Abstract
Candida albicans infection produces elongated hyphae resistant to phagocytic clearance compelling alternative neutrophil effector mechanisms to destroy these physically large microbial structures. Additionally, all tissue-based neutrophilic responses to fungal infections necessitate contact with extracellular matrix (ECM). Neutrophils undergo a rapid, ECM-dependent mechanism of homotypic aggregation and NETosis in response to C. albicans mediated by the β2 integrin, Complement Receptor 3 (CR3, CD11b/CD18, αMβ2). Neither homotypic aggregation nor NETosis occurs when human neutrophils are exposed either to immobilized fungal β-glucan or to C. albicans hyphae without ECM. The current study provides a mechanistic basis to explain how matrix controls the anti-fungal effector functions of neutrophils under conditions that preclude phagocytosis. We show that CR3 ligation initiates a complex mechanism of integrin cross-talk resulting in differential regulation of the β1 integrins VLA3 (α3β1) and VLA5 (α5β1). These β1 integrins control distinct anti-fungal effector functions in response to either fungal β-glucan or C. albicans hyphae and fibronectin (Fn), with VLA3 inducing homotypic aggregation and VLA5 regulating NETosis. These integrin-dependent effector functions are controlled temporally whereby VLA5 and CR3 induce rapid, focal NETosis early after binding Fn and β-glucan. Within minutes, CR3 undergoes inside-out auto-activation that drives the down-regulation of VLA5 and the up-regulation of VLA3 to support neutrophil swarming and aggregation. Forcing VLA5 to remain in the activated state permits NETosis but prevents homotypic aggregation. Therefore, CR3 serves as a master regulator during the antifungal neutrophil response, controlling the affinity states of two different β1 integrins which in turn elicit distinct effector functions.
Keywords: PHAGOCYTES, GRANULOCYTES, MYELOPOEISIS
Introduction
Deep-seated fungal infections are a major cause of morbidity and mortality due largely to a growing patient population with clinically impaired host defenses (1-6). Many cases are secondary to immunosuppression following organ transplantation, cancer chemotherapy, and AIDS, although trauma and burn patients, diabetics, and the elderly are particularly susceptible, as well (1, 7). Increasingly complex surgical procedures have led to the increase use of invasive measures such as intravenous catheters and hyperalimentation which further contribute to risk of acquiring candidiasis particularly in patients maintained in the surgical ICU for extended periods of time during recovery (8-10). Candida infection was reported to be remarkably high in non-trauma emergency surgical patients with prolonged hospital stays reaching a rate of 21.7/100 discharges, higher than other established high-risk patient populations (11). If the infection progresses to invasive, systemic candidiasis, the associated mortality ranges between 63-85% in untreated cases and 33-54% in those receiving appropriate anti-fungal therapy (12).
Neutrophils are the primary effector cells in promoting destruction and preventing dissemination of fungal pathogens (13-15). A low peripheral blood neutrophil count or a genetic defect in neutrophil function is sufficient to create host susceptibility for severe invasive fungal infections (16). Dimorphic fungi such as C. albicans switch in vivo between the single cell blastoconidia form and the elongated hyphal form (17). Given its relatively small size, the single cell form of C. albicans can be cleared by host neutrophils via phagocytosis. However, the large size of the hyphae obviates ingestion and thereby poses a challenge to the anti-fungal defense mechanisms available to the neutrophil (18).
Since mycotic infections occur within tissues, the response of extravasated neutrophils to fungal pathogens must take place in the context of the extracellular matrix (ECM). Recent work from this lab demonstrated a significant regulatory role for ECM in mediating the anti-fungal response of neutrophils (19). Specifically, we found that fibronectin (Fn) was required for neutrophil swarming and homotypic aggregation and a rapid release of neutrophil extracellular traps (NETs) with exposure to either immobilized soluble β-glucan or C. albicans hyphae. Neutrophil aggregation and NET release did not occur in response to either immobilized β-glucan, C. albicans hyphae, or Fn alone. The current study was undertaken to provide a mechanistic explanation for the coordinated response to the fungal pathogen associated molecular pattern (PAMP) β-glucan and the matrix protein Fn.
On human neutrophils, Complement Receptor 3 (CR3, CD11b/CD18, αMβ2), a β2 integrin, serves as a pattern recognition receptor for the fungal PAMP β-glucan (20, 21). Our previous work has shown that neutrophil clustering and NETosis to β-glucan in the context of Fn is requires CR3, but not Dectin-1, and is independent of both respiratory burst (19). CR3 is a unique surface receptor capable of recognizing ligands at two spatially distinct domains, the I-domain and the lectin-like domain. The promiscuous I-domain recognizes many ligands including Fn, fibrinogen and Intercellular Adhesion Molecule 1 (ICAM-1) (22). The lectin-like site has been shown to bind β-glucan (23). The simultaneous binding of both I-domain ligand and lectin-like domain ligand has been proposed to regulate cellular function not seen with ligation of either domain alone. In this study, we report a novel, complex and temporally regulated integrin cross-talk pathway in which dual ligation of CR3 signals the differential regulation of VLA3 (α3β1) and VLA5 (α5β1), β1 integrins not often associated with anti-fungal activity. Neutrophil swarming was determined by activated VLA3 while NETosis required activated VLA5; the activation state of CR3 regulated the activation states of both VLA3 and VLA5. Findings are consistent with a two stage temporal model where VLA5 and CR3 are initially activated by ligand binding upon contact with Fn and fungal β-glucan leading to rapid, matrix-dependent NET formation. Over time, dually ligated CR3 undergoes an inside-out auto-activation that drives the down-regulation (suppression) of VLA5 and the up-regulation (activation) of VLA3 to allow for neutrophil swarming and cluster formation. If VLA5 is maintained in a high state of activation, VLA3 cannot be engaged. These data provide the first direct evidence for integrin cross-talk between the β2 integrin CR3 to the β1 integrins VLA3 and VLA5 in human neutrophils exposed to fungal determinants. Taken together with previous reports, this study highlights the essentiality of considering the regulatory role of extracellular matrix and cognate integrins in efforts to understand host defense as it occurs within a tissue microenvironment.
Materials and Methods
Reagents
Abs used were as follows: blocking anti-CD11b (clone 44abc) hybridoma from American Type Culture Collection (Manassas, VA), APC-conjugate IgG (BioLegend, San Diego, CA), APC-conjugate CD11b (CBRM1/5; Biolegend, San Diego, CA), human integrin alpha5/CD49e antibody (clone P1D6 R&D Systems, Minneapolis, MN), Mouse IgG1 Isotype Control (R&D Systems), Mouse anti-human integrin α3 (VLA3) preservative-free mAb (clone M-KID2; EMD Millipore, Temecula, CA), anti-VLA6 (NKI-GoH3, EMD Millipore, Temecula, CA), FITC-conjugated anti-CD11b (CBRM1/5, BioLegend, San Diego, CA), FITC-conjugated mouse IgG1 (679.1Mc7, Beckman Coulter Brea, CA), FITC-conjugated anti-CD11b (ICRF44, Ancell, Bayport, MN). Anti-alpha5 (preservative-free) (SNAKA51, EMD Millipore, Temecula, CA), anti-Integrin alpha3, azide-free (ASC-1, EMD Millipore, Temecula, CA), Pharmaceutical-grade purified, endotoxin-free, soluble yeast β-glucan (ImPrime PGG) was kindly provided by Biothera (Eagan, MA). This is a β (1,3) (1,6) glucan from the cell wall of Saccharomyces cerevisiae with an average molecular weight of 150 kDa and 4.1% branching (24). The β-glucan preparation contained no more than 0.02% protein, 0.01% mannan, and 1% glucosamine. Purified, endotoxin-free Human fibronectin (Fn) was from BD Biosciences (Bedford, MA). Dulbecco’s PBS, Lebovitz’s L15 medium (L-15), Hank’s Balanced Salt Solution (HBSS), and Sytox Green were from Invitrogen (Carlsbad, CA). Accutase ™ was from Innovative Cell Technologies (San Diego, CA). Leukadherin-1 (LA1), a small molecule CR3 receptor agonist with function-blocking properties, was a gift from Dr. Vineet Gupta, Rush Medical Center, Chicago, IL. VLA3 blocking peptide (LXY1) and the β2 integrin allosteric antagonist (XVA143) were from Dr. Minsoo Kim, University of Rochester, Rochester, NY. All other reagents were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated, and were of highest quality available.
Neutrophil isolation
Blood was obtained from healthy human volunteers with approval of the Rhode Island Hospital Institutional Review Board. Blood was collected in EDTA-containing Vacutainer tubes (BD Biosciences, San Jose, CA) and used within 5 min of venipuncture. Histopaque-1077 was used for initial cell separation followed by sedimentation through 3% dextran (400–500 kDa molecular mass). Contaminating erythrocytes were removed by hypotonic lysis, yielding a 95% pure neutrophil preparation of 90% viability by trypan dye exclusion. Neutrophils were suspended in HBSS (without Ca2+/Mg2+) and placed on ice until use.
Neutrophil adhesion assay
Six-well tissue culture plates (Fisher Scientific, Waltham, MA) were coated overnight at 4°C with Fn at a concentration of 6 μg/ml in TBS (25mM Tris, 150 mM NaCl) pH 9.0 and/or 1 mg/ml β-glucan. Plates were moved to 37°C for 1 hour, washed twice with PBS, and air-dried. Neutrophils were resuspended to a concentration of 3.5× 106 cells/ml in L-15 medium supplemented with 2 mg/ml glucose and 2 ml was added to each well. Where indicated, cells were preincubated with 25 μg/ml blocking or activating mAb or isotype control on ice for 30 min. Cells were pretreated on ice with 10−9 M fMLP for 20 min and 1 mM Mn2+ was added to cells immediately before plating. Cells were exposed to either Fn alone or Fn + β-glucan for 30 min at 37°C. In a subset of experiments, 150 U/ml of DNase I was added at 0, 10, or 25 min, as noted. Cells were then imaged and scored for cluster formation and NETosis or harvested for FACS analysis.
Scanning electron microscopy
Samples for scanning electron microscopy (SEM) were fixed by gently layering 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer, rinsed with buffer and post fixed with 1% osmium tetroxide. Slides were rinsed, dehydrated and covered with resin and placed over Epox 812 filled slide-duplicating molds (Electron Microscopy Sciences, Hatfield, PA) overnight. Following dehydration in ethanol, samples were subsequently dried in a critical point dryer. The samples were then coated with 20nm of gold palladium (60:40) in an Emitech K550 sputter coater (Emitech, Ashford, UK). Cells were imaged with a Hitachi S-2700 scanning electronic microscope (Hitachi High Technologies America, Pleasanton, CA) and collected with Quartz PCI software (Quartz Imaging Corporation, Vancouver, BC).
