of 2 weeks) on the total number of lung ILC2s was determined by flow cytometry. Depletion of NK cells was associated with a significant increase in the number of Lin−CD45+CD90.2+ IL-33Rα ILC2s in the lungs of IP−/− mice to a level similar to WT counterparts (Fig 8C). These data are consistent with the possibility that the decreased HDM responses in IP−/− mice arises as a consequence of reduced lung ILC2 numbers caused by NK cell-mediated regulation.
FIGURE 8.
The elevated number of pNK cells in IP−/− mice is associated with depressed lung ILC2 numbers. Lung tissue was collected from WT and IP−/− mice (6 per group) and LMC prepared by dispersion of lung tissue. (A) ILC2 numbers in the LMC were determined by enumerating Lin−CD45CLA+CD90.2+ cells (after removing Lin− cells from the analysis) that expressed either IL-33Rα or IL-13 (intracellular staining) using 5-color flow cytometry. (B) The number of pNK cells was determined by enumerating NK1.1+DX5+ cells in CD3−CD19− gated LMC. (C) The effect of depleting NK1.1+ NK cells by administration of anti-NK1.1 mAb or control Ig to naïve WT or IP−/− mice (twice weekly over a period of 2 weeks) on the number and phenotype of lung ILC2s was determined by enumerating Lin−CD90.2+IL-33Rα+ cells. Results are expressed as cell number per mouse and are mean ± SEM (n = 6), *p < 0.05 and **p < 0.01. Data are representative of 3 independent experiments.
DISCUSSION
HDM is a clinically relevant allergen that is capable of conferring immunity following inhalation without the need to resort to the use of immunological adjuvants to prime the response. In mice, repeated inhalation of airborne HDM allergen results in the activation of adaptive immunity characterized by a pronounced CD4+ Th2 response, generation of specific IgE and infiltration of eosinophils into the airway (40). The mucosal allergic response in the airway is typified by increased mucus production and AHR, analogous to inflammatory processes observed in atopic asthma. The surprising finding in this study was that the inflammatory response elicited by inhaled HDM allergen was compromised in mice lacking IP receptors. In order to explain the observed defect in allergic sensitization in IP−/− mice, deficiencies in the resident lung mucosal lymphoid and myeloid cells were sought. The most notable immunological characteristic of IP−/− mice was that their lung tissues contained two-fold more NK cells when compared to WT littermates and these pNK cells produced IFN-γ. The increased size of the NK cell pool in IP−/− mice was only observed in the lungs since NK cell numbers present in lymphoid sites and other tissues, such as liver, remained normal. The elevated number of pNK cells in IP−/− mice could conceivably arise from expansion of these cells in the lung tissue and/or increased recruitment of NK cells to this site. NK cells in IP−/− mice expressed high levels of NKp46 compared to WT NK cells. NKp46 is a 46-kDa protein type I glycoprotein natural cytotoxicity receptor (NCR) that associates with the CD3ζ and FcεRIγ (41). It serves as a receptor for several ligands which includes vimentin (42) tumor antigens (43) or influenza viral hemagglutinin (44). Of these ligands, only vimentin is ubiquitous and its expression appeared normal in null mice, raising the possibility that elevated NKp46 expression arise from an increased frequency of mature NK cells (45).
PGI2 biosynthesis is promoted during allergic lung inflammation (46) and this mediator is known to have several immunoregulatory properties (29, 46, 47). That IP null mice have an immunological defect where NK cells are selectively affected is noteworthy. NK cells are responsive to a range of endogenously produced eicosanoids which include Resolvin-E1 (18), Lipoxin-A4 (19), PGD2 (20), PGE2 (21) and leukotrienes (22). In the present study, no gross changes in eicosanoid levels in BALF of IP−/− mice was observed, suggesting that the altered phenotype in these animals was linked with lack of IP expression. A notable characteristic of the null mice was the augmented production of CX3CL1 in the BALF that likely contributed to the altered phenotype of IP−/− mice. Our study confirms previous work that CX3CL1 is highly expressed by airway epithelial cells (35). Unusually CX3CL1 exists as both trans-membrane and soluble forms, the latter being generated following cleavage from the cell surface by the action of the proteases ADAM10, ADAM17 or γ-secretase (48). CX3CL1 appeared not to be responsible for the increased size of the pNK pool observed in IP−/− mice (49). However, administration of blocking antibodies to the chemokine reduced IFN-γ production by pNK cells in IP−/− mice, suggesting that CX3CL1 promotes cytokine production by pNK cells rather than their recruitment. In this context, CX3CL1 has been shown to augment both NK cell cytolytic activity (50, 51) and the release of IFN-γ (51, 52). The relative contribution of cell-associated and soluble forms of CX3CL1 in augmenting pNK cell function remains unclear.
