Abstract
Canonical Wnt signaling induces the stabilization of β-catenin, its translocation to the nucleus and the activation of target promoters. This pathway is initiated by the binding of Wnt ligands to the Frizzled receptor, the association of the LRP5/6 coreceptor and the formation of a complex comprising Dvl-2, Axin and protein kinases CK1α, ε, γ and GSK3. Among these, activation of CK1ε, constitutively bound to LRP5/6 through p120-catenin, is required for the association of the rest of the components. We describe here that CK1ε is activated by the PP2A/PR61ε phosphatase. Binding of Wnt ligands promotes the interaction of LRP5/6-associated CK1ε with Frizzled-bound PR61ε regulatory subunit, facilitating the access of PP2A catalytic subunit to CK1ε and its activation, what enables the recruitment of Dvl-2 to the receptor complex and the initiation of the Wnt pathway. Our results uncover the mechanism of activation of the canonical Wnt pathway by its ligands.
INTRODUCTION
The physiological relevance of Wnt/β-catenin pathway has been extensively demonstrated; this signaling cascade is essential for embryonic development and stem cells maintenance and is altered in human diseases such as cancer.1 Upon their binding to membrane receptors, canonical Wnt ligands trigger a cellular pathway that leads to an increase in nuclear and transcriptionally active β-catenin. This is a consequence not only of the stabilization of β-catenin protein but of its enhanced transport to the nucleus.2
The cellular receptor complex for the canonical Wnt ligands is composed by the Wnt high-affinity transmembrane protein Frizzled (Fz) and the co-receptors LRP5 or 6, two highly homologous proteins.2-4 LRP5/6 is constitutively bound to cadherins (E-cadherin in epithelial cells, N-cadherin in neurons or fibroblasts), and through these proteins to p120-catenin and CK1ε.5,6 Binding of Wnt ligands promotes the association of Fz with LRP5/6, cadherin and the p120-catenin/CK1ε complex. Subsequently, this kinase is activated7 which facilitates the association of Dvl-2 to LRP5/6.3,6 Accordingly, depletion of CK1ε or p120-catenin prevents the association of Dvl-2 to the receptor complex and interrupts the Wnt pathway. p120-catenin or CK1ε deficiency can be overcome by the ectopic expression of a truncated form of LRP5/68 that works as a constitutive active version (CA-LRP5/6).9
The interaction of the receptor with Dvl-2 facilitates the clustering of the LRP5/6-Fz complexes, the recruitment of CK1γ and the LRP5/6 phosphorylation by CK1γ on Thr1479 and Thr1493.2,10 LRP5/6 phosphorylation by CK1γ depends on other factors, such as phosphatidyl inositol 4,5 bisphosphate, transmembrane protein 198, Amer1, Prorenin receptor and vacuolar ATPase,11-14 required either for the activation of the kinase or for the correct assembly of the multi-protein complex called Wnt signalosome. Besides CK1γ several other kinases can phosphorylate LRP5/6 acting either as activators or inhibitors of Wnt signaling.4,10 LRP5/6 Thr1479 phosphorylation promotes the binding of Axin and the associated protein kinases CK1α and GSK3 to the complex,15,16 what correlates with the inhibition of GSK3 activity, therefore preventing phosphorylation by this kinase of β-catenin Ser37, poly-ubiquitination by β-TrCP1 ubiquitin ligase and β-catenin proteosomal degradation. Several mechanisms have been proposed to explain the regulation of β-catenin half-life after LRP5/6 phosphorylation and Axin recruitment: (1) the activity of GSK3 is blocked due to the direct interaction of GSK3 with phosphoSer1490 in LRP5/6;17,18 (2) GSK3 is sequestered into multivesicular bodies, preventing the access of GSK3 to β-catenin.19 This internalization of the signalosome into acidic vesicles requires the previous separation of p120-catenin from cadherin, a release that is dependent on the activity of the axin-associated CK1α.8 (3) Other authors suggest that β-catenin phosphorylation is not affected but Wnt inhibits the poly-ubiquitination of phosphorylated proteins.20 It is likely that these mechanisms can coexist, either in different cells or at different times after Wnt activation and being affected by the stimulation of other cellular signaling pathways, such as that involving YAP and TAZ.21
In this article we have analyzed one of the first responses to Wnt ligands: the activation of CK1ε. Differently to CK1α, this isoform does not show constitutive activity due to the presence of a 13 kDa carboxy-terminal sequence: intramolecular autophosphorylation of this element strongly inhibits CK1ε activity.22 This autoinhibition is reversed by the action of serine/threonine phosphatases that dephosphorylate these residues.23 Among them, type 2 protein phosphatase (PP2A) is the most promising candidate to act on CK1ε in the Wnt signaling as this phosphatase enhances several responses of this pathway, such as secondary axis formation in Xenopus embryos or activation of Engrailed and Siamois genes24-28 although some other authors have also reported negative actions.29,30 We reported here the involvement of a PP2A complex in CK1ε activation and describe the mechanism underlying it.
