Abstract
Platelets are the chief effector cells in hemostasis. However, recent evidence suggests that platelets have multiple roles in host defense against infection. Reports by us and others showed that platelets functionally contribute to protection against Staphylococcus aureus (S. aureus) infection. In the current study, the capacity of mouse platelets to participate in host defense against S. aureus infection was determined by assessing two possibilities. First, we determined the ability of platelets to kill S. aureus directly; and second, we tested the possibility that platelets enhance macrophage phagocytosis and intracellular killing of S. aureus. We report here evidence in support of both mechanisms. Platelets effectively killed two different strains of S. aureus. A clinical isolate of methicillin resistant S. aureus (MRSA) was killed by platelets (>40% killing in 2 h) in a thrombin-dependent manner while a methicillin sensitive strain (MSSA) was killed to equal extent but did not require thrombin. Interestingly, thrombin-stimulated platelets also significantly enhanced peritoneal macrophage phagocytosis of both MRSA and MSSA by >70%, and restricted intracellular growth by >40%. Enhancement of macrophage anti-S. aureus activities is independent of contact with platelets but is mediated through releasable products, namely IL-1β. These data confirm our hypothesis that platelets participate in host defense against S. aureus both through direct killing of S. aureus and enhancing the antimicrobial function of macrophages in protection against S. aureus infection.
Keywords: Platelets, Macrophages, S. aureus, Sepsis, IL-1β
Introduction
Platelets are the second most abundant blood cell, outnumbered only by erythrocytes in the circulation (approximately 1.5–4.0 × 1011 platelets per liter of blood in healthy adult humans). The classical role attributed to platelets has been in coagulation, prevention of bleeding, initiation of wound healing and maintenance of vascular integrity. However, it is becoming increasingly clear that platelets contribute to diverse immunological processes extending beyond the traditional view of platelets as fragmentary mediators of haemostasis and thrombosis (1). Platelets are now appreciated for their contributions to both innate and adaptive immunity.
The capacity of platelets to participate in immunity is largely due to their ability to store and release a myriad of inflammatory and bioactive molecules, and express a wide range of functional immunoreceptors. Platelets store these bioactive molecules in three types of intracellular storage granules: dense (δ-), alpha (α-), and lysosomal (λ-) granules whose contents are released into the circulation or translocated to the surface upon platelet activation (1, 2). These mediators attract and modulate effector cells of the immune system. In addition, platelets themselves demonstrate direct effector function and should be regarded as effector immune cells (3).
Platelets appear to have multiple roles in host defense against infection. Platelets are among the first cells to detect endothelial injury and microbial pathogens as they gain access to or invade the bloodstream or tissues (4, 5). Platelets interact with various bacterial species, viruses, fungi, and protozoa, and demonstrate anti-microbial functions (6, 7). The mechanisms of platelet-bacteria interactions are complex, reflecting the diversity of platelet receptors involved in the recognition of bacteria. These bacterial receptors include complement receptors, FcγRIIa, TLRs, GPIIb-IIIa, and GPIb, and the interaction of platelets and bacteria is mediated through direct or indirect binding to these and other receptors [reviewed in (8)]. Upon contact with certain bacteria, platelets can become activated, aggregate and de-granulate (9–13). Activated platelets release over 300 known secretory products including anti-microbial products collectively known as platelet microbicidal proteins (PMPs) (14).
S. aureus is an opportunistic Gram-positive bacterium that is a main cause of skin infection in both community and hospital settings (15, 16). S. aureus is also among the most predominant endovascular pathogens and can lead to life-threatening conditions because it can efficiently disseminate from those sites of infection, causing invasive diseases: these include bacteremia, pneumonia, and infective endocarditis as well as organ damage and septic shock (17, 18). Thus, platelet interactions with S. aureus is rapidly becoming a model system, as the consequences of these interactions likely play significant roles in shaping infection and host defense.
Recent reports by us and others showed that platelets functionally contribute to protection against S. aureus infection (19–23). Using our novel inducible platelet-depleted mouse model, we demonstrated that platelet-depleted mice have high bacterial burden in kidneys, a more severe cytokine storm, and decreased survival compared to WT counterparts (19). However, the mechanistic detail of how platelets participate in host defense against S. aureus infection is not clearly understood. In the current study, the capacity of mouse platelets to participate in host defense against S. aureus infection was determined by addressing two questions: First, can platelets directly kill S. aureus? Second, do platelets enhance macrophage phagocytosis and intracellular killing of S. aureus? Our evidence supports both of these defense mechanisms.
Materials and Methods
Mice
Six to eight week old male and female C57BL/6 (B6) mice were maintained in the animal facility at the University of Toledo Health Science Campus, in a specific pathogen-free environment. All mouse experiments were performed according to NIH guidelines with approval of the Institutional Animal Care and Use Committee at the University of Toledo.