Quantification of neutrophil cluster formation
Cluster formation was quantified using custom MatLab software. Multiple images were taken per well and clusters of neutrophils were identified by eye as regions of interconnected or overlapping cells contained within a field of view (most fields of view were 410 × 410mm). Only clusters regions with areas greater than 400mm2 were considered, approximately the size of four tiled, non-overlapping neutrophils. To maximize consistency, a single person was used to identify every cluster region in each field of view captured. To minimize human bias, experimental conditions associated with each field of view were coded and the person doing the analysis was blinded. Fields of view were numerically characterized by the number of clusters per mm2 and the average area of each cluster in μm2. Data sets from each well were averaged and all wells for each condition were ensemble averaged and plotted as average clusters per mm (x-axis) vs. average cluster area in μm2 (y-axis) per condition. Ellipses represent 2D-SEM. Data represents 3-20 wells per condition.
Visualization and quantification of neutrophil NETs
Neutrophils were adhered as previously described plates coated with Fn ± β-glucan. NETs were visualized on adherent neutrophils by addition of 5 μM Sytox green and multiple images were taken per well. To minimize bias, experimental conditions associated with each field of view were coded and the person doing the analysis was blinded. Images were thresholded using the default thresholding algorithm in ImageJ (NIH, Bethesda, MD) and gated to include NETs and exclude nuclei. NET formation was quantified as a percent area of the totaled imaged field. Well averages were ensemble averaged to generate this data. Data represents 3-20 wells per condition.
Microscopy
Images were captured using a Nikon TE-2000U inverted microscope (Nikon, Melville, NY) coupled to an iXonEM+ 897E back illuminated EMCCD camera (Andor, Belfast, U.K.) outfitted with a Bioptechs (Butler, PA) stage heater and 20x, Nikon Plan Apochromat objective. Bright field images were captured using Elements software (Nikon). For fluorescence microscopy, a xenon lamp illuminated cells through a 33-mm ND4 filter and 20x Nikon Plan Apochromat objective using a Nikon B2-A long pass emission filter set cube.
FACS analysis
Following isolation procedure, neutrophils were resuspended to a concentration of 3.5× 106 cells/ml in L-15 medium supplemented with 2 mg/ml glucose and 2 ml was added to each well. Where indicated, cells were preincubated with integrin specific mAbs, peptides, or small molecules and vehicle or isotype controls. Cells were pretreated on ice with 10−9 M fMLP for 20 min and 1 mM Mn2+ was added to cells immediately before plating. Cells were exposed to either Fn alone or Fn + β-glucan for 30 min at 37°C. Following incubation time, 2mls of Accutase and 150U/ml DNase I (Sigma-Aldrich) was added to each well and plate was incubated at 37°C for an additional 7 min. To remove cells, contents of wells were first pipetted up-and-down with serological pipettes and then gently scraped from the well surface using cell scrapers (Costar, Corning, NY) and collected in 15 mL conical tubes. Wells were examined by microscope and 1 mL of L-15 was added and wells re-scraped if needed. Cells were spun down at 4°C for 10 min at 1400 RPM in a clinical centrifuge and resuspended at 1×107 cells/ml in BSS+ buffer (0.1% BSA, 1mM CaCl2, 1mM MgCl2) 100 μl cell aliquots were incubated with FITC-IgG control (10 μg/ml), FITC-ICRF44 (9.8 ug/ml), FITC-CBRM1/5 (15 μg/ml), FITC-ASC-1(10 μg/ml) , FITC-P1D6 (10 μg/ml), or FITC-SNAKA51 (2.5μg/mL) and Fc block (Accurate Chemical, Westbury, NY) for 60 min on ice at 4°C in foil. (Unconjugated mAb were labeled using a Molecular Probes Alexa fluor 488 antibody labeling kit per manufacturer’s instructions.) Cells were washed 3 times with BSS+ at 4°C and resuspended in 1% paraformaldehyde in PBS. Data was acquired on either a BD FACScan (Becton, Dickinson and Company, Franklin Lakes, NJ) using Lysis II software or by the Brown University Flow Cytomery and Sorting Facility on a BD FACSAria IIu using FACS DiVa software. Analysis was performed in FlowJo software (FlowJo, Ashland, OR) gating on neutrophils.
FACS-based FRET assay for extracellular extension of CR3 or VLA3
Experiments were performed as described in previous literature (25, 26). Briefly, neutrophil adhesion assays were performed as described above. 2 ml of thawed Accutase and 150U/ml DNaseI (Sigma-Aldrich) were added per well, sides of plates were tapped for 30 s and, placed at 37°C for 7 min. To remove cells, contents of wells were first pipetted up-and-down with serological pipettes and then gently scraped from the well surface using cell scrapers (Costar, Corning, NY) and collected in15 mL conical tubes. Wells were examined by microscope and 1 mL of L15 was added and wells re-scraped if needed. Cells were spun down at 4°C for 10 min at 1400 RPM in a clinical centrifuge and resuspended at 1×107 cells/ml in BSS+ buffer (0.1% BSA, 1mM CaCl2, 1mM MgCl2) 100 μl cell aliquots were incubated with FITC-IgG control (10 μg/ml), FITC-ICRF44 (9.8 ug/ml), FITC-CBRM1/5 (15 ug/ml), or FITC-ASC-1 (10 μg/ml) for 50 min with rotation at 4°C in foil. Cells were washed 3 times with BSS+ at 4°C and split into four 125μl aliquots in FACS compatible snap cap tubes on ice. Octadecylrodamine B (ORB) was added to a final volume of 250μl and concentrations of 0, 75, 200, and 400 nM. Samples were incubated on ice for 20 min and data was acquired by the Brown University Flow Cytomery and Sorting Facility on a BD FACSAria IIu using FACS DiVa software. Analysis was performed in FlowJo software gating on neutrophils.
Statistical Analysis
Data was pooled from a minimum of three independent experiments representing at least three different donors, as indicated. ANOVA analysis with Newman-Keuls posthoc analysis or paired-sample Student’s t-test as appropriate were performed using MATLAB (Mathworks, Natick, MA) or Excel (Microsoft, Redmond, WA) running the statistiXL data package (statistiXL, Nedlands, Australia). The null hypothesis was rejected if p < 0.001.
Results
Homotypic aggregation of neutrophils in response to C. albicans hyphae or immobilized β-glucan in the context of fibronectin
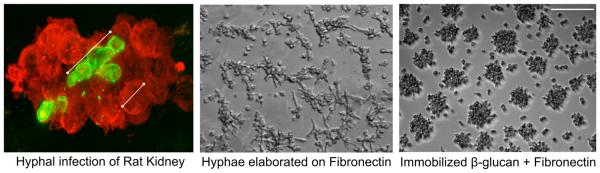
C. albicans infection instilled into a rat kidney shows robust aggregation of neutrophils (stained in red using an anti-neutrophil antibody) about a hyphal filament (stained in green using an anti-β-glucan antibody specific for β-(1-3)- and β-(1-6)-linked glucose (Fig. 1, left panel). This characteristic neutrophil aggregation around fungal hyphae is also observed in vitro when neutrophils are co-incubated with hyphae that have been elaborated on surfaces coated with Fn (Fig. 1, center panel). When this in vitro system is reduced to its minimal recognition elements of immobilized fungal β-glucan and Fn, cluster formation is conserved (Fig. 1, right panel). We have previously used both of these in vitro systems to characterize a rapid, matrix-dependent NET response of human neutrophils to fungal β-glucan (19). This study uses these model systems to characterize the integrin regulation of neutrophil cluster formation and NETosis to provide a mechanistic explanation for the dependence of matrix for the response to a fungal PAMP or hyphal filaments.
Fig. 1. Immobilized β-glucan + Fn supports neutrophil aggregation as seen in vivo in response to intact hyphae in tissue.
(Left panel) C. albicans infection within a rat kidney shows robust aggregation of neutrophils (stained in red with an anti-neutrophil antibody) about a hyphal filament (stained in green an anti-β-glucan antibody specific for β-(1-3), β-(1-6)-linked glucose. (Center panel) Aggregation also forms in vitro on intact C. albicans hyphae elaborated on Fn. 20x bright field image. (Right panel) β-glucan immobilized with Fn supports robust neutrophil aggregation. 20x bright field image. Bar = 100μm.