The elevated number of NK cells present in the lungs of IP−/− mice prompted speculation that they may be responsible for the reduced allergic lung inflammation elicited by HDM in these animals. Critically, the unresponsiveness of IP−/− mice to inhaled HDM was strictly dependent on the presence of NK cells since anti-NK1.1 antibody treatment restored the lung inflammatory response. Moreover, the suppression of allergic inflammation was demonstrable by the oropharyngeal transfer of purified IP−/− NK cells into WT mice. Interestingly, both WT and IP−/− NK cells were equally effective at suppressing the development of allergic pulmonary inflammation, suggesting that the NK-mediated regulation evident in IP−/− mice was primarily a consequence of the preponderance of pNK cells in these animals. These observations reveal that NK cells in IP−/− mice play a pivotal role in preventing the onset of inflammation elicited by inhaled allergen. Previous reports studying the impact of NK cells on the development of allergic lung inflammation have yielded contradictory findings. In certain instances, NK cells inhibited allergic lung inflammation (14, 15) and in others they promoted eosinophilic inflammation (16, 53) reviewed in (54). In some studies, the antigen sensitization phase was more susceptible to NK cell-mediated regulation compared to the subsequent lung inflammatory response elicited by OVA inhalation (55). It is noteworthy that these reports did not use HDM to elicit lung inflammation and the effect of endogenous eicosanoids was not considered. Certainly, NK cells have been shown to prevent the development of T cell immunity by multifarious mechanisms that typically fall into either impacting the antigen-presenting cell or the responding T cells (56).
IP−/− mice are not overtly immune-compromised, however, in studies utilizing protein antigens such as OVA, inflammatory responses are typically elevated (57, 58), often after systemic immunization using adjuvants (29). An important aspect of the present study is that it examines the responses elicited by the clinically relevant HDM allergen instilled directly into the airways, without the use of adjuvants (47). A notable difference between OVA and HDM responses in the lung is that ILC2s contribute to the lung inflammatory responses to allergens, such as papain (59, 60) and HDM (61–64), but have a less prominent role in OVA responses following systemic immunization (61, 64). ILC2s are a potent source of innate type 2 cytokines IL-5 and IL-13 in allergic lung inflammation (64, 65). ILC2s are present in human respiratory tissue and studies from mouse models of asthma demonstrate a role for these innate cells in promoting allergic inflammation (66, 67). ILC2s secrete IL-5 constitutively and are induced to express IL-13 during inflammatory processes and promote eosinophil homeostasis (68). Indeed, the raised frequency of NK cells in IP−/− mice was coincident with a reduction in the number of ILC2s present in the lung tissues, and NK cell depletion resulted in a rise in ILC2 numbers. These findings are consistent with the possibility that tissue NK cells, either directly or indirectly, limit ILC2 responses and subsequent allergic inflammation. Importantly, ILC2 function is susceptible to regulation by the action of IFN-γ and IFN-α/β (69–71). This form of regulation could arise from either the direct effect of NK cells on ILC2 cells (likely by the action of IFN-γ) or, alternatively, by inhibiting IL-33 release in the lung mucosa. Such regulation likely would impact the development of T cell-mediated immunity since ILC2s promote the development of CD4+ Th2 responses (72, 73).