Platelet Preparation
Mouse blood was obtained via cardiac puncture under isoflurane anesthesia. Blood was collected using a 1–3 ml syringe with 25 G needle containing anticoagulant (22.0 g/L trisodium citrate, 8.0 g/L citric acid, and 24.5 g/L dextrose), and was pooled into 1.5 ml microcentrifuge tubes. Platelets were isolated and purified by centrifugation as previously described (24).
Bacteria Preparation
A derivative of a previously characterized clinical isolate of MRSA USA300 (25) was provided by Dr. R.M. Blumenthal (University of Toledo, Toledo, OH). MSSA strain NRS72 (Sanger 476) was obtained from Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). Bacteria were grown overnight at 37 °C in tryptic soy broth (TSB), centrifuged at 10,000 x g for 5 min and suspended in 1 ml of Dulbecco’s Phosphate Buffered Saline (DPBS) buffer without calcium or magnesium. Bacteria were washed with DPBS and enumerated using a hemacytometer.
Bacteria Killing Assay
S. aureus (1× 107/100 μl) were placed into a microcentrifuge tube and mixed with quiescent platelets or platelets activated with 0.1U/ml thrombin (Chronolog, Havertown, PA) at a Multiplicity of Infection (MOI) of 1. The solution was centrifuged at 300 x g for 1 min to maximize platelet-bacteria contact, followed by 2 h incubation in a 37 °C water bath. Following incubation, a 1% saponin solution was added to lyse platelets. Solutions were serially diluted and plated on tryptic soy agar (TSA) plates, incubated overnight at 37 °C and colony forming units (CFU) were tallied for each plate. When inhibitors were used, platelets were pre-treated with the inhibitor for 15 min at 37 °C prior to activation and mixing with bacteria. Cytochalasin D (Sigma Aldrich, St. Louis, MO) was used at a final concentration of 20 μM, and diphenyleneiodonium chloride (DPI) (Sigma Aldrich) was used at 10 μM. Each experiment was performed in triplicate and serial dilutions were plated in triplicate. Each experiment was performed at least three times on different days.
Macrophage preparation and phagocytosis/ intracellular killing assays
To obtain fresh peritoneal macrophages, mice were injected intraperitoneally with 1ml thioglycollate (3%) and peritoneal fluids were obtained after 4 days. Peritoneal lavage was performed by injecting 10 ml DPBS, and removed using a syringe. The fluid was centrifuged to isolate peritoneal macrophages which were resuspended in RPMI-1640 medium. A sample of collected cells was stained using fluorescently conjugated anti-CD45, CD11b and F/480 (Biolegend, San Diego, CA), and analyzed by flow cytometry. We observed that >90% of peritoneal cells were CD45+, CD11b+, and F4/80+, indicating that they are of the macrophage lineage (data not shown). Phagocytosis of S. aureus by peritoneal macrophages was performed in the presence or absence of thrombin-activated platelets or releasates from thrombin-activated platelets. S. aureus (107 CFU) was mixed with macrophages (106) in microcentrifuge tubes followed by the addition of platelets (107) or equal volume of DPBS. Thrombin (0.1 U/ml) was used to activate platelets. The suspension was then incubated for 1 h in 37 °C water bath. At this time (t = 0h) the cells were washed with DPBS three times by centrifugation at 200 x g for 1 min to clear non-phagocytosed bacteria, as well as platelets or their products. A fraction of the macrophage population was lysed with 0.2% triton X-100, serially diluted and plated to record the CFU of S. aureus as a measure of phagocytosis/internalization. For bacteria killing assays, the remaining cells continued incubating for 3 h (t = 3h) in the presence or absence of fresh thrombin-activated platelets, after which the macrophages were washed as at t = 0h. The cells were lysed with 0.2% triton X-100 and CFU were counted to determine the intracellular killing efficiency. In some experiments, macrophages were incubated with recombinant IL-1β (10ng/ml), sCD40L (100ng/ml), or RANTES (10ng/ml) for this assay (all from PeproTech, Rocky Hill, NJ).
Microscopy
S. aureus (107) were stained with SYTO9 dye (1:50, Life Technologies, Grand Island, NY) for 15 min at room temperature per manufacturer’s instruction, before washing two times at 10,000 x g for 10 min. Platelets (107) were stained with anti CD42b-PE (1:50, Biolegend, San Diego, CA) for 20 min at room temperature. Platelets and bacteria were then mixed with or without 0.1U/ml thrombin and incubated for 2 h at 37°C protected from light. After incubation, the platelet- S. aureus suspension was centrifuged at 300 x g for 10 min, resuspended in 10 μl of DPBS, and mounted using Fluoromount G (Southern Biotech, Birmingham, AL).