Homotypic aggregation on Fn + β-glucan is dependent on CR3 and VLA3 and independent of VLA5
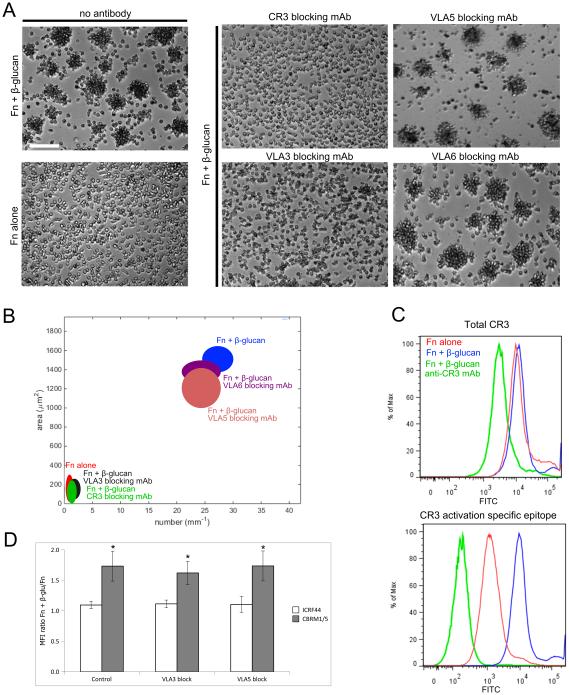
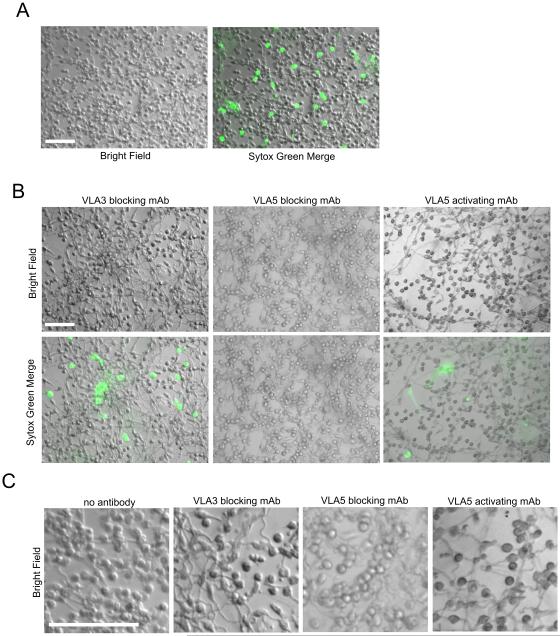
To identify the specific integrins involved in neutrophil aggregation, human neutrophils were pretreated on ice with 10−9 M fMLP and incubated for 30 min at 37°C on tissue culture plastic coated with either 6 μg/ml Fn (Fn alone) or 6 μg/ml Fn + 1mg/ml β-glucan (Fn + β-glucan) in L-15 supplemented with 2 mg/ml glucose and 1 mM Mn++. Previously, we showed that these conditions were optimal for primed neutrophils to exhibit rapid, ROS-independent NETosis and homotypic aggregation. To identify integrins required for NET release and/or aggregation, neutrophils were additionally pretreated either with specific blocking mAbs to CR3 (clone 44abc), VLA5 (P1D6), VLA3 (M-KID2), or VLA6 (NKI-GoH3). Confirming our previous report, antibody blocking of CR3 blocked cluster formation (Fig. 2A). Surprisingly, we found that blocking the VLA5, the canonical Fn receptor had no effect on aggregation in spite of the presence of Fn in the assay, but blocking of VLA3 completely inhibited cluster formation (Fig. 2A). VLA6 had no effect on clustering. Quantification of aggregate formation as average number of clusters per mm vs. average area of clusters confirmed significant inhibition of neutrophil clusters in response to Fn + β-glucan to levels indistinguishable from Fn alone upon antibody blocking of either CR3 or VLA3 (p<0.001), but with no significant change in cluster formation upon antibody blocking with isotype control (data not shown), VLA5, or VLA6 (Fig. 2B). The VLA5 independence of aggregate formation was confirmed using a second VLA5-blocking mAb AF1864 (data not shown).
Fig. 2. Neutrophil clustering on Fn + β-glucan is dependent on CR3 and VLA3.
(A) Human neutrophils, pretreated on ice with 10−9 M fMLP, were incubated at 37°C on tissue culture plastic coated with either 6 μg/ml Fn (Fn alone) or 6 μg/ml Fn + 1mg/ml β-glucan (Fn + β-glucan) in L-15 supplemented with 2 mg/ml glucose and 1 mM Mn++. Where indicated, neutrophils were additionally pretreated either with specific blocking mAbs to CR3 (clone 44abc), VLA5 (P1D6), VLA3 (MKID-2) or VLA6 (NKI-GoH3). After 30 min incubation, cells were fixed and multiple images were acquired per well. Bright field images acquired at 20x; bar = 100μm. (B) Neutrophil cluster formation was quantified using ImageJ and custom MatLab software and plotted as average clusters per mm (x-axis) vs. average cluster area in μm2 (y-axis) per condition. Ellipses represent 2D-SEM. Cells on Fn + β-glucan (blue) have a significant increase in both cluster number and area vs. cell on Fn alone (red). Cells on Fn + β-glucan pretreated with VLA5 blocking mAb (pink) or VLA6 blocking mAb (purple) showed no significant difference in clustering parameters vs. Fn + β-glucan (blue). Cells on Fn + β-glucan pretreated with VLA3 blocking mAb (black) or CR3 blocking mAb (green) a significant decrease in clustering parameters vs. Fn + β-glucan (blue) to levels statistically indistinguishable from cells on Fn alone (red). p < 0.001; ANOVA full factorial, post hoc Newman-Keuls. (C) CR3 adhesion to Fn + β-glucan results in auto-activation of CR3 but no significant change in surface expression. Representative histograms of neutrophils assayed as described on Fn (red), Fn + β-glucan (blue) or Fn + β-glucan in the presence of CR3 blocking mAb (green). After 30 min incubation, neutrophils were isolated and stained with directly conjugated mAbs for both total CR3 (top panel) and a CR3 activation specific epitope (bottom panel) and assayed by FACS. (D) The bar graph shows the average ratio of the MFI for cells adhered to Fn + β-glucan vs. Fn alone. Control conditions show no significant difference in staining for total CR3 (white bars) but a significant increase in CR3 activation specific epitope staining (grey bars) on Fn + β-glucan vs. Fn alone. Antibody blocking of VLA3 or VLA5 does not significantly influence the expression or activation of CR3 in response to Fn + β-glucan vs. Fn alone. Error bars represent SD. * p<0.001 Fn + β-glucan vs. Fn alone.
CR3 adhesion to Fn + β-glucan drives an inside-out auto-activation of CR3
Given that CR3 is the dominant receptor for β-glucan recognition by human neutrophils, we sought to understand whether CR3 expression and activation state was differentially modulated on Fn+β-glucan vs. Fn alone. Using flow cytometric analysis, CR3 surface expression and activation was quantified after adhesion on Fn ± β-glucan. Using a CR3 expression reporter antibody (ICRF44), no significant difference in CR3 surface expression was found as a function of ligand exposure (Fig. 2C, top panel). Using a CR3 activation reporter antibody, (CBRM 1/5), a significant increase in CR3 activation on Fn + β-glucan vs. Fn alone was identified (Fig. 2C, bottom panel). This increase was lost on Fn +β-glucan when CR3 was blocked (Fig. 2C) supporting a mechanism of inside-out auto-activation of CR3 on Fn + β-glucan.
CR3 surface expression and activation on neutrophils was quantified after adhesion on Fn ± β-glucan in the presence or absence of VLA3 or VLA5 blocking mAbs (Fig. 2D). After 30 min incubation, neutrophils were isolated and stained with directly conjugated mAbs for both total CR3 (ICRF44) and a CR3 activation specific epitope (CBRM1/5) and assayed by FACS. The average ratio of the MFI for cells adhered to Fn alone vs. cells adhered to Fn + β-glucan showed no significant increase in CR3 expression or activation on Fn + β-glucan after VLA3 or VLA5 blocking. That blockade of the β1 integrins does not affect CR3 expression or activation suggests that the integrin cross-talk pathway under study takes place in the β2-to-β1, but not in the β1-to-β2, direction.
Neutrophil homotypic aggregation and NETosis are dependent on CR3 but are differentially regulated with VLA3 driving homotypic aggregation and VLA5 driving NET formation
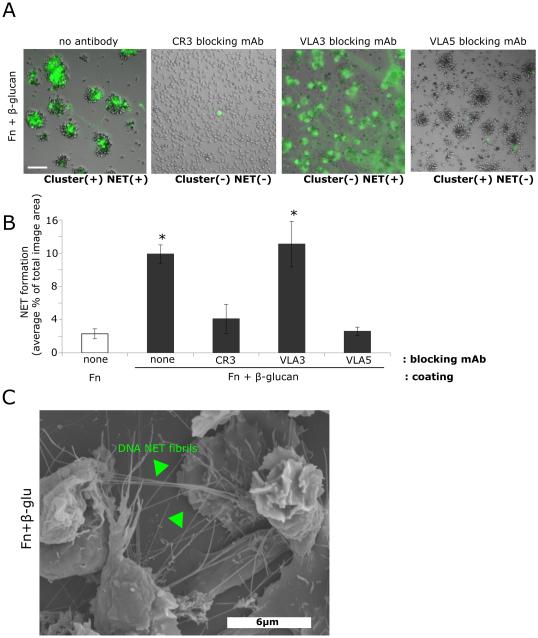
After identifying differential roles for VLA3 and VLA5 in neutrophil clustering, we determined the role of these β1 integrins in CR3-mediated rapid NET formation. Neutrophils were pre-incubated with blocking antibodies to CR3 (clone 44abc), VLA3 (M-KID2), or VLA5 (P1D6) and then allowed to adhere to Fn or Fn + β-glucan-coated surfaces and incubated at 37°C for 30 min, as above. NET formation was visualized using the cell impermeable DNA intercalating dye, Sytox green. Whereas CR3 was required for both cluster formation and NETosis to Fn + β-glucan, VLA3 engagement was required only for cluster formation and VLA5 was required only for NET production (Fig. 2A,B and Fig. 3A). Indeed, when NETs were quantified as an average percent area of the total imaged field, VLA5 blocking significantly reduced NET formation to levels indistinguishable from either CR3 blocking or Fn alone while VLA3 blocking did not significantly reduce NET formation (Fig. 3B). Scanning electron microscopy of neutrophils on Fn + β-glucan confirm of the presence of DNA fibril structures between aggregated cells (Fig. 3C, arrowheads) supporting the presence of extracellular DNA visualized by Sytox green staining of cells on Fn + β-glucan. These data support the hypothesis that CR3-dependent neutrophil cluster formation and NET response to Fn + β-glucan are decoupled with VLA5 being required for NETosis and VLA3 for homotypic aggregation.
Fig. 3. Neutrophil cluster formation and NETosis are regulated differentially by VLA3 and VLA5.
(A) Human neutrophils assayed as in Fig. 2. After 30 min incubation, NET formation in the presence or absence of CR3, VLA3, or VLA5 blocking mAbs was visualized using Sytox green, imaged, and scored for NETs. Bright field and FITC images acquired at 20x; bar = 100μm. (B) Quantification of NET formation. NETs were visualized with Sytox green and multiple images were taken per well. Images were thresholded and gated to include NETs and exclude nuclei. NET formation was quantified as a percent area of the total imaged field. Well averages were ensemble averaged to generate this data. * p<0.001 vs. Fn alone; ANOVA full factorial, post hoc Newman-Keuls; Error bars represent SEM. (C) 80x magnification Scanning Electron Microscopy images of neutrophils demonstrating NET elaboration. Neutrophils were prepared as described above, adhered to Fn + β-glucan coated wells for 30min at 37° C, then fixed and prepared for Scanning Electron Microscopy. DNA NET fibrils spanning aggregated neutrophils indicated by arrowheads.
