Abstract
Increasing amounts of pathogen replication usually leads to a proportionate increase in size and effector differentiation of the CD8+ T cell response, attributed to increased antigen and inflammation. Using a murine cytomegalovirus (MCMV) that is highly sensitive to the antiviral drug famcyclovir to modulate virus replication, we found that increased virus replication drove increased effector CD8+ T cell differentiation, as expected. Paradoxically, however, increased virus replication dramatically decreased the size of the CD8+ T cell response to two immunodominant epitopes. The decreased response was due to type-I interferon-dependent depletion of conventional dendritic cells (cDC), and could be reproduced by specific depletion of DCs from day 2 post-infection, or by sterile induction of type-I IFN. Increased virus replication and type I IFN specifically inhibited the response to two immunodominant epitopes that are known to be dependent on antigen cross-presented by DCs, but did not inhibit the response to “inflationary” epitopes whose responses can be sustained by infected non-hematopoietic cells. Our results show that type I IFN can suppress CD8+ T cell responses to cross-presented antigen by depleting cross-presenting cDCs.
Materials and Methods
All studies were performed under the approval of the Institutional Biosafety Committee. All animal work was performed in accordance with the NIH guidelines and the OHSU Institutional Animal Care and Use Committee. The OHSU department of Comparative Medicine and Division of Animal Resources have the accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Mice and Famcyclovir treatment
C57BL/6, CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ), BALB/c, and CD11-c DTR (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) were purchased from Jackson Laboratory. C57BL/6 mice were bred to CD45.1 mice, in-house, to generate CD45.2 x CD45.1 F1 mice used for adoptive transfer experiments. Mice used were between the ages of 6–36 weeks. Famcyclovir [250mg] pills were crushed and dissolved in water at a 2mg/mL concentration (Roxane Laboratories). Famcyclovir treatment was administered continuously starting at 3 days prior to infection.
Virus strains
All mice were inoculated with 2*105 PFU i.p. of MCMV-TK or MCMV-delta m157 (dm157) virus. Preparation of MCMV-TK and MCMV-dm157 mice is previously described by Snyder et al. In short, the thymidine kinase (TK) gene was amplified from a plasmid (derived from HSV-1) and subcloned into a plasmid containing kanamycin and flanked with FRT recombination sites. The TK-Kan construct was amplified with MCMV sequences of the m157 region and homologous recombined with wild-type MCMV cloned into an artificial bacterial chromosome (BAC, strain MW9701). The result was the replacement of the m157 gene with TK.
Detection of antigen specific CD8 T cells
Antigen-specific CD8 T cells were detected by peptide stimulation or through staining by epitope specific tetramers (made by the NIH tetramer core). Peptides used to stimulate or bound to tetramers were M45 (HGIRNASFI), M57 (SCLEFWQRV), m139 (TVYGFCLL), and M38 (SSPPMFRV).
For Intracellular Cytokine Staining (ICS), cells were cultured for 6 hours at 37°C with peptides and brefeldin A [10μg/ml]. DMSO was cultured with cells as an experimental negative control. Cells where then stained for cell surface markers followed by a cell fixation and permabolization with BD Cytofix/Cytoperm kit (according to manufacture’s protocol). Following permabolization, intracellular markers where stained with fluorochrome conjugated antibodies.
Tetramer and surface phenotype of CD8 T cells were performed at 4°C, 1 hour. The following fluorescently labeled antibodies used in this study were: KLRG1 (Biolegend, clone:2F1/KLRG1), CD127 (Biolegend, clone: A7R34), PD-1 (Biolegend, clone: RMP1-30), CD8α (Biolegend, clone: 53–6.7), CD3 (clone 145-2C11), CD11b (eBioscience, clone M1/70), CD11c (eBioscience, clone: N418), Live/Dead Fixable Aqua Dead Cell Stain Kit 405 (Invitrogen). All samples were measured on a BD LSRII flow cytometer. Data was analyzed by Flowjo software (Tree Star Inc., Ashland, OR).
Dendritic cell isolation
Spleens were harvested and minced. Samples were incubated at 37 degrees with collagenase D and DNase for 30 minutes, mixing every 10 minutes. Tissue was smashed and filtered through a 70 μm mesh filter and washed with 10% FBS RPMI.
Dendritic cell populations were gated from the following gating scheme: singlets [FSC-A/FSC-H], live cells [Amine aqua-], CD3-, CD11c+, MHCII+. Conventional DCs were identified as CD11chiMHCII+ and plasmacytoid DCs were identified as CD11cintPDCA-1+.
Adoptive Transfer
CD45.1 x CD45.2 F1 mice were bred in house. CD8 T cells were purified from spleens by a magnetic negative selection kit (EasySep™ Mouse CD8+ T Cell Isolation Kit, StemCell Technologies) and 3*106 pure CD8 T cells (>95%) were transferred by i.v. injection.
Cytokine Array
Quantification of circulating cytokines and chemokines was measured in the plasma of infected mice. Plasma was collected on multiple days and frozen at −80°C until all specimens were collected. Plasma was thawed and analyzed by a multiplex ELISA assay. (Quansys Biosciences: Mouse Cytokine Screen cat#110951MS or Life Technologies: Cytokine Mouse Magnetic 10-PLEX Kit cat# LMC001M). IFN-α was measured by a single-plex ELISA (Life Technologies).
