Abstract
Background
Delirium is common in post-acute care (PAC) patients with dementia; its treatment is not established. We hypothesized that cognitively-stimulating activities would reduce the duration and severity of delirium and improve cognitive and physical function to a greater extent than usual care.
Design
Single-blind randomized clinical trial.
Setting
eight PAC facilities.
Participants
283 community-dwelling older adults with dementia and delirium.
Intervention
Cognitively-stimulating activities delivered daily for up to 30 days.
Measurements
Primary outcomes were delirium duration (Confusion Assessment Method), and delirium severity (Delirium Rating Scale). Secondary outcomes were cognitive function (Digits Forward, Montreal Cognitive Assessment and CLOX) and physical function (Barthel Index).
Results
Mean percentage of delirium-free days were similar in both groups: 64.8% (95% CI: 59.6–70.1) (intervention) vs. 68.7% (95% CI: 63.9–73.6) (control), p = 0.37, Wilcoxon's rank sums test. Delirium severity was similar in both groups: 10.77 (95% CI: 10.10–11.45) (intervention) vs. 11.15 (95% CI: 10.50–11.80) (control), a difference of 0.37 (95% CI: 0.56–1.31, p= 0.43). Significant differences for secondary outcomes favoring intervention were found: executive function: 6.58 (95% CI: 6.12–7.04) vs. 5.89 (95% CI: 5.45–6.33), a difference of −0.69 (95% CI: 1.33– −0.06, p=0.03); and constructional praxis: 8.84 (95% CI: 8.83–9.34) vs. 7.53 (95% CI: 7.04–8.01), a difference of − 1.31 (95% CI: 2.01– −0.61, p=0.0003). After adjusting for baseline constructional praxis the group comparison was no longer significant. Average length of stay was shorter in intervention (36.09 days vs. 53.13 days, SE = 0.15, p = 0.01, negative binomial regression).
Conclusion
Cognitively-stimulating activities did not improve delirium but did improve executive function and reduced length of stay. Resolution of delirium may require more intense non-pharmacological management when the patient has dementia.
Keywords: Delirium, Dementia, Post-acute Care, Non-pharmacological Intervention
INTRODUCTION
Delirium is a state of confusion characterized by an acute decline in cognitive function.1 It is common and deadly in older adults with dementia.2 The effects of delirium persist long after hospitalization3, 4 and manifest as worsening global cognition.5 Discharges to post-acute care (PAC) following a hospitalization have risen sharply in recent years. Because of the temporal proximity to medical illness, these settings carry a high delirium burden that if unresolved, reduces rehabilitation potential,6 accounts for high medical costs,7 and predicts new institutionalization.8 It is important to determine if intervening on delirium in PAC can improve clinical outcomes for patients at highest risk for poor outcomes, those with delirium superimposed on dementia (DSD).3
The treatment of delirium is not established. Current practice is directed toward symptom management using antipsychotic or sedative medications.1 These medications carry an increased risk for adverse outcomes in patients with dementia and may actually prolong the duration of delirium.1 Multi-component non-pharmacological interventions that modify delirium risk factors are efficacious for prevention,9 but the evidence for their efficacy in treatment is lacking,10 although they are recommended in current guidelines.11 With few exceptions, there is no indication that the individual components making up these interventions were implemented in a consistent fashion, or in a “dose” that was likely to produce an effect. Because there is no widely accepted intervention for delirium,12 we sought to determine whether our theoretically-based, non-pharmacological intervention would demonstrate efficacy for the symptom management of DSD. Because delirium resolution may be independent of improvements in specific cognitive domains,13 we also examined these outcomes.
Our intervention uses individualized cognitively-stimulating activities to restore the disrupted cognitive function that is found in delirium regardless of precipitating cause.14 Several lines of evidence support a strong relationship between delirium and dementia;1, 15 interventions that improve symptoms in one of these conditions may also be effective in the other. For example, cognitive stimulation therapy can improve cognitive function in dementia.16 In our pilot work we found that PAC patients with DSD who engaged daily in cognitively-stimulating activities had less decline in global cognition and physical function compared to the control group.17 Cognitive stimulation is often one component of efficacious delirium prevention protocols, although its independent effects are not know and much of this research did not include patients with dementia.9 In a recent clinical trial, an intervention of combined cognition-focused and physical therapy improved executive function and instrumental activities of daily living in cognitively impaired survivors of critical illness when compared to physical therapy only.18
We hypothesized that individualized cognitively-stimulating activities would reduce the duration and severity of delirium and improve cognitive and physical function in PAC patients with DSD to a greater extent than usual care.
METHODS
In this single-blind clinical trial we enrolled 283 patients who were admitted to PAC settings following a hospitalization between February, 2011 and October, 2014. Follow-up was completed in January, 2015. Written consent was obtained prior to enrollment in the study. The study was approved by the Penn State institutional review board, was registered (ClinicalTrials.gov identifier: NCTO1267682), and had a data safety and monitoring committee that convened annually.
Participants
Participants were recruited at admission to one of eight PAC settings in Pennsylvania. Interested individuals were screened for eligibility within 72 hours of admission by trained research nurses.
Eligible participants were 65 years of age or older, community-dwelling prior to hospitalization, had a knowledgeable informant, and had both mild to moderate stage of dementia, and full or subsyndromal delirium. We admitted those with full or subsyndromal delirium because of the poor outcomes observed in both.19 The presence of dementia was based on a score of three or greater on the Modified Blessed Dementia Rating Scale (MBDRS)20 and a Clinical Dementia Rating (CDR)21 score ranging from 0.5 to 2.0. The presence of delirium was based on the presence of two or more positive features on the Confusion Assessment Method (CAM).22 All dementia and delirium diagnoses were adjudicated by a panel of three experts: behavioral neurologist, neuropsychologist, and geriatrician.
