Abstract
Children and adults with Neurofibromatosis type 1 (NF1), a common autosomal dominant condition, manifest a variety of ophthalmologic conditions. Plexiform neurofibromas involving the eyelid, orbit, periorbital and facial structures (termed OPPN) can result in significant visual loss in children. Equally important, OPPNs can cause significant alteration in physical appearance secondary to proptosis, ptosis, and facial disfigurement, leading to social embarrassment and decreased self-esteem.
Despite NF1 being a relatively common disease in which routine ophthalmologic examinations are required, no formal recommendations for clinical care of children with OPPNs exist. While medical and surgical interventions have been reported, there are no agreed upon criteria for when OPPN require therapy and which treatment produces the best outcome. Since a multi-disciplinary team of specialists (oculofacial plastics, pediatric ophthalmology, neuro-ophthalmology, medical genetics and neuro-oncology) direct management decisions, the absence of a uniform outcome measure that represents visual and or aesthetic sequelae complicates the design of evidence based studies and feasible clinical trials.
In September 2013, a multi-disciplinary task force, composed of pediatric practitioners from tertiary care centers experienced in caring for children with OPPN, was convened to address the lack of clinical care guidelines for children with OPPN. This consensus statement provides recommendations for ophthalmologic monitoring and outlines treatment indications, forthcoming biologic therapy, while also discussing challenges to performing clinical trials in this complicated condition.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is a relatively common oncogenic condition that occurs in approximately 1:3,500 births.1, 2 NF1 has an autosomal dominant inheritance pattern with roughly 50% of all new cases due to sporadic mutations. Children with NF1 manifest a variety of ophthalmologic conditions, including low grade gliomas of the afferent visual pathway (termed optic pathway gliomas), glaucoma, choroidal nodules, Lisch nodules and plexiform neurofibromas (PNs) involving the eyelid, orbit, periorbital and facial structures.1, 3, 4 All of these manifestations, except for Lisch nodules and choroidal nodules, can result in visual loss in children, frequently during the age of visual maturation.2 Ectropion uveae alone should not cause vision loss, although it has been associated with glaucoma.4 While most cases of NF1-related vision loss are secondary to optic pathway gliomas, PNs of the orbit and face frequently cause vision loss secondary to deprivational or anisometropic amblyopia as well as glaucoma. 5-8 Equally important is the alteration in physical appearance secondary to proptosis, ptosis, and facial disfigurement, leading to social embarrassment and decreased self-esteem.
Terminology that describes neurofibromas in NF-1 can be confusing. Discrete neurofibromas arise from small nerves or nerve endings, and include dermal neurofibromas that protrude from the surface of the skin, or subcutaneous neurofibromas that present as firm nodules just below the surface of the skin; these tumors tend to be small and generally appear in the second decade of life, becoming more frequent as the patient ages. They have no risk of malignant transformation and rarely cause neurologic deficits. In contrast, PNs are complex nerve sheath tumors that follow multiple nerve branches. Most PNs are diagnosed in early childhood and may demonstrate rapid growth during this period. PNs can result in substantial morbidity due to their appearance and proclivity to cause functional and neurologic deficits, and are at risk for malignant transformation.2 PNs involving the eyelid, orbit, periorbital and facial structures have been described using a variety of names including orbitotemporal PNs,7, 9 orbitopalpebral neurofibromatosis,10, 11 orbitotemporal neurofibromatosis,5, 12-14 orbital neurofibromas,15 and orbitofacial neurofibromatosis.8, 16 PNs in these areas are most appropriately labeled as orbital-periorbital PN (OPPN) to encompass all locations where they occur. To provide clarity and consistency within the medical literature, we propose that the medical and research community adopt the term OPPN.
Ophthalmic and clinical characteristics of OPPNs have not been routinely described in the relatively small number of case reports or case series. In clinical trials assessing treatment of all PN locations, OPPN comprise a small portion of those subjects and ophthalmologic outcome measures are typically not reported. Surgical case series, primarily in adults, also have not focused on ophthalmic characteristics or outcomes.12, 14, 17-20 While the surgical techniques described in these case series are important, without a well-defined indication for treatment or a formal definition of “therapeutic success,” it is difficult to determine if and when intervention is indicated, and whether an intervention is actually beneficial. Improvement in physical appearance and visual outcomes (i.e., avoiding or decreasing amblyopia) are the most common indications for medical and or surgical treatment, yet neither has been well studied nor have they been included in clinical trials.
In this review, we will describe the biologic mechanism, provide a formal definition of OPPN, describe the natural history of PN growth, discuss treatment options and conclude with consensus recommendations for OPPN management.
Biology of Plexiform Neurofibromas
NF1 is caused by a mutation in the NF1 tumor suppressor gene on chromosome 17q11.2 - 350 kb, 60 exons.2, 21 The gene product neurofibromin (2818 amino acids) contains a domain with significant homology to Ras GTPase-activating proteins (GAP) and thus regulates Ras activity. Lack of functional neurofibromin leads to dysregulated Ras signaling and tumorigenesis.22 Plexiform neurofibromas are composed of neoplastic Schwann cells, fibroblasts, perineural cells, and mast cells.23 Neoplastic Schwann cells lack NF1 gene expression, and loss of neurofibromin is associated with elevated levels of activated Ras.24, 25 Activated Ras results in the initiation of a cascade of signaling events such as activation of Raf and mitogen-activated protein kinase (MAPK) that lead to increased cell proliferation.26, 27 In addition, activation of the mTOR pathway has been identified in benign and malignant NF1 tumors,28-30 and the tumor microenvironment contributes to the pathogenesis of PN. Schwann cells have been shown to secrete kit ligand which recruits mast cells and results in abnormal growth.31-33 Additional cooperating events such as increased expression of growth factors and growth factor receptors, including EGFR, PDGFR, and VEGF may contribute to PN development and progression.34-37 Many of the potential treatment targets for PNs are shared with cancers, such as Ras, cKIT, angiogenesis, and mTOR.