In summary, our data demonstrate a hitherto unknown role for PGI2 in regulating the number and properties of NK cells resident in lung tissue and reveal a role for NK cells in preventing allergic inflammation by limiting the number of HDM-responsive ILC2s in the lung. In recent years, development of novel therapeutic strategies that selectively target the Th2 response has proven difficult. We propose that the improved understanding of the role of eicosanoids, such as PGI2, in promoting NK cell maturation and expansion in the lung is critical, not only for potentiating innate immunity in the mucosal site, but also preventing sensitization to airborne allergens. Certainly, approaches that seek to prevent allergic inflammation by specifically promoting mucosal NK cell response would form a novel immunotherapeutic approach.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01-HL079189 (to K.R.), R15-HL112073 (to Z.J.) and the Center for Environmental Health Sciences Core Facilities (Fluorescence Cytometry, Histology and Inhalation) supported by NIGMS, NIH grant P30GM103338.
We thank Britt Postma, Pam Shaw (FACS Core), Lou Herritt and Diane Brooks (Histology Core) for their valuable technical assistance.
Abbreviations used in this article
- BALF
bronchoalveolar lavage fluid
- EPO
eosinophil peroxidase
- HDM
house dust mite
- ILC2s
type 2 innate lymphoid cells
- IP−/−
PGI2 IP receptor-deficient
- LMC
lung mononuclear cells
- MoDC
monocyte-derived dendritic cells
- pNK cells
pulmonary natural killer cells
- WT
wild type
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends in molecular medicine. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills T. Dust mite allergens and asthma--a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 3.Svensson L, Redvall E, Bjorn C, Karlsson J, Bergin AM, Rabiet MJ, Dahlgren C, Wenneras C. House dust mite allergen activates human eosinophils via formyl peptide receptor and formyl peptide receptor-like 1. Eur J Immunol. 2007;37:1966–1977. doi: 10.1002/eji.200636936. [DOI] [PubMed] [Google Scholar]
- 4.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 5.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature medicine. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke DL, Davis NH, Campion CL, Foster ML, Heasman SC, Lewis AR, Anderson IK, Corkill DJ, Sleeman MA, May RD, Robinson MJ. Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation. Mucosal Immunol. 2014;7:558–567. doi: 10.1038/mi.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama WM. Fundamental Immunology. 7. 2013. Natural Killer Cells; pp. 395–431. [Google Scholar]
- 9.Yokoyama WM. Natural killer cell receptors. Curr Opin Immunol. 1995;7:110–120. doi: 10.1016/0952-7915(95)80036-0. [DOI] [PubMed] [Google Scholar]
- 10.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Nakayama M, Sakaki M, Hayakawa Y, Imawari M, Ogasawara K, Okumura K, Smyth MJ. IFN-gamma production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J Leukoc Biol. 2011;90:777–785. doi: 10.1189/jlb.0411208. [DOI] [PubMed] [Google Scholar]
- 12.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin Immunol. 2014;26:127–131. doi: 10.1016/j.smim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Fan Y, Wang S, Jiao L, Qiu H, Yang X. NK cells contribute to intracellular bacterial infection-mediated inhibition of allergic responses. J Immunol. 2008;180:4621–4628. doi: 10.4049/jimmunol.180.7.4621. [DOI] [PubMed] [Google Scholar]
- 15.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker C, Checkel J, Cammisuli S, Leibson PJ, Gleich GJ. IL-5 production by NK cells contributes to eosinophil infiltration in a mouse model of allergic inflammation. J Immunol. 1998;161:1962–1969. [PubMed] [Google Scholar]
- 17.Farhadi N, Lambert L, Triulzi C, Openshaw PJ, Guerra N, Culley FJ. Natural killer cell NKG2D and granzyme B are critical for allergic pulmonary inflammation. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Perussia B, Campbell KS. Prostaglandin D2 suppresses human NK cell function via signaling through D prostanoid receptor. J Immunol. 2007;179:2766–2773. doi: 10.4049/jimmunol.179.5.2766. [DOI] [PubMed] [Google Scholar]
- 21.Zielinski CC, Gisinger C, Binder C, Mannhalter JW, Eibl MM. Regulation of NK cell activity by prostaglandin E2: the role of T cells. Cell Immunol. 1984;87:65–72. doi: 10.1016/0008-8749(84)90130-8. [DOI] [PubMed] [Google Scholar]