TLR agonist, Diphtheria Toxin, and blocking antibody administration
Diphtheria Toxin (Sigma) was giving in a single i.p. injection at a concentration of 3 ng per gram of body weight. Poly(I:C) was a gift from Dr. Obrahi at OHSU (Invivogen Cat: tlrl-pic) and was administered i.p. to mice at 100μg/mouse on days 1 and 2 post-infection. MAR1-5A3 mAb and GIR-2308 isotype control IgG1 was purchased from BioXcell and administered i.p. at 1mg/mouse, 12 hours post-infection. PD-L1 blocking antibody (10F.9G2) was purchased from BioXcell. Anti-PD-L1 treatment started at day 0 pi (administered at 200ug/mouse i.p., every three days).
Statistics
Prism software was used for statistical analysis (GraphPad Software, Inc.).
Introduction
Cytomegalovirus (CMV) is a ubiquitous virus, infecting most mammalian species with species-specific CMV strains. As a member of the herpesvirus family, it infects its host for a lifelong persistent/latent infection, which is usually asymptomatic. One of the most remarkable aspects of CMV immunobiology is the size of the CD8+ T cell response to it, particularly during the chronic phase of infection. In order to understand the role of chronic virus replication in driving the response, we generated a recombinant murine (M)CMV in which virus replication could be completely inhibited by drug treatment (1). During our initial characterization of acute infection with that reagent, we observed a striking phenomenon: when virus replication was completely inhibited by commencing drug treatment prior to infection, there was a paradoxical dramatic increase in the peak size of the CD8+ T cell response. Here we describe our investigation of that phenomenon.
Larger amounts of pathogen during acute infection generally elicit larger CD8+ T cell responses, triggered both by more antigen and more inflammation (1,2). This has been clearly dissected in studies of the CD8+ T cell response to listeria monocytogenes (LM), where increasing bacterial dose increased the response (2), and, conversely, antibiotic treatment shortly after infection severely inhibited the response (3).
For MCMV, the relationship between virus load and the acute CD8+ T cell response has mostly been investigated in the context of NK cells, where altered efficacy of NK cells led to a difference in virus load during acute infection. The results have been mixed, with either positive or negative impacts of NK cells on the kinetics and peak CD8+ T cell response (4–7), reviewed in (8). These studies are complicated by use of virus strains that differ in virulence, and by use of mouse strains that differ in susceptibility to MCMV. BALB/c and C57BL/6 mice differ at the Ly49H (cmv1) locus that determines the ability of NK cells to control MCMV, but also in other poorly understood ways that impact both CMV control and the T cell response. More importantly, the complex cross-talk between NK cells and dendritic cells that occurs during infection, with each cell type supporting the other, makes it difficult to determine whether NK cells impact the T cell response through immunological mechanisms or via their effect on virus titers (6, 7, 9). In addition to profoundly impacting virus titers, NK cells can influence the CD8+ T cell response in multiple ways, including killing virus-infected APCs (10) while promoting survival of uninfected DCs for effective T cell stimulation (6). We decided to remove NK cell control as a variable by using a virus lacking the NK-stimulatory protein encoded by m157, and to vary virus titer by pharmacological inhibition of virus replication, in order to cleanly dissect the relationship between virus replication and the CD8+ T cell response to MCMV.
In this study, we used a recombinant MCMV expressing the thymidine kinase (TK) gene from Herpes Simplex Virus (MCMV-TK) (1). This rendered the MCMV-TK exquisitely sensitive to the anti-viral drug famcyclovir in mice, enabling us to block viral replication without relying on NK cell-mediated resistance. Surprisingly, we found that completely blocking MCMV replication during acute infection had the paradoxical effect of augmenting certain CD8 T cell responses. Specifically, the already large responses to two immunodominant epitopes were augmented, while other responses were unaffected. This effect was directly attributable to a reduction in type I IFN and the consequent increased survival of cDCs when viral replication was blocked. Importantly, the magnitude of the anti-viral CD8 T cell populations could be enhanced by blocking type I IFN signaling, or reduced by the depletion of DCs or induction of type I IFN. These data show that type I IFN-dependent loss of cDCs during acute viral replication can limit the magnitude of some anti-viral CD8 T cell populations.
Results
Blocking viral replication enhances the acute CD8+ T cell response to MCMV
Native MCMV is only moderately sensitive to inhibition by nucleoside analog antivirals. In order to investigate the impact of virus replication on the ensuing CD8+ T cell response, we generated an MCMV that is sensitive to nucleoside analog antiviral drugs by inserting the thymidine kinase (TK) gene from herpes simplex virus (HSV) into the m157 locus of MCMV. The m157 locus is the preferred locus for insertion of transgenes since it removes the ability of C57BL/6 mice to sense MCMV through the activating NK receptor Ly49H, and in consequence makes handling of MCMV by C57BL/6 mice similar to most wild mouse strains (11). HSV-TK renders MCMV sensitive to the nucleoside analog famcyclovir, and we have previously shown that MCMV-TK replication is completely inhibited by famcyclovir in vitro and in vivo (1).