Dissolution of NETs by DNase I disrupts early, but not late, neutrophil clusters. Neutrophil clusters form in the absence of intact NET structures
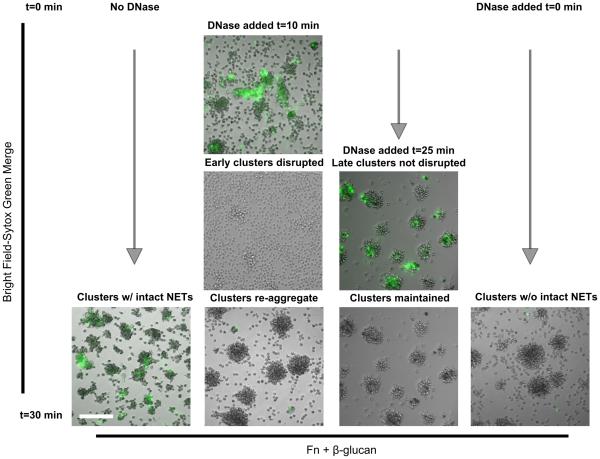
In a previous study, we reported that treatment with DNase I was able to dissolve Sytox green-staining NET structures and disrupt neutrophil clusters on Fn + β-glucan which was consistent with the hypothesis that the NET structures played a physical role in seeding the neutrophil clusters (19). We now show in Fig. 3A that the CR3-dependent cluster formation and NET response to Fn + β-glucan are decoupled. To place this finding in the context of our previous work, we examined the temporal and longitudinal influence of DNase I in the formation of neutrophil clusters on Fn + β-glucan. Neutrophils were allowed to adhere to Fn + β-glucan-coated surfaces and incubated at 37°C, as above. After 30 min, in the absence of DNase, NETs can be visualized with Sytox green at the center of neutrophil aggregates (Fig. 4, first column). If we allow the neutrophils to incubate at 37°C for 10 min, early clusters can be visualized aggregating around NETotic foci (Fig. 4, second column, top panel). Addition of DNase I dissolved both Sytox-green staining NET structures and neutrophil clusters within 30 sec (Fig. 4, second column, center panel). After an additional 20 min incubation, clusters were reformed in similar field locations in the absence of physical NET structures (Fig. 4, second column, bottom panel). Addition of DNase I after 25 min dissolved NET structures but mature clusters were not disrupted and persisted at 30 min (Fig. 4, third column). If DNase I is included in the assay medium from time 0 min and NET structures are not allowed to persist, cluster formation is still observed (Fig. 4, fourth column). These data suggest that although at early time points NETs can support the aggregation of neutrophils, that the physical NET structures can be decoupled from neutrophil cluster formation.
Fig. 4. Dissolution of NETs by DNase I disrupts early, but not late, neutrophil clusters. Neutrophil clusters form in the absence of intact NET structures.
(A) Human neutrophils assayed as in Fig. 2 on Fn + β-glucan. DNase I was added either at time 0 before any neutrophil clusters had formed, after 10 min when early clusters had formed and associated NETs could be visualized, or after 25 min with matured clusters and associated NETs could be visualized. After 30 min incubation, cluster formation and NETs in the presence or absence of DNase I was visualized using Sytox green and imaged. Bright field and FITC images acquired at 20x; bar = 100μm.
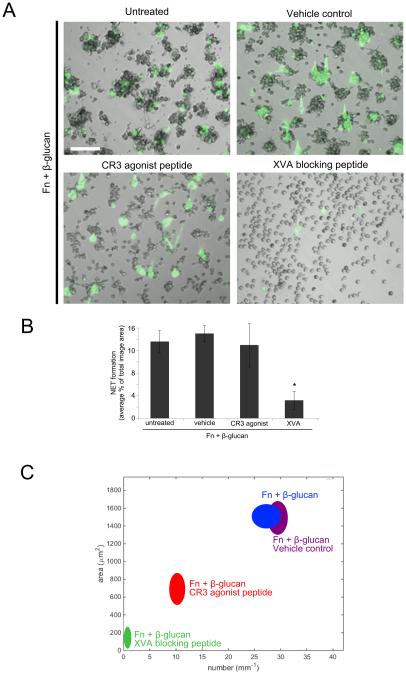
A CR3 agonist leukadherin-1, that decouples CR3 binding from some downstream signaling, supports NET formation but partially inhibits cluster formation. Integrin affinity blocker, XVA143, blocks both cluster formation and NETosis
To further interrogate the role of CR3 identified in our antibody blocking studies, neutrophils were pretreated with either a small molecule CR3 agonist, leukadherin-1 (LA1) or small molecule β2 allosteric antagonist, XVA143 (XVA). The XVA antagonist binds to the β2 I domain MIDAS near a key regulatory interface with the α I domain and blocks communication of conformational change to the I domain and at the same time inducing conformational rearrangements elsewhere in the integrin, including swing-out of the hybrid domain, resulting in an extended conformation with an inactive I domain (27, 28). Cluster formation and NETosis in response to Fn + β-glucan was completely abrogated in neutrophils pretreated with 10 μM XVA antagonist (Fig. 5). This indicates that the low affinity I domain conformation of CR3 does not support aggregate or NET formation. Interestingly, we found that neutrophils pretreated with 15 μM LA1, a small molecule CR3 agonist that prevents CR3 from leaving its high affinity conformation while decoupling the receptor from some of its intracellular signaling (29), showed significantly decreased neutrophil clusters in both number and area (Fig. 5A, 5C) while having no significant effect on NETosis (Fig. 5A-B). These data indicate that flexibility in the conformational state of CR3 determines neutrophil cluster formation and supports the involvement of CR3 intracellular signaling in CR3-to-VLA3 cross-talk. The preservation of NET formation after CR3 agonist treatment here suggests that the VLA5 role in NETosis is independent of CR3-to-VLA5 cross-talk,
Fig. 5. Leukadherin-1 allows NET formation but attenuates cluster formation. XVA143 blocks both cluster formation and NETosis.
(A) Human neutrophils assayed as in Fig. 2. Where indicated, neutrophils were additionally pretreated either with CR3 agonist LA1, β2 allosteric antagonist XVA, or vehicle control. After 30 min incubation, NET formation was visualized using Sytox green, imaged, and scored for NETs. Bright field and FITC images acquired at 20x; bar = 100μm. (B) Quantification of NET formation. NETs were visualized with Sytox green and multiple images were taken per well. Images were thresholded and gated to include NETs and exclude nuclei. NET formation was quantified as a percent area of the total imaged field. Well averages were ensemble averaged to generate this data. * p<0.001 XVA vs. all other conditions; ANOVA full factorial, post hoc Newman-Keuls; Error bars represent SEM (C) Neutrophil cluster formation was quantified using ImageJ and custom MatLab software and plotted as average clusters per mm (x-axis) vs. average cluster area in μm2 (y-axis) per condition. Ellipses represent 2D-SEM. Cells on Fn + β-glucan pretreated with the XVA β2 antagonist (green) have a significant decrease in both cluster number and area vs. cells on Fn + β-glucan (blue). Cells on Fn + β-glucan pretreated with vehicle control (purple) showed no significant difference in clustering parameters vs. Fn + β-glucan (blue). Cells on Fn + β-glucan pretreated with the CR3 agonist LA1 (red) showed an intermediate phenotype with a significant decrease in clustering parameters vs. Fn + β-glucan (blue) but a significant increase in clustering parameters vs. cells pretreated with the XVA blocking peptide (green). p < 0.001; ANOVA full factorial, post hoc Newman-Keuls.
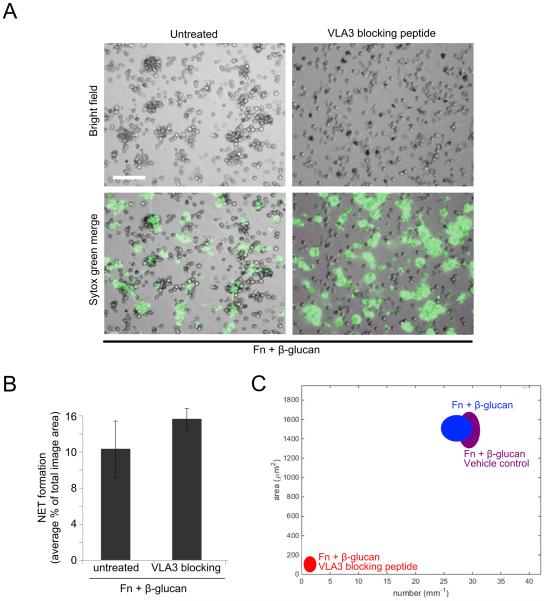
VLA3 blocking peptide inhibits cluster formation but not NETosis
To confirm the regulatory role of VLA3 in cluster formation suggested by our VLA3 antibody blocking experiments, neutrophils were pretreated with 25 μg/ml of a cyclic VLA3 blocking peptide, LXY1, identified for its ability to block binding of VLA3 to laminin and shown to block VLA3-mediated leukocyte elongation (30, 31). Cells treated with the VLA3 blocking peptide then adhered them to Fn + β-glucan coated surfaces for 30 min at 37°C showed no significant change in NET formation when compared to untreated cells (Fig. 6A-B) but showed a complete abrogation of cluster formation (Fig. 6A, 5C). These data confirm antibody blocking data in supporting both a regulatory role for VLA3 in cluster formation and the β1-mediated decoupling of cluster formation and NETosis.
Fig. 6. VLA3 blocking peptide, LXY1, blocks cluster formation but not NETosis.
(A) Human neutrophils assayed as in Fig. 2 in the presence or absence of pretreatment with 25μg/ml VLA3 blocking peptide LXY1. After 30 min incubation, NET formation was visualized using Sytox green, imaged, and scored for NETs. Bright field and FITC images acquired at 20x; bar = 100μm. (B) Quantification of NET formation. NETs were visualized with Sytox green and multiple images were taken per well. Images were thresholded and gated to include NETs and exclude nuclei. NET formation was quantified as a percent area of the total imaged field. Well averages were ensemble averaged to generate this data. Error bars represent SEM. (C) Quantification of cluster formation. Cluster formation was quantified using custom MatLab software and plotted as average clusters per mm (x-axis) vs. average cluster area in μm2 (y-axis) per condition. Ellipses represent 2D-SEM. Cells on Fn + β-glucan pretreated with the LXY1 VLA3 blocking peptide (red) have a significant decrease in both cluster number and area vs. cells on Fn + β-glucan (blue). Cells on Fn + β-glucan pretreated with vehicle control (purple) showed no significant difference in clustering parameters vs. Fn + β-glucan (blue). p < 0.001; ANOVA full factorial, post hoc Newman-Keuls.
