Abstract
Genomic risk information for potentially actionable complex diseases and pharmacogenomics communicated through genomic counseling may motivate physicians and patients to take preventive actions. The Ohio State University-Coriell Personalized Medicine Collaborative is a randomized trial to measure the effects of in-person genomic counseling on chronic disease patients provided with multiplex results. Nine personalized genomic risk reports were provided to patients through a web portal, and to physicians via electronic medical record (EMR). Active arm participants (98, 39% female) received genomic counseling within one month of report viewing; control arm subjects (101, 54% female) could access counseling 3-months post-report viewing. We examined whether genomic counseling affected documentation of physician-patient communication by reviewing the first clinical note following the patient’s genomic counseling visit or report upload to the EMR. Multivariable logistic regression modeling estimated the independent effect of genomic counseling on physician-patient communication, as Intention to Treat (ITT) and Per Protocol (PP), adjusted for physician educational intervention. Counselees in the active arm had more physician-patient communications than control subjects (ITT, OR: 3.76 (95% CI: 1.38 – 10.22, P<0.0094); PP, OR: 5.53 (95% CI: 2.20 – 13.90, P=0.0017). In conclusion, genomic counseling appreciably affected physician-patient communication following receipt of potentially actionable genomic risk information.
Keywords: complex disease, counseling, electronic medical record, genetic, genomic, pharmacogenomics, physician-patient communication
Graphical Abstract
Genomic counseling of patients with chronic disease receiving potentially actionable complex disease and pharmacogenomics results in an academic medical center was associated with increased physician- patient communication regarding test results
INTRODUCTION
Genomic medicine is an emerging medical discipline that involves using a patient’s family history, genetic, protein, metabolic and other biological marker profiles in the clinical setting. Knowledge of a patient’s genomic profile could help identify and manage health risks, aid in the diagnosis of existing disease, determine what interventions (e.g. pharmacologic, surveillance) will have the greatest benefit, and improve patient-centered health outcomes. To reach this goal, a number of complex issues must be resolved, including optimizing patient genomic testing result delivery, preparation of the physician workforce, and evidence-based research to systematically evaluate the translation of genomics into clinical care (1–7).
To date, there have been relatively few studies examining the potential effects of delivery of actionable genomic risk information on physician-patient communication (8, 9), or how genomic counseling might affect this process. Physician-patient communication, including patient activation, is associated with greater adherence to health care provider recommendations and greater patient satisfaction (10, 11). However, even when actionable genomic risk information is available, participants often keep the results to themselves. Kaufman et al., surveying participants of direct to consumer genomic services, found that only 28% discussed actionable test results with a health care provider, most often a primary care physician, and seldom with a genetic counselor (12). Similar physician involvement was seen by Bloss et al. (13), and more recently, by van der Wouden et al. (14) (26.5% and 27%, respectively). Bloss et al. also found that 1) speaking with a physician or genetic counselor about results was not associated with a change in anxiety level, and 2) those who discussed results had a higher completion rate of recommended health screening tests (e.g. diabetes) at long term follow-up (9).
We sought to determine whether in-person genomic counseling offered to patients receiving potentially actionable genomic risk information as part of a randomized trial affected patient communication with their physician team. Specifically, we examined whether genomic counseling affected documentation of physician-patient communication about genomic test results, determined by review of the first clinic note following test report upload to the electronic medical record, or after the patient met with a genomic counselor.
METHODS
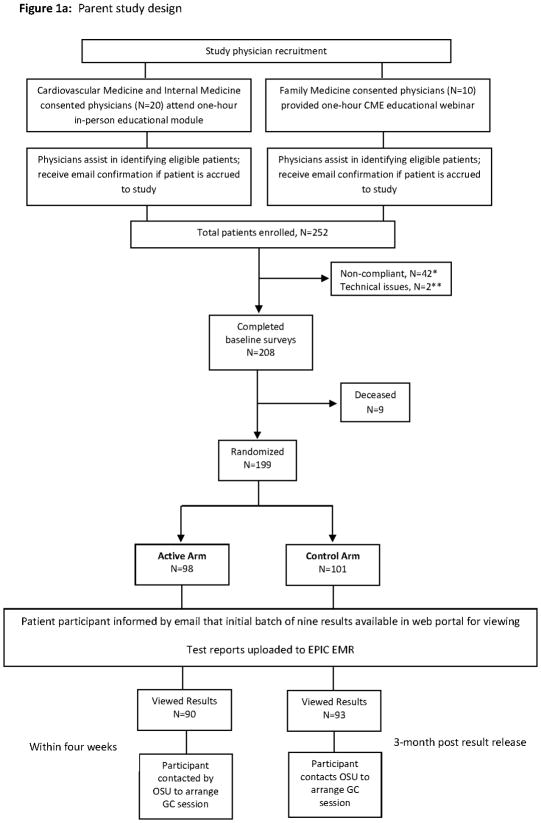
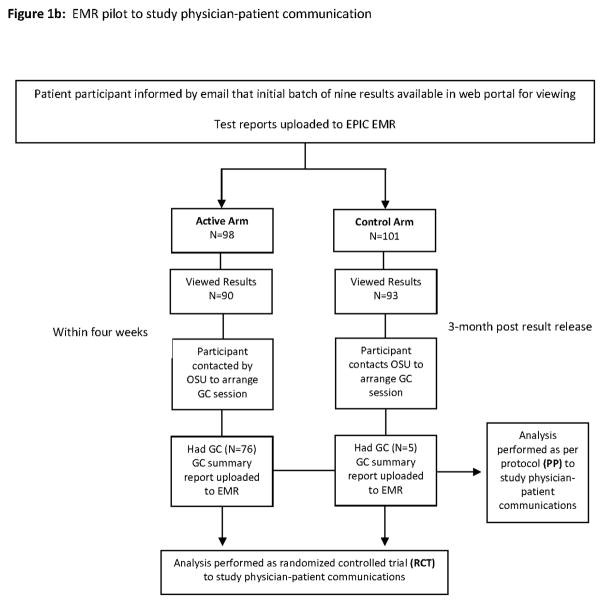
The Ohio State University-Coriell Personalized Medicine Collaborative (OSU-CPMC) parent study is a randomized clinical trial of in-person genomic counseling (GC) for patients with chronic disease (heart failure, hypertension) receiving potentially actionable results in an academic medical center setting (Figure 1a). The primary study aim was to determine whether genomic counseling impacts risk perception and genomic test result comprehension (15). As part of the parent study, we recruited physicians taking care of OSU-CPMC patients into a pilot study to explore test result utilization and physician-patient communication regarding results (Figure 1b) (15). The study was approved by the institutional review boards at Ohio State and the Coriell Institute for Medical Research.