Definition
Most OPPN track along the distribution of the trigeminal nerve. PNs will occasionally involve other facial and head structures. OPPN can be categorized by their current anatomic location:
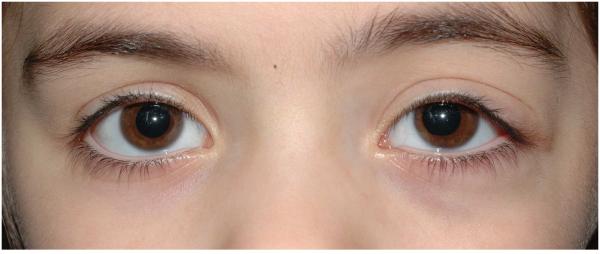
Isolated upper eyelid —frequently assume an “S” shape (see Figure 1) and can result in mild ptosis without obscuration of the visual axis. Future progression into the peri-orbit/orbit is highly unlikely.
Figure 1.
Small plexiform neurofibroma restricted to the left upper eyelid causing a mild degree of ptosis.
Eyelid and peri-orbital region — extending across the V1 and V2 distribution of the trigeminal nerve. On occasion, the ptosis can be profound causing deprivational or refractive amblyopia (see Figure 2). Future progression into the orbit is possible.
Figure 2.
Left upper and lower eyelid plexiform neurofibroma causing ptosis and subsequent deprivational amblyopia.
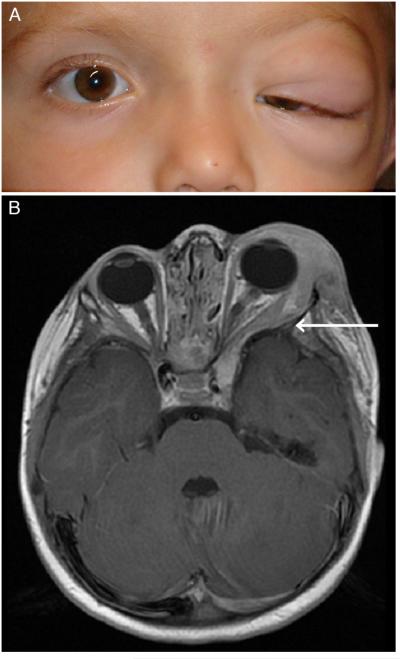
Orbit with/without eyelid involvement — invades lateral orbit and can invade toward the cavernous sinus, deemed infiltrative (see Figure 3a and 3b).
Figure 3.
a. Infiltrative orbital and peri-orbital plexiform neurofibroma resulting in anisometropic and deprivational amblyopia.
b. MRI of infiltrative orbital/peri-orbital plexiform neurofibroma and sphenoid wing dysplasia. Arrow indicates the forward protrusion of the anterior temporal lobe and displacement of the orbital contents.
The frequency of OPPN, as categorized above, is roughly equal across anatomic locations (i.e., one-third per location).7
Associated structural findings
Absence or marked reduction of the sphenoid bone that comprises the posterolateral wall of the orbit, termed sphenoid wing dysplasia, is a congenital abnormality that commonly occurs on the same side as the OPPN (see figure 3b). Sphenoid wing dysplasia can permit protrusion of the anterior temporal lobe into the orbit causing compression of the extra-ocular muscle and the optic nerve. This also likely contributes to proptosis, pulsatile exophthalmos and strabismus that can be associated with the OPPN.
Diagnosis
An infant or young child presenting with peri-orbital asymmetry or unilateral proptosis, with or without elevated intraocular pressure, should be evaluated for an OPPN. While most OPPN are congenital, they may not be obvious immediately after birth. Although not formally studied, the initial identification of OPPN typically occurs before 5 years of age.7 The palpable mass of OPPN can be either firm or soft; sometimes the cluster of nodules resembles what’s described as a “bag of worms”. Concurrent eyelid edema can be present. In children diagnosed with NF1, the suspected OPPN should not be biopsied. If the diagnosis of NF1 has not been established, a formal consultation with a physician expert in the care of children with NF1 is recommended before biopsy is considered.
All children with a newly identified OPPN, regardless of whether a diagnosis of NF1 has been confirmed or excluded, should undergo MR imaging of the brain and orbits to confirm the diagnosis of PN and to better define its extent. Even when if the OPPN is suspected to be isolated to eyelid, MRI should still be performed since the entire portion of the OPPN may not be completely visualized on external examination.
Clinical Characteristics
Incidence
The incidence of OPPN in children with NF1 is likely less than 10%. Oystreck and colleagues described 55 patients examined over a 28 year period.8 Avery et al reported 21 children from two institutions over a 10 year period, although they excluded children with concurrent glaucoma and/or optic pathway gliomas.7 Of children with PNs enrolled in clinical treatment trials, 9% were classified as having head PN.38 Nearly 43% of cases in the surgical series by Needle and colleagues included the head, neck or face.39 Based on our combined clinical experience from multiple large NF1 clinics, OPPN likely occur in fewer than 10% of children with NF1.
Age at Presentation
Most OPPN are identified within the first few years of life. Small OPPN restricted to the eyelid may go unnoticed until later in childhood—especially if the ptosis is mild or unrecognized.