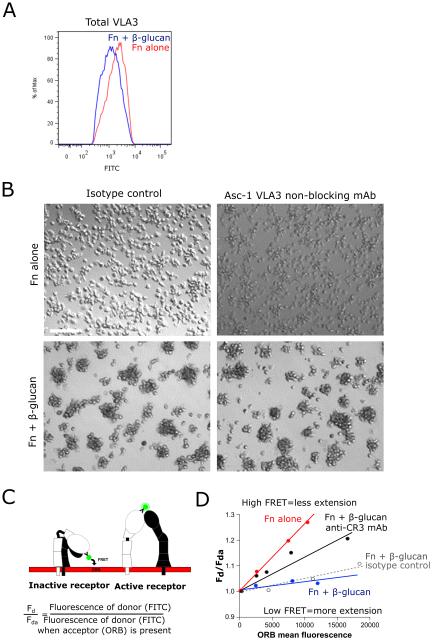
Activation of VLA3 is significantly increased by CR3 on Fn + β-glucan-coated surfaces
Total VLA3 surface expression in neutrophils after exposure to Fn + β-glucan was unaltered as determined by staining using a directly conjugated antibody to VLA3 (ASC-1) with an average MFI ratio of Fn to Fn + β-glucan of 1.08 ± 0.13 (Fig. 7A). To determine if CR3 activation in turn caused activation of VLA3, we needed to develop an assay for VLA3 activation as there are no commercially available antibodies that bind a VLA3 activation-specific epitope. To quantify conformational changes in VLA3 induced by Fn + β-glucan, we adapted a FRET-based headpiece extension assay previously described for CR3 (25, 26).This FACS-based assay reports the relative distance between a FITC-labeled mAb to an epitope on the integrin headpiece and octadecyl rhodamine B (ORB), a membrane partitioning fluorophore. To measure the conformational change of VLA3, we used the anti-VLA3 antibody clone ASC-1, which has been shown to block VLA3 binding to laminin but not to Fn or collagen type IV (32). Use of ASC-1 in our cell aggregation assay confirmed that it had no blocking function (Figure 7B) with regard to clustering on Fn + β-glucan. Neutrophils assayed on Fn ± β-glucan coated surfaces were isolated and stained with FITC-conjugated ASC-1 and aliquots were co-stained with increasing concentrations of ORB and assayed by FACS. Energy transfer from the FITC donor to the ORB acceptor causes a decrease in FITC intensity (quenching) that is proportional to the distance between the donor and acceptor fluorophores. The degree of this quenching gives a readout of VLA3 activity measured as headpiece extension (schematized in Fig. 7C). FACS data is analyzed by plotting the ratio of FITC mean fluorescence intensity in the absence of ORB acceptor (Fd) to that in the presence of ORB acceptor (Fda) versus ORB mean fluorescence (Fig. 7D). The slope of these lines quantitatively represents the extent of energy transfer from the donor to the acceptor, with a steep slope reporting significant quenching of the FITC reporter antibody characteristic of an inactive receptor with its headpiece in close proximity to the cell membrane acceptor fluorophore and a shallow slope characteristic of an active receptor with its headpiece and associated FITC reporter antibody extended away from the cell membrane and unable to undergo FRET transfer to the cell membrane receptor (Fig. 7C-D).
Fig. 7. VLA3 activation is significantly increased on Fn + β-glucan vs. Fn-coated surfaces and is mediated by CR3.
(A) Human neutrophils assayed as in Fig. 2. After 30 min incubation, neutrophils were isolated and stained with a directly conjugated mAb for a VLA3. FACS histogram showing total VLA3 on Fn (red) and Fn + β-glucan (blue). (B) Human neutrophils assayed as in Fig. 2 in the presence of the non-function blocking VLA3 mAb ASC-1 or isotype control. After 30 min incubation, bright field images acquired at 20x; bar = 100μm. (C) Shown is a schematic demonstrating the loss of FRET between ORB membrane dye and FITC-conjugated VLA3 mAb upon the extension of the extracellular domain. (D) Human neutrophils assayed as in Fig. 2 in the presence or absence of CR3 blocking mAb (44abc) or isotype control. After 30 min incubation, neutrophils were isolated and stained with a directly conjugated mAb for a VLA3 (Asc-1). Neutrophils were then incubated with 0, 75, 200, or 400 nM ORB and then analyzed by FACS. Representative data are plotted as the fraction of donor mean fluorescence intensity in the absence of acceptor fluorophore (Fd) to that in the presence of acceptor fluorophore (Fda) on the y-axis (Fd/Fda) vs. ORB mean fluorescence on the x-axis.
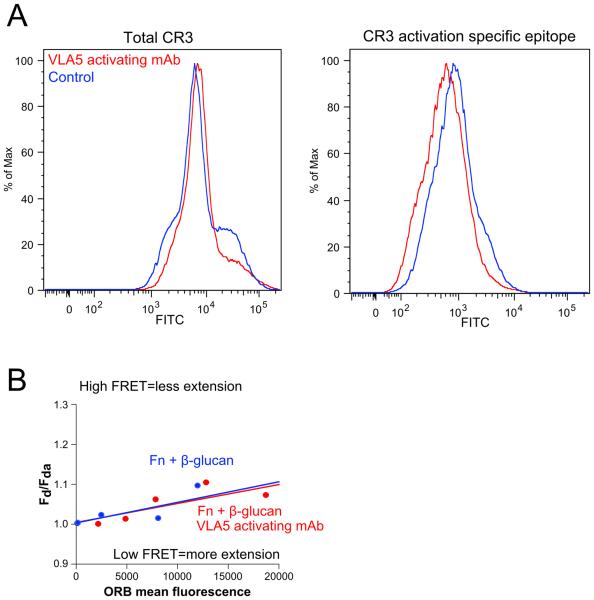
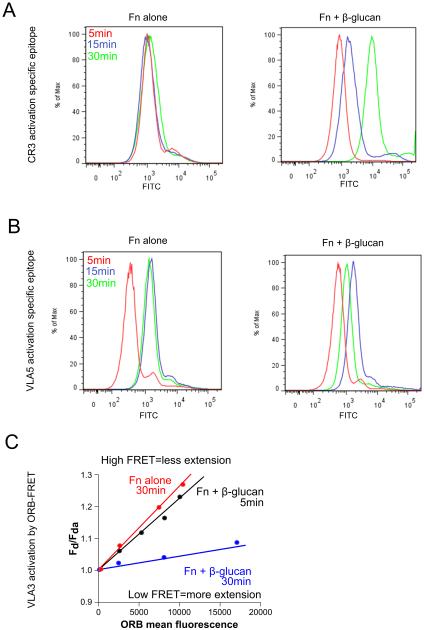
Using FITC-ASC-1, we found decreased slope reported for neutrophils adhered on Fn+β-glucan than reported for neutrophils adhered on Fn alone for 30 min at 37°C and an average distance ratio of 1.67 ± 0.13, indicating that VLA3 is activated on Fn + β-glucan (Fig. 7D). This VLA3 activation is significantly reduced to an average distance ratio of 1.31 ± 0.05 when neutrophils are pre-treated with a CR3 blocking mAb (44abc) providing evidence for a CR3-to-VLA3 cross-talk mechanism that drives neutrophil aggregation to Fn + β-glucan (Fig. 7D).
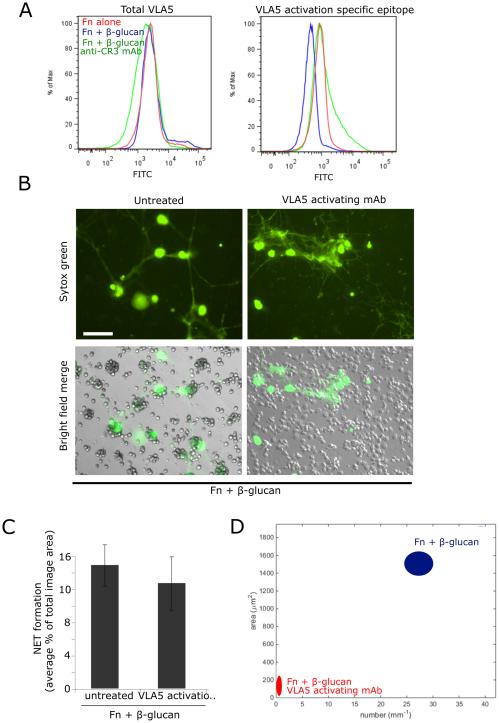
Activation of VLA5 is significantly decreased by CR3 on Fn + β-glucan and is required to permit VLA3 cluster formation
We determined if there was differential expression or activation of VLA5 on Fn + β-glucan vs. on Fn alone during NETosis. FACS analysis using a directly conjugated mAb to total VLA5 (P1D6) showed no significant change in VLA5 expression over the conditions tested (Fig. 8A, left panel). However, staining with an activation specific epitope of VLA5 (SNAKA51) suggested that neutrophils that were adhered on Fn + β-glucan surfaces for 30 min at 37°C had significantly less activated VLA5 than those adhered cells adhered to surfaces coated with Fn alone and this decrease in activity was seen in neutrophils pretreated with an CR3 blocking mAb (Fig. 8A, right panel).
Fig. 8. VLA5 shows a CR3-dependent reduction in activity on Fn + β-glucan vs. Fn-coated surfaces. Driving VLA5 activation with an activating mAb blocks cluster formation but not NETosis, suggesting that an active inhibition of VLA5 is required for cluster formation.
(A) Human neutrophils assayed as in Fig. 2. After 30 min incubation, neutrophils were isolated and stained with a directly conjugated mAb for either total VLA5 (P1D6) or an activation specific epitope of VLA5 (SNAKA51). FACS histogram showing VLA5 staining of cells adhered to Fn (red), Fn + β-glucan (blue), or Fn + β-glucan after pretreatment with a CR3-blocking mAb (green). (B) Human neutrophils assayed as above in the presence or absence of pretreatment with VLA5 activating mAb. After 30 min incubation, NET formation was visualized using Sytox green, imaged, and scored for NETs. Bright field and FITC images acquired at 20x; bar = 100μm. (C) Quantification of NET formation. NETs were visualized with Sytox green and multiple images were taken per well. Images were thresholded and gated to include NETs and exclude nuclei. NET formation was quantified as a percent area of the total imaged field. Well averages were ensemble averaged to generate this data. Error bars represent SEM. (D) Quantification of cluster formation. Cluster formation was quantified using custom MatLab software and plotted as average clusters per mm (x-axis) vs. average cluster area in μm2 (y-axis) per condition. Ellipses represent 2D-SEM. Cells on Fn + β-glucan pretreated with the VLA5 activating mAb (red) have a significant decrease in both cluster number and area vs. cells on Fn + β-glucan (blue). p < 0.001; ANOVA full factorial, post hoc Newman-Keuls.