In some experiments, S. aureus were stained with FITC-conjugated anti-S. aureus polyclonal primary antibody (Abcam, Cambridge, MA) for 30 min at 37 °C. Peritoneal macrophages were incubated with FITC labeled S. aureus in RPMI-1640 medium in the presence or absence of 0.1U/ml thrombin-activated platelets for 1 h (t = 0). Peritoneal macrophages were washed with DPBS to clear non-phagocytosed bacteria and platelets. Peritoneal macrophages were then stained with PE-conjugated anti-IgG secondary antibody, plated on coverslips, and observed under confocal microscopy to distinguish intracellular (FITC+, PE−) from extracellular (FITC+, PE+) bacteria.
Microscopy was performed using an Olympus FV1000 confocal microscope. Slides were imaged using the Olympus PlanAPO60x/1.42 oil immersion objective at 1x optical zoom (macrophages/bacteria) or 2x optical zoom (platelets/bacteria). Images were acquired using the FV10-ASW v2.1 software. The following fluorescence filter sets were used for image acquisition: FITC (Ex. 488 nm/ Em. 519 nm), TRITC (Ex. 543 nm/ Em. 578 nm).
ELISA
Peritoneal macrophages (107/ml) were incubated with unstimulated or thrombin-activated platelets (108/ml) in DPBS in the presence or absence of S. aureus (107 CFU/ml) and the mixtures were incubated for 1 h. Supernatants were collected and stored at −20°C until used for ELISA analysis for IL-1β. IL-1β mini ELISA kit was purchased from PeproTech (Rocky Hill, NJ) and the assay was preformed according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as mean ± S.E. Significant differences between two groups were determined using the two-tailed, unpaired student’s t test (Figures 2A and 3A). All other group comparisons were analyzed using one-way ANOVA with Tukey multiple comparison test. Bartlett’s test for equal variances were also performed. Statistical significance was set at p < 0.05.
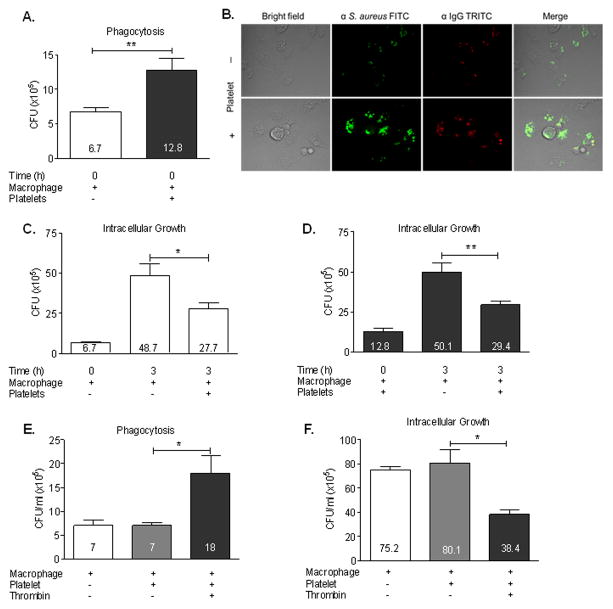
Figure 2. Platelets enhance macrophage phagocytosis and restrict intracellular growth of S. aureus.
Peritoneal macrophages were incubated with MRSA in RPMI medium in the presence or absence of 0.1U/ml thrombin-activated platelets for 1 h (t = 0). Peritoneal macrophages were washed with PBS to clear non-phagocytosed bacteria and platelets. (A) MRSA CFU collected from macrophage lysates at t = 0 (B) Representative confocal images of macrophages at t = 0. Peritoneal macrophages were incubated with FITC labeled MRSA in RPMI medium in the presence or absence of 0.1U/ml thrombin-activated platelets for 1 h (t = 0), washed with PBS, and stained with PE-conjugated anti-IgG secondary antibody. (C and D) Macrophages from white or black bars in (A) were further incubated for 3 h (t = 3) in the presence or absence of freshly activated platelets, intracellular MRSA were collected from macrophage lysates, and CFUs were determined. (E) MRSA CFU collected from macrophage lysates at t = 0. (F) Macrophages from white, gray, or black bars in (E) were further incubated for 3 h (t = 3) with platelets in the presence or absence of thrombin, intracellular MRSA were collected from macrophage lysates, and CFUs were determined. All measures are presented as mean ± SE (n=3), *P<0.05, **P<0.005.
Figure 3. Releasates from thrombin-stimulated platelets enhances macrophage uptake and restrict intracellular growth of S. aureus.