Signs and symptoms
Blepharoptosis is the most common and notable sign of an OPPN with an incidence of nearly 100%, followed by proptosis, eyelid edema and strabismus.16 Although not reported in an all studies, the report by Chaudry and colleagues documented proptosis in over 50% of patients with OPPN.16
An OPPN should be considered in infants and young children who present with buphthalmos and or glaucoma. The exact frequency of glaucoma discovered during the initial diagnosis of an OPPN has not been adequately studied. A cross-sectional study which included both children and adults with OPPN secondary to NF1 reported that nearly 25% of them had glaucoma.8 It is uncommon that a child’s symptom will lead to the initial discovery of an OPPN, as most are visible. Symptoms such as eye pain from exposure keratopathy and diplopia from strabismus do occur in OPPNs that demonstrate significant growth.
Natural History
The introduction of volumetric MRI analysis of PNs has allowed investigators to closely monitor small changes in PN volume over time and has provided a better understanding of their natural history.40 This method, which requires imaging of the entire PN with axial and coronal STIR sequences without a gap between slices, has been used in several natural history trials and also in multiple treatment trials directed at PN.41-47 Guidelines for the measurement of response and the imaging of PNs in clinical trials were recently issued by the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS), Tumor Measurement Working Group.48
Young children (age ≤8 years) appear to have the fastest PN growth rates,49, 50 and there does not appear to be acceleration in the PN growth rate during puberty.51 In adults PNs typically grow minimally. While PN growth rates vary between patients, PN growth was relatively constant over prolonged time periods within patients.49 Spontaneous slow decrease in PN volumes (median 3.4% decrease in tumor volume per year) has been described in one study,52 and was in large part attributed to measurement error. These studies support the need for treatment intervention directed at PNs in early childhood. Surgery is one option for the treatment of PN, but complete OPPN removal is feasible in only a small subset of patients. Continued PN growth after surgery has been described, particularly in patients with head and neck tumors39 and in younger patients.39, 53
Management of OPPN
What is the appropriate Ophthalmologic Assessment in Children with OPPN?
A comprehensive ophthalmologic exam should be performed at a minimum every 6 months throughout the period of visual development (i.e., before the age of 8 years) during which time amblyopia may develop. The mechanisms by which children with an OPPN are susceptible to vision loss are complex. Refractive amblyopia from anisometropia induced by ptosis or increased axial length occurs in up to 43% of children with OPPN.7, 8 Deprivation amblyopia from significant ptosis occurs in roughly one-third to one-half of patients. 7, 8 Amblyopia due purely to strabismus occurs in only 10-20%.7, 8 Nearly one-quarter of patients may demonstrate elevated intraocular pressure, however a rigorous longitudinal analysis comparing the incidence among children and adults has not been completed.8 Proptosis has been reported to occur in approximately 50%,16 yet surgical treatment for exposure keratopathy is reported to occur much less frequently (i.e., 5%).7 Although the frequency of compressive optic neuropathy is unknown, it has been considered as another mechanism of visual compromise in the setting of an OPPN. The frequency of exams in children with a rapidly growing OPPN should be increased at the discretion of the provider in order to monitor for amblyopia, glaucoma, strabismus, or optic nerve disease. While it is still important to provide ophthalmologic monitoring after the age of 8, the frequency of exams should be based on the patient’s clinical course.
Approach to Strabismus
There is a high prevalence of OPPN-associated eye misalignment or strabismus with reported rates ranging from 26-75% in several large case series of children and adults with OPPNs as compared with a rate of approximately 3-5% for the more common forms of strabismus in school-aged children.5, 8, 54-56 Strabismus may develop from mechanical restriction of the globe by tumor infiltration or compression of orbital tissues and extraocular muscles, by globe displacement from increased axial length, and globe displacement secondary to orbital and sphenoid wing dysplasia.10, 55 Further, in the setting of severe vision loss, a sensory strabismus may also develop. In their detailed examination of ocular motility in 49 patients with OPPNs, Oystreck et al. 55 found that the mechanisms for eye misalignment were multifold and that no specific factor was highly predictive. As expected, more severe ocular misalignment was associated with poorer visual acuity; 37% of patients with strabismus had vision less than 20/200 in the affected eye compared with 0% of patients without strabismus.55
The onset of strabismus during the period of visual development in a child has the potential to compromise binocular vision and places a child at risk for strabismic amblyopia. Although the data are limited, strabismic amblyopia occurs less frequently than refractive amblyopia; vision loss from strabismus has been documented to account for 10-22% of vision loss associated with OPPNs.5, 7, 8 The management of strabismus in these children is complex and no studies exist to support early versus late surgical treatment of strabismus. Rather, the provider should focus on non-surgical treatment for strabismic amblyopia, including correcting any induced refractive error, conventional occlusion therapy with patching or atropine penalization, and consideration of prisms for smaller angle eye misalignment. If the severity of strabismus precludes the effectiveness of amblyopia treatment, then surgical correction may be considered at an earlier stage in the disease process. A conservative approach to management would advocate later surgery once the growth phase of the OPPN has attentuated and the overall disease process is more stable.18, 57
Imaging Evaluation
A MRI scan of the brain and orbits should be performed in all children with a suspected OPPN. High resolution MRI sequences with and without contrast should be acquired through the orbit, face and cavernous sinus. The radiation exposure from CT scans should be avoided whenever possible in all children with NF1.