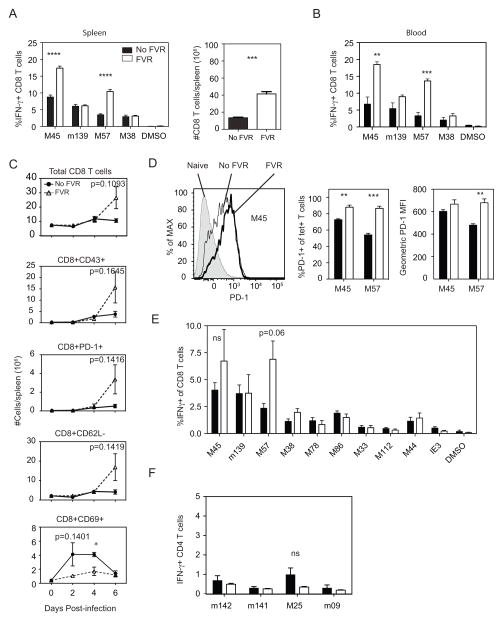
We examined the impact of blocking MCMV replication on the CD8+ T cell response to immunodominant epitopes, expecting that the much lower antigenic load would lead to a reduction in the CD8+ T cell response. Indeed, previous work has shown that blocking replication of Listeria monocytogenes leads to a truncated effector CD8+ T cell response and early formation of memory T cells (3). Strikingly, however, pre-treating mice with famcyclovir before MCMV-TK infection resulted in an increase in the number of CD8 T cells and the frequency of MCMV-specific CD8+ T cells in the spleen and blood at day 7 post-infection (Figure 1A and B). The increase was particularly dramatic in responses to M45 and M57, two responses that are immunodominant at the peak of the acute response (12, 13). This increase in the frequency of antigen-specific T cells was accompanied by a marked expansion of total CD8+ T cells in the spleen as early as day 6 post-infection (Figure 1C), consistent with the known ability of uncontrolled MCMV infection to deplete lymphocytes from the spleen (14). Moreover, significantly larger numbers of splenic CD8+ T expressed the activation markers CD43 and PD-1 while losing CD62L expression (Figure 1C). These phenotypic changes are characteristic of antigen-driven activation and T cell clonal expansion. Notably, the frequency and intensity of expression of the inhibitory receptor PD-1 was not reduced by famcyclovir treatment: in fact, it was as high or higher on M45- and M57-specific cells in famcyclovir-treated mice compared to untreated mice (Figure 1D). Thus, inhibition of clonal expansion by PD-1-mediated T cell suppression cannot explain the difference in antigen-specific CD8+ T cell numbers. Interestingly, CD69 expression was affected by famcyclovir in the opposite direction to the other activation markers (Figure 1C). CD69 expression peaked earlier and was more marked on T cells in the untreated animals. Although expression of CD69 is frequently interpreted as indicating antigen-driven T cell activation, because it is transiently induced on T cells by TCR signaling, it is also known to be upregulated in an antigen-independent manner by cytokines on bystander cells (15). Thus, collectively, these data are consistent with the notion that, when virus replication is inhibited there is less non-specific cytokine stimulation, and hence less CD69 expression. However, activation markers associated with TCR signaling (CD43, PD-1, CD62L downreguation), as well as numbers of virus-specific CD8+ T cells were increased, which suggests that when virus replication is inhibited, there is a paradoxical increase in antigen-driven T cell stimulation.
Figure 1. Enhanced Ag-specific CD8 T Cells in C57BL/6 mice with Replication-deficient MCMV-TK.
C57BL/6 mice were treated with Famcyclovir-fortified water, starting 3 days prior to infection. Mice were infected with 2*105 PFU of virus i.p. and after 7 days of infection splenocytes and blood were analyzed for CD8 T cell activation and the frequency of antigen-specific (Ag) cells by flow cytometry. (A) The IFN-γ response to peptide stimulation identifies Ag-specific CD8 T cells and total numbers of CD8 T cells from the spleen. (B) The IFN-γ response to peptide stimulation identifies Ag-specific CD8 T cells in the blood. (C) The frequency of CD8 T cells from the total lymphocyte population, as well as the expression of CD43, PD-1, CD62L, and CD69 on CD8 T cells. Data points were measured at 2, 4, and 6 days pi. (D) Shown is the PD-1 expression on tetramer+ M45 specific cells. The representative graphs show the frequency of PD-1 on M45 and M57-specific T cells and the geometric mean fluorescence intensity (MFI) from tetramer+ PD-1 CD8 T cells. MCMV produces a wide breath of antigenic peptides. (E) Shown is the IFN-γ response after peptide stimulation to dominant and subdominant peptide epitopes. (F) To identify if Famcyclovir treatment enhances CD4 T cells response during acute infection, splenocytes were stimulated with known CD4 specific peptides. Graphs represent the average with the SEM and significance as determined by a student t-test (*p<0.05, **p<0.005, ***p<0.0005). Bars represent 3 mice per group. Figure 1C and 1E experiments were performed once, all other experiments have been repeated at least 2 times.
We analyzed the T cell response in the spleen to a broader spectrum of epitopes (Figure 1E). This revealed that the increased response in famcyclovir-treated mice was largely restricted to the M45 and M57 epitopes, two of the most immunodominant responses during acute infection. Moreover, the frequency of antigen-specific CD4+ T cell responses was largely unaffected, and certainly not increased, by famcyclovir treatment (Figure 1F). In sum, our data show that inhibiting virus replication resulted in the selective increase of certain CD8+ T cell responses. The responses that were increased (M45 and M57) are immunodominant during acute infection (12); subdominant responses were not enhanced by famcyclovir. However, the frequency of CD8+ T cells responding to m139, which is also an immunodominant epitope, was not increased by famcyclovir. At this point, the basis for the selective impact of famcyclovir was quite unclear. We decided to focus our efforts on understanding the mechanism by which the M45 and M57 responses were increased by famcyclovir.
