Abstract
Type I interferons (IFNs) are key mediators of immune defense against viruses and bacteria. Type I IFNs have also been implicated in protection against fungal infection, but their roles in anti-fungal immunity have not been thoroughly investigated. A recent study demonstrated that bacterial and fungal β-glucans stimulate IFN-β production by dendritic cells (DCs) following detection by the Dectin-1 receptor, but the effects of β-glucan-induced type I IFNs have not been defined. We investigated whether type I IFNs regulate CD8 T cell activation by fungal β-glucan particle-stimulated DCs. We demonstrate that β-glucan-stimulated DCs induce CD8 T cell proliferation, activation marker (CD44 and CD69) expression, and production of IFN-γ, IL-2 and granzyme B. Moreover, we show that type I IFNs support robust CD8 T cell activation (proliferation, and IFN-γ and granzyme B production) by β-glucan-stimulated DCs both in vitro and in vivo due to autocrine effects on the DCs. Specifically, type I IFNs promote antigen presentation on MHC I molecules, CD86 and CD40 expression, and the production of IL-12 p70, IL-2, IL-6 and TNF-α by β-glucan-stimulated DCs. We also demonstrate a role for autocrine type I IFN signaling in bacterial lipopolysaccharide (LPS)-induced DC maturation, although in the context of LPS stimulation, this mechanism is not so critical for CD8 T cell activation (promotes IFN-γ production, but not proliferation or granzyme B production). This study provides insight into the mechanisms underlying CD8 T cell activation during infection, which may be useful in the rational design of vaccines directed against pathogens and tumors.
Introduction
CD4 T cells have been shown to play key roles in the control of pathogenic fungi (1, 2). Th1 cells yield interferon (IFN)-γ to promote fungal killing by macrophages and neutrophils, while the Th17 cytokines IL-17 and IL-22 recruit and activate neutrophils. The role of CD8 T cells in anti-fungal defense is less well defined, although several studies have demonstrated that they are important. For example, depletion of CD8 T cells renders mice more susceptible to pulmonary infection with Crypotococcus neoformans and Paracoccidioides brasiliensis (3, 4). Some fungi have been shown to be facultative intracellular parasites (5) and thus infected cells may represent targets for CD8 T cell-mediated cytotoxicity. However, most fungi grow in yeast and filamentous forms that must be targeted for destruction by internalization (phagocytosis) or by extracellular mechanisms including neutrophil extracellular traps. CD8 T cell-dependent anti-fungal defense is therefore likely due in large part to the IFN-γ-mediated activation of macrophages and neutrophils.
β-glucans are glucose polymers that are commonly found in the cell walls of fungi, as well as some bacteria. β-glucans in particulate form (e.g. exposed on the surface of a yeast cell) activate the C-type lectin receptor (CLR) Dectin-1, which plays key roles in anti-fungal defense (6). Dectin-1, which is predominantly expressed by myeloid phagocytes (including DCs), signals via an ITAM-like motif to activate signaling pathways that trigger phagocytosis, an oxidative burst, and inflammatory cytokine production (6). Bacterial and fungal β-glucans have also been shown to induce the Dectin-1-dependent maturation of DCs, which enables them to efficiently activate both CD4 T cells (Th17 polarization in particular) and CD8 T cells (1, 7-9).
The caspase activation and recruitment domain (CARD) 9 adaptor protein plays a central role in anti-fungal defense due to its ability to activate NF-κB downstream of Dectin-1 and other CLRs that detect fungal components (6). Dectin-1 signaling via the CARD9-NF-κB pathway leads to DC production of inflammatory cytokines, including IL-6, IL-12 and TNF-α (10). A recent paper showed that CARD9 also transduces signals via interferon regulatory factor (IRF)5 to induce the expression of IFN-β by DCs (11).
Type I IFNs (including IFN-α and IFN-β) are key mediators of immune defense against viruses and also bacteria, largely due to their ability to activate cytotoxic effector cells (NK and CD8 T cells) to kill infected host cells (12). More recently, type I IFNs have been implicated in protection against fungal infection (12). For example, DCs have been shown to produce IFN-β upon stimulation with C. neoformans and Candida albicans, and mice deficient in the type I IFN receptor (IFNAR) are more susceptible to infection with these fungi (11, 13, 14). A recent study demonstrated that DCs are a major source of IFN-β following C. albicans infection (11).
The type I IFN receptor, which comprises IFNAR1 and IFNAR2 subunits, is broadly expressed on hematopoietic and non-hematopoietic cells, and type I IFNs have been shown to act via diverse mechanisms (12). The roles of type I IFNs in anti-fungal immunity have not yet been thoroughly investigated, although type I IFNs have been implicated in the promotion of fungicidal responses, the recruitment and activation of neutrophils, and production of the cytokines IFN-γ and TNF-α (11, 13, 14).
In the current study we investigated whether type I IFNs produced by DCs in response to stimulation with fungal β-glucan particles regulate DC-mediated CD8 T cell activation. Using neutralizing antibodies and IFNAR1-deficient mice, we show that type I IFNs are required for robust CD8 T cell proliferation and production of IFN-γ and granzyme B upon co-culture with fungal β-glucan-stimulated DCs. However, in contrast to the influence of other cytokines that orchestrate T cell activation and polarization, the direct action of type I IFNs on CD8 T cells is not required. Instead, our in vitro and in vivo studies demonstrate that autocrine IFNAR signaling in the β-glucan-stimulated DCs is required for efficient CD8 T cell activation. We show that autocrine type I IFN signaling promotes antigen presentation, upregulation of the co-stimulatory molecules CD40 and CD86, and production of certain inflammatory cytokines (IL-12 p70, IL-6 and TNF-α). We also report a similar role for autocrine type I IFN signaling in DC maturation following LPS stimulation, although in that context, autocrine type I IFN signaling promotes IFN-γ production, but not proliferation or granzyme B production by CD8 T cells.