No studies have been informative about the frequency of follow up MRIs; therefore clinical progression should be the primary indication. OPPN involving the orbit and or moving towards infiltrating the cavernous sinus should be imaged frequently (i.e., at least every 3-6 months) until clinical stability and lack of further growth can be confirmed. If the child experiences progressive OPPN growth or demonstrates continued vision loss not related to amblyopia, repeat imaging at higher frequency may be warranted.
Treatment Indications for OPPN
Newly diagnosed OPPN are best managed by close observation with serial ophthalmological and MRI evaluations since many OPPN will not progress or cause significant symptoms. Identifying which OPPN to treat and identifying the optimal time to initiate therapy is challenging. Considerations prior to initiating treatment include: age of the patient and extent of visual maturation, growth rate of the tumor, presence of a concurrent optic pathway glioma, presence of symptoms or a functional deficit (i.e., vision loss, strabismus, proptosis, ptosis, amblyopia or glaucoma), and extent of OPPN infiltration into other structures (i.e., cavernous sinus).
In the absence of significant tumor growth, initial intervention should be directed toward management of specific symptoms. For growing tumors, indications for debulking surgery or consideration for enrolling in a clinical trial include visual decline, progressive tumor that may soon invade a critical structure (e.g. cavernous sinus), or is likely to cause a new or worsening functional deficit, and potentially progressive disfigurement. Malignant transformation should be considered if the increase in OPPN exceeds the rate of increase typically seen in patients of similar age,49 along with referral to an oncologist experienced in caring for patients with PN secondary to NF1. Most malignant transformation of PN secondary to NF1 occur in adults,58 and are infrequently located in the head and neck region.59, 60 Radiation therapy to the orbital region is a known risk factor for malignant transformation.59
For adults, an aggressive/more definitive surgical approach might be considered before medical therapy, as OPPN at this age are less likely to have continued growth. It is always crucial to balance present function with the risk to future function imposed by surgical intervention.
Although improvement in visual outcomes and physical appearance are the most common indications to initiate treatment, neither has been well studied or included in clinical trials, making evidence based recommendations for treatment impossible. In all cases, management decisions should include the input from a multi-disciplinary team including neuro-oncology, ophthalmology / neuro-ophthalmology, oculofacial plastics, craniofacial surgery and genetics.
Surgical Treatment of OPPN
“All results (of surgery in neurofibromatosis) are compromised by the very nature of the tumor, its diffuse position, its widespread involvement of all the constituents of the region or organ, and its tendency to recur” - Dr. J. Conley.61
Timing of Surgical Intervention
Optimal timing of surgical intervention is uncertain, as the rate and extent of growth of tumors is unpredictable. Adnexal and orbital deformities often continue developing after initial excision and repair. These are not really recurrences but rather a progression of remaining peri-orbital and orbital tumors. Progression is more rapid in childhood and puberty but can still occur later in life. Rapidly growing OPPN can indicate the possibility of a malignant peripheral nerve sheath tumor, in which surgical and medical therapy may be indicated. Families are extremely motivated for surgery but must be cautioned regarding the likelihood of multiple procedures required for long term tumor management.
Surgical Management Concerns: Are multiple procedures really worth the effort?
In a 20 year study done at the NF1 Clinic at The Children’s Hospital of Philadelphia, 131 patients had 302 procedures for head and neck tumors. The overall freedom from progression was 56%, but head, neck and facial tumors had double the probability of recurrence as compared to tumors excised from the extremities.39 The conclusion drawn from this study was that patients less than 10 years of age with lesions of head, neck, and face were unlikely to have long term benefits from surgery since OPPN recurrence was so common. Development of an effective medical therapy was the recommended goal.
There are a number of concerns with these recommendations. While medical therapy is a major goal and ideally would be the preferred treatment, 20 years later, we are still searching for an effective medical treatment for OPPN. Furthermore, avoiding surgery does not take into account either the functional or the psychosocial appearance concerns of these younger patients. Nevertheless, the absence of data supporting a functional or psychosocial benefit from surgical repair—especially in cases of OPPN recurrence—obviates it from being universally recommended.
Functional Concerns
Protecting against amblyopia is the most common functional concern. This problem can be due to occlusion by a ptotic lid (< age 8 years) or anisometropia from pressure of orbital or lid tumors on the eye or from strabismus. Corneal exposure problems from proptosis and epiphora from lid malposition are other common functional problems.
Appearance Concerns
Periorbital soft tissue complications include: ptosis, lid contour and asymmetry of the eyelids, canthal abnormalities and skin changes. Facial descent from the weight of OPPNs can also produce cheek and oral commissure deformities. Orbital PNs can produce proptosis leading to a significant alterations in appearance.
Indications and Timing of Surgical Intervention
Both the timing and degree of surgical intervention are controversial subjects. Early surgery before age 10 years may be necessary due to functional concerns regarding amblyopia and the effects of expansion on both the periorbital soft tissues and the bony orbit. Early surgery is also important in attempting to maximize the child’s cosmetic outcome and minimize psychosocial concerns from the deformities of OPPN growth as it affects not only the child but also the entire family constellation.
Current Recommendations: Soft Tissues
Management of any associated congenital nasolacrimal problems can be treated early. Intubation of the nasolacrimal system may offer protection from inadvertent damage during more extensive orbital surgery. Debulking OPPN invading the cheek early is somewhat controversial due to concerns for possible facial nerve damage. Balloon expansion can provide tissue replacement for large areas with skin deformities. Exenteration for use of a facial prosthesis is an option, but should be avoided as it has other limitations affecting quality of daily life in hot weather or for other functions such as swimming, for example.62
Current Recommendations: Bone
Sphenoid bone defect repair may be needed for progressive proptosis with pulsating exophthalmos. Calvarial bone grafts with titanium mesh are useful in order to cover the sphenoid defect and minimize bone resorption. This should be considered on a case by case basis and is a more critical issue when associated with a seeing eye.63
Measuring Surgical Outcomes
At present, surgical management is still largely dependent on clinical judgment. Unfortunately, there are no data-driven recommendations that can be made with confidence. Most studies have a relatively limited follow up. In addition, limited progression after the 2nd decade may be a myth as there are patients with continued progression into late adulthood. The problems to address in measuring surgical outcomes relate to the diversity of facial abnormalities, a limited sample size for randomization and the further difficulty of studying changes in appearance over time following multiple interventions.