Peritoneal macrophages were incubated for 1 h (t = 0) with MRSA in RPMI medium in the presence or absence of releasates collected from 0.1U/ml thrombin-activated platelets. Peritoneal macrophages were washed with PBS to clear non phagocytosed bacteria. (A) MRSA CFU from macrophage lysates at t = 0. (B) Representative confocal images of macrophages at t = 0. Peritoneal macrophages were incubated with FITC labeled MRSA in RPMI medium in the presence or absence of releasates collected from 0.1U/ml thrombin-activated platelets for 1 h (t = 0), washed with PBS, and stained with PE-conjugated anti-IgG secondary antibody. (C and D) Macrophages from white or black bars in (A) were further incubated for 3 h (t = 3) in the presence or absence of fresh releasate collected from 0.1U/ml thrombin-activated platelets and intracellular MRSA from macrophage lysates was determined. All measures are presented as mean ± SE (n=3), *P<0.05, **P<0.01.
Results
Direct killing of S. aureus by platelets
Previous reports have shown that platelets can kill S. aureus, but the bactericidal mechanism(s) involved are not well understood. To establish the capacity of mouse platelets for direct killing of S. aureus, freshly washed mouse platelets were co-cultured with bacteria and the bactericidal activity quantified by measuring viable bacteria CFU after 2 h incubation. Figure 1A shows that unstimulated platelets did not reduce the number of MRSA CFUs, while thrombin-stimulated (0.1U/ml thrombin) platelets effectively reduced the number of CFUs of MRSA compared to the input numbers (>40% killing in 2 h). In comparison, platelets did not require thrombin activation to kill MSSA (Supplemental Figure 1A). These data suggest that killing of different S. aureus species have different dependency on platelet activation.
Figure 1. Bactericidal activity of platelets against S. aureus.
MRSA was incubated with unstimulated or thrombin-activated platelets (MOI=1) or their releasate, for 2 h at 37 C in triplicate microfuge tubes. When indicated, platelets were pre-treated with inhibitor for 15 min prior to incubation with bacteria. (A) Effect of platelet activation on MRSA survival. (B) Representative images of platelets binding MRSA after staining with anti CD42b PE (red, α-platelet) and SYTO9 FITC (green, bacteria) respectively. (C and D) Effect of Cyto D on bactericidal activity of platelets or platelet releasate respectively. (E) Effect of DPI on platelet bactericidal activity. All measures are presented as mean ± SE (n=3), *P<0.05, **P<0.001, ***P<0.0001.
Platelet-mediated killing of S. aureus is dependent on Cytochalasin D and independent of DPI
Data from previous reports suggest that S. aureus killing most likely occurs extracellularly due to the release of PMPs and/or β-defensins (22, 23). However, S. aureus can be internalized by human platelets (26). To assess whether mouse platelet-mediated bactericidal activity depends on internalization of S. aureus, co-cultures were directly observed using confocal imaging. Platelets could bind bacteria and appeared to form clusters (Fig. 1B), but internalized bacteria were rarely observed. Furthermore, the bactericidal activity was actin dependent, as the addition of cytochalasin D was able to block killing (Fig. 1C). Although cytochalasin D is known to inhibit phagocytosis, it also prevents secretion in platelets. Thus, since internalization of S. aureus by platelets was seldom observed, killing activities may be attributed to a secreted product. To further test this hypothesis, we repeated the killing assay in the presence or absence of platelet releasate. Only releasate from activated platelets mediated killing and no killing was observed by releasate from cytochalasin D pre-treated platelets (Fig. 1D). Collectively, these data support the notion that platelets mediate S. aureus killing independent of internalization.
Platelets are known to produce modest quantities of reactive oxygen metabolites (27). Therefore, we tested the effect of reactive oxygen metabolites (ROM) on platelet bactericidal activity. To do this, platelets were pre-treated with DPI to inhibit ROM production for 15 min prior to incubation with S. aureus. Surprisingly, DPI had no effect on bacterial killing (Fig. 1E). These data suggest that mouse platelets do not require ROMs for bactericidal activity.
Platelets enhance macrophage uptake and restricted intracellular growth of S. aureus
In our previous studies, platelet-depleted mice rapidly succumbed to S. aureus blood infection compared to wild-type mice, which survived the infection. This observation may seem at odds with previous reports showing the importance of neutrophils in host-defense against S. aureus infection. However, platelets enhance many neutrophil activities, including bacterial killing and NET production (28). These findings suggest that the roles of platelets extend beyond the direct killing of S. aureus, and that platelets are important for the function of other phagocytic cells for clearing S. aureus. There is also strong evidence of macrophage activity in S. aureus infection (29). To test whether platelets enhance macrophage uptake and intracellular killing of S. aureus, peritoneal macrophages were incubated with MRSA (1:10 ratio) for 1h in the presence or absence of thrombin-activated platelets before lysis to assess intracellular CFU. As shown in Fig. 2A, co-culture with activated platelets doubled the internalization of MRSA by macrophages, as indicated by the higher CFU/ml at time = 0 h. MSSA internalization by macrophages was also enhanced by platelets (Supplemental Figure 1B).