RESULTS
PP2A is required for CK1ε activation and Dvl-2 recruitment to the signalosome
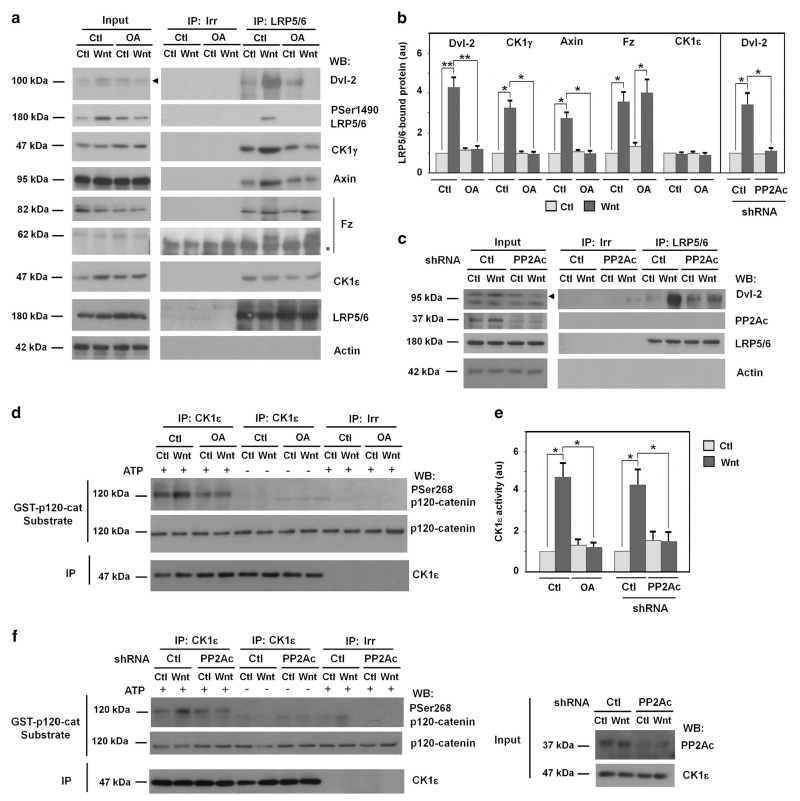
At nanomolar concentrations okadaic acid (OA) specifically inhibits PP2A.31 We analyzed the impact of this compound on very early Wnt3a responses in the widely used cell system of HEK293 cells. As shown in Supplementary Figure S1A, Wnt3a induced a rapid Dvl-2 phosphorylation, visualized as the shift in the molecular weight of this protein, and increased its interaction with LRP5/6, an interaction mediated by its binding to Fz (see Introduction section). As expected, Wnt3a also caused a later β-catenin upregulation (Supplementary Figure S1B). Both responses were sensitive to the addition of DKK1, a well-characterized inhibitor of this pathway.32 OA also prevented the Wnt3a-induced Dvl-2 phosphorylation and the association of Dvl-2 to LRP5/6 analyzed by co-immunoprecipitation experiments (Figures 1a and b). Other responses, such as CK1γ binding to LRP5/6 and the phosphorylation of Ser 1490 in this protein were also blocked by OA, as well as the recruitment of Axin. On the contrary, formation of Fz-LRP5/6 complex by Wnt3a was not altered (Figures 1a and b). The constitutive interaction of LRP5/6 with CK1ε was not affected either.
Figure 1.
PP2A is required for CK1ε activation and Dvl-2 recruitment to the signalosome. (a and d) HEK293 cells were treated with 10 nM of okadaic acid (OA) and control or Wnt3a-conditioned medium for 30 min (a) or 15 min (d). Five hundred microgram of protein from total cell extracts were immunoprecipitated (IP) with anti-LRP5/6 (a) or anti-CK1ε (d). Associated proteins were analyzed by WB in a and CK1ε activity assay was performed in d as described6 using p120-catenin as substrate. Phosphorylation of Ser268 was analyzed by WB with a specific PSer268 p120-catenin antibody. In a, the arrowhead indicates the migration of phosphorylated Dvl-2 and the asterisk marks the IgG that hinders the visualization of the 64 kDa Fz isoform in immunoprecipitates. Fz was detected in these cells as two bands of 85 and 64 kDa; the higher band corresponds to the glycosylated form of the protein since it was converted in the 64 kDa band upon Endoglycosydase H digestion. (c and f) PP2A catalytic subunit (PP2Ac)-depleted HEK293 cells were incubated with control or Wnt3a-conditioned medium for 30 min (c) or 15 min (f) and LRP5/6 in C or CK1ε in f were immunoprecipitated from total cell extracts. Associated proteins were analyzed by WB in c and a CK1ε activity assay was performed in f. Autoradiograms from three or four different experiments performed in a and c were quantified and represented in b; from CK1ε activity assays (d and f), in e. The mean ± s.d. was obtained for each condition and Unpaired t-test statistics were performed. **P<0.01,*P<0.05.
CK1ε activity was also determined as previously described.6 This protein kinase was immunoprecipitated from control or Wnt-treated cell extracts and its activity was assayed using p120-catenin as substrate; Ser268 in this protein is phosphorylated in vitro by this protein kinase and can be easily analyzed using a phospho-specific antibody.6 Addition of Wnt3a to the cells increased CK1ε activity; this upregulation was markedly decreased by incubation with OA (Figures 1d and e). As controls, Ser268 phosphorylation was not detected when ATP was omitted from the protein kinase reaction or when the immunoprecipitation was performed with an irrelevant antibody.
To verify the involvement of PP2A in CK1ε activation by Wnt3a, the same assays were repeated in cells depleted of the catalytic subunit of this phosphatase (PP2Ac) using a specific short hairpin RNA (shRNA). Downregulation of PP2Ac blocked the increase in Dvl-2 phosphorylation caused by Wnt and prevented the upregulation in Dvl-2/LRP5/6 binding (Figures 1b and c). Depletion of PP2Ac also prevented the upregulation in CK1ε activity caused by Wnt3a (Figures 1e and f). Therefore, interference in PP2A activity causes a defect in the initial responses to Wnt, similar to that shown when specifically blocking CK1ε (see below).
PR61ε is necessary for the Wnt-induced activation of CK1ε
PP2A is a heterotrimeric complex composed by a catalytic subunit, a scaffold subunit and a variable regulatory subunit that controls the specificity and activity of PP2A.33 Among the different regulatory subunits, the members of the PR61 family have been related to the Wnt pathway.33 Therefore, we selectively eliminated the expression of the PR61 isoforms α, β or ε using specific shRNAs in SW-480 cells and determined their relevance in the Wnt-induced Dvl-2 association to LRP5/6. As shown in Supplementary Figure S2A only depletion of PR61ε (also known as B56ε or B’ε) prevented Dvl-2 phosphorylation and the interaction of LRP5/6 with this protein, although all the shRNAs similarly affected its targeted gene (Supplementary Figure S2B). PR61ε shRNA downregulated the levels of this protein; however, it did not affect those of PP2Ac (Supplementary Figure S2C).