Exclusion criteria included: having any neurological, or neurosurgical disease associated with cognitive impairment, including Parkinson’s disease with Lewy bodies, acute stroke, Huntington’s disease, normal pressure hydrocephalus, seizure disorder, subdural hematoma, head trauma or known structural brain abnormalities; nonverbal; having a life expectancy of six months or less; acute major depression or psychosis; and severe hearing, or vision impairment.
Participants were randomly assigned to cognitive stimulation (intervention), or usual care (control). Randomization was concealed until after enrollment and was conducted using SAS Release 9.3 using randomly permuted blocks of sizes 2, 4 and 6 to ensure approximately balanced intervention group sizes across the length of the study, and to control for possible temporal effects. All outcomes were measured by trained assessors, blind to randomization. We maintained blinding by keeping assessment and intervention teams separate in the clinical area and during research team meetings. At the completion of the study we asked assessors if there were any instances when blinding was broken; none were reported.
Procedure
The study had three phases: baseline, intervention, and follow-up. Baseline assessments were conducted by the research nurse at enrollment. Data included: demographic information; mental status assessment using the Mini-Mental State Exam, a 30-item cognitive screen23; medical diagnoses, number of prescribed medications, including those with anticholinergic properties identified using the Anticholinergic Cognitive Burden Scale,24 and therapies obtained from the medical chart; APOE genotyping by extracting DNA from buccal swabs25 ; and for the intervention group, an assessment of activity preferences. The intervention period began within 24 hours of baseline and continued for 30 days, or until discharge. Daily assessments of delirium, cognitive function, and physical function were completed in both groups, and daily intervention sessions were conducted with the intervention group. We used two separate teams of trained research assistants: one team delivered the intervention and the other team conducted the outcome assessments. Phone follow-up with the participant's legally authorized representative occurred three months after admission and was completed by the research nurse.
Conditions
The protocol for the intervention has been published and includes a training video illustrating implementation.26 The goal of the intervention is to elicit active engagement in simple activities that provide cognitive stimulation in a non-regimented way and promote processing supportive of function in the domains of attention, memory, orientation and executive function. The PI (AK), in consultation with the neuropsychologist (LC), and the research nurse prescribed the activities. Fifteen activities of increasing difficulty were individually selected for each participant based on assessments of their leisure interests, physical function, and mental status. For example, if the participant was an older male with mild cognitive impairments, no visual impairments but some difficulty hearing, and who was a former high school chemistry teacher who enjoyed woodworking and movies, the following activities might be prescribed and delivered using a hearing amplifier: name three elements that are gases, metals; replicate building a block design; and identify Famous Faces. The match to interest and ability was done to provide intrinsic motivation for engagement and to capture attention,27 the most prominent domain affected by delirium. Activities were selected from a large database of activities previously tested in older adults with dementia.28 The advantage of activities, such as word searches or puzzles, is that they offer stimulation in multiple cognitive domains, combined in novel ways, and unobtrusively provide the opportunity for cognitive processing. Participants received the intervention, delivered by trained research assistants, in individual sessions for up to 30 minutes each day, five days per week for 30 days or until discharge. The dosage was based on studies that have demonstrated the efficacy of daily, 20-minute recreational therapy for the behavioral symptoms of dementia,27 and our pilot work with DSD patients.17
Prior to the activity session, the interventionists corrected any potentially confounding conditions (i.e., poor lighting, noise etc.). During the session they used principles for maximizing cognitive processing: active participation, verbal encouragement, variability in tasks, and increase in the level of difficulty as success occurred with simpler tasks.29 At the completion of the session, the activities attempted, time on task, and level of participation were recorded.
Treatment fidelity checks were conducted on 10% of all intervention sessions by trained research assistants (never members of the assessment team). These research assistants unobtrusively observed the assigned interventionist during the delivery of the intervention and rated adherence to critical aspects of treatment delivery, such as: used correct activities for participant; excluded extraneous/environmental factors that might influenced the delivery of the intervention (noise, poor lighting); and used the system of least restrictive prompts to engage the participant. Booster sessions for interventionists were held every three months and were conducted by the project director (PM) and the principal investigators (AK and DMF).
Because the management of DSD is variable across practitioners we conducted weekly medical chart reviews to characterize usual care: therapies attended; number of medications received; and number of documented nursing interventions delivered for delirium/confusion.
Outcomes
Inter-rater reliability was conducted on 10% of the outcome measures. Assessor booster sessions were held every three months and conducted by the project director (PM) and the principal investigators (AK and DMF).
Our primary outcomes were delirium duration, and severity. Secondary outcomes were cognitive and physical function.
Delirium duration was measured using the CAM.22 The CAM has been validated in dementia populations, has a sensitivity between 94% and 100%, and a specificity between 90% and 95%.22 In this study a weighted kappa of 0.88 was obtained for inter-rater reliability. The CAM includes the four features of acute and fluctuating course, inattention, disorganized thinking, and impaired level of consciousness. In this study, the presence of two or more features indicated subsyndromal delirium, and the presence of features 1 and 2 and either 3 or 4 indicated full delirium. Delirium duration was assessed in two ways: time to first delirium remission, i.e. the number of days until reaching two consecutive CAM features = 0 or 130 and percentage of total days delirium free (CAM= 0 or 1). Delirium severity was measured using the Delirium Rating Scale (DRS),31 a 13-item clinician-rated scale validated in both delirium and dementia groups,32 and having good sensitivity, specificity, and high interrater reliability (ICC 0.97). In this study an ICC of 0.72 was obtained for inter-rater reliability. Scores range from 0 to 39; higher scores indicate greater severity.