To delineate whether bacteria are not just bound to macrophages but truly internalized, we labeled bacteria with FITC-conjugated anti-S. aureus primary antibody prior to incubation with macrophages, followed by PE-conjugated anti-IgG secondary antibody after incubation, and then observed the cells by confocal microscopy to distinguish intracellular (FITC+, PE−) from extracellular (FITC+, PE+) bacteria. We observed that most S. aureus were FITC+ PE−, indicating that they are internalized by macrophages (Fig. 2B). Importantly, macrophages appear to ingest more bacteria in the presence of activated platelets (lower row) as compared to non-activated platelets (upper row). To provide a quantitative measure of internalization CFUs were recovered from lysed macrophages which show increased phagocytosis in the presence of activated platelets (Fig. 2E). It should also be noted that activated platelets enhanced phagocytosis and restricted intracellular growth of MSSA similarly to MRSA (Supplemental Fig. 1B and C).
To test whether platelets play any role in enhancing S. aureus killing by macrophages, peritoneal macrophages from Fig. 2A time = 0 h were co-cultured in the absence or presence of freshly prepared platelets for an additional 3 h (t = 3 h) and CFUs were enumerated. In the absence of platelets there was a 3–4-fold increase in the CFU collected from macrophages after 3 h, suggesting bacterial replication inside macrophages (Fig. 2C and D). However, in the presence of thrombin-activated platelets, MRSA replication was significantly reduced suggesting that platelet-stimulated macrophages are more efficient at restricting the intracellular growth of S. aureus. It should also be noted that platelet-mediated enhancement of phagocytosis and restriction of intracellular growth is thrombin dependent (Fig. 2E and F). This is consistent and support our observations in Fig 2C.
Releasates from thrombin stimulated platelets enhance macrophage uptake and restrict intracellular growth of S. aureus
Platelets can engulf bacteria and facilitate their transport to immune effector cells (26, 30). So it is possible that platelets capture blood-borne pathogens and deliver them through direct contact to macrophages for phagocytosis and killing, consistent with observations in Fig. 2. Another possibility is that enhanced uptake and killing occurs through soluble mediators released by platelets. To determine whether platelet-enhanced uptake and killing was due to direct interaction or through releasable products, parallel experiments were performed as in Fig. 2 except peritoneal macrophages were incubated with releasates collected from resting or activated platelets instead of intact platelets. Interestingly, platelet releasates alone significantly enhanced macrophage uptake of bacteria by over 60% (Fig. 3A). This uptake was supported by confocal images indicating enhanced S. aureus internalization (Fig. 3B). Additionally, platelet releasates restricted intracellular growth of MRSA, as indicated by significant inhibition of bacterial growth compared to control (Fig. 3C and D). The effect mediated by platelet releasates also appears similar to that mediated by platelets (compare Fig. 2 and Fig. 3). Collectively, these data indicate that platelets mediate this process through releasable products rather than through direct interactions with macrophages.
Platelets enhance macrophage uptake and restrict intracellular growth of S. aureus through IL-1β
Activated platelets release many bioactive molecules with immune functions that affect other immune cells, including macrophages. For example, platelets are a rich source of such cytokines and chemokines as IL-1β, sCD40L, and RANTES (14, 31–33). To assess this, phagocytosis and killing assays were performed as above except peritoneal macrophages were incubated with S. aureus in the presence or absence of recombinant IL-1β, sCD40L, RANTES, or a combination of all three. Unexpectedly IL-1β, but not sCD40L or RANTES, significantly increased the uptake of S. aureus by macrophages by ~33% (Fig. 4A), and the addition of all mediators combined did not add to the effect observed by IL-1β alone (data not shown). Intracellular killing assays were also performed in the presence or absence of these recombinant cytokines to determine if they can mimic the effects of intracellular killing of S. aureus by macrophages. Again, only IL-1β, but not sCD40L or RANTES, significantly inhibited intracellular growth of S. aureus (Fig. 4C and D). Dose dependency studies determined that the optimal dose of IL-1β was 10 ng/ml for both internalization and reducing intracellular growth (Fig. 5).
Figure 4. IL-1β enhanced S. aureus phagocytosis by peritoneal macrophages and restricted their intracellular growth.
Peritoneal macrophages were incubated with MRSA in RPMI medium in the presence or absence of IL-1β, CD40L, or RANTES for 1 h (t = 0). Peritoneal macrophages were washed with PBS to clear non-phagocytosed bacteria. (A) MRSA collected from macrophage lysates at t = 0 were plated and CFUs enumerated. (B–D) Macrophages from black or gray bars in (A) were further incubated for 3 h (t = 3) in the presence or absence of IL-1β, CD40L, RANTES, washed with PBS, intracellular MRSA were recovered from macrophage lysates, and CFU were determined. All measures are presented as mean ± SE (n=4), *P<0.05, NS=not significant.