Findings in Fig. 3A show that VLA5 blocking does not impede neutrophil clustering on Fn + β-glucan and Fig. 8A indicates that VLA5 undergoes a suppression of the high affinity state prior to clustering. To determine if reduced activation of VLA5 is required for neutrophil cluster formation, we took advantage of a VLA5 activating mAb (SNAKA51) that binds an epitope mapped to the calf-1/calf-2 domains of the α5 subunit and drives VLA5 into a high affinity state (33). Neutrophils pretreated with the VLA5-activating mAb and adhered to Fn + β-glucan-coated surfaces for 30 min at 37°C showed no significant difference in NETosis when compared to control cells (Fig. 8B-C) but had a complete abrogation of neutrophil cluster formation (Fig. 8B, 8D). This suggests that VLA5 must be actively inhibited to allow for neutrophil aggregation in response to Fn+β-glucan.
Driving VLA5 activation does not affect CR3 expression or activation
We determined CR3 activity in neutrophils that had been pre-treated with the VLA5 activating mAb (SNAKA51) and adhered to Fn + β-glucan for 30 min at 37°C. By staining neutrophils with directly conjugated mAbs reporting both CR3 expression (ICRF44) and CR3 activation (CBRM1/5), we found that driving activation of VLA5 had no significant effect on CR3 expression or activation status (Fig. 9A), indicating that activating VLA5 blocks aggregate formation in a CR3 independent mechanism and providing additional evidence against a mechanism of β1-to-β2 cross-talk in this system.
Fig. 9. Driving VLA5 activation does not affect CR3 expression or activation, providing additional evidence dismissing β1 to β2 cross talk in this system. Additionally, driving VLA5 activation does not affect VLA3 activation, suggesting that VLA5’s role in the regulation of cluster formation is downstream of CR3 and VLA3.
Human neutrophils assayed as in Fig. 2 in the presence or absence of pretreatment with VLA5 activating mAb (SNAKA51). (A) After 30 min incubation, neutrophils were isolated and stained with directly conjugated mAbs for both total CR3 (ICRF44) and a CR3 activation specific epitope (CBRM1/5). FACS histograms showing total CR3 (left) and CR3 activation (right) on Fn and Fn + β-glucan for control cells (blue) and cells treated with the VLA5 activating mAb (red). (B) After 30 min incubation, neutrophils were isolated and stained with a directly conjugated mAb for a VLA3 (ASC-1). Neutrophils were then incubated with 0, 75, 200, or 400 nM ORB and then analyzed by FACS. Representative data are plotted as the fraction of donor mean fluorescence intensity in the absence of acceptor fluorophore (Fd) to that in the presence of acceptor fluorophore (Fda) on the y-axis (Fd/Fda) vs. ORB mean fluorescence on the x-axis.
To determine if there was a VLA5-to-VLA3 mechanism of VLA3 activation, we examined the activation state of VLA3 using the VLA3 ORB-FRET activation assay described previously (Fig. 7C,D). Neutrophils pretreated with VLA5 activating mAb (SNAKA51) for 30 min at 37°C, were isolated and stained with a directly conjugated mAb for a VLA3 (ASC-1), then aliquoted, incubated with increasing amounts of ORB, and analyzed by FACS. The ratio of FITC mean fluorescence intensity in the absence of ORB acceptor (Fd) to that in the presence of ORB acceptor (Fda) versus ORB mean fluorescence was plotted (Fig. 9B). We observed no significant differences in slope or average distance ratios reported for neutrophils treated with VLA5 activating mAb (SNAKA51) adhered on Fn + β-glucan than reported for control neutrophils adhered on Fn+β-glucan (Fig. 9B), suggesting that any role of VLA5 in cluster formation is downstream of VLA3 and not a mechanism of VLA5-dependent activation of VLA3.
Behavior of human neutrophils in response to intact hyphae elaborated in the context of Fn show that NET formation and clustering can be decoupled in response to intact hyphae
To determine if the neutrophil integrin cross-talk response to Fn + β-glucan translates to the neutrophil response to intact hyphae in the context of matrix, we elaborated C. albicans hyphae on tissue culture plastic coated with 10 μg/ml Fn in YPD at 37°C. Hyphae were washed and human neutrophils, pretreated on ice with 10−9 M fMLP, were added and incubated at 37°C in L-15 supplemented with 2 mg/ml glucose and 1 mM Mn++. Where indicated, neutrophils were additionally pretreated either with specific mAbs to block VLA5 (P1D6) or VLA3 (M-KID2) or to activate VLA5 (SNAKA51). After 30 min at 37°C, NET formation was visualized using Sytox green (Fig. 10). Consistent with the neutrophils response to Fn + β-glucan, neutrophils clustered around hyphal filaments and elaborated NETs (Fig. 10A, 10C). Also consistent with findings using immobilized β-glucan, blocking VLA5 inhibited NETs but not clustering, and blocking VLA3 inhibited clustering but not NETs (Fig. 10B-C). Additionally, activation of VLA5 blocked clustering but not NETs (Fig. 10B-C). These data show that neutrophil NET formation and clustering can be decoupled in response to intact hyphae as found for immobilized β-glucan.
Fig. 10. Behavior of human neutrophils in response to intact hyphae elaborated in the context of Fn show that NET formation and clustering can be decoupled in response to intact hyphae.
C. albicans blastoconidia were allowed to elaborate into hyphae on tissue culture plastic coated with 10 μg/ml Fn in YPD at 37°C. Hyphae were washed and human neutrophils, pretreated on ice with 10−9 M fMLP, were added and incubated at 37°C in L-15 supplemented with 2 mg/ml glucose and 1 mM Mn++. After 30 min incubation, NET formation was visualized using Sytox green, imaged, and scored for cluster formation and NETs with (A) control cells and (B) neutrophils that were additionally pretreated either with specific mAbs to block VLA5 (P1D6) or VLA3 (MKID-2) or to activate VLA5 (SNAKA51). Bright field and FITC images acquired at 20x; bar = 100μm. (C) Enlarged images showing cluster aggregation to hyphae.
A time course analysis of integrin activation in neutrophils adhered to Fn ± β-glucan supports a temporal model of integrin cross-talk
We reported that the neutrophil response to Fn + β-glucan NET formation occurs very rapidly, with cells undergoing this rapid NETosis serving as foci for neutrophil cluster formation. To extend these data presented to the current study, we undertook a time course analysis of integrin activation in neutrophils adhered to Fn ± β-glucan. Human neutrophils assayed as described previously were isolated after 5, 15, and 30 min incubation, stained with directly conjugated mAbs for a CR3 activation specific epitope (CBRM1/5) or a VLA5 activation specific epitope (SNAKA51) and assayed by FACS. Neutrophils adhered to Fn + β-glucan showed a significant time dependent increase in CR3 activation that was not seen in cells adhered to Fn alone (Fig. 11A). Cells adhered to Fn ± β-glucan both showed a significant increase in VLA5 activation between 5min and 15min that was sustained through 30min on Fn alone (Fig. 11B). Cells adhered to Fn + β-glucan then showed a significant reduction in VLA5 activation at 30 min when compared to 15 min (Fig. 11B, right panel). To examine VLA3 activation, after 5 min and 30 min incubation on Fn ± β-glucan, neutrophils were isolated and stained with a directly conjugated mAb for VLA3 (ASC-1). Neutrophils were then incubated with 0, 75, 200, or 400 nM ORB and then analyzed by FACS. Representative data are plotted as the fraction of donor mean fluorescence intensity in the absence of acceptor fluorophore (Fd) to that in the presence of acceptor fluorophore (Fda) on the y-axis (Fd/Fda) vs. ORB mean fluorescence on the x-axis. Quenching of the VLA3 reporter mAb of neutrophils adhered to Fn alone had a steep slope indicating low activity at 5 min (data not shown) that was maintained through 30 min with no significant difference in average distance ratios (Fig. 11C). Cells adhered to Fn + β-glucan, however, had a steep slope not significantly different than that of cells on Fn alone at 5 min indicating low activity, but a significantly shallower slope at 30min indicating increased VLA3 activity with an average distance ratio of 1.53 ± 0.13. These data suggest that integrin regulation in response to Fn + β-glucan is a temporally-coordinated process.
Fig. 11. A time course analysis of integrin activation in neutrophils adhered to Fn + β-glucan supports temporal model of regulation.
Human neutrophils assayed as in Fig. 2 (A) Human neutrophils, pretreated on ice with 10−9 M fMLP, were incubated at 37°C on tissue culture plastic coated with either Fn alone or 6 Fn + β-glucan in L-15 supplemented with 2 mg/ml glucose and 1 mM Mn++. After 5, 15, and 30 min incubation, cells were isolated and stained with directly conjugated mAbs for a CR3 activation specific epitope (CBRM1/5) or a VLA5 activation specific epitope (SNAKA51) and assayed by FACS. FACS histograms showing CR3 activation (A) and VLA5 activation (B) for cells adhered to Fn alone (left panel) and Fn + β-glucan (right panel) for 5 min (red), 15 min (blue), or 30 min (green). (C) Neutrophils assayed as described above. After 5 min and 30 min incubation, neutrophils were isolated and stained with a directly conjugated mAb for VLA3 (ASC-1). Neutrophils were then incubated with 0, 75, 200, or 400 nM ORB and then analyzed by FACS. Representative data are plotted as the fraction of donor mean fluorescence intensity in the absence of acceptor fluorophore (Fd) to that in the presence of acceptor fluorophore (Fda) on the y-axis (Fd/Fda) vs. ORB mean fluorescence on the x-axis.
Discussion
Systemic infection caused by candida species is the fourth leading cause of nosocomial bloodstream infection in modern hospitals and carries high morbidity and mortality rates in spite of antifungal therapy. In the last three decades, there has been an increase in fungal infections in patients who are immunosuppressed or neutropenic (1, 7). Current studies of fungal infection trends show resistance is increasing among fungal species susceptible to these drugs (34, 35). To combat rising fungal infection rates and growing resistance, an increased focus on additional ways to enhance the innate immune anti-fungal response may offer alternative treatments.