Figure 1.
*Non-compliance was when an individual had not completed the baseline surveys within a 45 day time limit.
**Technical issues means the saliva DNA sample failed.
Physician participants
Patients were enrolled with the assistance of Cardiovascular Medicine, Internal Medicine, and Family Medicine physicians. Physician leaders, one each from Cardiovascular Medicine and Internal Medicine, arranged informational group meetings among their physician colleagues. For these two groups, physician participation included attending a one-hour in-person educational module on the study randomization component, genetics/genomics/pharmacogenomics, single nucleotide polymorphisms and associated relative risks, test report composition, case examples and the process of genomic counseling. In all, we had twenty physicians (response rate, 57%; 12/27 Internal Medicine; 8/8 Cardiovascular Medicine) who consented to participate and worked with investigators to recruit patients. As study design required a sufficient number of physicians to be involved in order to accrue an adequate number of patients, leadership in the Department of Family Medicine were approached. However, this group was not interested in having their physician teams participate in the one-hour in-person educational module given work time constraints. Thus, a one-hour educational webinar accredited by Ohio State Wexner Medical Center for a maximum of 1.5 AMA PRA Category 1 Continuing Medical Education Credit(s)™ was made available. In total, 10 Family Medicine physicians participated in recruiting patients to study; however, none chose to view the webinar.
All study physicians were informed that each patient participant was provided access to 9 personalized CPMC risk reports (coronary artery disease, type 2 diabetes, hemochromatosis, melanoma, prostate cancer, age related macular degeneration, type 1 diabetes and lupus as well as impact of the CYP2C19 gene on clopidogrel metabolism) through a private web portal. These eight conditions were chosen given the relative high frequency of the genetic variant used to assess risk; varied effect size of each variant on risk; and that each condition is potentially actionable via lifestyle modification or medical intervention (Table 1) (17). The reports present personalized risk information as relative risk for each of the 8 health conditions, based on genetic variant, family history and health behavior risk factors individually, in both graphical and numeric format (Supplemental Figure 1). To ensure readability, the report design was informed by multiple rounds of pilot testing conducted by allowing individuals with no scientific background to review report drafts and provide feedback.
Table 1.
All possible reportable disease risk values*
| Disease | Genetic Variant RR | Family History RR** | BMI RR | Smoking RR | Diabetes RR | Number of risk variables |
|---|---|---|---|---|---|---|
| AMD | 2.4, 6.0 | 4.0 | NA | 1.4, 2.0 | NA | 5 |
| CAD | 1.3, 1.7 | 1.2, 1.4 | NA | 2.1*** | 1.7 | 6 |
| DM1 | 0.08, 0.3 | 2.3; 6.6 | NA | NA | NA | 4 |
| DM2 | 1.2, 1.3 | 1.3, 1.9 | 2.3, 5.9 | NA | NA | 6 |
| HH | 27.0**** | NA | NA | NA | NA | 1 |
| LUP | 1.4, 2.0 | 4.1, 11.3 | NA | 1.5 | NA | 5 |
| MEL | 1.7, 3.0 | 2.2 | NA | NA | NA | 3 |
| PRO | 1.5 | 1.9 | NA | NA | NA | 2 |
| Total | 14 | 11 | 2 | 4 | 1 | 32 |
RR: Relative Risk
NA: not applicable risk factor
Values for Caucasian population represented
- AMD: one or more first-degree relatives with age-related macular degeneration
- CAD: one or both parents diagnosed with coronary artery disease
- DM2: one or both parents with type 2 diabetes
- LUP: one or two or more first-degree relatives with a history of any of 11 autoimmune diseases
- MEL: one or more first-degree relatives with melanoma
- PRO: biological father or any brothers diagnosed with prostate cancer
RR varies by gender and race
RR only provided to males. Male heterozygotes and homozygote wild type received an RR of 1.0; females got absolute risk: homozygotes received 16% lifetime risk, heterozygotes and wild type homozygotes received a lifetime risk of 1%.
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Physicians were made aware that their patient’s test reports would be made available following patient completion of required baseline surveys, genomic testing completion by the Coriell CLIA certified genotyping lab, report transfer from Coriell to Ohio State, and direct uploading to an EPIC® electronic medical record (EMR). The CPMC reports were accessible by the study physician or any health care team member through hyperlinks to the report content via the EPIC®/Labs tab. Likewise, physicians were made aware that if a patient participant was seen for genomic counseling, the summary letter would be made available in the EPIC® EMR as a research encounter. Per study design, there was no active notification of physicians for test report upload, or summary letter upload to the EMR.