Figure 5. IL-1β dose response relationship.
Peritoneal macrophages were incubated with MRSA in RPMI medium in the presence different concentration of IL-1β. Peritoneal macrophages were washed with PBS to clear non phagocytosed bacteria. (A) MRSA CFU from macrophage lysates at t = 0. (B) Macrophages from (A) were further incubated for 3 h (t = 3) in the presence (●) or absence (▲) of IL-1β, washed with PBS, intracellular MRSA were collected from macrophage lysates, and CFUs were determined. All measures are presented as mean ± SE (n=3),*P<0.05, **P<0.005, ***P<0.0005.
To further test this hypothesis, uptake and intracellular killing assays were repeated with platelet releasates in the presence or absence of anti- IL-1β neutralizing antibody. Internalization of S. aureus by macrophages showed a modest 25% decrease in the presences of IL-1β neutralizing antibody, which did not reach statistical significance. However, neutralization of IL-1β from platelet releasates significantly abrogated the reduced intracellular growth of S. aureus by macrophages by 81% (Fig. 6).
Figure 6. Effect of neutralizing IL-1β on platelet releasate-mediated phagcytosis and restriction of intracellular growth of S. aureus by peritoneal macrophages.

Peritoneal macrophages were incubated with MRSA in RPMI medium with releasate collected from 0.1U/ml thrombin-activated platelets for 1 h (t = 0) in the presence or absence of anti-IL-1β neutralizing antibody. Peritoneal macrophages were washed with PBS to clear non phagocytosed bacteria. (A) MRSA collected from macrophage lysates at t = 0 were plated and CFUs enumerated. (B) Macrophages from white bar in (A) were further incubated for 3 h (t = 3) with freshly added platelets releasate in the presence or absence of anti-IL-1β and intracellular bacteria CFUs from macrophage lysates were determined. All measures are presented as mean ± SE (n=3), *P<0.05, **P<0.005, ***P<0.0005.
Finally, IL-1β levels present in the solution before and after the addition of platelet releasates were quantified. As shown in Fig 7, neither platelets (non-activated or thrombin-activated) nor macrophages produced detectable amounts of IL-1 β. However, in the presence of MRSA, both cell populations produce marginal amounts of IL-1β (all mice show increases but statistical significance only in 3 of 4 mice tested). Macrophages only produced measurable amounts of IL-1β when co-cultured with MRSA and platelets. However, the addition of thrombin-activated platelets caused a robust increase in the IL-1β produced by macrophages thus implying that thrombin-activated platelets are required for optimal IL-1β production.
Figure 7. Platelets secret IL-1β and synergistically induce macrophages secretion of IL-1β.

Peritoneal macrophages (107/ml) were incubated with unstimulated or thrombin-activated platelets (108/ml) in PBS in the presence or absence of MRSA (107 CFU/ml) and the mixtures were incubated for 1 h. Supernatants were collected and analyzed by ELISA for quantification of IL-1β. All measures are presented as mean ± SE (n=4), *P<0.05, ***P<0.0001.
Discussion
Accumulating evidence suggests that platelets contribute to diverse immunological processes, extending beyond their classical role in haemostasis and thrombosis. Platelets engage the immune system by interacting with various immune cells (34, 35), and participate in both innate and adaptive immune responses. Our recent publication indicate that platelets provide protection to S. aureus infection (19). Based on this observation, the goal of the present study was to further elucidate the mechanistic details of how platelets promote protection against S. aureus infection and whether this occurs directly and/or through the potentiation of leukocytes antimicrobial functions.
In this study, platelets were observed to (i) kill S. aureus directly in an activation dependent manner, (ii) activated platelets bind, but rarely internalize, S. aureus and force the pathogens into clusters, (iii) cytochalasin D was able to block killing, and (iv) platelet bactericidal activity appears to be independent of ROM. The inhibition of killing by cytochalasin D suggests that bactericidal activity is actin dependent. Actin polymerization and rearrangement is an important process for phagocytosis as well as for fusion of intracellular granules with plasma membrane which is common during platelet activation. Because (i) internalization of S. aureus was not observed; (ii) we detected killing of S. aureus using releasates from thrombin-activated platelets, and (iii) cytochalasin D inhibited killing by intact platelets and platelet releasates; we interpret these data to suggest that release of granule contents is responsible for killing of S. aureus. Thus, our data are consistent with previous observations suggesting that S. aureus killing most likely occurs extracellularly (22).