Materials and Methods
Microbial components
Fungal β-glucan particles were prepared by treating Saccharomyces cerevisiae zymosan A (Sigma) to remove TLR agonists (depleted zymosan) as previously described (15). Briefly, zymosan particles were boiled in 10 M NaOH for 30 minutes and then thoroughly washed with PBS and sonicated prior to use. Salmonella minnesota lipopolysaccharide (LPS) was from Invivogen.
Mice
All animal procedures were performed with the prior approval of the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center. Wild-type C57BL/6 mice (CD45.2 and the congenic CD45.1 strain), IFNAR1-deficient mice and OVA peptide (SIINFEKL)-specific OT-I mice were purchased from The Jackson Laboratory. Dectin-1-deficient mice, originally obtained from Gordon Brown (University of Aberdeen, UK), were bred in house.
Cells
Dendritic cells were derived from the bone marrow of mouse femurs and tibias by culture with 20 ng/ml mGM-CSF for 8 days. Adherent cells (>95% CD11b+ CD11c+) were harvested from the cultures, plated and rested for 4 hours prior to stimulation. Naïve CD8 T cells were isolated from the spleens and lymph nodes of OT-I mice by negative selection (>90% CD8+ CD44-/lo) using a kit from Stem Cell Technologies, according to the manufacturer's instructions.
DC:T cell co-culture
DCs were treated with OVA peptide (SIINFEKL; 1.1 nM) for 30 minutes and then stimulated with β-glucan particles or LPS for 24 hours. DCs were then washed thoroughly and CFSE-labeled naïve CD8 T cells were added at a 1:5 ratio (8×104 DCs plus 40×104 T cells) and cultures were supplemented with 1 M sodium pyruvate and 50 mM β-mercaptoethanol. In some experiments, anti-IFNAR1 blocking antibodies, anti-CD86 blocking antibodies or isotype control antibodies (200 μg/ml; BioLegend) were added to DC cultures and/or DC:T cell co-cultures as described in the figure legends. Following 3 days of co-culture, T cells were harvested for flow cytometry (CFSE, CD44, CD69) and for re-stimulation to assess cytokine and granzyme B production. T cells were re-stimulated with 50 ng/ml PMA plus 500 ng/ml ionomycin for 6 hours with GolgiPlug and GolgiStop (Biolegend) for the last 4 hours in order to assess cytokine and granzyme B production by intracellular flow cytometry, and for 24 hours (without the protein export inhibitors) for cytokine assessment by ELISA.
In vivo CD8 T cell activation
Naïve CD8 T cells from OT-I mice (CD45.2+) CD8 T cells were injected i.v. into CD45.1+ recipient mice (5×105 T cells/recipient). The same day, DCs plated with GM-CSF were given OVA peptide 257-264 (SIINFEKL; 550 pM) and then stimulated for 24 hours with fungal β-glucan particles (100 μg/ml). The following day, the DCs were lifted and washed with PBS prior to i.v. delivery into the same recipient mice (5×105 DCs/mouse). Spleens were harvested 4 days later, and donor-derived CD8 T cells (CD45.2+ CD8+ cells) were evaluated by flow cytometry to assess numbers and maturation. Splenocytes were also plated and re-stimulated with OVA peptide (SIINFEKL; 1.1 nM) for 6 hours (with GolgiPlug and GolgiStop for the last 4 hours) for assessment of cytokine and granzyme B production by intracellular flow cytometry.
Flow cytometry and Western blotting
Flow cytometry was performed as described previously (16) using a BD Fortessa Analyzer, and data were analyzed using FlowJo software. Antibodies were from BioLegend (MHC I, MHC IOVA (SIINFEKL), CD8α, CD44, CD80, CD86) and BD Bioscience (CD8α, CD40, CD69, IFN-γ, IL-2) and eBiosciences (granzyme B). Western blotting was performed as described previously (16) using the NuPAGE Novex gel system from Invitrogen and antibodies from Cell Signaling Technologies. A LI-COR Odyssey imaging system was used for visualization and densitometric analysis.
Cytokine measurements
Type I IFN production was assessed indirectly using interferon-stimulated response element (ISRE)-luciferase L929 reporter cells from Bruce Beutler's laboratory (17) and a luciferase assay system kit from Promega. All other cytokines were assessed using ELISA kits from BioLegend.
Statistical analysis
Statistical significance was assessed using t-tests (Figures 1 and 2) or ANOVA with Bonferroni correction (Figures 3-5, 7-8 and Supplemental Figure 1).
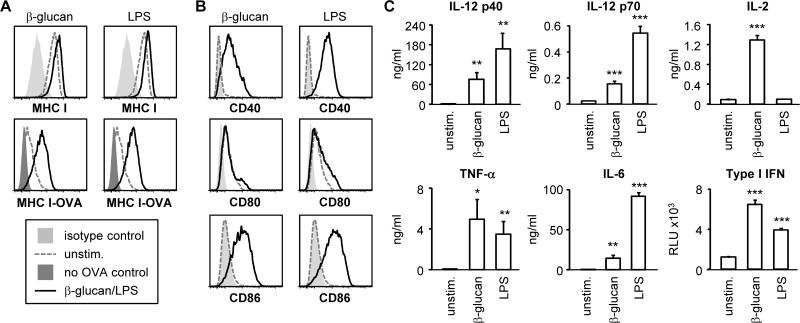
Figure 1. Fungal β-glucan particles promote antigen presentation and induce DC maturation.