Famcyclovir does not increase the CD8 T cell response to MCMV-TK in the presence of simultaneous uninhibited viral replication
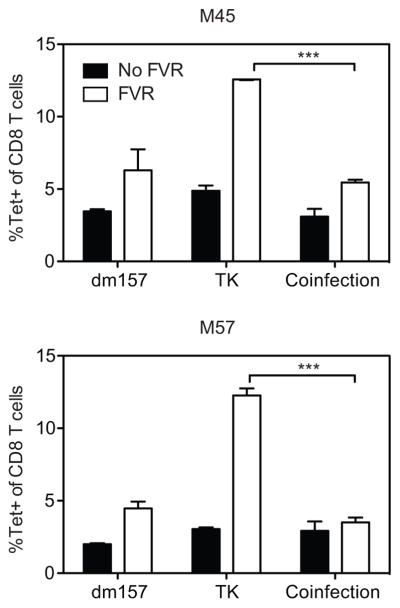
There are two basic ways in which famcyclovir treatment could result in an enhanced CD8+ T cell response to MCMV-TK. First, famcyclovir (in the presence of TK) could specifically boost the response: for example, viral DNA incorporating famcyclovir could act as a previously unappreciated adjuvant. Alternately, in untreated animals, uninhibited viral replication could suppress the potential response. If the first explanation were true, famcyclovir+TK should boost the response even in the presence of uninhibited (wild-type MCMV) viral replication. Conversely, if the second explanation were true, the presence of active virus replication should prevent the ability of famcyclovir to augment the CD8+ T cell response to MCMV-TK. To distinguish these possibilities, we performed a co-infection experiment, in which mice were infected with the virus that could be inhibited by famcyclovir (MCMV-TK), which lacks m157, at the same time as a wild-type virus that was similarly deficient in m157 but resistant to famcyclovir (MCMV-dm157). As shown in Figure 2, famcyclovir treatment had only a very small impact on the frequency of M45- or M57-specific T cell populations in mice infected with MCMV-dm157 lacking HSV TK. Wild-type MCMV replication is inhibited, although only modestly, by famcyclovir, suggesting that a modest inhibition of virus replication resulted in a small increase in T cell responses. In contrast, as shown previously, famcyclovir treatment strongly boosted the frequency of M45- and M57-specific T cells when mice were infected with the drug-sensitive MCMV-TK. Strikingly, co-infection with both viruses prevented famcyclovir from boosting the frequency of M45- and M57-specific populations above the level seen in famcyclovir-treated WT-infected mice. Therefore, these data argue that famcyclovir is not acting as an adjuvant and instead suggest that viral replication directly or indirectly suppresses the CD8 T cell response to MCMV infection.
Figure 2. Mice co-infected with replicating dm157-TK lack enhanced M45 and M57-specific responses with famcyclovir treatment.
At day 7pi, the frequency of splenic M45 and M57-tetramer specific CD8 T cells was measured from a single or co-infection of mice with dm157-TK (2*105 PFU) and MCMV-TK (2*105 PFU). Graphs represent the average with the SEM and significance as determined by a student t-test (***p<0.0005). Bars represent three mice per group. Experiments were done three times.
Impact of famcyclovir on CD8+ T cell expansion, effector phenotype, contraction and memory
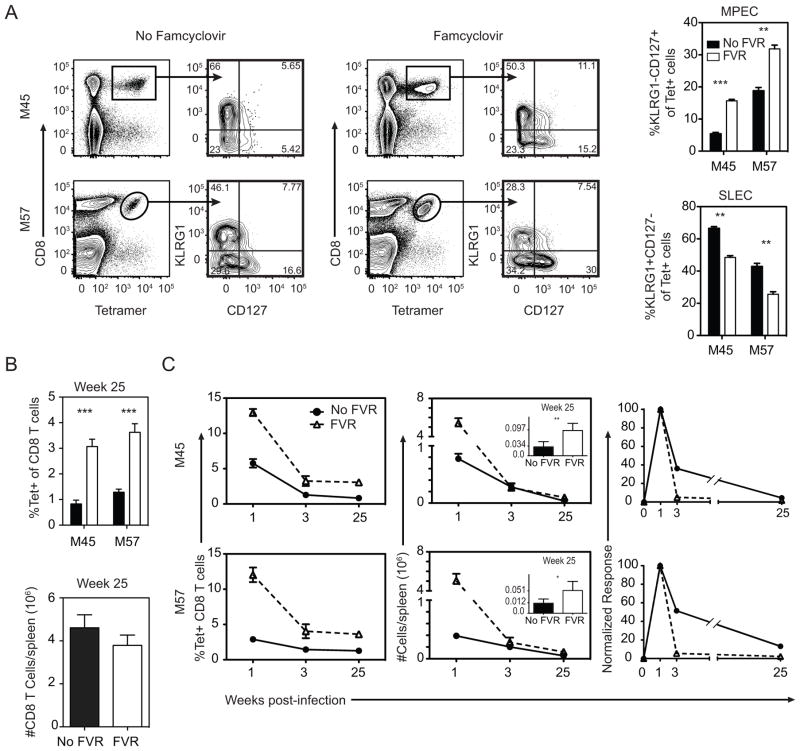
The primary CD8+ T cell response to acute infection results in two populations of cells: short lived effector cells (SLEC), that are destined to die, and memory precursor effector cells (MPECs) that can form long-lived memory cells (16). These populations are distinguished by the markers KLRG-1 and CD127 (16). Previous work has shown that truncating acute pathogen replication reduces inflammation, which in turn results in accelerated T cell memory formation (17). In line with this, famcyclovir treatment increased the proportion of M45- and M57-specific cells that displayed an MPEC phenotype, and decreased the percentage of cells with a SLEC phenotype (Figure 3A). We predicted that this increase in MPEC cells at acute time points would result in significantly higher numbers of memory cells at late times after infection. Indeed, by 25 weeks post-infection, famcyclovir-treated mice had significantly more M45- and M57-specific CD8+ T cells compared to untreated mice (Figure 3B). Interestingly, however, the differential between the two conditions was not as high as expected from their difference at the peak of the response, particularly taking into account the difference in MPEC frequency. Analyses of the kinetics revealed that M45- and M57-specific T cells underwent a more rapid rate of contraction in famcyclovir-treated mice (Figure 3C). In sum, however, these data indicate that famcyclovir treatment enabled a larger primary T cell response and also increased memory T cell numbers.