Our studies also suggest an indirect role of platelets in pathogens clearance through potentiation of phagocytic and killing capacity of phagocytes. It was observed that (i) activated platelets or their releasates enhance S. aureus phagocytosis and restrict intracellular growth within peritoneal macrophages, (ii) platelet-secreted IL-1β, but not CD40L or RANTES, mediated enhancement of S. aureus phagocytosis and restricted intracellular growth in peritoneal macrophages, and that (iii) IL-1β neutralizing antibody blocked platelet releasate-mediated enhancement of S. aureus phagocytosis and allowed S. aureus intracellular survival in peritoneal macrophages. These data suggest that the role of platelets extends beyond direct encounter of pathogens, and that platelets play a critical role in mediating phagocytic leukocyte antimicrobial functions during infection.
The IL-1 family of proteins has been implicated for its ability to regulate functional properties of many immune cell types and is an important mediator of host response to infection (36, 37). Activated platelets secret IL-1β and mediate inflammatory signaling by regulating IL-1β synthesis (38). Additionally, IL-1β is secreted by activated murine macrophages upon exposure to LPS (39). A recent study has shown that IL-1β stimulates antimicrobial immunity in macrophages through the recruitment of other antimicrobial effector molecules (40). In that study, recombinant IL-1β restricted intracellular replication and growth of Mycobacterium tuberculosis in murine and human macrophages. Our results are, collectively, consistent with these previous studies.
Our data indicate that platelet-produced IL-1β induced macrophages to further secrete additional IL-1β, amplifying growth restriction of S. aureus. Macrophages produce marginal levels of IL-1β in response to S. aureus, (Fig. 7). However, upon the addition of activated platelets, IL-1β levels increased dramatically. The effect of IL-1β on the uptake of S. aureus by macrophages did not seem to be as significant as its effect on restricting intracellular growth. However, we observed that activated platelets surround S. aureus and force bacteria into clusters which has previously been reported (23). The enhanced aggregation and clustering of bacteria will likely facilitate the engulfment of increased bacteria numbers by macrophages, thus enhancing phagocytosis and promoting rapid clearance of bacteria from circulation. In parallel to direct encounter of pathogens, platelets mediate bactericidal activities indirectly through enhancement of phagocytosis and restricted intracellular growth by other leukocytes.
Our work supports other recent evidence that platelets assist in clearance of S. aureus (and potentially other bacterial species) by liver Kupffer cells. Kupffer cells are macrophage-like cells that have been shown to interact with platelets during septic infection with S. aureus (30). In light of our current observations, the S. aureus captured by Kupffer cells and subsequently surrounded by platelets may be enhancing the clearance of S. aureus in vivo through several mechanisms. First, interaction between Kupffer cells and platelets is mediated by GPIb (CD42) and GPIIb (CD41) which are known to activate platelets. This may provide an activating signal required for platelets to kill S. aureus localized to Kupffer cell surfaces. Secondly, platelet release of IL-1β may enhance Kupffer cell activities (uptake and reduced intracellular survival) similarly to peritoneal macrophages in our study. Therefore, platelets and Kupffer cells work in coordinated fashion to localize S. aureus to defined areas where platelets can accumulate, kill S. aureus and activate Kupffer cells.
In total, platelets appear to be an emerging central player in host-defense through direct bactericidal activities and by orchestrating innate immune cells to perform more efficiently in clearing infectious pathogens.
Supplementary Material
Footnotes
This work was supported by NIH RO1 HL122401 (to R.G.W).
References
- 1.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC P For The Platelet Colloquium. Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 2.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Micro. 2014;12:426–437. doi: 10.1038/nrmicro3269. [DOI] [PubMed] [Google Scholar]
- 3.Garraud O, Cognasse F. Are platelets cells? And if yes, are they immune cells? Frontiers in Immunology. 2015;6:70. doi: 10.3389/fimmu.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Dieri R, de Laat B, Hemker HC. Thrombin generation: What have we learned? Blood Reviews. 26:197–203. doi: 10.1016/j.blre.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner EE, Andrews RK. Platelets: Envoys at the infection frontline. Journal of Infectious Diseases. 2013;208:871–873. doi: 10.1093/infdis/jit305. [DOI] [PubMed] [Google Scholar]
- 6.Klinger MHF. Platelets and inflammation. Anat Embryol. 1997;196:1–11. doi: 10.1007/s004290050075. [DOI] [PubMed] [Google Scholar]
- 7.Yeaman MR. The Role of Platelets in Antimicrobial Host Defense. Clinical Infectious Diseases. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 8.Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections - complex interactions with bacteria. Frontiers in immunology. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulrehman AY, Jackson EC, McNicol A. Platelet activation by Streptococcus sanguinis is accompanied by MAP kinase phosphorylation. Platelets. 2013;24:6–14. doi: 10.3109/09537104.2012.661105. [DOI] [PubMed] [Google Scholar]