- 22.Bray RA, Brahmi Z. Role of lipoxygenation in human natural killer cell activation. J Immunol. 1986;136:1783–1790. [PubMed] [Google Scholar]
- 23.Liu T, Laidlaw TM, Feng C, Xing W, Shen S, Milne GL, Boyce JA. Prostaglandin E2 deficiency uncovers a dominant role for thromboxane A2 in house dust mite-induced allergic pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:12692–12697. doi: 10.1073/pnas.1207816109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebbins KJ, Broadhead AR, Correa LD, Scott JM, Truong YP, Stearns BA, Hutchinson JH, Prasit P, Evans JF, Lorrain DS. Therapeutic efficacy of AM156, a novel prostanoid DP2 receptor antagonist, in murine models of allergic rhinitis and house dust mite-induced pulmonary inflammation. Eur J Pharmacol. 2010;638:142–149. doi: 10.1016/j.ejphar.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Herrerias A, Torres R, Serra M, Marco A, Pujols L, Picado C, de Mora F. Activity of the cyclooxygenase 2-prostaglandin-E prostanoid receptor pathway in mice exposed to house dust mite aeroallergens, and impact of exogenous prostaglandin E2. Journal of inflammation. 2009;6:30. doi: 10.1186/1476-9255-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catella-Lawson F, Crofford LJ. Cyclooxygenase inhibition and thrombogenicity. The American journal of medicine. 2001;110(Suppl 3A):28S–32S. doi: 10.1016/s0002-9343(00)00683-5. [DOI] [PubMed] [Google Scholar]
- 27.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, Ueno A, Oh-ishi S, Narumiya S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 28.Koo GC, Peppard JR. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984;3:301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- 29.Jaffar Z, Ferrini ME, Shaw PK, Fitzgerald GA, Roberts K. Prostaglandin I2 Promotes the Development of IL-17-Producing gammadelta T Cells That Associate with the Epithelium during Allergic Lung Inflammation. J Immunol. 2011;187:5380–5391. doi: 10.4049/jimmunol.1101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gevrey JC, Isaac BM, Cox D. Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine) J Immunol. 2005;175:3737–3745. doi: 10.4049/jimmunol.175.6.3737. [DOI] [PubMed] [Google Scholar]
- 34.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Molecular interventions. 2010;10:263–270. doi: 10.1124/mi.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korsgren M. NK cells and asthma. Current pharmaceutical design. 2002;8:1871–1876. doi: 10.2174/1381612023393738. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Liu M, Liu Y, Wang C, Yoshimura T, Gong W, Le Y, Tessarollo L, Wang JM. Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J Biol Chem. 2013;288:16262–16273. doi: 10.1074/jbc.M113.450635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 39.Klein Wolterink RG, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Current allergy and asthma reports. 2013;13:271–280. doi: 10.1007/s11882-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 40.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–110. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westgaard IH, Berg SF, Vaage JT, Wang LL, Yokoyama WM, Dissen E, Fossum S. Rat NKp46 activates natural killer cell cytotoxicity and is associated with FcepsilonRIgamma and CD3zeta. J Leukoc Biol. 2004;76:1200–1206. doi: 10.1189/jlb.0903428. [DOI] [PubMed] [Google Scholar]
- 42.Harris DT, Kapur R, Frye C, Acevedo A, Camenisch T, Jaso-Friedmann L, Evans DL. A species-conserved NK cell antigen receptor is a novel vimentin-like molecule. Dev Comp Immunol. 1992;16:395–403. doi: 10.1016/0145-305x(92)90041-a. [DOI] [PubMed] [Google Scholar]
- 43.Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol. 2009;182:2221–2230. doi: 10.4049/jimmunol.0801878. [DOI] [PubMed] [Google Scholar]
- 44.Mandelboim O, Porgador A. NKp46. The international journal of biochemistry & cell biology. 2001;33:1147–1150. doi: 10.1016/s1357-2725(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 45.Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118:5439–5447. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaffar Z, Wan KS, Roberts K. A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J Immunol. 2002;169:5997–6004. doi: 10.4049/jimmunol.169.10.5997. [DOI] [PubMed] [Google Scholar]