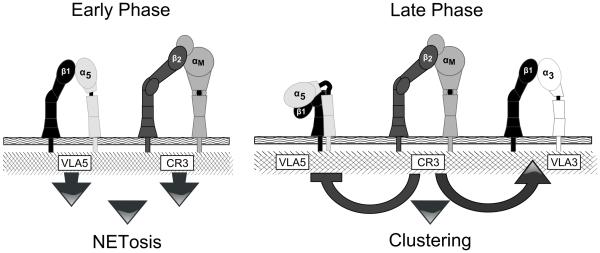
Neutrophils are the primary cells responsible for the elimination of fungi in invasive tissue infections, yet the mechanisms by which they respond to virulence-associated, non-ingestible hyphal forms of fungi are poorly understood. The aim of this current study was to investigate the surface receptors involved in the neutrophil response to fungal β-glucan in the context of the ECM protein, Fn. This is motivated by the widespread tissue distribution of Fn and the contact with this matrix component by neutrophils responding to deep-seated mycosis. Prior work from our laboratory showed that neutrophil swarming and clustering as well as NET release to β-glucan and intact hyphae are dependent on Fn. The swarming of neutrophils and eventual clustering around hyphal filaments, along with NET release, are regarded as essential components for effective host defense against this dimorphic opportunist. In pursuit of a better understanding of this matrix-dependent immune response, we now demonstrate a novel, complex, and temporally regulated cross-talk pathway in which the β2 integrin CR3, signals the differential regulation of individual β1 integrins not often associated with anti-fungal activity. We show that NETosis and swarming/clustering are controlled by the activation state of distinct β1 integrins, with both effects ultimately regulated by CR3. Our data support a two-stage temporal model (schematized in Fig. 12) where VLA5 and CR3 are activated by ligand binding immediately following contact with Fn and β-glucan leading to rapid, matrix-dependent NET formation. Over time, CR3 undergoes inside-out auto-activation that drives the down-regulation (suppression) of VLA5 and the up-regulation (activation) of VLA3 to allow for swarming/cluster formation (Fig. 11). If VLA5 is not down-regulated, clustering is obviated. This study not only reinforces the need to account for matrix to fully understand mechanisms of anti-fungal host defense, but also reveals new roles for β1 integrins in host defense to candida. We also show a previously unappreciated level of complexity and inter-family communication among β1 and β2 integrins.
Fig. 12. Two- stage model of integrin cross-talk in mediating neutrophil clustering and NETosis on Fn + β-glucan that decouples clustering and NET formation.
We propose an early stage of VLA5 and CR3 adhesion to Fn + β-glucan that triggers NETosis and subsequent CR3 inside-out auto activation that leads to a later stage β2 to β1 cross-talk that inactivates VLA5 and activates VLA3 leading to characteristic neutrophil clustering around NETotic foci.
A significant finding of this paper is the ability of CR3 to coordinate a simultaneous up-regulation and down-regulation of two distinct β1 integrins in response to fungi via integrin cross-talk. Integrin cross-talk was first described as "trans-dominant inhibition" in which activation of one integrin prevented the activity of another integrin through intracellular signaling and has since evolved to encompass many types of inter-integrin regulation (36, 37). For example, on T-cells, the β2 integrin LFA-1 down-regulates the VLA4 response to ECM while up-regulating the VLA5 response to Fn to allow for stronger adhesion (38). Our findings offer new insight into the molecular mechanisms underlying the neutrophils response to fungal β-glucan in the context of tissue matrix. We propose CR3 as the master regulator, coordinating the trans-dominant inhibition of VLA5 and the up-regulation of VLA3, resulting in neutrophil swarming and homotypic aggregation.
Our initial observation of neutrophils swarming into clusters on immobilized Fn and β-glucan or hyphae led us to question the importance of cluster formation in the immune response to fungi. Others have suggested that, as hyphal forms of fungi are much too large to be phagocytosed, neutrophils circumvent this problem by enclosing microbes together with other neutrophils (39, 40). The encompassment of the microbe may provide areas of concentrated neutrophilic effector activity better able to eliminate the infection than would occur by binding of single cells along a hyphal filament (18). We identify VLA3 activity as necessary for clustering. This is consistent with previous work in our laboratory that has shown that upon dual ligation of CR3 by Fn and β-glucan, neutrophils demonstrated enhanced chemotaxis mediated by a shift in Fn binding from VLA5 to VLA3 (41).
However we also consider an alternative to the essentiality of VLA3 activity and swarming to the anti-fungal response. It is plausible that the early NETotic response is responsible for hyphal killing and perhaps physical containment of blastoconidia which serves to “wall off” the infectious focus. In addition to NETosis, some early responding neutrophils likely elaborate chemokines (i.e., IL-8, LTB4) to attract the next wave of neutrophils into organized clusters with the initiating cell located centrally. It is not yet clear if NETosis and chemokine release results from the same cell or from neighboring early responders. Importantly, whether swarming is essential or dispensible to host defense remains to be confirmed. We recognize that VLA3-induced clustering could be a detrimental aspect of the inflammatory response and contribute to collateral tissue damage as a result of gathering a high concentration of neutrophils into a focal point. This allows for the hypothesis that, by targeting VLA3 activity, collateral tissue damage may be attenuated while maintaining the biologically important immune defense mechanism of NETosis. In support, it has been shown that VLA3-high expressing cells from septic animals displayed hyper-inflammatory phenotypes that when blocked or conditionally knocked down showed reduced extravasation and increased survival rates as compared to healthy animals (42). Future work will test the host response of mice lacking VLA3 upon fungal challenge.
The role of VLA5 in matrix-dependent rapid NET formation has not been previously described. Whereas CR3 cross-talk affecting the affinity states of VLA3 and VLA5 is necessary for swarming and aggregation, whether cross-talk between CR3 and VLA5 regulates NETosis is less evident. Antibody blocking of CR3 or VLA5 clearly prevents NETosis suggesting a non-redundant role for these receptors in initiating NET release. The reason NET release on Fn + β-glucan necessitates binding of both receptors is not yet known. The requirement for CR3 was further supported by the β2 integrin allosteric antagonist XVA143. The mechanism of action of CR3 small molecule agonist LA1, locking activated CR3 receptor into its structurally high affinity state while decoupling it from some of its intracellular communication within the cell, generated a slightly different result, affecting clustering more profoundly than NETosis for reasons yet to be discerned. That NET accumulation persisted over the 30 min observation period, even as VLA5 was becoming down regulated, suggests that NET triggering requires VLA5 activation but perhaps persistence of formation and release of NETs becomes constitutive after an initialization event. Of course this scenario is difficult to discern at the population level and would necessitate determining the activation status of each NETotic cell to formally determine if VLA5 high affinity state is only needed initially to trigger NET release. By identifying VLA5 as having a role in NET formation, we have uncovered a new pharmaceutical molecular target for diseases exhibiting excessive NET formation while showing that different structural conformations of CR3 may mediate different cellular functions. Our results are also consistent with the reported finding that post-NETotic, anuclear neutrophils are capable of chemotaxis and bacterial phagocytosis perhaps representative of multitasking antimicrobial effector functions against fungal filaments (43). These authors concluded that NETosis is not limited to immobile and incapacitated cells and this may serve to aid the spread of microbiocidal NETs around the area around a focus of infection to an extent greater than would be possible if NETosis was limited to nonmigratory neutrophils.
VLA5 binds Fn with high affinity at two locations: its RGD sequence, an Arg-Gly-Asp motif contained by many ECM proteins, and its PHSRN site, a Pro–His–Ser–Arg–Asn synergy sequence specific to Fn (44). Specifically, residue Asp154 distinguishes α5 from other α subunits and results in its strong preference for Fn over other RGD ligands (45). VLA5 is unique in that the integrin conformation and the ligand binding are not mutually related. A recent study showed RGD-bound VLA5 structure conformation was similar to the non-ligated structure conformation. Furthermore, when bound with an inhibitor mAb, VLA5 was able to bind ligand (45). Taken together, this indicates VLA5 can bind Fn in what is considered the inactive confirmation. As inhibition of VLA5 extension does not necessarily mean it is unable to recognize ligand and communicate with the cell, perhaps a ligated VLA5 in a low affinity conformation induces intracellular signaling not possible in its high affinity state. Furthermore, antibody blockade of VLA5 receptor has no effect on the neutrophil response to Fn + β-glucan, indicating the addition of β-glucan inhibits the activity of VLA5 towards its canonical ligand Fn. The switch from VLA5 to VLA3 Fn affinity may facilitate a type of cellular migration that is not possible with the strong adhesion between VLA5 and Fn.
The recognition of immobilized β-glucan in the context of Fn shifts CR3 in to a more activated state as determined by FACS using a CR3 activation reporter antibody (Fig. 2C). This activity is critical, as treatment with a CR3 blocking mAb (44abc) not only decreases CR3 activation, as measured by staining of a CR3 activation specific epitope (Fig. 2C), but also decreases the neutrophil response of swarming (Fig. 2A) and NET release (Fig. 3A-B). Additionally, we show with the use of a CR3 receptor agonist that locks activated CR3 into an extended conformation and decouples intracellular signaling, that CR3 must be able to move between its physical conformational states in order to allow for swarming (Fig. 5A). We demonstrate that CR3 and VLA3 activity, but not expression, is increased in the presence of β-glucan (Figs. 2C, 7A, 7D). In addition, VLA5 activity must be down regulated to allow for cluster formation (Fig. 8). This is so because of the reduction in binding of the activation reporter antibody SNAKA51 observed on cells exposed to Fn+glucan while no such reduction in total VLA5 expression is noted by the anti-VLA5 antibody P1D6 which binds to all VLA5 molecules regardless of activation state. Both SNAKA51 and P1D6 are well characterized to bind to VLA5 at epitopes that map outside the ligand-binding domain so expression of total and activated VLA5 receptors can be measured without interference by the presence of ligand. Taken together, CR3 serves as the master regulator of a full blown neutrophilic response to fungal β-glucan in the context of matrix under conditions where phagocytosis is not available in host defense.
Previous work in our lab has previously suggested that dual ligation of CR3 affects β1 subfamily integrins, VLA5 and VLA3 and can have effects on CR3-mediated anti-fungal responses via integrin cross-talk. Harler et al. showed migration on a Fn substrate was mediated by the integrins VLA5 and CR3, however, supplementation of the Fn with β-glucan led to a shift in integrin-mediated chemotaxis to one that is determined by VLA3 (41). VLA3 contains binding domains for both laminin and RGD and it is this later functionality that controls migration on glucan when VLA5 is blocked. In addition, Lavigne, O’Brien et al. proved blocking VLA5 or VLA3 was sufficient to relieve the Fn-dependent suppression of respiratory burst induced by the β-glucan activation of CR3 (46). In the present study, we demonstrate CR3 participates in β2 to β1 cross-talk with both VLA5 and VLA3 to determine the neutrophil response to either immobilized β-glucan or fungal hyphae in the context of Fn. We further show that β1 integrins differentially regulate neutrophil function with VLA3 driving clustering and VLA5 being required for NETosis. Our body of work show the essential role of matrix-to-integrin interactions in mediating the multiple neutrophil anti-fungal effector functions including chemotaxis, oxidative burst, clustering and NETosis. All of these functions are initiated upon ligation of CR3 at the I-domain by fibronectin and at the lectin-like site by fungal β-glucan and fully executed upon proper temporal regulation of the activation state of VLA3 and VLA5.