- 10.Kalvegren H, Skoglund C, Helldahl C, Lerm M, Grenegard M, Bengtsson T. Toll-like receptor 2 stimulation of platelets is mediated by purinergic P2X1-dependent Ca2+ mobilisation, cyclooxygenase and purinergic P2Y1 and P2Y12 receptor activation. Thromb Haemost. 2010;103:398–407. doi: 10.1160/TH09-07-0442. [DOI] [PubMed] [Google Scholar]
- 11.Sun D, Popescu NI, Raisley B, Keshari RS, Dale GL, Lupu F, Coggeshall KM. Bacillus anthracis peptidoglycan activates human platelets through FcgammaRII and complement. Blood. 2013;122:571–579. doi: 10.1182/blood-2013-02-486613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keane C, Tilley D, Cunningham A, Smolenski A, Kadioglu A, Cox D, Jenkinson HF, Kerrigan SW. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. Journal of thrombosis and haemostasis : JTH. 2010;8:2757–2765. doi: 10.1111/j.1538-7836.2010.04093.x. [DOI] [PubMed] [Google Scholar]
- 13.Arman M, Krauel K, Tilley DO, Weber C, Cox D, Greinacher A, Kerrigan SW, Watson SP. Amplification of bacteria-induced platelet activation is triggered by FcgammaRIIA, integrin alphaIIbbeta3, and platelet factor 4. Blood. 2014;123:3166–3174. doi: 10.1182/blood-2013-11-540526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2003;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 15.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1β Is Sufficient for Abscess Formation in Immunity against Staphylococcus aureus in Mice. PLoS Pathogens. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ. Comparison of Staphylococcus aureus From Skin and Soft-Tissue Infections in US Emergency Department Patients, 2004 and 2008. Clinical Infectious Diseases. 2011;53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 17.Gordon RJ, Lowy FD. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infectious Disease Clinics. 16:297–318. doi: 10.1016/s0891-5520(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 19.Wuescher LM, Takashima A, Worth RG. A novel conditional platelet depletion mouse model reveals the importance of platelets in protection against S. aureus bacteremia. Journal of thrombosis and haemostasis : JTH. 2015;13:303–313. doi: 10.1111/jth.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeaman MR, Norman DC, Bayer AS. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman MR, Puentes SM, Norman DC, Bayer AS. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infection and immunity. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trier DA, Gank KD, Kupferwasser D, Yount NY, French WJ, Michelson AD, Kupferwasser LI, Xiong YQ, Bayer AS, Yeaman MR. Platelet antistaphylococcal responses occur through P2X1 and P2Y12 receptor-induced activation and kinocidin release. Infection and immunity. 2008;76:5706–5713. doi: 10.1128/IAI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, Zimmerman GA, Weyrich AS. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcγRIIA binds and internalizes IgG-containing complexes. Experimental Hematology. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 25.McCullough AC, Seifried M, Zhao X, Haase J, Kabat WJ, Yogev R, Blumenthal RM, Mukundan D. Higher incidence of perineal community acquired MRSA infections among toddlers. BMC Pediatrics. 2011;11:1–6. doi: 10.1186/1471-2431-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youssefian T, Drouin A, Massé JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV andStaphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 27.Wachowicz B, Olas B, Zbikowska HM, Buczynski A. Generation of reactive oxygen species in blood platelets. Platelets. 2002;13:175–182. doi: 10.1080/09533710022149395. [DOI] [PubMed] [Google Scholar]
- 28.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, DeVinney R, Doig CJ, Green FHY, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature medicine. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 29.Flannagan RS, Heit B, Heinrichs DE. Antimicrobial mechanisms of macrophages and the immune Evasion strategies of staphylococcus aureus. Pathogens. 2015;4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong CHY, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14:785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawrylowicz CM, Santoro SA, Platt FM, Unanue ER. Activated platelets express IL-1 activity. The Journal of Immunology. 1989;143:4015–4018. [PubMed] [Google Scholar]
- 32.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 33.Klinger MH, Wilhelm D, Bubel S, Sticherling M, Schröder JM, Kühnel W. Immunocytochemical localization of the chemokines RANTES and MIP-1 alpha within human platelets and their release during storage. International Archives Of Allergy And Immunology. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 34.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thrombosis Research. 2013;131:191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Li N. Platelet–lymphocyte cross-talk. Journal of Leukocyte Biology. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 36.Oppenheim JJ, Kovacs EJ, Matsushima K, Durum SK. There is more than one interleukin 1. Immunology Today. 1986;7:45–56. doi: 10.1016/0167-5699(86)90124-6. [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual Review of Immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 38.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. The Journal of Cell Biology. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beuscher HU, Günther C, Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. The Journal of Immunology. 1990;144:2179–2183. [PubMed] [Google Scholar]
- 40.Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, Behar SM. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation() Journal of immunology (Baltimore, Md : 1950) 2013;190:4196–4204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.