Cognitive function was measured with three instruments. Attention was measured using Digit Span Forward (range: 0 to 16). Memory (range: 0–3) and orientation (range: 0–7) were measured using the corresponding items from the Montreal Cognitive Assessment (MoCA).33 We obtained weighted kappas of 0.95, 0.96 and 0.97 for the attention, memory, and orientation measures, respectively. Executive function and constructional praxis were measured using the CLOX,34 a clock drawing task that elicits impairment in executive function (CLOX 1), and discriminates it from non-executive constructional failure (CLOX 2). We obtained weighted kappas of 0.92 and 0.93 for the CLOX 1 and CLOX 2 measures, respectively.
Physical Function was measured using the Barthel Index (BI),35 an ordinal scale for assessing activities of daily living in patients receiving inpatient rehabilitation; scores range from 0 (totally dependent) to 100 (fully independent). In this study an ICC of 0.87 was obtained for inter-rater reliability.
Other outcomes were length of stay and discharge disposition at the 3 month follow-up (community, nursing home, or death). The study was not powered to detect differences in these outcomes.
Statistical Analysis
Power to detect a difference in mean levels of severity and duration of delirium between the groups was estimated a priori, assuming a total sample size of 256 participants after attrition, and adjusting for cluster effects due to multiple observations made on the same subjects.
Assuming 30 observations per subject, and an intraclass correlation coefficient equal to 0.25, this sample size would provide > 99% power to detect a medium effect size, or 0.5 times the within-group standard deviation (sd). The difference detectable with 80% power would be 0.185 sd.
Within-group standard deviations and effect sizes were estimated from pilot data with up to 30 days’ observation on 16 subjects. Based on these pilot data, DRS and CAM showed effect sizes of 0.45, and 0.47, respectively. The study would provide at least 94%, and 96% power to detect the differences, respectively.
The intention-to-treat principle was used for analysis, and the statistician was blind to group assignment until all analyses were complete. Descriptive statistics including frequencies, means and standard deviations were calculated separately by intervention group. Cross-sectional analyses of patient characteristics and other variables having a single observation per subject were compared between the groups using Analysis of Variance for continuous variables, and chi-square analysis for categorical variables. Sample distributions were examined for all analysis variables. Variables showing substantial deviation from normality were rank-transformed for analysis.
Variables having a single observation per subject were compared between the groups using Analysis of Variance. Normality was evaluated by analysis of residuals. Length of stay was a single observation per subject (i.e., count of the number of days the patient received PAC), and was not normally distributed (intervention skew = 3.67; control skew= 2.7). To account for the variable type and non-normal distribution, group comparison of length of stay was analyzed using a negative binomial mixed model due to the significant contribution of facility to these values (χ2(1) = 71.77, p < .01). For variables with multiple observations per subject, groups were compared using mixed linear models in order to account for correlations among repeated measurements made on the same individuals. Categorical dependent variables with multiple observations per participant were analyzed using generalized linear models to implement mixed-model logistic regression analysis. To evaluate the sensitivity of the results to this analytic choice, the primary analyses were repeated with the inclusion of a facility term as a blocking variable in the model. The results show generally slight changes in the p-values for the treatment group comparisons, and no statistical decision would be altered. We also adjusted the analyses for the baseline difference in CLOX 2 and report both unadjusted and adjusted results. Time to resolution was evaluated using the Kaplan-Meier product limit survival estimator. The log-rank test was used for comparisons between groups.
Means and standard deviations are presented, as well as least-squares (marginal) means and standard errors. Means and least-squares means will be equal for the ANOVA analyses; least-squares means are reported in order to get estimated standard errors for comparability with those that are obtained from the mixed model analyses. Note that the standard deviations calculated for variables with more than one observation per individual include both between- and within-subject variation.
RESULTS
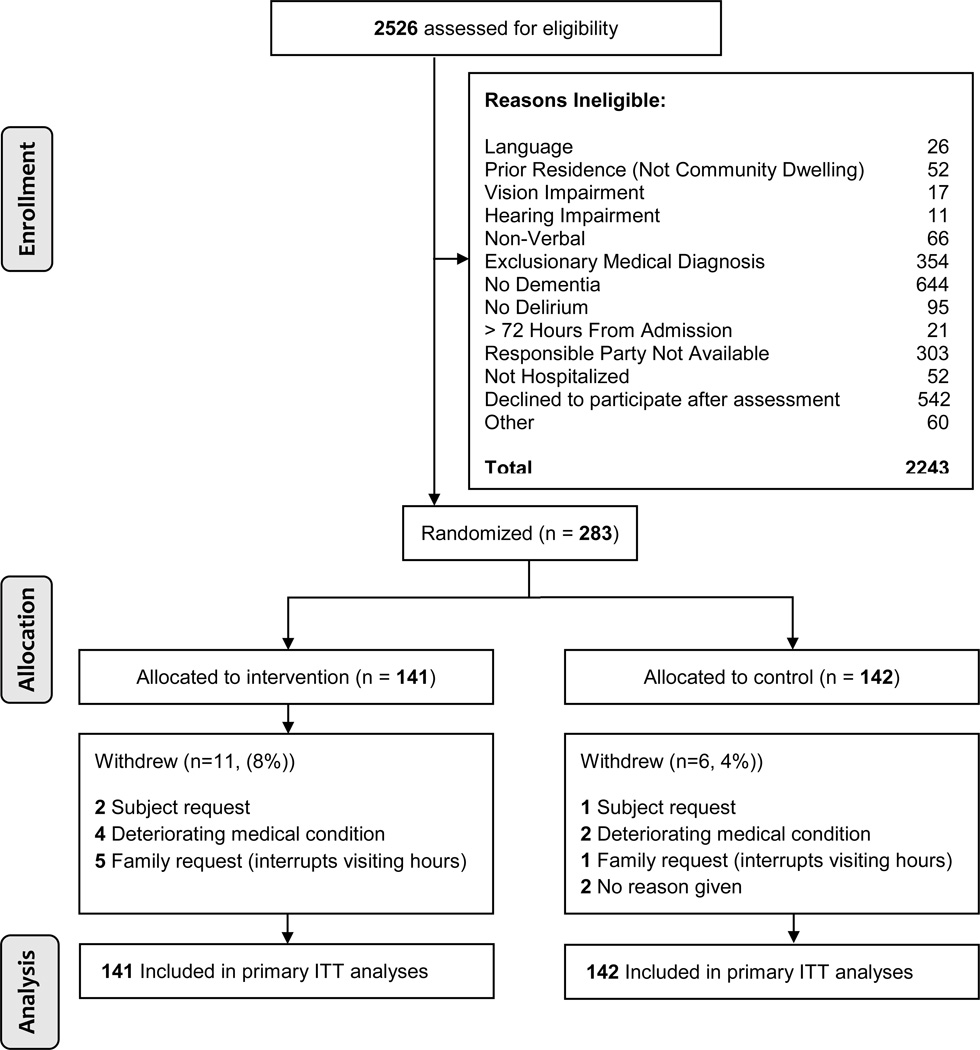
Participant flow through the study is depicted in Figure 1. A total of 17 (6%) participants withdrew from the study. Reasons for withdrawal were similar in both groups. Table 1 lists the demographic, baseline clinical characteristics, and usual care received by total sample and group. Baseline values for cognitive and physical function outcomes are published online as supplemental material. Overall, participants were elderly, white and female with mild to moderate dementia. The adjudication panel reached agreement on 98.9% and 100% of the dementia and delirium diagnoses, respectively. There were no differences between the groups for any demographic or clinical characteristic at baseline or usual care received with the exception of CLOX 2 (constructional praxis) which was significantly greater in intervention (p = 0.02). No adverse events were reported in either group.