- 47.Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–6203. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- 48.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 49.Inngjerdingen M, Rolstad B, Ryan JC. Activating and inhibitory Ly49 receptors modulate NK cell chemotaxis to CXC chemokine ligand (CXCL) 10 and CXCL12. J Immunol. 2003;171:2889–2895. doi: 10.4049/jimmunol.171.6.2889. [DOI] [PubMed] [Google Scholar]
- 50.Robinson LA, Nataraj C, Thomas DW, Cosby JM, Griffiths R, Bautch VL, Patel DD, Coffman TM. The chemokine CX3CL1 regulates NK cell activity in vivo. Cell Immunol. 2003;225:122–130. doi: 10.1016/j.cellimm.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Yoneda O, Imai T, Nishimura M, Miyaji M, Mimori T, Okazaki T, Domae N, Fujimoto H, Minami Y, Kono T, Bloom ET, Umehara H. Membrane-bound form of fractalkine induces IFN-gamma production by NK cells. Eur J Immunol. 2003;33:53–58. doi: 10.1002/immu.200390007. [DOI] [PubMed] [Google Scholar]
- 52.Fraticelli P, Sironi M, Bianchi G, D’Ambrosio D, Albanesi C, Stoppacciaro A, Chieppa M, Allavena P, Ruco L, Girolomoni G, Sinigaglia F, Vecchi A, Mantovani A. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathias CB, Guernsey LA, Zammit D, Brammer C, Wu CA, Thrall RS, Aguila HL. Pro-inflammatory role of natural killer cells in the development of allergic airway disease. Clin Exp Allergy. 2014;44:589–601. doi: 10.1111/cea.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karimi K, Forsythe P. Natural killer cells in asthma. Frontiers in immunology. 2013;4:159. doi: 10.3389/fimmu.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ple C, Barrier M, Amniai L, Marquillies P, Bertout J, Tsicopoulos A, Walzer T, Lassalle P, Duez C. Natural killer cells accumulate in lung-draining lymph nodes and regulate airway eosinophilia in a murine model of asthma. Scand J Immunol. 2010;72:118–127. doi: 10.1111/j.1365-3083.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 56.Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–349. doi: 10.1016/j.it.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, Nagai H. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am J Respir Cell Mol Biol. 2003;29:314–320. doi: 10.1165/rcmb.2003-0035OC. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, Inagaki N, Narumiya S, Nagai H. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br J Pharmacol. 2002;137:315–322. doi: 10.1038/sj.bjp.0704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halim TY. Group 2 innate lymphoid cells in disease. Int Immunol. 2016;28:13–22. doi: 10.1093/intimm/dxv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Rijt L, von Richthofen H, van Ree R. Type 2 innate lymphoid cells: at the cross-roads in allergic asthma. Seminars in immunopathology. 2016 doi: 10.1007/s00281-016-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, Takei F, McNagny KM. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–1148. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 62.Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24:707–712. doi: 10.1016/j.coi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Everaere L, Ait-Yahia S, Molendi-Coste O, Vorng H, Quemener S, LeVu P, Fleury S, Bouchaert E, Fan Y, Duez C, de Nadai P, Staels B, Dombrowicz D, Tsicopoulos A. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, Gorentla B, Liu W, Gorska MM, Chu H, Martin RJ, Alam R. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68 e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 68.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat JF, Qureshi ST, Mazer BD, Mossman KL, Malo D, Gamero AM, Vidal SM, King IL, Sarfati M, Fritz JH. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17:65–75. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stehle C, Saikali P, Romagnani C. Putting the brakes on ILC2 cells. Nat Immunol. 2015;17:43–44. doi: 10.1038/ni.3353. [DOI] [PubMed] [Google Scholar]
- 72.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 73.Liu B, Lee JB, Chen CY, Hershey GK, Wang YH. Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia. J Immunol. 2015;194:3583–3593. doi: 10.4049/jimmunol.1400951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.