Bone marrow-derived DCs were stimulated with 100 μg/ml fungal β-glucan particles or 100 ng/ml LPS for 24 hours, and surface expression of MHC I, MHC I-OVA peptide (SIINFEKL) and co-stimulatory molecules was assessed by flow cytometry (A-B). For MHC IOVA measurement, DC cultures were supplemented with 5.5 μM OVA peptide (SIINFEKL) 1 hour prior to β-glucan/LPS stimulation. Cytokine levels in culture supernatants harvested 24 hours after DC stimulation were assessed by ELISA (all except type I IFN) or a luciferase reporter assay (type I IFN) (C). Cytokine data are mean plus standard deviation of triplicate culture. All data are representative of at least 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001 (relative to unstimulated DCs)
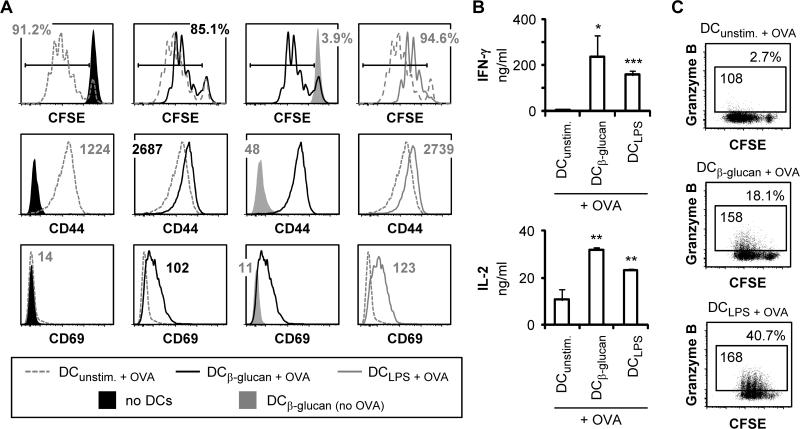
Figure 2. Fungal β-glucan particle stimulation of DCs enhances their ability to activate CD8 T cells.
DCs cultures were supplemented with 1.1 nM OVA peptide (SIINFEKL) for 1 hour prior to stimulation with 100 μg/ml fungal β-glucan particles or 100 ng/ml LPS for 24 hours. DCs were then washed with PBS prior to the addition of CFSE-labeled naïve OT-I CD8 T cells (1:5 DC:T cell ratio). Co-cultures were incubated for 3 days, and T cells were then harvested for assessment of proliferation (CFSE dilution; gated on CD8+ cells) and surface expression of CD44 and CD69 (gated on proliferating CD8+ cells) by flow cytometry (A). T cells were also re-stimulated with PMA + ionomycin to assess cytokine production by ELISA (B; 24 hour supernatants, mean plus standard deviation of triplicate culture) and granzyme B production by intracellular flow cytometry (C; 6 hour stimulation with protein export inhibitors for the final 4 hours, gated on CD8+ cells). % granzyme B-producing T cells and granzyme B MFI (gated on granzyme B-producing T cells) are indicated (C). All data are representative of at least 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001 (relative to T cells activated by unstimulated DCs)
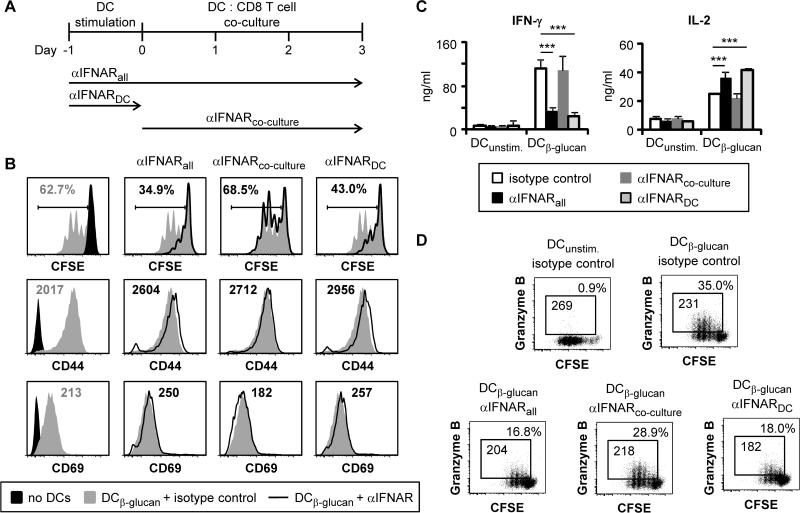
Figure 3. Blockade of IFNAR1 signaling during DC stimulation suppresses CD8 T cell activation by β-glucan-stimulated DCs.
DCs were loaded with OVA peptide (SIINFEKL; 1.1 nM) and stimulated with 100 μg/ml fungal β-glucan particles for 24 hours. DCs were then washed prior to co-culture with CFSE-labeled naïve OT-I CD8 T cells (1:5 DC:T cell ratio) for 3 days. Cultures were supplemented with anti-IFNAR1 antibodies (or isotype control antibodies; 200 μg/ml) during the DC stimulation stage (αIFNARDC), the DC:T cell co-culture stage (αIFNARco-culture), or throughout both culture stages (αIFNARall; antibodies replaced after DC washing) (A). Proliferation (CFSE dilution; gated on CD8+ cells) and surface expression of CD44 and CD69 (gated on proliferating CD8+ cells) were assessed by flow cytometry (B). T cells were also re-stimulated with PMA + ionomycin to assess cytokine production by ELISA (C; 24 hour supernatants, mean plus standard deviation of triplicate culture), and granzyme B production by intracellular flow cytometry (D; 6 hour stimulation with protein export inhibitors for the final 4 hours, gated on CD8+ cells). % granzyme B-producing T cells and granzyme B MFI (gated on granzyme B-producing T cells) are indicated (D). All data are representative of at least 3 independent experiments. ***p<0.001
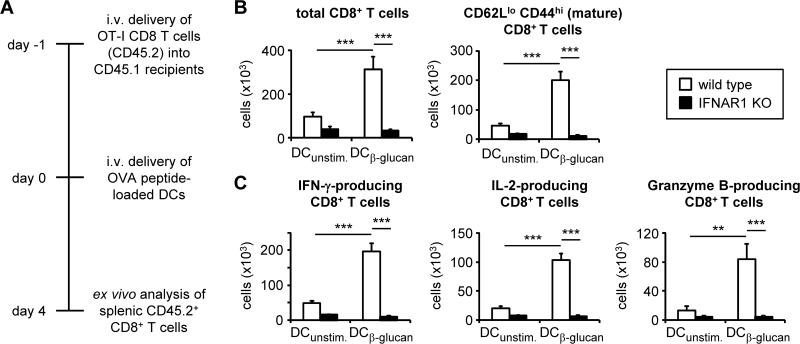
Figure 5. IFNAR1 deletion compromises the ability of β-glucan-stimulated DCs to activate CD8 T cells in vivo.