Figure 3. Impact of virus replication on expansion and contraction of Ag-specific populations.
Mice were infected with MCMV-TK and monitored for expansion, contraction, and memory-effector phenotype over time. (A) The dot plots and graphs show the frequency of short-lived effector cells (CD127lo, KLRG1hi) and memory-precursor effector cells (CD127hi, KLRG1lo) of tetramer+ cells at day 7 pi. At week 25 pi the (B) frequency of M45 and M57-specifc and total number of CD8 T cells in the spleen were quantified. (C) The graphs show the frequency and absolute number kinetics of the M45 and M57-specific cells at weeks 1, 3, and 25 pi (inset graph: Week 25 numbers). Absolute numbers of M45 and M57-tetramer+ cells were normalized to week one data. Graphs represent the average with the SEM and significance as determined by a student t-test (*p<0.05, **p<0.005, ***p<0.0005). Bars represent three mice per group. Experiments were done twice.
Famcyclovir suppresses the inflammatory response
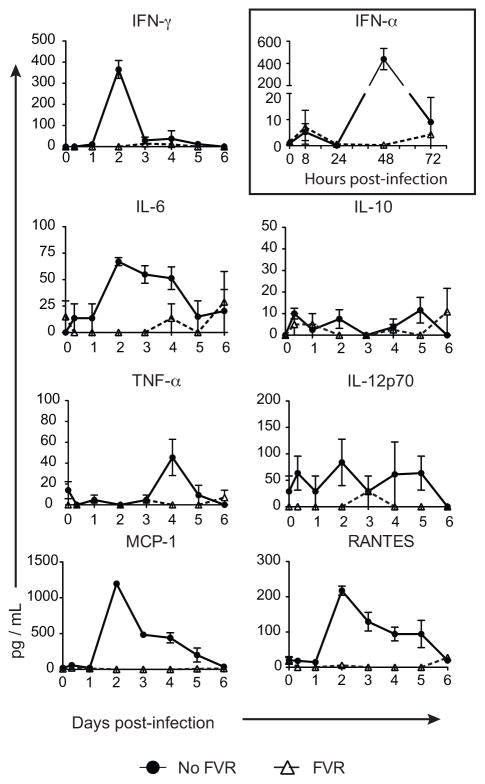
In published models of other infections, inflammatory cytokines increase the magnitude of the CD8+ T cell response (18). However, at the same time, inflammatory cytokines drive the responding T cells towards a more differentiated SLEC phenotype(16). Since famcyclovir inhibits viral replication, it would be expected to limit the acute inflammatory response stimulated by MCMV infection. Thus, the results reported above are discordant with the expected relationship between inflammation, CD8+ T cell population size and the production of SLECs. To investigate the impact of famcyclovir on inflammation, we measured key inflammatory cytokines over the first few days of infection (Figure 4). As expected, the very earliest cytokine response to MCMV– an increase in type I IFN at 8 hours post-infection (19, 20)– was identical in the presence or absence of drug. However, subsequent cytokine responses were absent or severely muted in the drug-treated animals. Both type I IFN and IL-12 act on CD8+ T cells to promote proliferation (“signal 3”), yet these cytokines were expressed at much higher levels in animals that did not receive famcyclovir, in which the CD8+ T cell responses were lower. IL-10 can suppress immune responses, yet this cytokine was not found in significant amounts in either infection condition, and is thus unlikely to account for the difference. Overall, inflammatory responses were similar to those described in the literature for the infected animals that did not receive famcyclovir, and were, as expected, very low in the drug-treated animals.
Figure 4. Famcyclovir Treatment Abrogates Acute Inflammatory Cytokines.
Mice were infected with MCMV-TK and plasma was collected at multiple time-points post-infection and subjected to a single or multiplex cytokine detection assay. The shown graphs represent the quantity of peripheral cytokines and chemokines (IFN-γ, IL-6, IL-10, IL-12p70, MCP-1, and RANTES) on days 0–6 pi. The graph in the box shows the peripheral IFN-α response measured at times 0, 8, 24, 48, and 72 hours post-infection. Mice without Famcyclovir (FVR) treatment are in the closed circles and the FVR treated mice are represented by open triangles. Each time point represents three mice per group. Experiments were done twice.