Our experiments demonstrate the mechanisms that form the early events that lead to the anti-fungal response by neutrophils which includes aggregation into an attack complex around a fungal hypha. The earliest responding neutrophils, upon multifocal contact with matrix and fungal hyphae (or β-glucan) undergo NETosis followed by aggregation. Our findings show that these NETotic neutrophils represent a minor population of the assayed cells; most cells are not associated with NET release. We presume these NETotic cells release an as yet undefined chemoattractant to which other PMNs respond and gather as a subsequent wave of chemotactic neutrophils. This does not in any way preclude neutrophils that enter clusters at a later time from releasing NETs and in previous work we have shown that NETs released by neutrophils in our system are indeed cytotoxic for candida hyphae (19). One may speculate that there is benefit to the host to have neutrophils perform multiple antimicrobial events in defense including NETosis to allow containment and clearance of a complex infection involving both yeast and hyphal forms in vivo. Consistent with this notion, we define the integrin-based mechanism that controls the early events that lead to an amplification of a neutrophil-based cluster response that is likely to be a more efficient anti-fungal response than neutrophils responding individually.
Ultimately, this report provides insights on a newly recognized pathway of integrin cross-talk between the β2 integrin CR3 and the β1 integrins VLA3 and VLA5 and its role in the neutrophilic responses to fungal β-glucan. Whereas our current experimental focus is on the response to fungi, integrin cross-talk future work can be proposed to determine whether this is a universal component of the neutrophilic response to all microbial infections of tissues. These results may find new pathways to identify novel targets for anti-fungal therapy and immune cell enhancement as well as increase our understanding of the complex regulation of integrins. Target outcomes may provide an adjuvant or alternative therapy to combat the increasing fungal resistance to azole therapy and improve recovery time of patients with fungal infections.
Acknowledgements
We thank Dr. Meredith Crane and Allan Huang for technical assistance; Matthew Hirakawa and the Brown University Leduc Bioimaging Facility for SEM; and Kevin Carlson and the Brown University Flow Cytometry Facility for FACS acquisition.
Footnotes
This work was supported by the NIH grant GM066194 (JSR); HL125265 (JSR, MK); DK106512, HL109582, DK084195 (VG); UNCF/Merck Graduate Science Research Dissertation Fellowships (ASB and CMJ). Work was also supported by funds from the Department of Surgery, Rhode Island Hospital.
- CR3
- complement receptor 3
- ECM
- extracellular matrix
- Fn
- fibronectin
- NET
- neutrophil extracellular trap
- LA1
- Leukadherin-1
- PAMP
- pathogen-associated molecular pattern
- PMN
- polymorphonuclear leukocyte
- ROS
- reactive oxygen species
- SEM
- scanning electron microscopy
Conflicts-of-interest
Dr. Jonathan Reichner is a shareholder in Biothera (Eagan, MN) and reports the gifting of Imprime-PGG glucan for use in current studies from Biothera (Eagan, MN). Dr. Vineet Gupta is an inventor on pending patent applications and is also a co-founder of Adhaere Pharmaceuticals, Inc. (Skokie, IL) that has licensed these applications related to Leukadherin-1. This author has the potential for financial benefit from their future commercialization. The other co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 3.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 4.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 2005;41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 5.Miller LG, Hajjeh RA, Edwards JE., Jr. Estimating the cost of nosocomial candidemia in the united states. Clin. Infect. Dis. 2001;32:1110. doi: 10.1086/319613. [DOI] [PubMed] [Google Scholar]
- 6.Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J. The direct cost and incidence of systemic fungal infections. Value in Health. 2002;5:26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 7.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP, National Epidemiology of Mycoses Survey(NEMIS) Study Group Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. 2001;33:177–186. doi: 10.1086/321811. [DOI] [PubMed] [Google Scholar]
- 9.Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, Garnacho-Montero J, Leon MA, EPCAN Study Group A bedside scoring system ("Candida score") for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 2006;34:730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 10.Manolakaki D, Velmahos G, Kourkoumpetis T, Chang Y, Alam HB, De Moya MM, Mylonakis E. Candida infection and colonization among trauma patients. Virulence. 2010;1:367–375. doi: 10.4161/viru.1.5.12796. [DOI] [PubMed] [Google Scholar]
- 11.Kourkoumpetis T, Manolakaki D, Velmahos G, Chang Y, Alam HB, De Moya MM, Sailhamer EA, Mylonakis E. Candida infection and colonization among non-trauma emergency surgery patients. Virulence. 2010;1:359–366. doi: 10.4161/viru.1.5.12795. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA. Molecular epidemiology in the care of patients. Arch. Pathol. Lab. Med. 1999;123:1007–1010. doi: 10.5858/1999-123-1007-MEITCO. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt KR, Grimbacher B. Mendelian traits causing susceptibility to mucocutaneous fungal infections in human subjects. J. Allergy Clin. Immunol. 2012;129:294–305. doi: 10.1016/j.jaci.2011.12.966. quiz 306-7. [DOI] [PubMed] [Google Scholar]
- 14.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van't Wout JW, Van der Meer JW, Barza M, Dinarello CA. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. Eur. J. Immunol. 1988;18:1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012;80:1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014 doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 2013;190:4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Ross GD. Generation of Recombinant fragments of CD11b expressing the functional Beta-Glucan-binding lectin site of CR3 (CD11b/CD18) Journal of Immunology. 1999;162:7285–7293. [PubMed] [Google Scholar]
- 21.Forsyth CB, Plow EF, Zhang L. Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CD18 subunit. J. Immunol. 1998;161:6198–6205. [PubMed] [Google Scholar]
- 22.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I Domain is a Major Recognition Site on the Leukocyte Integrin Mac-1 (CD11b/CD18) for Four Distinct Adhesion Ligands. The Journal of Cell Biology. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J. Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 24.Bose N, Chan AS, Guerrero F, Maristany CM, Qiu X, Walsh RM, Ertelt KE, Jonas AB, Gorden KB, Dudney CM, Wurst LR, Danielson ME, Elmasry N, Magee AS, Patchen ML, Vasilakos JP. Binding of Soluble Yeast beta-Glucan to Human Neutrophils and Monocytes is Complement-Dependent. Front. Immunol. 2013;4:230. doi: 10.3389/fimmu.2013.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefort CT, Hyun YM, Schultz JB, Law FY, Waugh RE, Knauf PA, Kim M. Outside-in signal transmission by conformational changes in integrin Mac-1. 2009;183:6460–6468. doi: 10.4049/jimmunol.0900983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien XM, Heflin KE, Lavigne LM, Yu K, Kim M, Salomon AR, Reichner JS. Lectin site ligation of CR3 induces conformational changes and signaling. J. Biol. Chem. 2012;287:3337–3348. doi: 10.1074/jbc.M111.298307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welzenbach K, Hommel U, Weitz-Schmidt G. Small molecule inhibitors induce conformational changes in the I domain and the I-like domain of lymphocyte function-associated antigen-1. Molecular insights into integrin inhibition. J. Biol. Chem. 2002;277:10590–10598. doi: 10.1074/jbc.M110521200. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Carman CV, Kim M, Salas A, Shimaoka M, Springer TA. A small molecule agonist of an integrin, alphaLbeta2. J. Biol. Chem. 2006;281:37904–37912. doi: 10.1074/jbc.M606888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, Moita LF, Schurer S, Traver D, Ruiz P, Vazquez-Padron RI, Ley K, Reiser J, Gupta V. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci. Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. 2012;209:1349–1362. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao N, Xiao W, Wang X, Marik J, Park SH, Takada Y, Lam KS. Discovery of targeting ligands for breast cancer cells using the one-bead one-compound combinatorial method. J. Med. Chem. 2008;52:126–133. doi: 10.1021/jm801062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattaramalai S, Skubitz KM, Skubitz AP. A novel recognition site on laminin for the α3β1 integrin. Exp. Cell Res. 1996;222:281–290. doi: 10.1006/excr.1996.0036. [DOI] [PubMed] [Google Scholar]
- 33.Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J. Cell. Sci. 2005;118:291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 2005;43:5425–5427. doi: 10.1128/JCM.43.11.5425-5427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavanti A, Davidson AD, Fordyce MJ, Gow NA, Maiden MC, Odds FC. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J. Clin. Microbiol. 2005;43:5601–5613. doi: 10.1128/JCM.43.11.5601-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J. Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez AM, Bhattacharya R, deHart GW, Jones JCR. Transdominant Regualtion of Integrin Function: Mechanisms of Crosstalk. Cell Signal. 2010;22:578. doi: 10.1016/j.cellsig.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter JC, Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters alpha4beta1- and alpha5beta1-mediated function. J. Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 40.Diamond RD, Krzesicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am. J. Pathol. 1978;91:313–328. [PMC free article] [PubMed] [Google Scholar]
- 41.Harler MB, Wakshull E, Filardo EJ, Albina JE, Reichner JS. Promotion of Neutrophil Chemotaxis Through Differential Regulation of Beta 1 and Beta 2 Integrins. The Journal of Immunology. 1999:6792–6799. [PubMed] [Google Scholar]
- 42.Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality through exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is dependent on integrin alpha3beta1. Blood. 2014 doi: 10.1182/blood-2014-01-552943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front. Biosci. 1997;2:d126–46. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 45.Nagae M, Re S, Mihara E, Nogi T, Sugita Y, Takagi J. Crystal structure of alpha5beta1 integrin ectodomain: atomic details of the fibronectin receptor. J. Cell Biol. 2012;197:131–140. doi: 10.1083/jcb.201111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavigne LM, O'Brien XM, Kim M, Janowski JW, Albina JE, Reichner Integrin Engagement Mediates the Human Polymorphonuclear Leukocyte Response to a Fungal Pathogen-Associated Molecular Pattern. The Journal of Immunology. 2007:7276–7283. doi: 10.4049/jimmunol.178.11.7276. [DOI] [PubMed] [Google Scholar]