(A) Experimental scheme for CD8 T cell and DC adoptive transfer. 5×105 OT-I CD8 T cells (CD45.2) were injected i.v. into congenic (CD45.1) recipient mice. The same day, wild type and IFNAR1-deficient DCs were loaded with OVA peptide (SIINFEKL; 550 pM) and stimulated with 100 μg/ml fungal β-glucan particles for 24 hours. The following day, DCs were harvested and washed, and 5×105 DCs were injected i.v. into the same recipient mice. Spleens were harvested 4 days later. (B) Donor T cell maturation and expansion was assessed by flow cytometry of splenocytes (gated on CD45.2+ CD8+ cells). (C) Splenocytes were plated and stimulated with OVA peptide (SIINFEKL; 1.1 nM), and cytokine and granzyme B production was assessed by intracellular flow cytometry (6-hour stimulation with protein export inhibitors for the final 4 hours; gated on CD45.2+ CD8+ cells). Data are mean plus standard error of pooled data from 2 independent experiments (6-8 mice/group). **p<0.01, ***p<0.001
Figure 7. Autocrine type I IFN signaling promotes DC maturation following β-glucan stimulation.
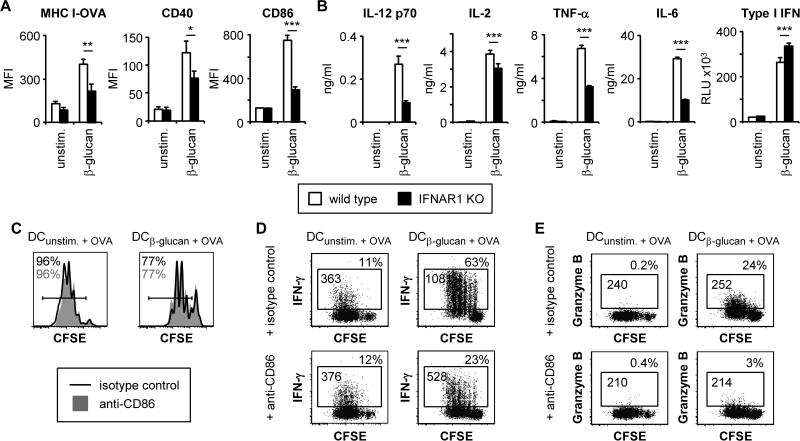
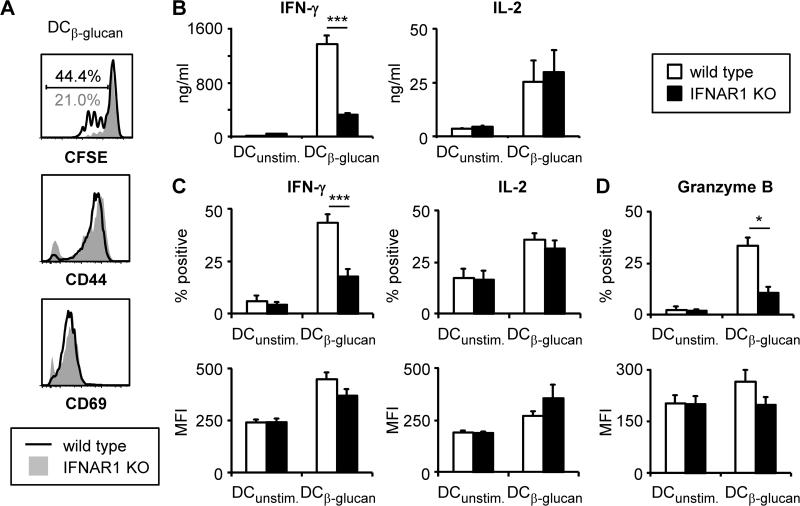
(A-B) Wild-type and IFNAR1-deficient DCs were stimulated with 100 μg/ml fungal β-glucan particles for 24 hours. Surface expression of MHC I-OVA and co-stimulatory molecules was assessed by flow cytometry (A). For MHC I-OVA measurement, DC cultures were supplemented with 5.5 μM OVA peptide (SIINFEKL) 1 hour prior to β-glucan stimulation. Cytokine levels in culture supernatants were assessed by ELISA (all except type I IFN) or a luciferase reporter assay (type I IFN) (B; mean plus standard deviation of triplicate culture). (C-E) Wild-type DCs were loaded with 1.1 nM OVA peptide (SIINFEKL) and stimulated as above prior to co-culture with naïve CFSE-labeled OT-I CD8 T cells for 3 days in the presence of anti-CD86 or isotype control antibodies (200 μg/ml). Proliferation (CFSE dilution) was assessed by flow cytometry (C). T cells were also re-stimulated with PMA + ionomycin (6 hour stimulation, with protein export inhibitors for the final 4 hours) to assess IFN-γ (D) and granzyme B production (E) by intracellular flow cytometry. % IFN-γ/granzyme B-producing T cells (gated on CD8+ cells) and IFN-γ/granzyme B MFI (gated on IFN-γ/granzyme B-producing CD8+ cells) are indicated (D-E). Data are mean plus standard error of 5-6 independent experiments (A), or representative of at least 3 independent experiments (B-E). *p<0.05, **p<0.01, ***p<0.001
Figure 8. Autocrine type I IFN signaling also regulates DC maturation and CD8 T cell activation following LPS stimulation.