Patient participants
252 patient participants were enrolled by study physicians with assistance of study recruiters over a two year period; 4 additional patients were recruited via Media/Research Match with physician involvement. Patient participants were administered a one-hour educational presentation including access to the CPMC web portal, background information on DNA, genes, and single nucleotide polymorphisms, CPMC test report composition, relative risk, the randomization component, and how they would be contacted regarding the availability of free GC. DNA samples and consent documentation were sent to Coriell, and unique CPMC web portal accounts were created for each patient participant. 53 patients were subsequently removed from study (51 failed to complete all required parent study questionnaires; 2 due to unsuccessful genotyping). Thus, of the original 252 study participants, 199 patient participants comprised the study population. These individuals were randomized to either the active or control arm, with each arm receiving email notification of the availability of the initial batch of nine CPMC test reports. Active arm participants were called to schedule a GC appointment within four weeks of online viewing of at least one test report. Control arm participants were notified by email that they could request in-person GC after a 3-month randomization period, and after the viewing of at least one test report. Contact information to schedule the GC appointment for control arm participants was provided in the email. Study participants in both the active and control arms, who received GC, comprised the “Received GC” group.
The in-person GC session, provided by one of two available licensed genomic counselors, focused on individualized risk assessment for all nine personalized CPMC risk reports. During each session, the genomic counselor reviewed and expanded the patient’s family history to obtain at least a 3-generation pedigree, and reviewed the patient’s medical and social history, environmental risk information, and current health promotion and screening practices (15). Sessions included active discussion of the major risk factors for a given disease, specific actions to prevent or lower disease risk, and recommendations for the patient participant to speak to their physician team regarding the recommended actions. A multi-page risk summary letter, developed by the investigation team, provided focused interpretation for each of the nine personalized CPMC risk reports, as well as recommendations based on the patient’s medical and family histories. The GC summary letter was mailed to the patient, and also uploaded directly to the EMR.
Procedures
Each patient’s EMR was reviewed from the date the genomic reports were uploaded (e.g. November 8, 2011 for the initial round of patients accrued to study), until the study was closed for analysis on August 22, 2014. Specifically, any documented interaction/note (e.g. office visit; phone call; Table 2a) that occurred between the patient participant, the study physician, or any member of the health care team was manually reviewed by KS for the following study related search terms: Coriell, CPMC, genetic, genomic, genomic counseling, pharmacogenomic and variant. All relevant physician-patient communications (e.g. discussion of test results) related to these search terms, either by the study physician, or any medical provider were recorded. The number of times the patient participant was seen by the study physician during the time frame of chart review, with documentation of the first clinic interaction after upload of CPMC test reports, was recorded. If a patient participant was seen for GC, the number of times the patient was subsequently seen by the study physician, and date of the first clinic interaction post-GC was recorded. A second investigator, AS, subsequently performed manual review of the EMR with use of the 7 study related search terms for concordance. Both investigators (KS, AS) agreed upon a subset of physician-patient communications. Following this initial round of manual chart review, investigators had opportunity to use a new EPIC® informatics-based text search. As such, a second round of record review was performed by KS utilizing the same list of 7 key search terms, and modifications were made to the original spreadsheet. Again, all recorded physician-patient communications were verified using the EPIC® search function by a second investigator (AS). Both reviewers agreed on the final subset of physician-patient communications, as well as the placement of each communication into specific categories. Examples of physician-patient communications are found in Table 2b. Review of the EMR took between 15 minutes and 3 hours per patient participant to complete.
Table 2a.
Electronic Medical Record categories reviewed for documentation
| Clinical support encounters |
| Documentation only (e.g. phone call; email correspondence) |
| Office visits |
| Patient letters |
| Problem list |
| Provider notes |
| Research encounters |
| Subspecialist referral |
Table 2b.
Communication Topics and Examples of Physician-Patient Result Communication on the First Interaction from the Electronic Medical Record (EMR)
| Physician-Patient Communication | Frequency | Quoted Examples from EMR |
|---|---|---|
| Discussion of Complex Disease Test Results | 9 | She had a number of questions about her genetic testing that was done during a recent study protocol. I have reviewed those test results and updated her records to indicate that she has a slight increased risk of melanoma. She also has a slight increased risk of macular degeneration and should be screened on a yearly basis. |
| Specialty Referral Based on Study Findings | 4 | A clinical genetics research assessment (Coriell database) was performed in November of 2012 through a genetic counselor; this revealed genetics risk factors for the following: Increased risk: Coronary artery disease (CAD): Type 2 diabetes: Systemic lupus erythematous (SLE); Age-Related Macular Degeneration (AMD). Family history is relevant based on clinical genetics assessment for these diseases. FH was updated. Note, pt already receiving regular screening for CAD and DM2, and had elevated HgbA1c on a few occasions prior to consultation and afterward; then was diagnosed and treated for abdominal cancer. Referred to Ophthalmology. |
| Risk Reduction Conversation | 3 | Regarding macular degeneration prevention - speak with your eye doctor about EYE-caps or a similar vitamin with the “AREDS” formula. |
| Acknowledgement of study results in EMR | 2 | Documented CYP2C19 results from Coriell study: Ultra-rapid metabolizer |
| Blood Testing/Screening for Lipids and Glucose | 1 | Genetic risk assessment: he has been completed as a part of a project with Coriell institute. He has genetic risk factors for Type I and Type II diabetes, as well as CAD. He is a rapid metabolizer for Plavix. To address risk factors, we are repeating fasting lipids and will obtain a fasting blood glucose. |
| Patient Sharing of Study Test Results via EPIC MyChart | 1 | Dr. ____: Thought I had done this but doing some questionnaires on the CPMC study today. Attached is the letter that I think you need to be able to access my test result from the Study. Physician reply: Thanks. |
Outcome of Interest
The primary study outcome of interest was defined as any physician-patient communication about the test results on the patient’s first interaction with their physician after EMR results were uploaded for control arm participants or after GC for active arm participants.