Famcyclovir prevents depletion of conventional DCs
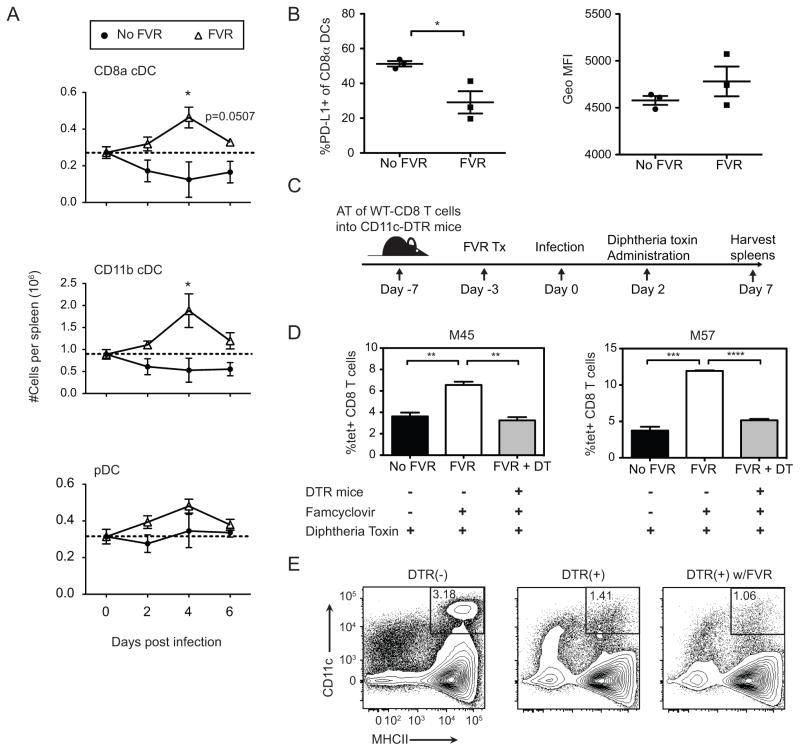
In the absence of effective NK cell control, MCMV is known to deplete conventional (CD11c+) dendritic cells (cDC) from the spleen during acute infection (4, 19, 21). Since our virus lacks m157, it is not susceptible to control by Ly49H+ NK cells. Therefore, we thought that MCMV-TK might deplete cDCs, and that famcyclovir might prevent this depletion. We therefore quantified splenic dendritic cell numbers on days 0, 2, 4, and 6 days post-infection, measuring two cDC subsets (CD11b+ and CD8+) as well as plasmacytoid DCs (pDC) (Figure 5A). Without famcyclovir treatment, both cDC subsets declined by day 2 pi and remained low. In contrast, in the presence of famcyclovir, cDCs were not depleted, and both subsets in fact increased in number by day 4 pi. Consistent with previous reports, pDC numbers were not depleted by MCMV infection, and famcyclovir had no additional effect. Thus, inhibiting viral replication rescued cDCs from MCMV-induced depletion. We also asked whether famcyclovir altered the phenotype of DCs, since PD-L1 expressing DCs can inhibit CD8+ T cell responses. Famcyclovir treated mice had a lower frequency of CD8+ DCs expressing PD-L1. However, the mean fluorescence intensity of PD-L1 cDCs did not differ between treated and untreated mice (Figure 5B).
Figure 5. Depletion of CD11c dendritic cells during acute MCMV-TK infection abrogates the M45 and M57 T cell response.
(A) The graphs show the absolute numbers of splenic conventional DCs (CD11b+ and CD8α+) and plasmacytoid DCs (pDCs) on days 0–6 pi in MCMV-TK infected mice with or without FVR treatment. The dotted line represents absolute numbers from naïve mice (n=4). (B) Graphs show PD-L1 frequency and MFI on CD8α+ DCs at 4 days pi. (C) CD11c-DTR and littermate control mice were recipients of bead purified wild-type (CD45.1/CD45.2) CD8 T cells at one week prior to infection and given one dose of diphtheria toxin 2 days pi. (D) The graph shows the frequency of the M45 and M57-specific response in CD11c-DTR and littermate control animals at 7 days pi. (E) Plots show DC depletion from DTR+ and WT littermate mice two days post DT treatment, gated on CD3- population. Graphs represent the average with the SEM and significance as determined by a student t-test (*p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001). Each time point and bar represent three mice per group. The experiments were done twice.