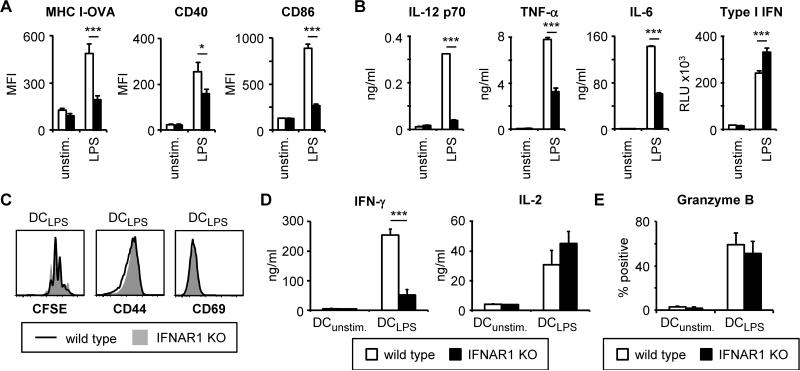
(A-B) Wild-type and IFNAR1-deficient DCs were stimulated with 100 ng/ml LPS for 24 hours. Surface expression of MHC I-OVA and costimulatory molecules was assessed by flow cytometry (A). For MHC I-OVA measurement, DC cultures were supplemented with 5.5 μM OVA peptide (SIINFEKL) 1 hour prior to LPS stimulation. Cytokine levels in culture supernatants were assessed by ELISA (all except type I IFN) or a luciferase reporter assay (type I IFN) (B; mean plus standard deviation of triplicate culture). (C-E) Wild-type and IFNAR1-deficient DCs were loaded with 1.1 nM OVA peptide (SIINFEKL) and stimulated as above prior to co-culture with CFSE-labeled OT-I CD8 T cells for 3 days. Proliferation was assessed by flow cytometry (C). T cells were also re-stimulated with PMA + ionomycin (6 hour stimulation, with protein export inhibitors for the final 4 hours) to assess IFN-γ and IL-2 production by ELISA (D). Granzyme B production following PMA + ionomycin stimulation was assessed by intracellular flow cytometry; % granzyme B-producing T cells (gated on CD8+ cells) are indicated (E). Data are mean plus standard error of 4-9 independent experiments (A, E), or representative of at least 3 independent experiments (B-D). *p<0.05, ***p<0.001
Results
Fungal β-glucan particle stimulation induces Dectin-1-dependent DC maturation
In order to determine how fungal β-glucans influence CD8 T cell activation by DCs, we first assessed antigen presentation, co-stimulatory molecule expression and cytokine production by bone marrow-derived DCs stimulated with β-glucan particles derived from Saccharomyces cerevisiae (see Materials and Methods). β-glucan stimulation did not significantly increase surface levels of MHC I, but did increase the presentation of ovalbumin (OVA) peptide on MHC I molecules at the cell surface (Figure 1A). Surface expression of the co-stimulatory molecules CD40 and CD86 (but not CD80) was also elevated following β-glucan stimulation (Figure 1B), and the DCs produced IL-6, TNF-α, IL-12 p40, IL-12 p70 and IL-2 (Figure 1C). Consistent with a recent report that β-glucan-containing particles (curdlan and zymosan) induce the production of type I interferons (IFNs) (11), we also observed type I IFN production by the fungal β-glucan particle-stimulated DCs (Figure 1C). All of the DC responses to β-glucan particles were Dectin-1-dependent (Supplemental Figure 1). The effects of fungal β-glucan particles on DC maturation were comparable with Gram-negative bacterial lipopolysaccharide (LPS) stimulation (Figure 1), except that LPS consistently induced higher levels of IL-6 and IL-12 p70 production, but did not stimulate IL-2 release (Figure 1C).
Fungal β-glucan particle-stimulated DCs activate CD8 T cells
We next examined the ability of the β-glucan particle-stimulated DCs to activate CD8 T cells. DCs were incubated with OVA peptide and stimulated with either fungal β-glucan particles or LPS prior to co-culture with naïve OVA-specific (OT-I) CD8 T cells. Consistent with previous reports that DCs stimulated via Dectin-1 can prime CD8 T cells (7-9), fungal β-glucan particle stimulation of DCs increased the expression of T cell activation markers (CD44 and CD69; Figure 2A) and the production of IFN-γ, IL-2 and granzyme B by CD8 T cells following re-stimulation with PMA and ionomycin (Figure 2B-C and Supplemental Figure 2A). CD8 T cell activation by the β-glucan-stimulated DCs was Dectin-1-dependent (Supplemental Figure 2B-C). The fungal β-glucan-stimulated DCs were comparable to LPS-stimulated DCs in their ability to activate CD8 T cells (Figure 2 and Supplemental Figure 2). Interestingly, the CD8 T cells co-cultured with unstimulated DCs proliferated more robustly than those co-cultured with β-glucan/LPS-stimulated DCs (Figure 2A) but were not fully activated (Figure 2 and Supplementary Figure 2). The β-glucan/LPS-stimulated DCs were particularly effective at inducing CD69, IFN-γ and granzyme B production (Figure 2 and Supplementary Figure 2).
Type I IFNs produced by fungal β-glucan-primed DCs regulate CD8 T cell activation via autocrine signaling in DCs
Type I IFNs have been shown to be important for anti-fungal defense and are known to have pleiotropic effects on the immune system (12). We therefore next investigated whether type I IFNs play a role in CD8 T cell activation by fungal β-glucan-stimulated DCs using an antibody that blocks type I IFN detection by its receptor (anti-IFNAR1). Continuous antibody supplementation throughout both the DC stimulation and DC:T cell co-culture stages (Figure 3A) inhibited CD8 T cell proliferation and reduced IFN-γ and granzyme B production, but did not alter the expression of the activation markers CD44 and CD69 or reduce IL-2 production by the proliferating CD8 T cells (Figure 3B-D).
Interestingly, the presence of the IFNAR1 antibody during the co-culture only (i.e. not during the DC stimulation phase; Figure 3A) had no effect on CD8 T cell proliferation, activation marker expression, or IFN-γ and granzyme B production (Figure 3B-D). We therefore investigated whether addition of the IFNAR1 antibody during the DC stimulation stage only, followed by thorough washing to remove the antibody prior to the co-culture stage (Figure 3A), was sufficient to alter CD8 T cell activation. We observed similar effects to the continuous blockade of IFNAR1 signaling throughout the DC stimulation and co-culture stages i.e. decreased T cell proliferation and reduced IFN-γ and granzyme B production (Figure 3B-D).