Statistical Analyses
Descriptive statistics for socio-demographic and clinical variables were generated and compared between study arms. Both the “Intention-To-Treat (ITT)” analysis and the “Per-Protocol (PP)” analysis were performed for a robust interpretation of study results (16). As there were 30 subjects who did not return for a physician visit after the EMR report upload or after GC, comparison on age, gender, race, education, insurance, disease groups, and number of elevated genetic variant risks (RR>1.2) between these 30 subjects with the rest of the sample population was performed to show no significant difference. Outcomes were analyzed on the assumption that physician communication could not be generated for those patients who did not come back for a clinical visit or have any documented physician encounter (null imputation). Multivariable logistic regression models were used to estimate the effects of GC and physician education intervention on any recorded physician-patient communication regarding genomic testing results. In order to capture the within-physician clustering arising from physicians who recruited more than one patient, these models were estimated using generalized estimating equations with an independent working correlation structure and robust standard errors. Possible confounder effects, including patient’s age, gender, education, and disease group were also examined. Due to the sample size limitation, these effects were examined in individual models. A two-sided significance level of α=0.05 was used for all tests. All analyses were carried out in SAS version 9.4 (SAS institute, Cary, NC).
RESULTS
Patient Characteristics
Table 3a depicts basic characteristics and socio-demographic information for each arm (ITT analysis; 98 active; 101 control). Table 3b describes each arm per protocol (i.e. had GC vs. did not have GC). There were no significant differences in demographics between the study groups based on randomization or receiving GC except more males were randomized into active arm. Of 199 study participants, 137 (68.8%) had an associate’s degree or higher; 180 (90.4%) were white. There were more male participants, 107 (53.8%), than female; 25 (12.5%) worked in a health care related occupation (e.g. nursing). Mean age was 58.1 years (range from 24 to 94). All 199 study participants received email notice of the availability of the initial batch of 9 test reports in their web portal, of which 183 participants viewed at least one CPMC report. There were 40 subjects with 1 elevated genetic variant risk variable; 68 subjects with 2 elevated genetic risk variables; and 87 subjects with 3+ elevated genetic risk variables. In all, 33 (16.6%) participants had intermediate and 3 poor metabolizer response to Clopidogrel. The average number of participants’ visits to physician after initial results uploaded to EMR was 2.8 (median: 2; range 0–9). Of 80 active arm participants scheduled for in-person GC, 76 (95%) were seen. In the control arm, 15/101 contacted investigators after the randomization period and received in-person GC three or more months after viewing at least one result. Thus, the Received GC group comprised 91 individuals. Comparisons on age, gender, race, insurance, education, disease groups, and number of elevated genetic risk variants did not show significant differences between the 22 subjects in the active arm who did not receive GC and the rest of the subjects in the active arm who did receive GC. No significant difference was found in the control arm between those who received GC and those did not. Participants in the GC arm were followed-up for an average of 222 days (median: 154; range: 36–1010), which was similar to an average of 175 days follow-up for participants in the control arm (median: 101; range: 30–739).
Table 3a.
Basic characteristics for each arm (ITT analysis)
| Variable | GC arm (N=98) | Non-GC arm (N=101) | Total |
|---|---|---|---|
| Age | 57.8 ± 13.46 | 58.5 ± 12.6 | 58.1 ± 13.0 |
| Gender a | |||
| Male | 60 (61.2%) | 47 (46.5%) | 107 (53.8%) |
| Female | 38 (38.8%) | 54 (53.5%) | 92 (46.2%) |
| Race | |||
| Caucasian | 87 (88.8%) | 93 (92.1%) | 180 (90.5%) |
| Other | 11 (11.2%) | 8 (7.9%) | 19 (9.6%) |
| Education | |||
| <= high school | 9 (9.2%) | 10 (9.9%) | 19 (9.5%) |
| Some college | 17 (17.3%) | 26 (25.7%) | 33 (16.6%) |
| Associate degree | 13 (13.3%) | 12 (11.9%) | 25 (12.6%) |
| Bachelor degree | 22 (22.4%) | 27 (26.7%) | 49 (24.6%) |
| Graduate degree | 37 (37.8%) | 26 (25.7%) | 63 (31.7%) |
| Income | |||
| < $25k | 6 (6.1%) | 12 (11.9%) | 18 (9.1%) |
| $25–50k | 17 (17.4%) | 20 (19.8%) | 37 (18.6%) |
| $50–75k | 24 (24.5%) | 14 (13.9%) | 38 (19.1%) |
| $75–100k | 22 (22.5%) | 17 (16.8%) | 39 (19.6%) |
| >$100k | 26 (26.5%) | 36 (35.6%) | 62 (31.2%) |
| Did not want to answer | 3 (3.1%) | 2 (2.0%) | 5 (2.5%) |
| Insurance | |||
| Yes | 90 (91.8%) | 95 (94.1%) | 185 (93.0%) |
| No | 8 (8.2%) | 5 (4.9%) | 13 (6.5%) |
| Disease | |||
| HF | 45 (45.9%) | 54 (53.5%) | 99 (49.8%) |
| HTN | 53 (54.1%) | 47 (46.5%) | 100 (50.2%) |
| Physician-patient result communication on first interaction | |||
| Yes | 13 (13.3%) | 4 (4.0%) | 17 (8.5%) |
| No | 85 (86.7%) | 97 (96.0%) | 182 (91.5%) |
P-value for comparison between the two arms = 0.04 (Chi-square test)
Table 3b.