Figure 1.
Flow of Primary Participants (N= 283) Through Study
Table 1.
Demographic and Baseline Clinical Characteristics of Sample and Description of Usual Care
| Variable | Total Sample (N= 283) |
Intervention (N= 141) |
Control (N= 142) |
|
|---|---|---|---|---|
| Age in years (mean ± SD) | 85.78± 6.8 | 85.28 ±6.8 | 86.28 ±6.7 | |
| Gender N (%): | Female | 183 (64.6) | 88 (62.4) | 95 (66.9) |
| Male | 100 (35.3) | 53 (37.6) | 47 (33.1) | |
| Race N (%): | White | 276 (97.5) | 136 (96.4) | 140 (98.6) |
| Black | 7 (2.4) | 5 (3.6) | 2 (1.4) | |
| Education N (%)1 | Grade school | 14 (5.0) | 5 (3.6) | 9 (6.6) |
| Some High School | 72 (26.0) | 33 (23.6) | 39 (28.7) | |
| High School | 107 (38.8) | 61 (43.6) | 46 (33.8) | |
| Post-secondary | 83 (30.0) | 41 (29.3) | 42 (30.9) | |
| Marital Status N (%): | Married | 108 (38.2) | 56 (39.7) | 52 (36.6) |
| Widowed | 148 (52.3) | 70 (49.6) | 78 (54.9) | |
| Single | 12 (4.2) | 6 (4.3) | 6 (4.2) | |
| Separated/Divorced | 15 (5.3) | 9 (6.4) | 6 (4.2) | |
| APOE status N(%)2 | One e4 allele | 77 (28.3) | 32 (23.5) | 45 (33.1) |
| Two e4 alleles | 10 (3.7) | 5 (3.7) | 5 (3.7) | |
| None | 185 (68.0) | 99 (72.8) | 86 (63.2) | |
| Charleson Comorbidity (mean ± SD) | 3.00 ± 1.93 | 2.97 ± 1.9 | 3.0 ± 1.9 | |
| CDR (mean ± SD)3 | 1.25 ± 0.5 | 1.24 ± 0.5 | 1.26 ± 0.5 | |
| MMSE (mean ± SD) at admission4 | 13.86 ± 6.0 | 14.48± 6.1 | 13.24± 5.8 | |
| Blessed (mean ± SD)5 | 5.96± 2.3 | 6.00 ± 2.4 | 5.92 ± 2.3 | |
| Full Delirium on Admission N (%) | 115 (40.6) | 55 (39.0) | 60 (42.3) | |
| Sub-syndromal Delirium on admission N (%) | 168 (59.4) | 86 (60.9) | 82 (57.7) | |
| Usual Care | ||||
| Therapies Received N (%): | Physical | 277(97.9) | 139 (98.6) | 138 (97.2) |
| Occupational | 268 (94.7) | 134 (95.0) | 134 (94.4) | |
| Speech | 177 (62.5) | 84 (59.6) | 93 (65.5) | |
| Other | 8 (2.8) | 3 (2.1) | 5 (3.5) | |
| Medications (mean ± SD)6 | ||||
| Total prescribed (includes scheduled & as needed) | 15.38 ±4.7 | 15.76 ±5.3 | 15.00 ±4.2 | |
| Medications administered with Anticholinergic Properties (level 1, 2 & 3) | 1.61 ± 1.1 | 1.60 ± 1.1 | 1.63 ±1.1 | |
| Number of Nursing Interventions for Delirium Documented/Week (mean ± SD) |
1.34 ± 3.9 | 1.31± 4.1 | 1.37 ± 3.8 | |
. Missing = 7 (intervention n = 140; control n = 136)
. Missing = 11 (Intervention n = 136; control n = 136)
. Clinical dementia rating Scale (CDR)- range (for this study) 0.5–2·0
. Mini-Mental Status Exam (MMSE)- range = 0–30
. Modified Blessed Dementia Rating Scale- range = 0–17
. Missing = 2 (Intervention n = 139; control n = 142)
Completion of outcome assessments was excellent. We obtained 92.6% and 95.3% of all possible CAM and DRS assessments; 86.3% and 79.1% of all possible MoCA and CLOX assessments; and 95.8% of all possible BI assessments. There were no differences in missing data between the groups, and we found no evidence that missing data were due to any substantive correlations with demographic, or clinical characteristics.