Despite these limitations, we offer four categories which may prove of use in assessing surgical outcomes (Table 1). Even though appearance considerations overlap between these categories, they can at least provide specific diagnostic groups for use in measuring surgical outcomes within the limitations and problems related to timing, randomization, multiple interventions and diversity of procedures
Table 1.
Categories to Assess Surgical Outcomes in Orbital/Periorbital Plexiform Neurofibromas.
| Vision Problems | Estimated Incidence | |
|---|---|---|
| Occlusive amblyopia | 32 - 43% | |
| Anisometropia amblyopia | 38 - 43% | |
| Strabismic amblyopia | 10 - 20% | |
| Motility disorders | 50% | |
| Corneal abnormalities | n/a | |
| Optic nerve compression | n/a | |
| Papilledema | n/a | |
| Optic nerve atrophy | n/a | |
| Appearance and Psychosocial | n/a | |
| Ptosis, lid contour and symmetry | 94% | |
| Motility disorders | 55% | |
| Corneal scarring | n/a | |
| Proptosis | 57% | |
| Canthal abnormalities | n/a | |
| Skin changes | n/a | |
| Facial descent | n/a | |
| Cheek deformities | n/a | |
| Oral commissure deformities | n/a | |
| Structural Problems | ||
| Bony orbital expansion | n/a | |
| Soft tissue expansion of eyelids | n/a | |
| Laxity of eyelids/canthi | n/a | |
| Facial descent from the weight of tumor | n/a | |
| Soft tissue expansion of cheek | n/a | |
| Other Concerns | n/a | |
| Pain or discomfort | n/a | |
| Suspicion of malignancy | n/a | |
| Overall quality of life | n/a |
n/a = published data not available
Medical Treatment of OPPN
The medical treatment of PNs has been frustrating with little evidence of efficacy. Standard chemotherapy has not been shown to be of benefit and is associated with the risk of treatment induced secondary malignant neoplasms. Because of the mutagenic nature of most chemotherapeutic agents, especially alkylator and topoisomerase inhibitors, chemotherapy is not used. Thalidomide demonstrated some activity in one small clinical trial.64
In efforts to reduce PN growth or shrink existing PNs, several clinical trials have used targeted agents, including tipifarnib,45 pirfenidone,41, 44, 65 sirolimus,43, 47 and peginterferon alfa-2b.42, 46, 66 The tipifarnib trial was double-blinded and included a placebo control group (29 patients), and the median time to progression (TTP) of progressive PNs treated with placebo was 10.6 months. Tipifarnib did not result in a doubling of the TTP compared to the placebo arm. 45 Three subsequent open label phase II trials used the tipifarnib trial placebo group as comparison; peginterferon alfa-2b was the only agent that resulted in more than a doubling of the median TTP.46 In these trials PN volume decrease ≥ 20% was only observed with peginterferon alfa-2b in 4 of 83 patients.
In a phase II trial of the c-kit and PDGFR inhibitor imatinib, PN volume decrease ranging from 20 to 40% was observed in 6 of 23 response evaluable patients, however the tumors that responded were all small (i.e., < 20 ml).67 Most recently, preliminary results of a phase I trial with the MEK inhibitor selumetinib (AZD6244 hydrogen sulfate) in children with inoperable substantial PNs reported partial responses (tumor volume decreases ≥ 20%) in 6/11 patients.68 Additional phase II trials with the receptor tyrosine kinase inhibitor cabozantinib (XL184) and the MEK inhibitor PD-0325901 are ongoing.
The completed and ongoing phase I/II studies demonstrate that PN specific clinical trials using biologic agents can be conducted safely in children and young adults. In addition, prolongations in the TTP of progressive PNs and PN shrinkage have been observed in several trials. Thus, there is reason to hope that early intervention with targeted agents in children with established, but small, and minimally disfiguring OPPN may prevent progression and the resultant facial disfigurement.
Current and Future Challenges of OPPN Clinical Trials
Challenges in the design of OPPN therapeutic trials for children include defining when to initiate medical treatments for OPPN, the availability of adequate pediatric drug formulations, and establishing the safety of the prolonged administration of targeted agents in young children. Standardized clinical trial design and selection of trial endpoints which represent clinical benefit as well as quality of life assessments will be required to meaningfully assess the efficacy of novel agents on OPPNs. The REiNS collaboration, an international initiative comprised of clinical (i.e., oncology, genetics, pediatrics, ophthalmology, neurology, neuro-surgery, radiology, psychology) and lab-based scientist with expertise in NF1, was established with the goal of achieving consensus within the NF1 community about the design and endpoints of future clinical trials for manifestations of NF.48, 69-73
Although recent molecular-targeted trials have overcome some of the limitations that have slowed the development of more effective medical therapies for OPPNs, significant challenges still remain. The wider availability of MRI volumetric analysis has made interpretation of clinical trials more reproducible and objective. However, since OPPNs reside in a critical anatomic location, functional deficits or therapeutic improvements can develop even in the absence of measurable size changes. Objective functional and patient reported outcome measures will be essential to meaningfully assess benefit of novel therapeutics for OPPNs.