Basic characteristics for each arm as per protocol
| Variable | Had GC (N=91) | Did not have GC (N=108) | Total |
|---|---|---|---|
| Age | 58.5 ± 12.8 | 57.9 ± 13.2 | 58.1 ± 13.0 |
| Gender | |||
| Male | 47 (51.7%) | 60 (55.6%) | 107 (53.8%) |
| Female | 44 (48.3%) | 48 (44.4%) | 92 (46.2%) |
| Race | |||
| Caucasian | 82 (90.1%) | 98 (90.7%) | 180 (90.5%) |
| Other | 9 (9.9%) | 10 (9.3%) | 19 (9.6%) |
| Education | |||
| <= high school | 5 (5.5%) | 14 (13.0%) | 19 (9.5%) |
| Some college | 18 (19.8%) | 25 (23.2%) | 33 (16.6%) |
| Associate degree | 14 (15.4%) | 11 (10.2%) | 25 (12.6%) |
| Bachelor degree | 26 (28.6%) | 23 (21.3%) | 49 (24.6%) |
| Graduate degree | 28 (30.8%) | 35 (32.4%) | 63 (31.7%) |
| Income | |||
| < $25k | 8 (8.8%) | 10 (9.3%) | 18 (9.1%) |
| $25–50k | 15 (16.5%) | 22 (20.4%) | 37 (18.6%) |
| $50–75k | 19 (20.9%) | 19 (17.6%) | 38 (19.1%) |
| $75–100k | 20 (22.0%) | 19 (17.6%) | 39 (19.6%) |
| >$100k | 26 (28.6%) | 36 (33.3%) | 62 (31.2%) |
| Did not want to answer | 3 (3.3%) | 2 (1.9%) | 5 (2.5%) |
| Insurance | |||
| Yes | 82 (90.1%) | 103 (95.4%) | 185 (93.0%) |
| No | 8 (8.8%) | 5 (4.6%) | 13 (6.5%) |
| Disease | |||
| HF | 39 (42.9%) | 60 (55.6%) | 99 (49.8%) |
| HTN | 52 (57.1%) | 48 (44.4%) | 100 (50.2%) |
| Physician-patient result communication on first interaction | |||
| Yes | 16 (17.6%) | 4 (3.7%) | 20 (10.1%) |
| No | 75 (82.4%) | 104 (96.3%) | 179 (89.9%) |
Physician-patient result communication on first clinic interaction (ITT analysis)
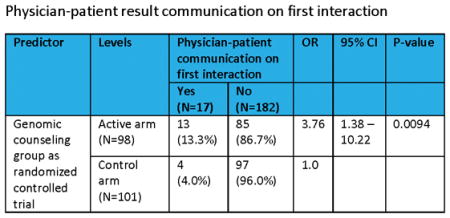
Using an ITT framework, there were a total of 17 physician-patient communications regarding genomic testing results (e.g. specialty referral; discussion of test results; Table 2b) on the patient’s first clinic interaction (13, active arm; 4, control arm). Median time to first clinic interaction was 198.5 days (range: 44–651) in the active arm and 78.5 days (range: 1–644) in the control arm. Active arm participants receiving GC as randomized was a significant predictor of physician-patient communication regarding testing results on the first clinic interaction (Table 4a). The odds of having this communication exchange on the patient’s first interaction after EMR report upload or after GC for active arm participants was 3.76 times higher than for control subjects (95% CI: 1.38 – 10.22, P<0.0094). Neither physician educational intervention nor its interaction with the GC arm was a significant predictor. The ORs were not significantly modified after adjusting for patient’s age, gender, education, disease group, or number of elevated genetic variant risks in separate modeling (Table 4b). However, the odds of having communication exchange on the patient’s first interaction for GC arm as randomized was greatly increased after adjusting for the number of days between the EMR results uploaded and the first clinic interaction (OR: 6.16, 95% CI: 2.07 – 18.37, P=0.003). The association between physician-patient result communication with age, gender, education, disease number of elevated risks, or the number of days between the EMR results uploaded and the first clinic interaction was not significant in any of the separate models.
Table 4a.
Physician-patient result communication on first interaction (ITT analysis)
| Predictor | Levels | Physician-patient communication on first interaction | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Yes (N=17) | No (N=182) | |||||
| GC group as randomized | Active arm (N=98) | 13 (13.3%) | 85 (86.7%) | 3.76 | 1.38 – 10.22 | 0.0094 |
| Control arm (N=101) | 4 (4.0%) | 97 (96.0%) | 1.0 | |||
| Physician educational intervention | Yes (N=122) | 10 (8.2%) | 112 (91.8%) | 0.82 | 0.24 – 2.80 | 0.76 |
| No (N=77) | 7 (9.1%) | 70 (90.9%) | 1.0 | |||
Table 4b.
Estimated OR of physician-patient result communication on first interaction adjusted for co-variants (ITT analysis)
| Effect | OR | 95% CI | P-value |
|---|---|---|---|
| Adjusted on patient’s age | |||
| GC group as randomized | 3.72 | 1.38 – 10.01 | 0.0095 |
| Physician educational intervention | 0.82 | 0.24 – 2.82 | 0.75 |
| Adjusted on patient’s gender | |||
| GC group as randomized | 3.47 | 1.24 – 9.70 | 0.020 |
| Physician educational intervention | 0.82 | 0.24 – 2.81 | 0.75 |
| Adjusted on patient’s education | |||
| GC group as randomized | 3.74 | 1.36 – 10.27 | 0.011 |
| Physician educational intervention | 0.81 | 0.25 – 2.68 | 0.74 |
| Adjusted on patient’s disease group | |||
| GC group as randomized | 3.67 | 1.36 – 9.90 | 0.011 |
| Physician educational intervention | 0.90 | 0.25 – 3.28 | 0.87 |
| Adjusted on patient’s number of elevated genetic variant risks | |||
| GC group as randomized | 3.70 | 1.34 – 10.22 | 0.011 |
| Physician educational intervention | 0.82 | 0.24 – 2.78 | 0.75 |
| Adjusted on the number of days between EMR results uploaded and the first interaction | |||
| GC group as randomized | 6.16 | 2.07 – 18.37 | 0.003 |
| Physician educational intervention | 0.65 | 0.19 – 2.25 | 0.52 |
Physician-patient result communication on first clinic interaction (PP analysis)
Using a “per protocol” framework, there were a total of 20 physician-patient communications regarding genomic testing results noted on the first clinic interaction after receiving GC (16, active arm; 4 control arm). Median time to first clinic interaction was 205 days (range: 44–833) for patients who received GC and 80 days (range: 1–534) for patients who did not receive GC. The distribution of physician-patient communications is shown in Figure 1. The odds for participants that received GC to engage in physician-patient communication regarding genomic testing results at the patient’s first clinic interaction after GC were 5.53 (95% CI: 2.20 – 13.90, P=0.0017) times higher than for patients who did not receive GC (Table 5a). Neither physician educational intervention nor its interaction with actual GC group was a significant predictor. The ORs were not significantly modified after adjusting for patient’s age, gender, education, disease group, the number of elevated genetic variant risks, or the number of days between EMR results uploaded and the first clinic interaction in separate models (Table 5b). The association between physician-patient result communication with age, gender, education, disease group, number of elevated risks, or the number of days between EMR results uploaded and the first clinic interaction was not significant in any of the separate models.