There was very good adherence to the intervention, and no withdrawals were due to the intervention itself. The intervention group participated in an average of 70.5% of the total sessions possible during the PAC stay. On average, 12.99 ± 8.2 intervention sessions that lasted for 19.1± 13.4 minutes were delivered to participants. Participation during these sessions was primarily active (mean= 2.7± 0.7 on 3 point scale: 3= active; 0= dozing). Omitted sessions were not due to any demographic, or clinical characteristics. Interventionists achieved between 95%, and 100% compliance on specified intervention delivery elements.
Outcomes
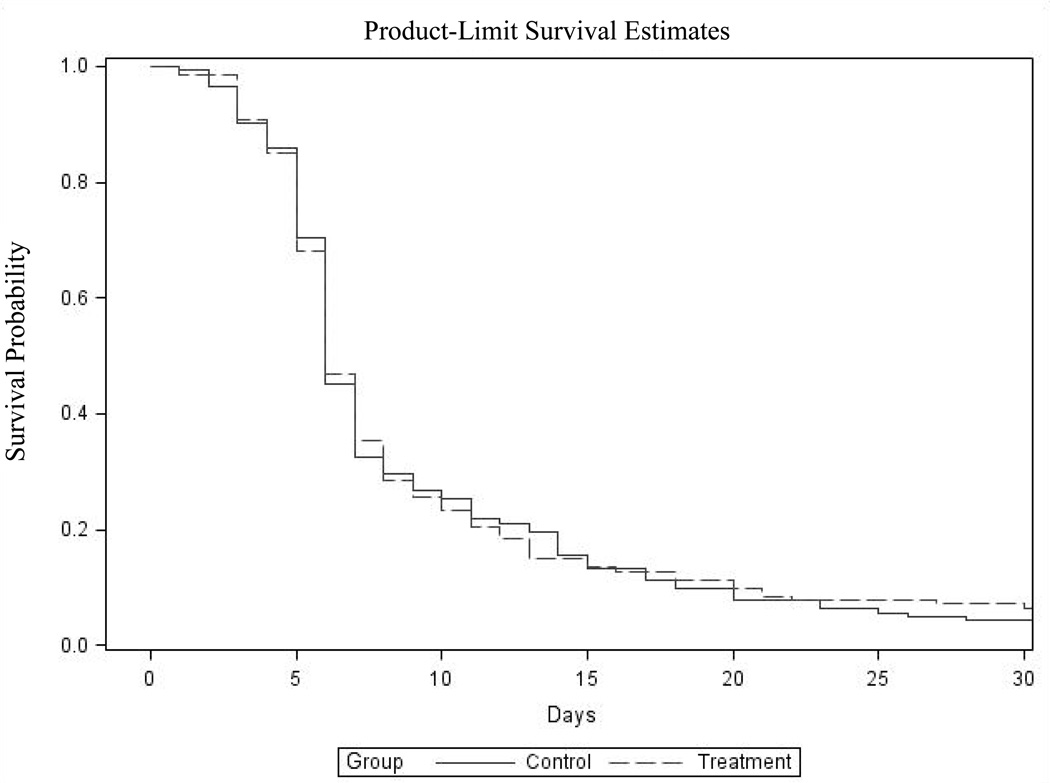
For participants with two or more PAC days, 86.3% (120/139) of intervention and 87.1% (122/140) of control participants experienced a remission of delirium at some point over the PAC stay (i.e., CAM= 0 or 1 for two or more consecutive days). Figure 2 is a graph of the Kaplan-Meier survival analysis depicting time to first remission by group. Intervention participants experienced a first remission on day 6.88 (95% CI: 6.14–7.61), and usual care participants on day 7.39 (95% CI: 6.47–8.31) (p=0.89, log-rank test). Despite a relatively early first remission, only 37.68% of intervention participants and 34.78% of usual care participants were in remission at discharge or completion of the intervention period. We also examined the mean percentage of delirium-free days and found that 64.8% (95% CI: 59.6–70.1) of intervention, and 68.7% (95% CI: 63.9–73.6) of control days were delirium free (p = 0.37, Wilcoxon's rank sums test).
Figure 2.
Time to First Remission
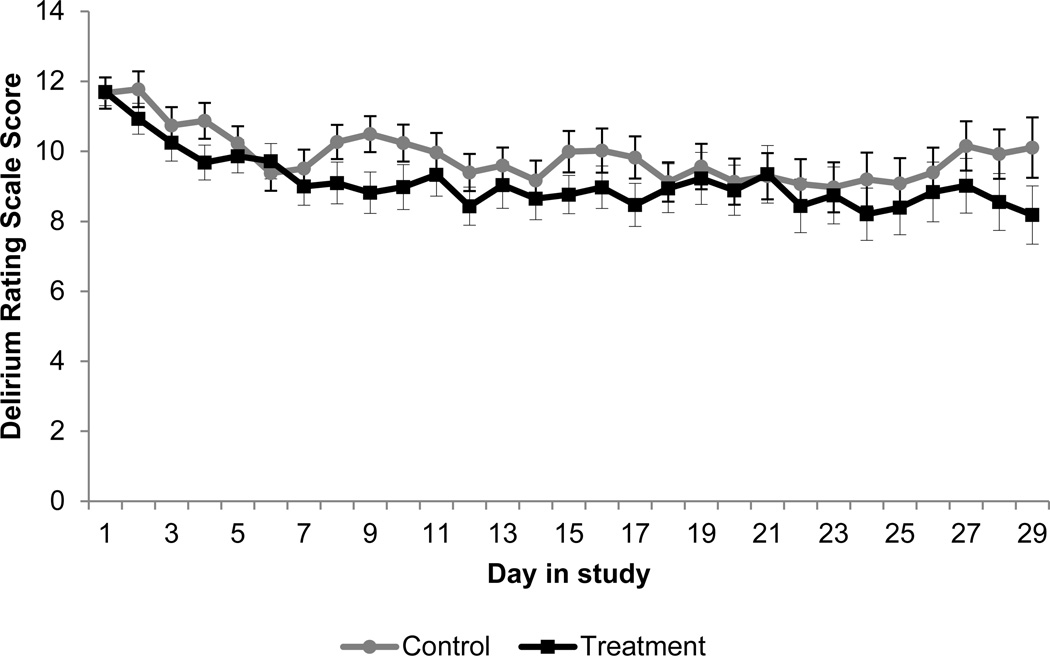
Figure 3 is a graph of the mean delirium severity score (DRS) over time by group. Participants exhibited primarily subsyndromal delirium, defined as a score between 8 and 15 on the DRS (10.77 (95% CI: 10.10–11.45) (intervention) vs. 11.15 (95% CI: 10.50–11.80) (control), a difference of 0.37 (95% CI: 0.56–1.31, p= 0.43). Full delirium was observed in 14.47% of intervention, and 14.82% of control days.