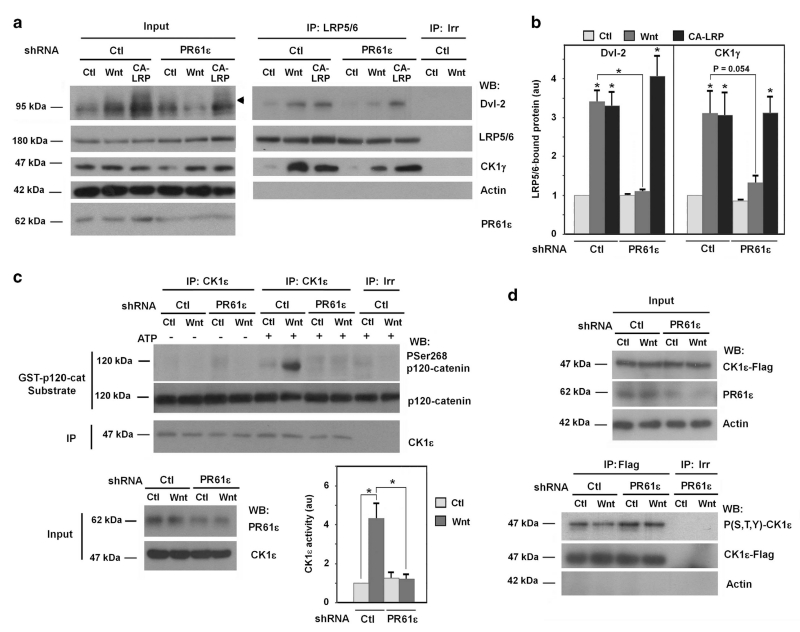
The same results were obtained in other cell lines, such as HT-29 M6 (Supplementary Figure S3) and HEK293, used in our assays. PR61ε down-modulation inhibited the Wnt-induced Dvl-2 phosphorylation and the recruitment of this protein and CK1γ to LRP5/6 (Figures 2a and b). The effect of this shRNA was also corroborated using a different shRNA decreasing PR61ε; this interference RNA also precluded the Wnt3a-induced Dvl-2 phosphorylation and interaction with LRP (Supplementary Figure S4B), as well as other later responses, such as β-catenin upregulation (Supplementary Figure S4A, see below). As described for PP2Ac, the upregulation in CK1ε activity by Wnt was also prevented by PR61ε depletion (Figure 2c).
Figure 2.
PR61ε is necessary for the Wnt-induced activation of CK1ε (a) PR61ε-depleted HEK293 cells (shRNA PR61ε #2558) were transfected with GFP or CA-LRP. Twenty-four hours post-transfection cells were treated with control or Wnt3a-conditioned medium for 30 min when indicated, and LRP5/6 was immunoprecipitated from total cell extracts. Associated proteins were analyzed by WB. (b) Autoradiograms from three different experiments performed in a were quantified and represented. When not indicated P-values were calculated with respect to the control. *P <0.05, other P-values between 0.1 and 0.05 are indicated. (c and d) PR61ε-depleted HEK293 cells were treated with control or Wnt3a-conditioned medium for 15 min. In C, CK1ε was immunoprecipitated from total cell extracts and the immunocomplex was incubated with 2 pmol of recombinant GST-p120-catenin in CK1 phosphorylation conditions. Phosphorylation of Ser268 was analyzed by WB with a specific PSer268 p120-catenin antibody. The quantification of four different experiments is represented. In d, CK1ε-Flag was expressed in PR61ε-depleted or control cells treated with Wnt3a-conditioned medium for 15 min when indicated. CK1ε-Flag was immunoprecipitated and phosphorylation was determined by WB with a specific phospho Ser,Thr,Tyr antibody.
We also verified that CK1ε activation was associated to its dephosphorylation. We transfected full-length and carboxy-terminus depleted (ΔC-CK1ε) forms of this enzyme; ectopic CK1ε was immunoprecipitated and analyzed with a polyclonal antibody detecting phosphoserine, phosphothreonine and phosphotyrosine. As shown in Supplementary Figure S5, this antibody shows the correct specificity and reacts with phosphorylated proteins. Using this tool we showed that in control cells the full-length protein was phosphorylated to a much higher extent than the ΔC-CK1ε form that lacks the polyphosphorylated tail23 (Supplementary Figure S6). CK1ε phosphorylation was decreased upon Wnt stimulation (Supplementary Figure S6 and Figure 2d). Downregulation of PR61ε with a specific shRNA prevented the Wnt-induced loss of phosphate on CK1ε (Figure 2d). This result indicates that PR61ε is required for CK1ε dephosphorylation, a modification associated to its activation.
In contrast to CK1γ, CK1ε is required for Wnt-induced Dvl-2 phosphorylation and interaction with LRP5/6 (Supplementary Figures S7A and B). This CK1ε requirement in Wnt/β-catenin pathway can be bypassed if this pathway is stimulated by expression of the constitutively active mutant CA-LRP5/6.8 Even in the absence of CK1ε, CA-LRP5/6 induced Dvl-2 phosphorylation and increased its interaction with LRP5/6 (Supplementary Figure S7C). Likewise, depletion of PR61ε did not affect Dvl-2 phosphorylation and Dvl-2/LRP5/6 interaction when the pathway was stimulated by CA-LRP5/6, contrary to what detected when stimulation was performed with Wnt3a (Figure 2a). Thus, PR61ε depletion mimics that of CK1ε in the Wnt3a pathway.
PR61ε binds to the Wnt-receptor Fz
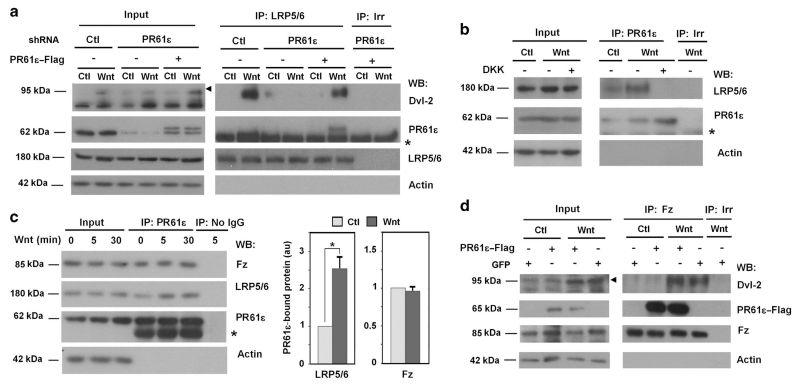
In order to fully demonstrate the requirement of PR61ε for Dvl-2 phosphorylation and binding to LRP5/6 we rescued the depletion of PR61ε transfecting an ectopic version of the protein not sensitive to the shRNA. As seen in Figure 3a, ectopic PR61ε-Flag was capable to reinstate Dvl-2 phosphorylation and interaction with LRP5/6 in endogenous PR61ε-downregulated cells. Moreover, we also detected the presence of PR61ε, either endogenous or ectopically expressed in LRP5/6 immunoprecipitates only upon Wnt stimulation. The interaction between endogenous LRP5/6 and PR61ε was verified by the inverse immunoprecipitation; thus, LRP5/6 was detected in PR61ε immunocomplexes (Figures 3b and c). This interaction was upregulated by Wnt stimulation and was prevented by supplementation to the cells of the Wnt inhibitor DKK (Figure 3b). Fz was also associated to PR61ε; however, in contrast to LRP5/6, Fz binding to PR61ε was constitutive and not stimulated by Wnt (Figure 3c). This suggests that PR61ε binding to LRP5/6 is dependent on the previous association of Fz to the co-receptor. Overexpression of PR61ε-Flag did not affect Dvl-2 phosphorylation or binding to Fz (Figure 3d), suggesting PR61ε and Dvl-2 do not compete for the interaction with the Wnt receptor. As expected, as it is a member of the PP2A complex PR61ε also interacted with PP2Ac (Supplementary Figure S8).