Table 5a.
Physician-patient result communication on first interaction as per protocol
| Predictor | Levels | Physician-patient communication on first interaction | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Yes (N=20) | No (N=179) | |||||
| GC group as per protocol | Active arm (N=91) | 16 (17.6%) | 75 (82.4%) | 5.53 | 2.20 – 13.90 | 0.0017 |
| Control arm (N=108) | 4 (3.7%) | 104 (96.3%) | 1.0 | |||
| Physician educational intervention | Yes (N=122) | 13 (10.7%) | 109 (89.3%) | 1.16 | 0.33 – 4.06 | 0.82 |
| No (N=77) | 7 (9.1%) | 70 (90.9%) | 1.0 | |||
Table 5b.
Estimated OR of physician-patient result communication on first interaction as per protocol adjusted for co-variants
| Effect | OR | 95% CI | P-value |
|---|---|---|---|
| Adjusted on patient’s age | |||
| GC group as per protocol | 5.73 | 2.38 – 13.83 | 0.0014 |
| Physician educational intervention | 1.19 | 0.34 – 4.21 | 0.78 |
| Adjusted on patient’s gender | |||
| GC group as per protocol | 5.62 | 2.27 – 13.91 | 0.0016 |
| Physician educational intervention | 1.13 | 0.31 – 4.03 | 0.85 |
| Adjusted on patient’s education | |||
| GC group as per protocol | 5.51 | 2.19 – 13.91 | 0.0018 |
| Physician educational intervention | 1.14 | 0.32 – 4.03 | 0.84 |
| Adjusted on patient’s disease group | |||
| GC group as per protocol | 5.31 | 2.16 – 13.06 | 0.0019 |
| Physician educational intervention | 1.29 | 0.34 – 4.91 | 0.70 |
| Adjusted on patient’s number of elevated genetic variant risks | |||
| GC group as per protocol | 5.61 | 2.24 – 14.06 | 0.0016 |
| Physician educational intervention | 1.11 | 0.32 – 3.83 | 0.86 |
| Adjusted on the number of days between EMR results uploaded and the first interaction | |||
| GC group as per protocol | 5.46 | 2.01 – 14.81 | 0.0028 |
| Physician educational intervention | 1.07 | 0.32 – 3.62 | 0.91 |
Physician-patient result communication for any interaction during the follow-up period
Secondary analyses were performed with any note documenting physician-patient communication about the study results throughout the follow-up period. Most of the physician-patient communication occurred on the first interaction, with only 3 additional communications (1 participant in the active arm who actually received GC; 2 in the control arm who actually received GC) noted during the remainder of the follow-up period. The effect of actual GC on physician-patient communication throughout the follow-up period was 5.03 (95% CI: 2.19 – 11.71) times the odds for participants who did not receive GC in PP analysis. The effect of receiving GC as randomized was not a significant predictor of such communication throughout the follow-up period in ITT analysis (OR: 1.69, 95% CI: 0.88 – 3.23, P=0.12). Neither ORs were significantly modified after adjusting for the total number of follow-up days.
DISCUSSION
In our study, genomic counseling of patients with chronic disease receiving potentially actionable complex disease and pharmacogenomics results in an academic medical setting was associated with increased physician-patient communication regarding testing results. The effect on increasing physician-patient communication may rest on the ability of the genetic/genomic counselor to convey appropriate risks to the patient, and how their risk can be modified by individual actions (e.g. lifestyle modification) and through interaction with their physician team.
A number of studies have shown that genetic counseling can improve individual basic genetic knowledge (17), produce more accurate risk perceptions (18) and greater perceived personal control (19–23). We found that participants in our parent chronic disease study receiving genomic counseling had enhanced objective understanding of the genetic variant risk contribution to potentially actionable complex disease reports (24). Another study found that among women who received comprehensive BRCA testing ordered by their clinician, those receiving pre-test genetic counseling demonstrated improved knowledge and understanding of the information received, and greater satisfaction than non-counselees (21).
Although it is not clear in our study who initiated conversations (patients or physicians) regarding genomic testing results, having access to their personalized genomic data may help activate patients to take independent actions to manage their disease risks (25, 26) particularly when accompanied by genomic counseling. Patients who are more activated are more likely to self-manage and take a more proactive role in their health care than patients who are less activated (27). Given that counselees were provided specific actions to prevent or lower disease risk, both in the counseling session and in the GC summary letter, and advised to speak to their study physician team regarding these recommended actions, it is likely these recommendations would have been fresh in the patient’s mind when they next saw or contacted their physician, which we found to be the case in our EMR review. Continued integration of genomic counselors to provide this necessary support and positive reinforcement can help patients discuss potentially actionable results with their physicians, who may not otherwise address them, and increase personal utility of the information.
We had a 21.9% rate of physician-patient communication in the setting of genomic counseling. This is somewhat low but not surprising, given the physician educational intervention occurred at the onset of patient recruitment, was a one-time event, and the test results were forthcoming for newly accrued patients over the course of more than two years. Moreover, we took a passive approach in uploading test reports and GC summary letters into the EMR without active notification of the physician team as per study protocol. It is possible that physicians may not have perceived much value or benefit in the results. Although concern remains that personal genome results could lead to unnecessary workup (28), significant post-test increases in the use of medical procedures (e.g. mammogram) are not well supported by the current literature (13, 21, 29), and were not found in our study (Table 2b). It is also likely that time was limited during visits with patients with at least one chronic disease, and therefore other medical concerns took precedence.