Figure 3.
Mean Delirium Severity Score
Table 2 contains the cognitive and physical function outcomes. Statistically significant differences between the groups were found for executive function and constructional praxis (CLOX 1 and 2). They favored intervention. Cohen's effect size value for CLOX 1 (d = .19) suggested a small effect and for CLOX 2 (d=.32) a small to moderate effect. After adjusting for baseline differences in CLOX 2 the group comparison for constructional praxis was no longer significant. There were no differences between the groups for other cognitive measures or physical function.
Table 2.
Cognitive and Physical Function Outcomes by Group*
| Outcome | Intervention | Control | Unadjusted P- value |
Adjusted P- value** |
|---|---|---|---|---|
| Cognition | ||||
| Attention | 7.99 [0.16] (7.69, 8.32) |
7.87 [0.16] (7·56, 8·18) |
0.17 | 0.19 |
| Memory | 0.78 [0.06] (0.66, 0.92) |
0.80 [0.06] (0.69, 0.92) |
0.76 | 0.72 |
| Orientation | 3.47 [0.12] (3.24, 3.69) |
3.25 [0.11] (3.03, 3.47) |
0.18 | 0.09 |
| Executive Function |
||||
| CLOX 1 | 6.58 [0.23] (6.12, 7.04) |
5.89 [0.22] (5.45, 6.33) |
0.03 | 0.009 |
| CLOX 2 | 8.84 [0.26] (8.83, 9.34) |
7.53 [0.25] (7.04, 8.01) |
0.0003 | 0.11 |
| Physical Function |
||||
| Barthel Index | 41.64 [1.76] (38.17, 45.11) |
43.07 [1.73] (39.67, 46.46) |
0.57 | 0.77 |
Data are expressed as Marginal (Least Squares) Mean [standard error] (95% CI) unless otherwise indicated
Adjusted for facility and baseline CLOX 2
Length of stay was non-normally distributed and depended on the facility (χ2(1) = 71.77, p < .01). Excluding withdrawals (n=17) and accounting for facility, the model estimated average length of stay was 36.09 days for intervention and 53.13 days for usual care (b = 0.39, SE = 0.15, p = 0.01). To avoid over-interpretation of these results we examined group assignments in sites and found that the groups were distributed across facilities equivalently (Chi-sq (7) = 4.35, p = .7385). We found no differences in mortality: 20 (15.4%) deaths in intervention vs. 17 (12.5%) in control (p=0.61, chi square). In addition to withdrawals, 15 participants were lost to the three-month follow-up. We examined discharge location in the 214 participants who were alive and remained in the study at that point. More in intervention returned to the community [46 (32.6%) vs. 39 (27.5%)] and fewer were institutionalized [60 (42.5%) vs. 69 (48.6%)] than in usual care, but the difference was not statistically significance (p= 0.54, chi square).
DISCUSSION
This is the first clinical trial to test a non-pharmacological intervention recommended in current guidelines for management of delirium.11 A daily 30-minute session of individualized cognitively-stimulating activities, delivered with high fidelity, did not improve delirium duration or severity. There was a small effect for executive function favoring intervention and intervention participants spent fewer days in PAC than control participants.
Most participants experienced subsyndromal delirium across PAC days and most days were delirium free. Delirium features, however, persisted through discharge for the majority of participants. In a recent prospective cohort study, Cole and colleagues found that DSD is more protracted than previously thought.13 The majority of patients in that study had no, or partial delirium recovery at three months post-enrollment, despite significant improvements in global cognition and function. These findings and our results underscore the need for longer follow-up to capture possible delayed effects of intervention on resolution of DSD.
Few studies have investigated whether specific cognitive domains improve more than others under intervention for delirium. We found a small effect for executive function that favored intervention. This was above gains that may be attributed to rehabilitation therapies, which can also improve this outcome, and which the majority of both groups received.
Even the small improvement we observed in executive function may be notable given the underlying cognitive impairments of our participants and literature that suggests an association of this higher-order function with delirium pathophysiology.36, 37 In clinical studies, fronto-executive dysfunction independently predicted postoperative delirium even in the absence of cognitive impairment.38 Patients who recover from delirium have short5 and long-term4 impairments in executive function. Jackson and colleagues18 reported greater benefits for executive function using cognition-focused and physical therapy verses physical therapy only in ICU survivors. Unlike our study, their combined intervention continued for 12 weeks, their subjects were younger, and none had dementia. Executive function may be a key cognitive domain reflecting central nervous system integrity following delirium, and the effect of interventions that aim to strengthen it.