Enhanced CD8+ T cell responses are dependent on cDCs
We postulated that preservation of cDCs in famcyclovir-treated mice was important for the enhanced CD8+ T cell response. Most available evidence indicates that, in common with other infections, initial priming of the CD8+ T cell response to MCMV depends on DCs (22, 23). CD8+ conventional dendritic cells are critical for cross-presentation of pathogen antigens, and the acute response to MCMV is driven by cross-presented antigen (24–26). However, it is not known whether DCs are involved after the initial priming event in further enhancing the extent of CD8+ T cell proliferation (i.e. the clonal burst size). Since replicating virus depletes conventional DCs, we considered that this loss could be responsible for the muted CD8+ T cell response to cross-presented epitopes. Indeed, Robbins et al have reported that virus-induced DC loss reduced the very early (day 4) CD8+ T cell responses to MCMV in BALB/mice, albeit without impacting the peak (day 7) response (4). To ask whether DC loss could be preventing a later amplification of the CD8+ T cell response in C57/BL6 mice, we used CD11c-Diptheria Toxin Receptor (CD11c-DTR) transgenic mice, in which DCs can be depleted by administration of diphtheria toxin (DT). To allow for CD8+ T cell priming, but remove DCs during the clonal expansion phase, CD11c-DTR transgenic mice and littermate controls were infected with MCMV-TK, and DT was administered 2 days later (Figure 5E). The CD8+ T cell response to M57 and M45 was measured at 7 days post-infection (Figure 5C and D). Because activated CD8+ T cells express CD11c, they might also be depleted by DT, distorting any interpretation. To avoid this, we adoptively transferred wild-type CD8 T cells prior to infection. However, we found no difference in the frequency of T cells responding in the donor and recipient populations (not shown), and so the data shown in Figure 5D is for the total CD8+ T cell population. As expected, littermate control mice developed enhanced responses to M45 and M57 when pretreated with famcyclovir. Remarkably, however, when DCs were depleted from CD11c-DTR positive mice by treatment with DT, the response to M45 and M57 was equivalent to mice that did not receive famcyclovir. This result indicates that the ablation of DCs after initial priming has a suppressive effect on the peak acute CD8 T cell response of MCMV-TK infection. Together, these data strongly imply that the preservation of the CD11c APC compartment is necessary for the enhancement of the M45- and M57-specific CD8 T cell responses in replication-deficient MCMV infection.
TLR agonist reduces CD8 T cell responses in famcyclovir treated mice
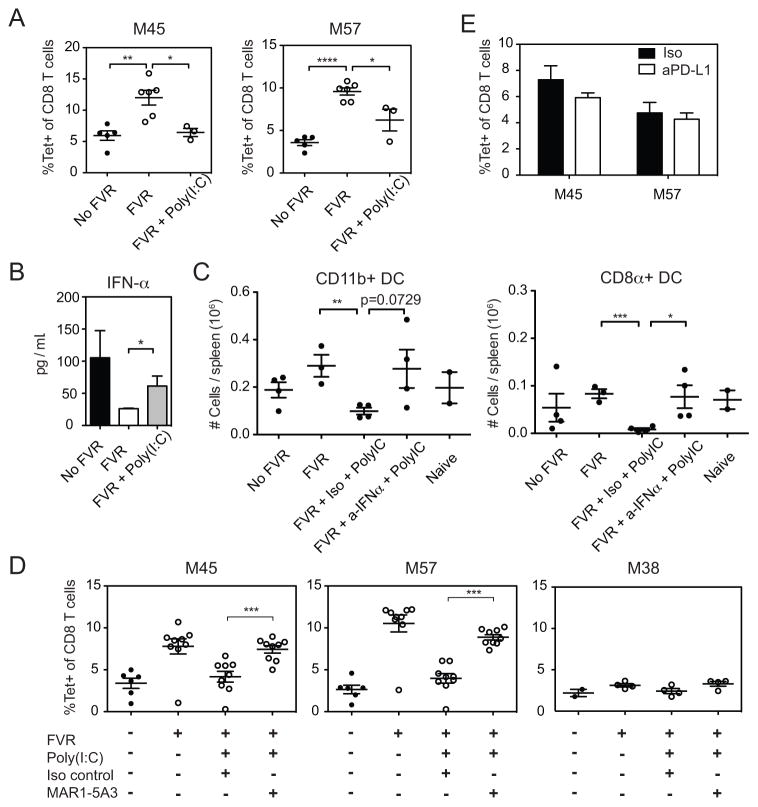
It seemed likely that the vastly increased cytokine response to replicating virus would be responsible for the ability of replicating virus to inhibit the T cell response to M45 and M57. For this reason, we asked whether a sterile induction of cytokines would replicate the impact of virus replication on the T cell response. MCMV is known to stimulate toll-like receptor 3 (TLR3) (27). Therefore, to mimic an MCMV-mediated inflammatory response in famcyclovir treated mice, we administered the TLR3 agonist Poly(I:C) on days 1 and 2 post-infection. Figure 6A shows that Poly(I:C) markedly reduced the ability of famcyclovir to enhance the CD8+ T cell response. Although Poly(I:C) induced multiple cytokines that were also induced by replicating CMV, we were particularly interested in the type I IFN response. Type I IFN has been reported to increase DC turnover in vivo (28, 29). Specifically, in MCMV infection, type I IFN depletes splenic DCs by preventing their replenishment from precursors (28, 29), resulting in the previously reported impact of DC loss on the very early CD8+ T cell response to MCMV in BALB/c mice (4). The type I IFN response to MCMV is biphasic: a small peak at 8 hours is followed by a larger peak at 48 hours (20, 30). Famcyclovir pretreatment prevents the second type I IFN peak (Figure 4, above). Therefore, we administered Poly(I:C) on days 1 and 2 post-infection to famcyclovir-treated mice in an attempt to recapitulate the second type I IFN peak. Figure 6B shows that Poly(I:C) treatment of famcyclovir-treated, MCMV-infected mice increased IFN-α levels at 48 hours, although not quite to the level seen in mice not treated with famcyclovir. Since we also know that cDCs are needed for the increased response, we next assessed the impact of Poly(I:C) and type I IFN on splenic cDC numbers (Figure 6C). Mice were infected with MCMV-TK in the presence of famcyclovir, and treated with Poly(I:C) on days 1 and 2 post-infection as before. In addition, they were treated with MAR1-5A3, an antibody against the IFNα/β receptor subunit 1, which effectively neutralizes the activity of type I IFN in vivo, or a matched isotype control. DC numbers were analyzed in mice sacrificed on day 4 post-infection, the time point at which the greatest difference was seen in famcyclovir-treated mice (Figure 5A). The number of conventional (CD11c+) DCs of both the CD11b and CD8+ subsets was increased by famcyclovir-treatment and Poly(I:C) prevented this increase, consistent with the interpretation that inflammatory cytokines suppress cDC numbers during MCMV infection. However, when type I IFN activity was neutralized, cDC numbers were restored to the levels seen in the absence of Poly(I:C).