Another major issue in proving efficacy is that OPPNs have an unpredictable natural history. PNs, including OPPNs, tend to maintain a relatively stable trajectory of growth in young children, but PN growth slows down at some point before reaching adulthood, and PN growth rates vary greatly between individuals.45 Whether single-arm studies are adequate to determine the efficacy of treatment for OPPNs is unclear, as factors such as age and when in the course of clinical growth patients are treated, likely confound results.49 Slowing of growth trajectory (increasing time to progression) as primary endpoint may thus require a randomized trial for meaningful evaluation.
In clinical trials for children with cancer, substantial and sustained tumor shrinkage has been at times accepted by the FDA as a surrogate measure for disease control and treatment efficacy. The definitions of response for children with cancer can be difficult to apply to OPPNs, especially for symptomatic tumors with minimal to no objective growth prior to initiation of therapy. The experience with most molecular-targeted agents utilized to date for patients with PNs is that they infrequently result in a large amount of tumor shrinkage; they are more likely to result in disease stabilization. This may not be the case with some of the new agents like MEK inhibitors. Thus, imaging response will likely only be part of a constellation of outcome measures that could be used for FDA approval for the use of molecularly-targeted agents in children with symptomatic and/or progressive OPPNs.
FDA approval, which is needed to get these novel agents to more patients and not only those entered on a clinical trial, will be dependent on demonstrating improvement in functional measures. What constitutes a validated functional outcome measure in children with OPPNs remains unclear.72 Measures of vision, cosmesis and overall quality of life will need to be looked at extremely carefully in these trials.70, 72, 73
Still another issue which must be considered is the relatively young age of patients who develop symptomatic OPPNs and the need to have these agents approved in the age range that would most benefit these patients. Because of the unknown long-term effects of almost all molecularly-targeted agents, including the MEK inhibitors, on normal body function and development (including brain development), it is critical to incorporate long-term measures of toxicity into ongoing studies. This will likely include sequential neurocognitive function and developmental assessments, especially in the very young child.73 To perform these trials adequately and efficiently, studies will most likely need to be multi-centered and performed by experienced working groups or by already formed neurofibromatosis translational consortia, such as the Department of Defense NF Clinical Trials Consortium.
Conclusion
The proposed nomenclature, clinical examination frequency, as well as indications for medical and surgical treatment of OPPN in children is a small, but necessary first step (See Table 2). The next step of defining therapeutic “efficacy” will be much more challenging as this will have to satisfy both industry and regulatory criteria needed for FDA approval, all the while considering what is most beneficial to the patient. Furthermore, multicenter clinical trials will need to include not only young patients who are at the greatest risk for irreversible vision loss, but also those vulnerable to lifelong reduced quality of life and self-esteem from their physical appearance.
Table 2.
Summary Table of OPPN Working Group Consensus Statement for Ophthalmic Monitoring and Management
|
Acknowledgements
OPPN working group: Children’s National Medical Center, Washington, DC: Kelly A. Hutcheson, MD and William P. Madigan, MD; Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL: Robert Listernick MD; University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, PA: Grant T. Liu, MD; Children’s Healthcare of Atlanta, Atlanta, GA: Jerry E. Berland, MD; National Eye Institute, Bethesda, MD: Edmond J. FitzGibbon, MD; University of Alabama-Birmingham, Birmingham, AL: Bruce R. Korf, MD, PhD
Funding/Support: This article was supported by “CureNFwithJack” and The Children’s Tumor Foundation. None of the study sponsors had a role in the design or content of this article. Authors have received additional support from the following sources: The National Eye Institute/National Institutes of Health grants K23-EY022673 (RAA), the National Cancer Institute, Center for Cancer Research intramural research program (BCW), and the Gilbert Family Neurofibromatosis Institute (RAA, RJP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicting relationship exists for any author.
Children and adults with Neurofibromatosis type 1 (NF1) manifest plexiform neurofibromas involving the eyelid, orbit, periorbital and facial structures (termed OPPN). This consensus provides recommendations for ophthalmologic monitoring and outlines treatment indications, and forthcoming biologic therapy.