In unadjusted analyses we observed a small to moderate effect for constructional praxis. These visual-spatial skills are important for hazard perception and deficits are found in patients with frontal dysfunction.39 However, after adjusting for baseline differences, the group comparison was no longer statistically significant.
While the intervention did not reduce delirium, it may have a role in future delirium prevention trials by way of strengthening important cognitive domains such as executive function and providing cognitive reserve as a protection against delirium risk factors. Impacting executive function also has implications for practice as these higher-order functions have importance for independence.40 In our analyses we found that groups were distributed equally across sites, and our length of stay analyses also controlled for the effect of facility on outcomes. We found that length of stay was significantly shorter in intervention compared to control. While there was no difference in discharge disposition, the ability to be transitioned more quickly to a lower-level of care represents cost savings that may be realized by even small effects on cognitive function. Additionally, as we discuss below, the outcome measures we used may not have been sensitive enough to demonstrate important differences between the groups attributed to the intervention. Instrument selection is an important issue for future research.
Attention, memory, and orientation may have been less amenable to intervention, but may also recover earlier, making improvement difficult to detect in PAC. Mark and colleagues5 found that one week following recovery from delirium, hospitalized older adults' performance on attention, memory and orientation was similar to those who did not experience delirium. The delirious group, however, remained significantly worse than the non-delirious group on executive function, praxis, and language, and in unadjusted analyses, our intervention had a small effect on two of these domains.
Both groups received physical and occupational therapies which may account for the lack of difference on physical function. While the aggressive rehabilitation available in PAC facilitates recovery, the large percentage of participants who had delirium features on discharge and who were admitted to long-term care (57.3% of those alive at three months) brings into question whether the PAC environment influences the persistence of delirium and higher-order cognitive symptoms.6
There are several limitations of this study. It is likely that cognition in PAC is linked to pre-illness cognition. Because we enrolled patients at admission to PAC, we were not able to test functioning prior to the illness that precipitated hospitalization. We addressed this limitation by using the Blessed and CDR at baseline to estimate preexisting cognitive function. By design only individuals with DSD were eligible for the study so we do not know if the intervention would be more effective in those without dementia. For patients with dementia, the duration of the intervention and length of follow-up may need to be extended for a longer period than a relatively-short PAC stay. The instruments we used for measuring our outcomes were selected to reduce participant burden; a larger effect might be found using a more in-depth neuropsychological test battery.
There are also several strengths of this study. We tested a theory-based intervention in a population lacking research on interventions that optimize rehabilitation. We also examined specific cognitive domains rather than one global outcome, thus adding to the literature on delirium resolution.
All interventions require staff time; using only those components with known benefits will improve the quality and cost-effectiveness of care. Cognitive stimulation did improve cognition and length of stay, having implications for both quality of life and cost of care. Resolution of delirium may require more intense non-pharmacological management when the patient has dementia.
Supplementary Material
Acknowledgments
Brief Explanation:
AK and DF were supported by National Institutes of Health/National Institute of Nursing Research grant number R01 NR012242.
Source of Funding: National Institutes of Health/National Institute of Nursing Research grant number R01 NR012242 awarded to AK and DMF.
Conflict of Interest
|
Elements of Financial/ Personal Conflicts |
AK | DF | ML | PM | LC | |||||
| Y | N | Y | N | Y | N | Y | N | Y | N | |
|
Employment or Affiliation |
X | X | X | X | X | |||||
| Grants/Funds | X | X | X | X | X | |||||
| Honoraria | X | X | X | X | X | |||||
|
Speaker Forum |
X | X | X | X | X | |||||
| Consultant | X | X | X | X | X | |||||
| Stocks | X | X | X | X | X | |||||
| Royalties | X | X | X | X | X | |||||
|
Expert Testimony |
X | X | X | X | X | |||||
|
Board Member |
X | X | X | X | X | |||||
| Patents | X | X | X | X | X | |||||
|
Personal Relationship |
X | X | X | X | X | |||||
|
Employment or Affiliation |
X | X | X | X | X | |||||
| Grants/Funds | X | X | X | X | X | |||||
| Honoraria | X | X | X | X | X | |||||
|
Speaker Forum |
X | X | X | X | X | |||||
| Consultant | X | X | X | X | X | |||||
| Stocks | X | X | X | X | X | |||||
| Royalties | X | X | X | X | X | |||||
|
Expert Testimony |
X | X | X | X | X | |||||
|
Board Member |
X | X | X | X | X | |||||
| Patents | X | X | X | X | X | |||||
|
Personal Relationship |
X | X | X | X | X | |||||
Footnotes
Authors Contributions:
Study concept and design: Kolanowski, Fick and Litaker
Acquisition, analysis or interpretation of data: Kolanowski, Fick, Litaker, Mulhall, Mogle, Hill, Yevchak-Sillner, Boustani, Gill, Clare
Drafting of manuscript: Kolanowski
Critical revision of the manuscript: Kolanowski, Fick, Litaker, Mulhall, Mogle, Hill, Yevchak-Sillner, Boustani, Gill, Clare
Statistical analysis: Litaker, Mogle
Obtained funding: Kolanowski and Fick
Sponsors Role:
The National Institute of Nursing Research/National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Ann Kolanowski, College of Nursing, 304 Nursing Sciences Building, Penn State University, University Park, Pa. 18704, USA.
Donna Fick, College of Nursing, 306 Nursing Sciences Building, Penn State University, University Park, Pa. 18704, USA.
Mark Litaker, School of Dentistry, 1720 2nd Avenue South, University of Alabama, Birmingham, AL 35294-0007, USA.
Paula Mulhall, Prevention Research Center, College of Health & Human Development, Penn State University, University Park, Pa. 18704, USA.
Linda Clare, The Centre for Research in Ageing and Cognitive Health, Washington Singer Laboratories, Exeter EX4 4QG, United Kingdom.