Figure 3.
PR61ε is required for Wnt signaling responses and binds to Wnt receptors. (a) PR61ε-depleted HEK293 cells (shRNA PR61ε #2558) were transfected with PR61ε-Flag when indicated and stimulated with control or Wnt3a-conditioned medium for 15 min. Total cell extracts were immunoprecipitated with anti-LRP5/6 antibody and associated proteins were analyzed by WB. The arrowhead indicates the migration of phosphorylated Dvl-2 and the asterisk indicates the IgG that hinders the visualization of PR61ε in the immunoprecipitates. (b) HEK293 cells were stimulated with control, Wnt3a or Wnt3a and DKK1-conditioned medium for 30 min. PR61ε was immunoprecipitated from total cell extracts and protein complexes were analyzed by WB. (c) HEK293 cells were treated with control or Wnt3a-conditioned medium for the indicated times. Total cell extracts were immunoprecipitated with anti-PR61ε antibody and associated proteins were analyzed by WB. The quantification of LRP5/6 and Fz proteins bound to PR61ε is also represented. *P<0.05. (d) PR61ε-Flag-overexpressing cells were stimulated with control or Wnt3a-conditioned medium for 15 min. Total cell extracts were immunoprecipitated with anti-Fz antibody and associated proteins were analyzed by WB.
Fz–PR61ε interaction was analyzed by pull-down assays performed with GST-fusion proteins. When GST-PR61ε was used as bait Fz was bound both using extracts from control or Wnt-treated cells, whereas LRP5/6 association of LRP5/6 with GST-PR61ε was highly increased in extracts from Wnt-treated cells (Figure 4a). The interaction of PR61ε and Fz was also analyzed by using the two recombinant purified proteins: PR61ε and the last intercellular domain of Fz1 or Fz3 fused to GST. Both proteins (GST-Fz1-C-tail or GST-Fz3-C-tail) retained recombinant PR61ε (Figure 4b), indicating that these sequences contain the binding site to the phosphatase.
Figure 4.
PR61ε directly binds to the Wnt-receptor Fz and interacts with CK1ε upon Wnt3a stimulation. (a) Pull-down assays were performed incubating 500 μg total cell extracts from HEK293 cells stimulated with control or Wnt3a-conditioned medium for 15 min, with 10 pmol of GST-PR61ε. Protein complexes were affinity purified and analyzed by WB. Levels of endogenous Fz and LRP5/6 proteins bound to PR61ε were quantified by analyzing four independent experiments (bottom). **P<0.01. (b) PR61ε binding assays were performed with 4 pmol of PR61ε and 2 pmol of GST-Fz-C-tail peptides or GST as a control. (c) Representation of amino acid sequence alignment of human Fz1, Fz3, Fz4 and Fz5-C-tail regions. The 25 first amino acids of the last cytosolic domain are indicated. (d) In vitro binding assays were performed incubating 1 pmol of recombinant PR61ε or GST as a control with 10 pmol of the indicated biotinylated-Fz-C-tail peptides. An irrelevant biotinylated-peptide was use as control. (e) A Pull-down assay was performed incubating 500 μg total cell extracts with 10 pmol of GST-PR61ε and the interaction was competed with 100 pmol of the indicated Fz-C-tail peptides. Protein complexes were affinity purified and analyzed by western blotting. (f and g) Total extracts from HEK293 cells expressing PR61ε-Flag WT or PR61ε-Flag ΔN were incubated with 10 pmol of GST-Fz-C-tail (F), GST-CK1ε (g) or GST as a control. A pull-down assay was performed and the protein complexes were analyzed by western blotting with the indicated antibodies. (h) CK1ε-Flag, either full-length or ΔC-tail was expressed in control cells; PR61ε was immunoprecipitated and immunocomplexes analyzed by WB.
The size of the cytoplasmic C-tail varies among the different members of the family, being the shortest in the Fz1 subfamily, comprising only 25 amino acids (623–647 in Fz1). This sequence presents similarity among representative elements of the different subfamilies: Fz1, 3, 4 and 5 (Figure 4c). Depletion of the first 40 amino acids in Fz3-C-tail, that extends much longer than Fz1 (up to 166 amino acids), compromised the interaction with PR61ε; a Fz3-C-tail deletion mutant (540–666) failed to interact with PR61ε (Figure 4b). Binding assays were also performed with biotinylated peptides encompassing the C-tail first 25 amino acids from Fz1, 4 and 5; all these peptides bound to PR61ε (Figure 4d). Moreover, they prevented the association of endogenous Fz with PR61ε, as they decreased the amount of the receptor pulled-down by GST-PR61ε (Figure 4e).
We analyzed the consequences of interfering in this association. Ectopic expression of GST-Fz1-C-tail in HEK293 did not affect the co-immunoprecipitation of Fz with LRP5/6 observed upon Wnt3a stimulation (Supplementary Figure S9A); however, it prevented the stimulation of CK1ε activity by Wnt3a (Supplementary Figure S9B). Thus, inhibition of the PR61ε interaction with Fz precluded the activation of CK1ε by Wnt3a.
The elements in PR61ε involved in binding to Fz were also investigated. A deletion mutant of PR61ε lacking the first 77 amino acids did not interact with GST-Fz1-C-tail (Figure 4f), although it associated with CK1ε as efficient as the full-length form (Figure 4g). Experiments ectopically overexpressing CK1ε indicated that PR61ε bound the full-length form much better than the C-tail-depleted mutant (Figure 4h). These results indicate that PR61ε uses different elements for binding to CK1ε and Fz1, being the N-tail required for the interaction with the receptor, and that the association with CK1ε takes place through the C-tail of the protein kinase, that contains the phosphorylated amino acids (Supplementary Figure S6).