References
- 1.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin. 2002;20:841–865. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 3.Viola F, Villani E, Natacci F, et al. Choroidal abnormalities detected by near-infrared reflectance imaging as a new diagnostic criterion for neurofibromatosis 1. Ophthalmology. 2012;119:369–375. doi: 10.1016/j.ophtha.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Ritch R, Forbes M, Hetherington J, Jr., Harrison R, Podos SM. Congenital ectropion uveae with glaucoma. Ophthalmology. 1984;91:326–331. doi: 10.1016/s0161-6420(84)34288-9. [DOI] [PubMed] [Google Scholar]
- 5.Greenwell TH, Anderson PJ, Madge SK, Selva D, David DJ. Long-term visual outcomes in patients with orbitotemporal neurofibromatosis. Clin Experiment Ophthalmol. 2014;42:266–270. doi: 10.1111/ceo.12179. [DOI] [PubMed] [Google Scholar]
- 6.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31:269–278. doi: 10.1097/WNO.0b013e31822aef82. [DOI] [PubMed] [Google Scholar]
- 7.Avery RA, Dombi E, Hutcheson KA, et al. Visual outcomes in children with neurofibromatosis type 1 and orbitotemporal plexiform neurofibromas. Am J Ophthalmol. 2013;155:1089–1094. doi: 10.1016/j.ajo.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oystreck DT, Morales J, Chaudhry I, et al. Visual Loss in Orbitofacial Neurofibromatosis Type 1. Ophthalmology. 2012;119:2168–2173. doi: 10.1016/j.ophtha.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson VM, Kyle PM. Orbital plexiform neurofibroma. Br J Ophthalmol. 1993;77:527–528. doi: 10.1136/bjo.77.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morax S, Herdan ML, Hurbli T. The surgical management of orbitopalpebral neurofibromatosis. Ophthal Plast Reconstr Surg. 1988;4:203–213. doi: 10.1097/00002341-198804040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Marchac D, Britto JA. Remodelling the upper eyelid in the management of orbitopalpebral neurofibromatosis. Br J Plast Surg. 2005;58:944–956. doi: 10.1016/j.bjps.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Erb MH, Uzcategui N, See RF, Burnstine MA. Orbitotemporal neurofibromatosis: classification and treatment. Orbit. 2007;26:223–228. doi: 10.1080/01676830600987227. [DOI] [PubMed] [Google Scholar]
- 13.Ziakas NG, Kanonidou ED, Boboridis KG. Childhood orbitotemporal neurofibromatosis masked by congenital glaucoma and buphthalmos. J Pediatr Ophthalmol Strabismus. 2011;48:e49–51. doi: 10.3928/01913913-20110712-06. [DOI] [PubMed] [Google Scholar]
- 14.Jackson IT, Carbonnel A, Potparic Z, Shaw K. Orbitotemporal neurofibromatosis: classification and treatment. Plast Reconstr Surg. 1993;92:1–11. doi: 10.1097/00006534-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Krohel GB, Rosenberg PN, Wright JE, Smith RS. Localized orbital neurofibromas. Am J Ophthalmol. 1985;100:458–464. doi: 10.1016/0002-9394(85)90514-8. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry IA, Morales J, Shamsi FA, et al. Orbitofacial neurofibromatosis: clinical characteristics and treatment outcome. Eye (Lond) 2012;26:583–592. doi: 10.1038/eye.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee V, Ragge NK, Collin JR. Orbitotemporal neurofibromatosis. Clinical features and surgical management. Ophthalmology. 2004;111:382–388. doi: 10.1016/j.ophtha.2003.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Lee V, Ragge NK, Collin JR. The surgical management of childhood orbito-temporal neurofibromatosis. Br J Plast Surg. 2003;56:380–387. doi: 10.1016/s0007-1226(03)00172-3. [DOI] [PubMed] [Google Scholar]
- 19.Van der Meulen JC, Moscona AR, Vandrachen M, Hirshowitz B. The management of orbitofacial neurofibromatosis. Ann Plast Surg. 1982;8:213–220. doi: 10.1097/00000637-198203000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Pessis R, Lantieri L, Britto JA, Leguerinel C, Wolkenstein P, Hivelin M. Surgical care burden in orbito-temporal neurofibromatosis: Multiple procedures and surgical care duration analysis in 47 consecutive adult patients. J Craniomaxillofac Surg. 2015;43:1684–1693. doi: 10.1016/j.jcms.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843. doi: 10.1016/S1474-4422(14)70063-8. [DOI] [PubMed] [Google Scholar]
- 22.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 23.Korf B, Widemann B, Acosta MT, Packer R. Translational/ Clinical studies in children and adults with neurofibromatosis type 1. In: Upadhyaya M, editor. Neurofibromatosis type 1: Molecular and cellular biology. Springer; Berlin Heidelberg: 2012. [Google Scholar]
- 24.Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999;24:283–285. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Serra E, Rosenbaum T, Winner U, et al. Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum Mol Genet. 2000;9:3055–3064. doi: 10.1093/hmg/9.20.3055. [DOI] [PubMed] [Google Scholar]
- 26.Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123:340–347. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet. 1999;89:14–22. [PubMed] [Google Scholar]
- 28.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 29.Johansson G, Mahller YY, Collins MH, et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–1245. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalaf WF, Yang FC, Chen S, et al. K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/-mast cells. J Immunol. 2007;178:2527–2534. doi: 10.4049/jimmunol.178.4.2527. [DOI] [PubMed] [Google Scholar]
- 32.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/-- and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang FC, Staser K, Clapp DW. The plexiform neurofibroma microenvironment. Cancer Microenviron. 2012;5:307–310. doi: 10.1007/s12307-012-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeClue JE, Heffelfinger S, Benvenuto G, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawachi Y, Xu X, Ichikawa E, Imakado S, Otsuka F. Expression of angiogenic factors in neurofibromas. Exp Dermatol. 2003;12:412–417. doi: 10.1034/j.1600-0625.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim HA, et al. Nf1-deficient mouse Schwann cells are angiogenic and invasive and can be induced to hyperproliferate: reversion of some phenotypes by an inhibitor of farnesyl protein transferase. Mol Cell Biol. 1997;17:862–872. doi: 10.1128/mcb.17.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling BC, Wu J, Miller SJ, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim A, Gillespie A, Dombi E, et al. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73:1273–1279. doi: 10.1212/WNL.0b013e3181bd1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Needle MN, Cnaan A, Dattilo J, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children's Hospital of Philadelphia experience, 1974-1994. J Pediatr. 1997;131:678–682. doi: 10.1016/s0022-3476(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 40.Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28:257–265. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Babovic-Vuksanovic D, Widemann BC, Dombi E, et al. Phase I trial of pirfenidone in children with neurofibromatosis 1 and plexiform neurofibromas. Pediatr Neurol. 2007;36:293–300. doi: 10.1016/j.pediatrneurol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Jakacki RI, Dombi E, Potter DM, et al. Phase I trial of pegylated interferon-alpha-2b in young patients with plexiform neurofibromas. Neurology. 2011;76:265–272. doi: 10.1212/WNL.0b013e318207b031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr Blood Cancer. 2014;61:982–986. doi: 10.1002/pbc.24873. [DOI] [PubMed] [Google Scholar]
- 44.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61:1598–1602. doi: 10.1002/pbc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widemann BC, Dombi E, Gillespie A, et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16:707–718. doi: 10.1093/neuonc/nou004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2016 doi: 10.1093/neuonc/now158. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015;17:596–603. doi: 10.1093/neuonc/nou235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81:S33–40. doi: 10.1212/01.wnl.0000435744.57038.af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68:643–647. doi: 10.1212/01.wnl.0000250332.89420.e6. [DOI] [PubMed] [Google Scholar]
- 50.Widemann BC, Acosta MT, Ammoun S, et al. CTF meeting 2012: Translation of the basic understanding of the biology and genetics of NF1, NF2, and schwannomatosis toward the development of effective therapies. Am J Med Genet A. 2014;164A:563–578. doi: 10.1002/ajmg.a.36312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dagalakis U, Lodish M, Dombi E, et al. Puberty and plexiform neurofibroma tumor growth in patients with neurofibromatosis type I. J Pediatr. 2013;164:620–624. doi: 10.1016/j.jpeds.2013.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. doi: 10.1186/1750-1172-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen R, Ibrahim C, Friedrich RE, Westphal M, Schuhmann M, Mautner VF. Growth behavior of plexiform neurofibromas after surgery. Genet Med. 2013;15:691–697. doi: 10.1038/gim.2013.30. [DOI] [PubMed] [Google Scholar]
- 54.Altan-Yaycioglu R, Hintschich C. Clinical features and surgical management of orbitotemporal neurofibromatosis: a retrospective interventional case series. Orbit. 2010;29:232–238. doi: 10.3109/01676831003660689. [DOI] [PubMed] [Google Scholar]
- 55.Oystreck DT, Alorainy IA, Morales J, Chaudhry IA, Elkhamary SM, Bosley TM. Ocular motility abnormalities in orbitofacial neurofibromatosis type 1. J AAPOS. 2014;18:338–343. doi: 10.1016/j.jaapos.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Friedman DS, Repka MX, Katz J, et al. Prevalence of Amblyopia and Strabismus in White and African American Children Aged 6 through 71 MonthsThe Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:2128–2134. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farris SR, Grove AS., Jr Orbital and eyelid manifestations of neurofibromatosis: a clinical study and literature review. Ophthal Plast Reconstr Surg. 1996;12:245–259. doi: 10.1097/00002341-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Widemann BC. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009;11:322–328. doi: 10.1007/s11912-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 60.Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma--clinical characteristics, survival, and response to therapy. Cancer. 1981;47:2503–2509. doi: 10.1002/1097-0142(19810515)47:10<2503::aid-cncr2820471033>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Conley J. Neurogenic tumours of the head and neck. J Otolaryngol Soc Aust. 1972;3:362–368. [PubMed] [Google Scholar]
- 62.Foster JA, Katowitz JA. Benign Eyelid Tumors. Springer-Verlag; New York: 2002. [Google Scholar]
- 63.Snyder BJ, Hanieh A, Trott JA, David DJ. Transcranial correction of orbital neurofibromatosis. Plast Reconstr Surg. 1998;102:633–642. doi: 10.1097/00006534-199809030-00005. [DOI] [PubMed] [Google Scholar]
- 64.Gupta A, Cohen BH, Ruggieri P, Packer RJ, Phillips PC. Phase I study of thalidomide for the treatment of plexiform neurofibroma in neurofibromatosis 1. Neurology. 2003;60:130–132. doi: 10.1212/01.wnl.0000042321.94839.78. [DOI] [PubMed] [Google Scholar]
- 65.Babovic-Vuksanovic D, Ballman K, Michels V, et al. Phase II trial of pirfenidone in adults with neurofibromatosis type 1. Neurology. 2006;67:1860–1862. doi: 10.1212/01.wnl.0000243231.12248.67. [DOI] [PubMed] [Google Scholar]
- 66.Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22:443–462. doi: 10.1517/13543784.2013.772979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13:1218–1224. doi: 10.1016/S1470-2045(12)70414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Widemann B, Leigh JM, Fisher MJ, et al. Phase I study of the MEK1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs) J Clin Oncol. 2014;32(suppl):10018. [Google Scholar]
- 69.Widemann BC, Blakeley JO, Dombi E, et al. Conclusions and future directions for the REiNS International Collaboration. Neurology. 2013;81:S41–44. doi: 10.1212/01.wnl.0000435748.79908.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81:S15–24. doi: 10.1212/01.wnl.0000435745.95155.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plotkin SR, Ardern-Holmes SL, Barker FG, 2nd, et al. Hearing and facial function outcomes for neurofibromatosis 2 clinical trials. Neurology. 2013;81:S25–32. doi: 10.1212/01.wnl.0000435746.02780.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plotkin SR, Blakeley JO, Dombi E, et al. Achieving consensus for clinical trials: the REiNS International Collaboration. Neurology. 2013;81:S1–5. doi: 10.1212/01.wnl.0000435743.49414.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolters PL, Martin S, Merker VL, et al. Patient-reported outcomes in neurofibromatosis and schwannomatosis clinical trials. Neurology. 2013;81:S6–14. doi: 10.1212/01.wnl.0000435747.02780.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]