The low rate of physician-patient result communication does suggest an opportunity for more active alerting of the physician team regarding genomic consultations about potentially actionable results, and that we should be more active in EMR documentation and routing of potentially actionable results and their resulting preventive recommendations. Knowing that physicians may not raise the topic of genomic testing results during consultations with patients, even when they are available in the EMR, suggests an opportunity for intervention. Genomic counselors and other health care providers trained in patient activation can facilitate this intervention by building patient confidence and encouraging patients to talk to their physicians about their results.
The study has some limitations. First, the data were extracted from electronic chart notes. The content of undocumented oral communications between providers and patients, and the providers’ unwritten thought processes, is unknown. It is possible that additional discussions about study results and/or the genomic counseling intervention did take place, was not recorded, or was recorded incorrectly by the physician. Second, due to sample size constraints, we were unable to adjust for all variables in a single logistic regression model. Moreover, these analyses are likely underpowered to allow comment about the possible effects on physician education. Lastly, these results may not generalize across practice settings, or more diverse populations.
Our data suggest that genomic counseling appreciably affected physician-patient communication post-receipt of genomic risk information for multiple complex disease risks. Results of a recent systematic review suggest that communicating DNA based disease risk estimates has little or no effect on risk-reducing health behaviors (30), however, none of these prior studies facilitated participant behavior modification by providing genomic counseling for a range of multifactorial disease risks (relative risks 0.08 – >6.0), and incorporating a summary plan with action steps provided to both the patient and provider team as was done in this study. Outcomes were primarily based on self-report of behavior change, and did not involve EMR review. Continued investigation of genomic counseling intervention in the genomic result delivery process, with the potential to motivate positive change in health-related measures is warranted (31).
Supplementary Material
Sample CPMC® Coronary Artery Disease Report
Solid discs represent the participant’s relative risk, and vertical cylinders depict the range of RR values possible for the risk variable. On-line risk reports are organized using a tabbed approach, with separate tabs for disease condition information, risk results, limitations, methods, and links to request genetic counseling, or review educational material. To ensure readability, the CPMC report design was informed by multiple rounds of pilot testing conducted by allowing individuals with no scientific background to review report drafts and provide feedback. The CPMC chose to report relative risks to study participants because this approach allows for the reporting of all disease risk factors (genetic, family history, and lifestyle) using the same metric and does not require population estimates of disease incidence. The 8 CPMC health condition risk reports included in this study often present genetic variant risk based on a single SNP because of the lack of validated multigenic models with robust prediction. Risk estimates provided for non-genetic risk factors (family history and lifestyle or environmental factors) were derived or reported from valid and representative peer-reviewed publications. Non-genetic risk factors were included if they meet two criteria: the risk factor must be collected by the baseline required lifestyle questionnaire and the risk factor must be an established disease risk, included in multiple disease review articles and consistently associated with disease.
Acknowledgments
We thank Albert de la Chapelle, Clay Marsh, Philip Binkley, Naraj Tayal, Randy Wexler, Mary Jo Welker, Anup Kanodia, Wolfgang Sadee, Karen Wernke, Samantha DeMarsh, Sarah Adelsperger, Jennifer Lehman, Sarah Mazzola, Regina Dudzik, Amy Ehrlich, Sofia Durrani, and Meagan Kane for their kind assistance with this study.
Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R21HG006575. This work was also supported in part by grant U01 GM92655 and a sub-award for the Translational Pharmacogenomics Project (TPP) via U01 HL105198 Pharmacogenomics of anti-platelet intervention-2 (PAPI-2) from NHLBI; a sub-award from the National Center For Advancing Translational Sciences via Award Number UL1TR001070; and the Ohio State University Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Center for Advancing Translational Sciences. The Coriell Personalized Medicine Collaborative was funded by the William G. Rohrer Foundation, the RNR Foundation and a grant from the endowment of the Coriell Institute for Medical Research.
Footnotes
Competing Interests: The authors have no conflicts of interest to disclose
AUTHOR CONTRIBUTIONS
KS, ACS, TS, AS and JSR conceived of and helped design the study. ACS, TS, AS, KM, SH, AET JSR, and MC participated in the trial design and conception, conceptualized these analyses and advised on statistical analyses, and served as overall co- investigators. JP, JM and LS helped conceptualize these analyses and advised on statistical analyses. KS served as the study principal investigator, and oversaw all aspects of study execution. All authors helped to draft the manuscript, and read and approve the final version.