PR61ε is required for β-catenin accumulation and other cellular responses to Wnt3a but not to CA-LRP5/6
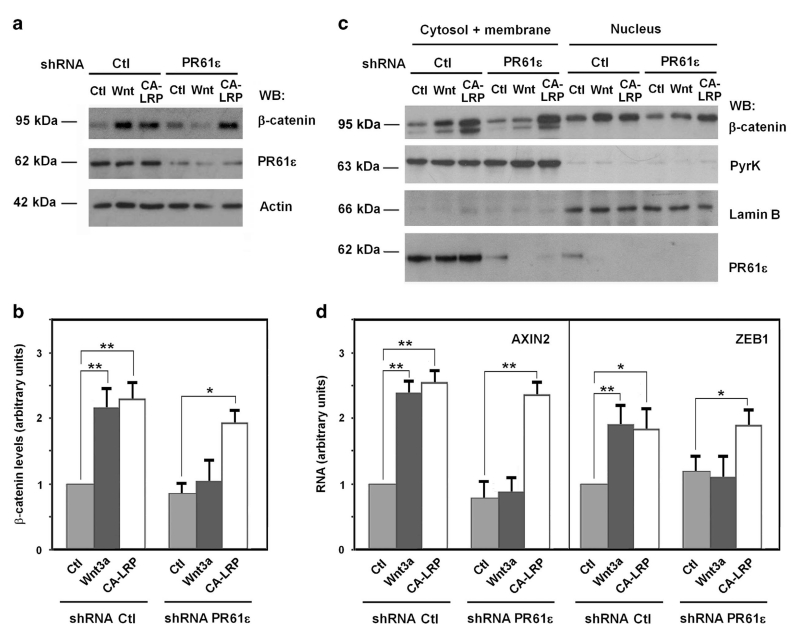
We analyzed the effect of PR61ε downregulation on later responses to Wnt activation. First, the sequestering of LRP5/6 into digitonin and proteinase K-resistant multivesicular bodies upon Wnt stimulation was prevented in cells with downregulated PR61ε (Supplementary Figure S10). In addition, the increase in β-catenin levels in response to Wnt3a was obliterated by PR61ε downregulation (Figures 5a and b). In contrast, depletion of this protein did not affect the upregulation in β-catenin caused by CA-LRP5/6 transfection. Similar results were observed when the cytosolic or nuclear fractions of β-catenin were analyzed: PR61ε depletion prevented the accumulation of β-catenin in both cellular compartments caused by Wnt3a but not by CA-LRP5/6 (Figure 5c). As a consequence, a shRNA specific for PR61ε but not the corresponding control prevented the Wnt3a induced increase in the RNA levels of two β-catenin transcriptional targets, AXIN2 and ZEB1.34,35 In accordance to our previous observations the RNA levels of these genes were stimulated by CA-LRP5/6 even in the absence of PR61ε (Figure 5d).
Figure 5.
PR61ε is necessary for the Wnt-induced upregulation in β-catenin transcriptional activity. (a and c) PR61ε-depleted HEK293 cells (shRNA PR61ε #2558), overexpressing CA-LRP when indicated, were stimulated with control or Wnt3a-conditioned medium for 6 h, and β-catenin accumulation was analyzed by WB from total cell extracts (a). (b) Autoradiograms from four different experiments performed in a were quantified using Image J software, and the mean ± s.d. was obtained for each condition. (c) The nuclear fraction was separated from the cytosolic and membrane-associated fraction as described.43,44 β-Catenin levels in each cellular fraction were analyzed by WB. Lamin B and pyruvate kinase were used as markers of nuclear and cytosolic-plus-membrane fractions, respectively. (d) RNA was isolated from PR61ε-depleted HEK293 cells overexpressing CA-LRP when indicated and treated with control or Wnt3a-conditioned medium for 16 h. Expression of AXIN2 or ZEB1 was assessed by quantitative RT-PCR. **P<0.01, *P<0.05.
Similar results were obtained when a cellular response to the stimulation of the Wnt pathway was determined. Wnt3a increases HEK293 cells invasion of Matrigel matrices (Figures 6a and b); this effect was sensitive to PR61ε depletion. Transfection of CA-LRP5/6 also upregulated HEK293 invasion; in this case, and as in other parameters shown above, stimulation of invasion was also observed in PR61ε-depleted cells (Figures 6a and b). Therefore, these results indicate that the activity of the PP2A-PR61ε complex is only required at the initial steps of the Wnt signaling, the same reactions that require CK1ε activity.
Figure 6.
PR61ε is required for stimulation of cell invasion by Wnt3a. Control and PR61ε-depleted HEK293T cells (shRNA PR61ε #2558), overexpressing CA-LRP when indicated, were placed in Matrigel-coated transwells and incubated with control or Wnt3a-conditioned medium for 36 h. Non-invading cells were removed from the upper surface of the membrane, whereas cells present at the lower surface were fixed and stained with DAPI. The DAPI-stained nuclei were counted in four different fields per filter by Image J software, and unpaired t-test statistics were performed. **P<0.01, *P<0.05, other P-values between 0.1 and 0.05 are indicated. (b) A representative 10 × picture of each condition is shown in a.
DISCUSSION
In this article we present new results describing the initiation of Wnt signaling. Upon binding, Wnt ligands promote the association of their receptor Fz to the co-receptor LRP5/6 that acts as a platform for assembling the signalosome complex. LRP5/6 is constitutively bound to cadherin proteins5,6 and, through these proteins, to p120-catenin and CK1ε. The activity of protein kinase is required for the initial steps of Wnt action; thus, for Dvl-2 association to LRP5/6.3,6 Regulation of CK1ε activity differs from other members of this family such as CK1α; whereas the activity of the latter is constitutive and its action on substrates just dependent on its accessibility to them, CK1ε must also be activated. This activation requires the dephosphorylation of a specific sequence in this protein that behaves as an internal inhibitor.23 We report here that dephosphorylation and activation of CK1ε is catalyzed by a PP2A complex formed by the catalytic and the regulatory PR61ε subunits. It is likely that this process of CK1ε activation by dephosphorylation cooperates with other effectors, such as DDX3, that increases the activity of this kinase through a direct interaction.36
The activity of PP2A phosphatase requires the action of constitutively active catalytic subunit, a structural common protein and the variable regulatory subunit that provides specificity for the substrate.33 Binding of the different regulatory subunits to the substrates is more stable whereas the catalytic subunit associates only transiently. As we show here, PR61ε is required for the canonical Wnt-induced activation of CK1ε. This regulatory subunit is constitutively associated to Fz; binding required the N-terminal sequence of PR61ε. This isoform has been detected both in the nucleus and in the cytosol.37 Presence in the nucleus is dependent on a NLS located in the same Fz-binding sequence; it is likely that the interaction with this protein prevents the interaction of PR61ε with the importin and its nuclear localization. In any case, through the association of Fz with LRP5/6, Wnt enhances the accessibility of the PR61ε/PP2Ac complex to CK1ε, facilitating its dephosphorylation and activation; thus, providing a simple mechanism for the initiation of the Wnt pathway.