To further corroborate these findings, we assessed the ability of IFNAR1-deficient DCs to activate CD8 T cells in vitro following β-glucan stimulation. Consistent with the data obtained using the neutralizing antibody, the CD8 T cells co-cultured with IFNAR1-deficient DCs exhibited reduced proliferation and IFN-γ and granzyme B production (Figure 4). Intracellular flow cytometry analysis revealed that levels of IFN-γ and granzyme B were not altered on a per cell basis, but that fewer T cells produced these mediators (Figure 4B-D).
Figure 4. IFNAR1-deficient DCs are less efficient than wild-type DCs at activating CD8 T cells following β-glucan stimulation.
Wild-type and IFNAR1-deficient DCs were loaded with OVA peptide (SIINFEKL; 1.1 nM) and stimulated with 100 μg/ml fungal β-glucan particles for 24 hours. DCs were then washed prior to co-culture with CFSE-labeled naïve OT-I CD8 T cells (1:5 DC:T cell ratio) for 3 days. T cells were then harvested and proliferation (CFSE dilution; gated on CD8+ cells) and surface expression of CD44 and CD69 (gated on proliferating CD8+ cells) were assessed by flow cytometry (A). T cells were also re-stimulated with PMA + ionomycin to assess cytokine production by ELISA (B; 24-hour supernatants, mean plus standard deviation of triplicate culture), and cytokine and granzyme B production by intracellular flow cytometry (C-D; 6-hour stimulation with protein export inhibitors for the final 4 hours, gated on CD8+ cells). % positive cells and MFI (gated on positive cells) are plotted (C-D). Data are representative of at least 3 independent experiments (A-B), or mean plus standard error of 3 independent experiments (C-D). *p<0.05, ***p<0.001
We next assessed the role of autocrine type I IFN signaling in CD8 T cell activation by β-glucan-stimulated DCs in vivo. Wild-type and IFNAR1-deficient DCs incubated with OVA peptide and stimulated with fungal β-glucan particles were adoptively transferred into recipient mice that had received naïve OVA-specific (OT-I) CD8 T cells the previous day (Figure 5A). The wild-type DCs induced strong OVA-specific CD8 T cell expansion and IFN-γ, IL-2 and granzyme B production, but the IFNAR1-deficient DCs failed to activate the CD8 T cells (Figure 5B-C).
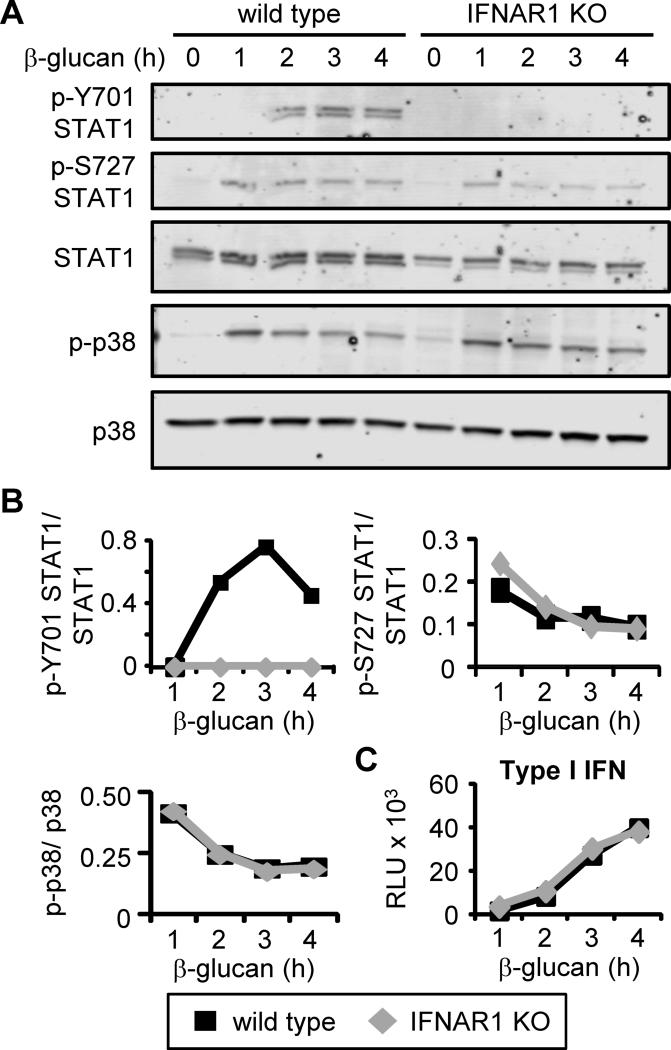
To confirm that type I IFNs act in an autocrine manner on the DCs we assessed the activation of signal transducer and activator of transcription 1 (STAT1), a key downstream mediator of IFNAR signaling. β-glucan stimulation induced phosphorylation of STAT1 on both Y701 and S727 (Figure 6A-B). Phosphorylation of Y701 is necessary for STAT1 to enter the nucleus and regulate transcription. The kinetics of STAT1 Y701 phosphorylation corresponded with the timing of type I IFN production (Figure 6C), and stimulation of DCs from IFNAR1-deficient mice confirmed that this activatory phosphorylation was type I IFN-dependent (Figure 6A). In contrast, STAT1 phosphorylation at S727 was induced at earlier time points and was type I IFN-independent (Figure 6A-B). Phosphorylation of p38 MAP kinase following β-glucan stimulation was also intact in the IFNAR1-deficient DCs (Figure 6A-B), indicating that although Dectin-1-mediated β-glucan responses were deficient, Dectin-1 signaling was not itself impacted by IFNAR1 deletion.