References
- 1.Biesecker LG. Opportunities and challenges for the integration of massively parallel genomic sequencing into clinical practice: lessons from the ClinSeq project. Genetics in medicine : official journal of the American College of Medical Genetics. 2012;14:393–398. doi: 10.1038/gim.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewey FE, Grove ME, Pan C, et al. Clinical interpretation and implications of whole-genome sequencing. Jama. 2014;311:1035–1045. doi: 10.1001/jama.2014.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feero WG, Manolio TA, Khoury MJ. Translational research is a key to nongeneticist physicians’ genomics education. Genetics in medicine : official journal of the American College of Medical Genetics. 2014 doi: 10.1038/gim.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 5.Hartzler A, McCarty CA, Rasmussen LV, et al. Stakeholder engagement: a key component of integrating genomic information into electronic health records. Genetics in medicine : official journal of the American College of Medical Genetics. 2013;15:792–801. doi: 10.1038/gim.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassy JL, Korf BR, Green RC. How to know when physicians are ready for genomic medicine. Science translational medicine. 2015;7:287fs219. doi: 10.1126/scitranslmed.aaa2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guttmacher AE, McGuire AL, Ponder B, et al. Personalized genomic information: preparing for the future of genetic medicine. Nature reviews Genetics. 2010;11:161–165. doi: 10.1038/nrg2735. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JS, Ostergren J. Direct-to-Consumer Genetic Testing and Personal Genomics Services: A Review of Recent Empirical Studies. Current genetic medicine reports. 2013;1:182–200. doi: 10.1007/s40142-013-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloss CS, Wineinger NE, Darst BF, et al. Impact of direct-to-consumer genomic testing at long term follow-up. Journal of medical genetics. 2013;50:393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- 10.Back A. Patient-physician communication in oncology: what does the evidence show? Oncology (Williston Park) 2006;20:67–74. discussion 77–68, 83. [PubMed] [Google Scholar]
- 11.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Medical care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman DJ, Bollinger JM, Dvoskin RL, et al. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. Journal of genetic counseling. 2012;21:413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 13.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. The New England journal of medicine. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Wouden CHCD, Maitland-van der Zee AH, Ruffin MT, 4th, Roberts JS, Green RC Impact of Personal Genomics Study Group†. Consumer Perceptions of Interactions With Primary Care Providers After Direct-to-Consumer Personal Genomic Testing. Ann Intern Med. 2016 doi: 10.7326/M15-0995. [DOI] [PubMed] [Google Scholar]
- 15.Sweet K, Gordon ES, Sturm AC, et al. Design and Implementation of a Randomized Controlled Trial of Genomic Counseling for Patients with Chronic Disease. Journal of personalized medicine. 2014;4:1–19. doi: 10.3390/jpm4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inter-Society Coordinating Committee for Practitioner Education in Genomics (ISCC) National Human Genome Research Institute; 2015. [Google Scholar]
- 17.Matloff ET, Moyer A, Shannon KM, et al. Healthy women with a family history of breast cancer: impact of a tailored genetic counseling intervention on risk perception, knowledge, and menopausal therapy decision making. J Womens Health (Larchmt) 2006;15:843–856. doi: 10.1089/jwh.2006.15.843. [DOI] [PubMed] [Google Scholar]
- 18.Smerecnik CM, Mesters I, Verweij E, et al. A systematic review of the impact of genetic counseling on risk perception accuracy. Journal of genetic counseling. 2009;18:217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson CL, Jouni H, Kruisselbrink TM, et al. Disclosing genetic risk for coronary heart disease: effects on perceived personal control and genetic counseling satisfaction. Clinical genetics. 2015 doi: 10.1111/cge.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkenstadt M, Shiloh S, Barkai G, et al. Perceived personal control (PPC): a new concept in measuring outcome of genetic counseling. American journal of medical genetics. 1999;82:53–59. doi: 10.1002/(sici)1096-8628(19990101)82:1<53::aid-ajmg11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong J, Toscano M, Kotchko N, et al. Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study. JAMA oncology. 2015:1–10. doi: 10.1001/jamaoncol.2015.3048. [DOI] [PubMed] [Google Scholar]
- 22.Boeldt DL, Schork NJ, Topol EJ, et al. Influence of individual differences in disease perception on consumer response to direct-to-consumer genomic testing. Clinical genetics. 2015;87:225–232. doi: 10.1111/cge.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sie AS, Spruijt L, van Zelst-Stams WA, et al. High Satisfaction and Low Distress in Breast Cancer Patients One Year after BRCA-Mutation Testing without Prior Face-to-Face Genetic Counseling. Journal of genetic counseling. 2015 doi: 10.1007/s10897-015-9899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweet KSA, Schmidlen T, McElroy J, Scheinfeldt L, Manickam K, Gordon E, Hovick H, Roberts JS, Toland AE, Christman M. Outcomes of a randomised controlled trial of genomic counselling for patients receiving personalized and actionable complex disease reports. 2015 doi: 10.1007/s10897-017-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibbard J, Greene J. What The Evidence Shows About Patient Activation: Better Health Outcomes And Care Experiences; Fewer Data On Costs. Health Aff (Millwood) 2013;32:207–214. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 26.Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genetics in medicine : official journal of the American College of Medical Genetics. 2009;11:570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- 27.Tzeng ATT, Vasdev S, Grindy A, Saleh J, Saleh KJ. The Role of Patient Activation in Achieving Better Outcomes and Cost-Effectiveness in Patient Care. JB-JS Reviews. 2015;3:1–10. doi: 10.2106/JBJS.RVW.N.00048. [DOI] [PubMed] [Google Scholar]
- 28.McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. Jama. 2008;300:2669–2671. doi: 10.1001/jama.2008.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darst BF, Madlensky L, Schork NJ, et al. Perceptions of genetic counseling services in direct-to-consumer personal genomic testing. Clinical genetics. 2013;84:335–339. doi: 10.1111/cge.12166. [DOI] [PubMed] [Google Scholar]
- 30.Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin J. The effect of genetic test-based risk information on behavioral outcomes: A critical examination of failed trials and a call to action. American journal of medical genetics Part A. 2015;167:2913–2915. doi: 10.1002/ajmg.a.37289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample CPMC® Coronary Artery Disease Report
Solid discs represent the participant’s relative risk, and vertical cylinders depict the range of RR values possible for the risk variable. On-line risk reports are organized using a tabbed approach, with separate tabs for disease condition information, risk results, limitations, methods, and links to request genetic counseling, or review educational material. To ensure readability, the CPMC report design was informed by multiple rounds of pilot testing conducted by allowing individuals with no scientific background to review report drafts and provide feedback. The CPMC chose to report relative risks to study participants because this approach allows for the reporting of all disease risk factors (genetic, family history, and lifestyle) using the same metric and does not require population estimates of disease incidence. The 8 CPMC health condition risk reports included in this study often present genetic variant risk based on a single SNP because of the lack of validated multigenic models with robust prediction. Risk estimates provided for non-genetic risk factors (family history and lifestyle or environmental factors) were derived or reported from valid and representative peer-reviewed publications. Non-genetic risk factors were included if they meet two criteria: the risk factor must be collected by the baseline required lifestyle questionnaire and the risk factor must be an established disease risk, included in multiple disease review articles and consistently associated with disease.