PR61ε interacts with the last cytosolic domain, the C-terminal tail of several members of the Fz family. Although we used in these assays the complete C-tail (25 amino acids) of Fz1 and peptides corresponding to equivalent sequences in other Fz proteins, it is likely that binding takes place through the first thirteen residues, as they are highly conserved in all the Fz isoforms. In contrast to Fz1, other Fz family members, such as Fz3, contain much longer C-tails, although, at least for Fz3 the amino acids relevant for PR61ε binding are located in the sequence conserved in Fz1. In any case, we cannot discard that other elements in the Fz cytoplasmic loops may also contribute to the binding, similarly to what has been described for Fz interaction with Dvl-2.38
As CK1ε acts at the initial steps of the pathway, PR61ε downregulation affects virtually all the responses to Wnt3a. The action of this phosphatase complex in the Wnt cascade takes place upstream CK1ε activation and a form of LRP5/6 that behaves as a gain-of function mutant bypasses the PR61ε and CK1ε requirements (this report, see also Vinyoles et al.8). This CA-LRP5/6 form is capable to upregulate β-catenin accumulation even in the absence of PR61ε and CK1ε indicating that they are dispensable for other reactions taken place downstream Dvl-2 binding to the receptor complex. We have detected that axin-β-catenin interaction is increased by PR61ε-depletion (MV, MD, unpublished observations). According to our data this interaction seems to be relevant for controlling the basal levels of β-catenin and not the Wnt-induced accumulation of this protein. It should be remembered that the final effect of this interaction, the phosphorylation of β-catenin by GSK3 is prevented in Wnt-treated cells.
In contrast to our results and some other reports demonstrating a positive role of PP2A in Wnt signaling,24-28 it has also been reported this phosphatase also acts negatively on this pathway.29,30 As phosphorylation controls multiple reactions in this pathway, it is possible that this phosphatase may also decrease phosphorylation having a positive action; this PP2A function would be governed by its interaction with regulatory proteins other than PR61ε. For instance, PR61α affects negatively this pathway.30 Therefore, the effects of PP2Ac overexpression or inhibition might be greatly dependent on the level of expression of the different regulatory proteins and the extent of constitutive phosphorylation of the proteins they control.
Our results also have other interesting implications. For instance, it is noteworthy that the CA-LRP5/6 form induces Dvl-2 phosphorylation independently of the levels of CK1ε; thus, in CK1ε-depleted cells (Supplementary Figure S6) and also in cells where the activity of this protein kinase is inhibited by PR61ε depletion (Figure 2a). Therefore, it is unlikely that the molecular shift caused by Dvl-2 phosphorylation is due to the action of CK1ε. Other protein kinases have been reported to associate with Dvl-2;39 it is possible that these (PAR-1, Pik1, protein kinase C, CK2, CK1δ, even some isoforms of Ck1γ not-targeted by our shRNA) are responsible or collaborate in this extensive phosphorylation. In any case this process takes place secondary to the multimerization of the receptor complexes, a process promoted by CA-LRP5/6, that constitutively interacts with Dvl-2 and endogenous full-length LRP5/6. Alternatively, it is possible that the relevant CK1ε substrate in Wnt signaling is Fz, and the modification of conserved S or T residues in Motif III of the receptor38 enhances its interaction with Dvl-2.
Finally, our experiments also show that, differently to other elements of the signalosome, neither Fz nor PR61ε are observed into the digitonin and proteinase K-resistant multivesicular bodies (Supplementary Figure S9). The localization of the signalosome in this compartment is a late response to Wnt (requires about 2 h, much longer than the rapid stimulation of CK1ε and Dvl-2 phosphorylation and binding to LRP5/6) and it has been suggested to contribute to a more extended response to these signaling factors.8 The absence of Fz and PR61ε in this compartment suggests that part of the signalosome is released from the complex before its sequestration into these vesicles, similarly to what we have described with cadherin, p120-catenin and CK1ε.8 It remains to be established the molecular basis for the Fz separation from the LRP5/6 complex. Maybe this release is related to the more unspecific action of the Fz proteins that also act as receptors for other Wnt factors inducing alternative pathways, such as the non-canonical Wnt ligands.40
MATERIALS AND METHODS
Cell culture
HEK293T, HT-29 M6 and SW-480 cells were obtained from the IMIM Cell Bank. Assays were performed at 60–70% confluence. Control L fibroblasts or stably transfected with a plasmid encoding Wnt3a were obtained from the ATCC (Manassas, VA, USA) (ref. CRL-2648 and CRL-2647, respectively). Wnt3a L fibroblasts were cultured in medium containing 0.4 mg/ml G-418. For obtaining DKK1-conditioned medium, HEK293 cells were transfected with pcDNA3-hDKK1-HA (kindly provided by Dr JM Gonzalez-Sancho, Universidad Autónoma de Madrid, Spain) or the control vector. Conditioned medium was collected from the indicated cells cultured for two days without antibiotic, centrifuged to remove cell debris, and stored at −80 °C. All cell lines were periodically tested to ensure that they stayed mycoplasma free.