Figure 6. β-glucan stimulation triggers IFNAR1-dependent STAT1 phosphorylation.
Wild-type and IFNAR1-deficient DCs were stimulated with 100 μg/ml fungal β-glucan particles for the indicated time points. STAT1 and p38 phosphorylation was assessed by Western blotting (A) and densitometric analysis was performed (B). Type I IFN levels in culture supernatants were assessed using a luciferase reporter assay (C; mean plus standard deviation of triplicate culture). All data are representative of at least 3 independent experiments.
Autocrine type I IFN signaling regulates DC maturation
In order to gain insight into how IFNAR1 signaling in DCs promotes CD8 T cell activation, we next investigated the roles of β-glucan-induced type I IFNs in DC maturation. β-glucan stimulation of MHC I-OVA, CD40 and CD86 surface expression was significantly reduced in IFNAR1-deficient DCs (Figure 7A), and production of IL-12 p70 (a known regulator of IFN-γ production), IL-6, TNF-α, and to a lesser extent IL-2, was also suppressed (Figure 7B). The importance of CD86 upregulation was revealed by the addition of an anti-CD86 blocking antibody to wild-type DC:T cell co-cultures, which demonstrated that CD86 co-stimulation is required for the induction of IFN-γ and granzyme B production by CD8 T cells activated by β-glucan-stimulated DCs, but not for T cell proliferation (Figure 7C-E).
Autocrine type I IFN signaling also regulates LPS-induced DC maturation and CD8 T cell activation
Finally, we observed a similar autocrine role for type I IFNs in the maturation of LPS-stimulated DCs, and the activation of CD8 T cells by LPS-stimulated DCs (Figure 8). LPS-induced MHC IOVA and CD86 upregulation was severely compromised in IFNAR1-deficient DCs (Figure 8A), and IL-12 p70, IL-6 and TNF-γ production was reduced (Figure 8B). LPS-stimulated IFNAR1-deficient DCs were also less efficient than wild-type DCs at inducing IFN-γ production by CD8 T cells in in vitro co-cultures, although T cell proliferation and granzyme B production were not compromised (Figure 8C-E).
Taken together, our data show that type I IFNs induced by microbial stimuli can promote DC maturation and thereby play a key role in CD8 T cell activation, although the precise role depends on the specific microbial stimulus.
Discussion
In this study we have shown that stimulation with fungal β-glucan particles induces DC maturation (including antigen presentation on MHC I, induction of the co-stimulatory molecules CD40 and CD86, and production of inflammatory cytokines) and that, like LPS-stimulated DCs, fungal β-glucan-stimulated DCs promote CD8 T cell activation (proliferation, expression of CD44 and CD69, and production of IFN-γ, IL-2 and granzyme B). Interestingly, consistent with previous observations (8), unstimulated DCs induced stronger CD8 T cell proliferation than β-glucan- or LPS-stimulated DCs. Nevertheless, both β-glucan- and LPS-stimulated DCs induced stronger T cell activation than the unstimulated DCs, and were particularly effective at inducing CD69, IFN-γ and granzyme B production. Our data are consistent with and extend the findings of previous studies demonstrating that mouse and human DCs activated via Dectin-1 with fungal and/or bacterial β-glucans can prime CD8 T cell responses in addition to activating CD4 T cells (7-9).
Moreover, we have demonstrated that type I IFNs produced by DCs in response to stimulation with fungal β-glucan particles or LPS regulate CD8 T cell activation (although with distinct roles in response to the different stimuli, perhaps due to the collaborative effects of other cytokines). Interestingly, unlike many of the other DC cytokines that act directly on T cells to regulate their activation and polarization, we have shown that in response to β-glucan or LPS stimulation, type I IFNs regulate CD8 T cell activation via autocrine effects on the DCs (Supplemental Figure 3). Specifically, type I IFNs induced by fungal β-glucan stimulation of Dectin-1 or by LPS stimulation of TLR4 promote antigen presentation, CD40 and CD86 expression, and the production of IL-12 p70, IL-6 and TNF-α. IL-12 p70 is a well characterized regulator of IFN-γ production by T cells, but the relevance of TNF-α and IL-6 to CD8 T cell activation by β-glucan-stimulated DCs is not yet clear. TNF-α has previously been reported to promote anti-viral CD8 T cell responses (18, 19). IL-6 has been shown to promote CD8 T cell activation via trans signaling (20) and in synergy with IL-7 or IL-15 (21), although other studies have suggested that it can negatively regulate DC maturation and CD8 T cell responses (22, 23).
Type I IFNs produced by mouse DCs following stimulation with TLR agonists (including LPS) have previously been shown to act in an autocrine manner to promote the expression of costimulatory molecules and inflammatory cytokines (including IL-6 and TNF-α) (24, 25). Type I IFNs have also been reported to accelerate the appearance of maturation markers, including HLA and CD86, when human DCs are derived from CD34+ progenitors using GM-CSF, TNF-α and IL-4 (26). Our study demonstrates that fungal β-glucan particle-induced type I IFNs have a similar influence on DC activation, and importantly, that these autocrine effects of both fungal β-glucan- and LPS-induced type I IFNs impact CD8 T cell activation.
Other cytokines induced upon microbial exposure have previously been reported to have autocrine effects on DCs that impact their ability to activate T cells. For instance, TNF-α has been shown to promote DC maturation in response to viral infection (18, 19). Moreover, IL-12 produced by Listeria-infected DCs has been reported to promote CD8 T cell activation by inducing the DCs to produce the chemokines CCL1 and CCL17, which support sustained cell contact between the DCs and the T cells (27). IL-10 can also act in an autocrine manner on DCs to suppress their ability to activate and polarize T cells (28).
In addition to shedding light on the mechanisms of activation of CD8 T cells in response to fungal infection, this study adds to a growing body of literature demonstrating the potential clinical utility of β-glucans. β-glucans are under evaluation for use as vaccine adjuvants, and their distinct signaling pathways and adjuvant properties may make them attractive alternatives to TLR agonists. It is interesting to note that CD8 T cell activation in response to several fungi has been reported to occur independently of CD4 T cell help (4, 29). Thus vaccination strategies that target CD8 T cells may be useful for promoting defense against opportunistic fungal pathogens in immunocompromised patients lacking CD4 T cells, such as AIDS patients.