Nikki Hill, College of Nursing, 201 Nursing Sciences Building, Penn State University, University Park, Pa. 18704, USA.
Jacqueline Mogle, College of Nursing, 307 Nursing Sciences Building, Penn State University, University Park, Pa. 18704, USA.
Malaz Boustani, School of Medicine and Aging Brain Center, Indiana University, Indianapolis, IN 46202, USA.
David Gill, Rochester Regional Health and Department of Neurology, University of Rochester, 655 Ridgeway Avenue #420, Rochester, NY 14626, USA.
Andrea Yevchak-Sillner, College of Nursing, 201 Nursing Sciences Building, Penn State, University Park, Pa. 18704, USA.
REFERENCES
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 3.Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mark RE, Muselaers N, Scholten H, et al. Short-term cognitive effects after recovery from a delirium in a hospitalized elderly sample. J Nerv Ment Dis. 2014;202(10):732–737. doi: 10.1097/NMD.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 6.Marcantonio ER, Simon SE, Bergmann MA, et al. Delirium symptoms in post-acute care: Prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4–9. doi: 10.1034/j.1601-5215.2002.51002.x. [DOI] [PubMed] [Google Scholar]
- 7.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morandi A, Davis D, Fick DM, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc. 2014;15(5):349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: A meta-analysis. JAMA Intern Med. 2015;175(4):512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP Series. PLoS One. 2015;10(6):e0123090. doi: 10.1371/journal.pone.0123090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence. Delirium: Diagnosis, prevention and management. [Accessed April 2016];2010 Available at: https://www.nice.org.uk/guidance/cg103. [Google Scholar]
- 12.Morandi A, Davis D, Taylor J, et al. Consensus and variations in opinions on delirium care: A survey of European delirium specialists. Int Psychogeriatr. 2013;25(12):2067–2075. doi: 10.1017/S1041610213001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole MG, Bailey R, Bonnycastle M, et al. Partial and no recovery from delirium in older hospitalized adults: Frequency and baseline risk factor. J Am Geriatr Soc. 2015;63(11):2340–2348. doi: 10.1111/jgs.13791. [DOI] [PubMed] [Google Scholar]
- 14.Kolanowski AM, Fick DM, Clare L, et al. An intervention for delirium superimposed on dementia based on cognitive reserve theory. Aging Ment Health. 2010;14(2):232–242. doi: 10.1080/13607860903167853. [DOI] [PubMed] [Google Scholar]
- 15.Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562. doi: 10.1002/14651858.CD005562.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Kolanowski AM, Fick DM, Clare L, et al. Pilot study of a nonpharmacological intervention for delirium superimposed on dementia. Res Gerontol Nurs. 2011;4(3):161–167. doi: 10.3928/19404921-20101001-98. [DOI] [PubMed] [Google Scholar]
- 18.Jackson J, Ely EW, Morey MC, et al. Cognitive and physical rehabilitation of ICU survivors: Results of the RETURN randomized, controlled pilot investigation. Crit Care Med. 2012;40(4):1088. doi: 10.1097/CCM.0b013e3182373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole MG, McCusker J, Voyer P, et al. Subsyndromal delirium in older long-term care residents: Incidence, risk factors, and outcomes. J Am Geriatr Soc. 2011;59(10):1829–1836. doi: 10.1111/j.1532-5415.2011.03595.x. [DOI] [PubMed] [Google Scholar]
- 20.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 22.Inouye S, Van Dyck C, Alessi C, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Boustani MA, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health. 2008;4(3):311–320. [Google Scholar]
- 25.Freeman B, Smith N, Curtis C, et al. DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33(1):67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- 26.Kolanowski AM, Hill N, Clare L, et al. Practical protocol for implementing cognitive stimulation in persons with delirium superimposed on dementia. Nonpharmacol Ther Dement. 2012;2(2):101–110. [PMC free article] [PubMed] [Google Scholar]
- 27.Kolanowski A, Litaker M, Buettner L, et al. A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc. 2011;59(6):1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buettner L, Kolanowski A, Yu F. Recreational games: Simple and effective cognitive stimulation programs for residents with dementia in long-term settings. Am J Recreat Ther. 2007;6(1):25–30. [Google Scholar]
- 29.Green C, Bavelier D. Exercising your brain: A review of human brain plasticity and training-induced learning. Psychol Aging. 2008;23(4):692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamis D, Devaney A, Shanahan E, et al. Defining ‘recovery’ for delirium research: A systematic review. Age Ageing. 2015;44:318–321. doi: 10.1093/ageing/afu152. [DOI] [PubMed] [Google Scholar]
- 31.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23(1):89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 32.Sepulveda E, Franco JG, Trzepacz PT, et al. Performance of the delirium rating scale-revised-98 against different delirium diagnostic criteria in a population with a high prevalence of dementia. Psychosomatics. 2015;56(5):530–541. doi: 10.1016/j.psym.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Nasreddine Z, Phillips N, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 34.Royall DR, Cordes JA, Polk M. CLOX: An executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64(5):588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahoney F, Barthel D. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 36.Culley DJ, Snayd M, Baxter MG, et al. Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: Possible implications for delirium. Front Aging Neurosci. 2014;6:107. doi: 10.3389/fnagi.2014.00107. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong TG, Hshieh TT, Wong B, et al. Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc. 2015;63(5):977–982. doi: 10.1111/jgs.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross RG, Grossman M. Update on Apraxia. Curr Neurol Neurosci Rep. 2008;8(6):490–496. doi: 10.1007/s11910-008-0078-y. PMCID: PMC2696397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martyr A, Clare L. Executive function and activities of daily living in Alzheimer’s disease: A correlational meta-analysis. Dement Geriatr Cogn Disord. 2012;33(2–3):189–203. doi: 10.1159/000338233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.