Antibodies
The following specific antibodies were used in this study: p120-catenin and β-catenin (all monoclonal antibodies from BD Biosciences, 610134 and 610153, respectively, Franklin Lakes, NJ, USA), phosphoSer268 in p120-catenin, Axin, Fz, Dvl-2 and LRP6 (Santa Cruz Biotechnology, sc-293000, sc-14029, sc-9169, sc-13974, sc-15399, respectively, Santa Cruz, CA, USA), PyrK (Chemicon AB-1235, Temecula, CA, USA), Lamin B (Abcam Ab 16048, Cambridge, UK), phosphoThr1490 in LRP6 (Cell Signaling 2568, Danvers, MA, USA), CK1ε and GSK3β (BD Biosciences 610445 and 610201, respectively), CK1γ and phosphoSer/Thr/Tyr (Abcam 64829 and 15556, respectively), GST (GE Healthcare, 27-4577-01, Little Chalfont, UK), PP2A catalytic subunit (PP2Ac) (Millipore 05-421, Billerica, MA, USA), Flag M2 (Sigma 3165, St Louis, MO, USA) and PR61ε regulatory subunit.37
Preparation of DNA constructs
The generation of the bacterial expression plasmid pGEX-6 P encoding the GST protein fused to p120-catenin wild type have been previously described.6,41 To obtain the C-tail domain of Fz1 (amino acids 623-647) fused to GST, PCR amplification was carried out using Taq polymerase (NE Biolabs, Ipswich, MA, USA), pcDNA3-Fz1 (a gift from Dr JM Gonzalez-Sancho) and the primers: forward 5′-AATAAGGGATCCGGCAAGACACTGAAT-3′ and reverse 5′-TTAATACTCGAGGCTAGCTTTCAGGCG-3′. The PCR product was digested with BamHI and XhoI and cloned into BamHI/XhoI linearized pGEX-6P3. The C-tail domain of Fz3 (amino acids 500-666) fused to GST was obtained by PCR amplification using retrotranscriptase kit (Roche 04379012001, Basel, Switzerland), mRNA extracts from HEK293 T cells and the primers: forward 5′-AAACCCGGGAAAAAGACATGCTTTGAATGG-3′ and reverse 5′-AAACTCGAGTTAAGCACTGGTTCCATCTTC-3′. The PCR product was digested with SmaI and XhoI and cloned into SmaI/XhoI linearized pGEX-6P2. Fz3 (amino acids 540-666) fused to GST was obtained by PCR amplification of pGEX-6P2-Fz3 C-tail using Taq polymerase and the primers: forward 5′-CGGAATTCCGCATCCACCCCAGCAGGAAG-3′ and reverse 5′-AAACTCGAGTTAAGCACTGGTTCCATCTTC-3′. The PCR product was digested with EcoR1 and XhoI and cloned into EcoR1/XhoI linearized pGEX-6P3. The full-length CK1ε was obtained by RT-PCR from mRNA extracts of HEK293 T cells using the primers: forward 5′-TAACCCGGGATGGAGCTACGTGTGGGGA-3′ and reverse 5′-TTAGCGGCCGCTCACTTCCCGAGATGGTCA-3′. The PCR product was digested with SmaI and NotI and cloned into SmaI/NotI linearized pGEX-6 P2. Fz1-C-tail pEbg2T construction was generated by digestion of PGEX-6P3-Fz1-C-tail plasmid with BamHI and NotI and cloned into the linearized BamHI/NotI pEbg2T. All the constructions were verified by sequencing. The plasmids codifying for CA-LRP6-GFP, a mutant that lacks the extracellular LRP domain,9 was provided by Dr A Kikuchi (Hiroshima University, Japan) and Flag-CK1ε and Flag-CK1εΔC 36 were a kind gift from Dr C Niehrs (Institute for Molecular Biology, Mainz, Germany). PR61ε WT and PR61ε ΔN cDNA in the eukaryotic vector pCS2, and PGEX-6P PR61ε were previously reported.37 Dvl-myc was provided by Dr A Bigas (IMIM, Barcelona, Spain).
Purification of recombinant proteins, pull-down and binding assays
GST-fusion proteins were expressed in Escherichia coli and purified by affinity chromatography on Glutathione-Sepharose as described.42 Pull-down assays were performed using 10 pmol of purified recombinant proteins fused to GST as bait and extracts from SW-480 cells. When indicated, binding was competed with Fz-C-terminal peptides corresponding to the equivalent last 25 amino acids in Fz1 sequence: Fz1-C-tail (aa 623-647), Fz4-C-tail (aa 497-521) or Fz5-C-tail (aa 523-547). Glutathione-Sepharose-bound proteins were analyzed by western blotting (WB) with specific monoclonal antibodies. Biotinylated-Fz-C-tail peptide binding was performed using the indicated N-terminal biotin-labeled peptides corresponding to the equivalent 25 amino acids in the C-terminal Fz1 sequence: Fz1-C-tail (aa 623-647) SGKTLNSWRKFYTRLTNSKQGETTV, Fz4-C-tail (aa 497-521) SAKTLHTWQKCSNRLVNSGKVKREK, Fz5-C-tail (aa 523-547) SGKTVESWRRFTSRCCCRPRRGHKS or an irrelevant peptide ARTKNQTARKSTGGKAPVT. Binding assays were also performed by incubating 2 pmol of biotinylated peptides with 4 pmol of recombinant PR61ε for 1 h at 4 °C; bound proteins were isolated with streptavidin-agarose (Sigma). Beads were washed and analyzed by WB. All binding and pull-down assays were repeated at least three times. Peptides were prepared at Peptide Synthesis Service, UPF, Barcelona, Spain.
Cell transfection and selection of transfectants
shRNA specific for PP2Ac (#TRCN2483), PR61ε (#TRCN2558 and #TRCN2559), PR61α (#TRCN10507), PR61β (#TRCN39935), CK1ε (#TRCN1837), CK1δ (#TRCN0598) CK1γ2 (#TRCN38671) and non-targeting (#SCH002) were all from Mission, Sigma. For stable expression of shRNA, SW-480 cells were infected as previously described.43 For transient expression in HEK293 cells, plasmids were transfected as reported.8
Immunoprecipitation assays
LRP5/6 immunoprecipitation assays were performed by homogenizing cells in 1% digitonin lysis buffer supplemented with protease and phosphatase inhibitors as described.6 RIPA buffer was used to prepare cell extracts for non-membrane protein immunoprecipitation assays.44 Proteins were immunoprecipitated from 500 μg cell extracts using 1 μg/ml of the appropriate antibody or an irrelevant IgG as control for 16 h at 4 °C; alternatively a control was used without IgG. Immunoprecipitated proteins were analyzed by WB using specific monoclonal antibodies.
RNA isolation and analysis
RNAs were obtained and analyzed by quantitative RT-PCR (qRT-PCR) as previously reported.8,45 The primers used are listed in Supplementary Table S1.