Induction of potent CD8 T cell responses is also of particular interest for immunotherapy against tumors. β-glucans are already being evaluated in clinical trials in combination with antibodies against tumor antigens, following a series of studies that demonstrated the efficacy of such combination therapies in mouse models (30). The adjuvant properties of β-glucans may also be beneficial for the development of DC vaccines designed to provoke T cell responses directed against tumor antigens. Sipuleucel-T (Provenge) was the first FDA-approved DC vaccine for the treatment of prostate cancer, and recent studies have stimulated further interest in the use of adjuvants in the development of DC vaccines to provoke more potent effector T cell responses (31, 32).
Finally, tumor cell-associated N-glycans detected by Dectin-1 on DCs have recently been demonstrated to induce upregulation of surface molecules that activate NK cell cytotoxic responses (33). This effect was IRF5-dependent, but type I IFN-independent. It would therefore be interesting to evaluate whether fungal β-glucans also promote NK cell activation by DCs (via Dectin-1 and IRF5, but not type I IFNs), and whether tumor cell N-glycans promote or modify the ability of DCs to prime CD8 T cell responses via the autocrine action of type I IFNs induced by Dectin-1-IRF5 signaling.
Supplementary Material
Acknowledgments
This project was supported by funds from the Samuel Oschin Comprehensive Cancer Institute (Eleanor and Glenn Padnick Discovery Fund in Cellular Therapy) and the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center (to HSG), and R01 grant AI071116 from the National Institutes of Health (to DMU). NH-K's PhD student stipend was funded in part by the Graduate Program in Biomedical Science and Translational Medicine at Cedars-Sinai Medical Center. AY's salary was supported by a Careers in Immunology fellowship from the American Association of Immunologists (to HSG).
References
- 1.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 2.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15:630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 3.Cano LE, Singer-Vermes LM, Costa TA, Mengel JO, Xidieh CF, Arruda C, Andre DC, Vaz CA, Burger E, Calich VL. Depletion of CD8(+) T cells in vivo impairs host defense of mice resistant and susceptible to pulmonary paracoccidioidomycosis. Infect Immun. 2000;68:352–359. doi: 10.1128/iai.68.1.352-359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Bliska JB, Casadevall A. Intracellular pathogenic bacteria and fungi--a case of convergent evolution? Nat Rev Microbiol. 2009;7:165–171. doi: 10.1038/nrmicro2049. [DOI] [PubMed] [Google Scholar]
- 6.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One. 2010;5:e13418. doi: 10.1371/journal.pone.0013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 9.Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y, Yannelli JR, Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 11.del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavin C. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity. 2013;38:1176–1186. doi: 10.1016/j.immuni.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 12.McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biondo C, Midiri A, Gambuzza M, Gerace E, Falduto M, Galbo R, Bellantoni A, Beninati C, Teti G, Leanderson T, Mancuso G. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J Immunol. 2008;181:566–573. doi: 10.4049/jimmunol.181.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, Bellantoni A, Malara A, Beninati C, Teti G, Mancuso G. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol. 2011;41:1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- 15.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanez A, Ng MY, Hassanzadeh-Kiabi N, Goodridge HS. IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Blood. 2015;125:1452–1459. doi: 10.1182/blood-2014-09-600833. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Yang W, Shi X, Du P, Su L, Qin Z, Chen J, Deng H. TNF receptor 1 mediates dendritic cell maturation and CD8 T cell response through two distinct mechanisms. J Immunol. 2011;187:1184–1191. doi: 10.4049/jimmunol.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, Falck-Pedersen E. TNF-alpha -dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci U S A. 2001;98:12162–12167. doi: 10.1073/pnas.211423598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottcher JP, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C, Beyer M, Schultze JL, Kastenmuller W, Rose-John S, Knolle PA. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 2014;8:1318–1327. doi: 10.1016/j.celrep.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J Immunol. 2008;180:7958–7968. doi: 10.4049/jimmunol.180.12.7958. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Dietze KK, Gibbert K, Lang KS, Trilling M, Yan H, Wu J, Yang D, Lu M, Roggendorf M, Dittmer U, Liu J. TLR ligand induced IL-6 counter-regulates the anti-viral CD8(+) T cell response during an acute retrovirus infection. Sci Rep. 2015;5:10501. doi: 10.1038/srep10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroder K, Spille M, Pilz A, Lattin J, Bode KA, Irvine KM, Burrows AD, Ravasi T, Weighardt H, Stacey KJ, Decker T, Hume DA, Dalpke AH, Sweet MJ. Differential effects of CpG DNA on IFN-beta induction and STAT1 activation in murine macrophages versus dendritic cells: alternatively activated STAT1 negatively regulates TLR signaling in macrophages. J Immunol. 2007;179:3495–3503. doi: 10.4049/jimmunol.179.6.3495. [DOI] [PubMed] [Google Scholar]
- 26.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 27.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181:8576–8584. doi: 10.4049/jimmunol.181.12.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 29.Nanjappa SG, Heninger E, Wuthrich M, Sullivan T, Klein B. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. J Clin Invest. 2012;122:987–999. doi: 10.1172/JCI58762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Gunn L, Hansen R, Yan J. Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp Mol Pathol. 2009;86:208–214. doi: 10.1016/j.yexmp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, Fromm PD, Hart DN, Van Tendeloo VF, Berneman ZN. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol Rev. 2015;67:731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 32.Constantino J, Gomes C, Falcao A, Cruz MT, Neves BM. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 2016;168:74–95. doi: 10.1016/j.trsl.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, Nishio J, Negishi H, Tamura T, Saijo S, Iwakura Y, Taniguchi T. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife. 2014;3:e04177. doi: 10.7554/eLife.04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.