Matrigel invasion assay
Seventy-thousand cells were resuspended in 75 μl DMEM 0.2% FBS-0.1% BSA supplemented with 75 μl of control or Wnt3a-conditioned medium. Cells were seeded on a Transwell filter chamber (Costar 3422, Thermo Fisher Scientific, Waltham, MA, USA) coated with 0.5 mg/ml Matrigel (BD Biosciences, 354230) and incubated for 36 h. DMEM 10% FBS was placed in the lower chamber and used as chemoattractant. Non-invading cells were removed from the upper surface of the membrane, while cells that adhered to the lower surface were fixed with PFA 4% for 20 min and stained with DAPI. The DAPI-stained nuclei were counted in four fields by Image J software (Public Domain developed at the NIH, Bethesda, MD, USA) and quantified.
Statistical analyses
All the results shown were representative from at least three independent experiments. Data are presented as mean ± s.d. Statistical analyses were conducted using GraphPad Prism software (GraphPad, La Jolla, CA, USA). Data were analyzed for significance using unpaired t-Test. P-values <0.01 are symbolized with two asterisks; P <0.05, with one asterisk; other P-values between 0.1 and 0.05 are indicated.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs González-Sancho, C Niehrs, A Bigas and A Kikuchi for providing reagents and N Ontiveros for excellent technical assistance. This work was funded by grants from the Ministerio de Economía y Competitividad (BFU2012-31554 and BFU2015-65153-R, both MINECO/FEDER, to MD and SAF2013-48849-C2-R1 to AGH) and Fundació La Marató de TV3 (120130) to MD and AGH. Support from ICREA Academia, 2014SGR-32 from Generalitat de Catalunya and ISCIII/FEDER (RD12/0036/005) is also appreciated. MV was a recipient of a predoctoral fellowship from FPI.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MD and AGdeH conceived the study. MV, BDV-P, JC, MP and AV performed the experiments. MV and BDV-P prepared the figures. MD and AGdeH designed the experiments and wrote the manuscript with inputs from MV and BDV-P and JY. All authors contributed to the interpretation and the discussion of the results.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruciat CM. Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol. 2014;31:46–55. doi: 10.1016/j.ceb.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Virshup DM. Updating the Wnt pathway. Biosci Rep. 2014;34:e00142. doi: 10.1042/BSR20140119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haÿ E, Laplantine E, Geoffroy V, Frain M, Kohler T, Müller R, et al. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling, osteoblast function, and bone formation. Mol Cell Biol. 2009;29:953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casagolda D, Del Valle-Pérez B, Valls G, Lugilde E, Vinyoles M, Casado-Vela J, et al. A p120-catenin-CK1epsilon complex regulates Wnt signaling. J Cell Sci. 2010;123:2621–2631. doi: 10.1242/jcs.067512. [DOI] [PubMed] [Google Scholar]
- 7.Swiatek W, Tsai I-C, Klimowski L, Pepler A, Barnette J, Yost HJ, et al. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 8.Vinyoles M, Del Valle-Pérez B, Curto J, Viñas-Castells R, Alba-Castellón L, García de Herreros A, et al. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol Cell. 2014;53:444–457. doi: 10.1016/j.molcel.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H, Sakane H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci. 2010;67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, Fu Y, Cruciat CM, Jia S, Wang Y, Tong Z, et al. Transmembrane protein 198 promotes LRP6 phosphorylation and Wnt signaling activation. Mol Cell Biol. 2011;31:2577–2590. doi: 10.1128/MCB.05103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, et al. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J. 2011;30:1433–1443. doi: 10.1038/emboj.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 15.Bilic J, Huang Y-L, Davidson G, Zimmermann T, Cruciat C-M, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 16.Del Valle-Pérez B, Arqués O, Vinyoles M, de Herreros AG, Duñach M. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol. 2011;31:2877–2888. doi: 10.1128/MCB.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mi K, Dolan PJ, Johnson GVW. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J Biol Chem. 2006;281:4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- 18.Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’ s phosphorylation of beta -catenin. Proc Natl Acad Sci USA. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taelman VF, Dobrowolski R, Plouhinec J-L, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Cegielska a, Gietzen KF, Rivers A, Virshup DM. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J Biol Chem. 1998;273:1357–1364. doi: 10.1074/jbc.273.3.1357. [DOI] [PubMed] [Google Scholar]
- 24.Hsu W, Zeng L, Constantini F. Identification of a domain of axin that binds to the serine/threonine proten phosphatase 2 A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 25.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases β-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratcliffe MJ, Itoh K, Sokol SY. A positive role for the PP2 A catalytic subunit in Wnt signal transduction. J Biol Chem. 2000;275:35680–35683. doi: 10.1074/jbc.C000639200. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Wu J, Tan C, Klein PS. PP2A:B56epsilon is required for Wnt/beta-catenin signaling during embryonic development. Development. 2003;130:5569–5578. doi: 10.1242/dev.00762. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Yang J, Liu Y, Yu T, Jia J, Liu C. PR55α, a regulatory subunit of PP2A, specifically regulates PP2A-mediated β-catenin dephosphorylation. J Biol Chem. 2009;284:22649–22656. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen P, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989;250:596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- 32.Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 33.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of Conductin/Axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Tilló E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. β-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA. 2011;108:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruciat CM, Dolde C, de Groot RE a, Ohkawara B, Reinhard C, Korswagen HC, et al. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-β-catenin signaling. Science. 2013;339:1436–1441. doi: 10.1126/science.1231499. [DOI] [PubMed] [Google Scholar]
- 37.Jin Z, Shi J, Saraf A, Mei W, Zhu G-Z, Strack S, et al. The 48-kDa alternative translation isoform of PP2A:B56epsilon is required for Wnt signaling during midbrain-hindbrain boundary formation. J Biol Chem. 2009;284:7190–7200. doi: 10.1074/jbc.M807907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tauriello DVF, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BaM, et al. Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci USA. 2012;109:E812–E820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao C, Chen Y-G. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 41.Castaño J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, et al. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, et al. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valls G, Codina M, Miller RK, Del Valle-Pérez B, Vinyoles M, Caelles C, et al. Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J Cell Sci. 2012;125:5288–5301. doi: 10.1242/jcs.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Valle-Pérez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, et al. Wnt controls the transcriptional activity of Kaiso through CK1ε-dependent phosphorylation of p120-catenin. J Cell Sci. 2011;124:2298–2309. doi: 10.1242/jcs.082693. [DOI] [PubMed] [Google Scholar]
- 45.Solanas G, Porta-de-la-Riva M, Agustí C, Casagolda D, Sánchez-Aguilera F, Larriba MJ, et al. E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J Cell Sci. 2008;121:2224–2234. doi: 10.1242/jcs.021667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.