Figure 6. Sterile Inflammation Reduces CD8 T Cell Responses in Famcyclovir Treated Mice.
MCMV-TK infected mice were given Poly(I:C) at day 1 and 2 post-infection. (A) Shown in the representative graphs are the frequency of M45 and M57-tetramer+ splenocytes at day 7 pi. (B) Plasma was collected from infected mice given Poly(I:C) and peripheral IFN-α was quantified by an ELISA at 48 hours pi. At day 4 pi, conventional DCs were shown to be preserved with FVR treatment (Figure 5). Shown is (C) the decrease in number of conventional DCs in an MCMV-TK infection with the administration of Poly(I:C) treatment and the recovery when these mice are treated with neutralizing IFN-αR1 mAb (MAR1-5A3). (D) Shown are frequency of M45, M57, and M38-tetramer specific CD8 T cell populations in response to Poly(I:C), along with the combination of MAR1-5A3 mAb or isotype control. (E) Antigen-specific T cells were measured at day 7 pi after MCMV infection with the addition of blocking PD-L1 mAb. Graphs represent the average with the SEM and significance as determined by a student t-test (*p<0.05, **p<0.005, ***p<0.0005). Individual plot points and bars represent two to nine mice per group. Experiments were done twice.
To test whether this increase in type I IFN could account for the impact of Poly(I:C) on the CD8+ T cell response in famcyclovir-treated MCMV-infected mice, mice were infected with MCMV-TK, administered Poly(I:C) and MAR1-5A3 as before, and the CD8+ T cell response was analyzed on day 7 (Figure 6D). While Poly(I:C) reduced the ability of famcyclovir to enhance the response to M45 and M57, the neutralization of type I IFN reversed this effect. Thus, neutralization of type I IFN in Poly(I:C) treated mice restored the elevated CD8+ T cell responses seen in mice treated with famcyclovir alone (Figure 6A). We also measured the response to M38, an inflationary epitope, which we previously observed to be little impacted by famcyclovir treatment (Figure 1F). Consistent with previous results, famcyclovir caused only a slight, non-significant increase in the response to M38. Similarly, Poly(I:C) treatment and IFN-α/βR blockade had little impact on the response to this inflationary epitope. These results suggest that the impact of famcyclovir on the T cell response to conventional epitopes is attributable to the fact that a large type I IFN response does not develop in famcyclovir-treated mice. Together, these results indicate that type I IFN, whether induced by replicating virus or by Poly(I:C), depletes cDCs, and that this negatively impacts the acute CD8+ T cell response to MCMV infection. During chronic lymphocytic choriomeningitis (LCMV, clone 13) infection, DCs that express PD-L1 and IL-10 can suppress the CD8+ T cell response. These suppressive DCs are dependent on type I IFN (43,44). It was possible that type I IFN could be inducing similarly suppressive DCs during acute MCMV infection. It has been shown that PD-L1 blockade reverses exhaustion during chronic LCMV infection (31, 32). To test whether PD-L1 on DCs is inducing a suppressive mechanism on M45 and M57-specific CD8 T cells, we used anti-PD-L1 antibody blockade during acute MCMV infection. Figure 6E shows PD-L1 blockade did not increase the CD8+ T cell response in MCMV infected mice and suggests that M45 and M57-specific CD8 t cells are not suppressed by a PD-1/PD-L1 mechanism.
Discussion
Increased virus replication usually results in an increased peak CD8+ T cell response by increasing the overall amount of antigen and inducing inflammatory cytokines that promote CD8+ T cell proliferation (signal 3). The inflammatory cytokines also promote CD8+ T cell differentiation to a short-lived effector phenotype. Here we describe a situation in which increased virus replication causes the expected increase in virus specific SLECs. However, its impact on virus-specific CD8+ T cell numbers is opposite to what would be expected, with 2–3 fold lower peak responses to major epitopes in the presence of virus replication. We show that a large type I IFN response elicited by replicating virus depletes cDCs, and that depletion of cDCs is responsible for the lower CD8+ T cell response.
In most experimental and clinical situations the profound impact of type I IFNs on viral load is the principle determinant of any immunological consequences. However, type I IFNs are pleiotropic cytokines with the potential to impact the immune response at multiple levels (33). They promote DC maturation for effective costimulation and cross-presentation (34, 35), and they act directly on TCR-activated CD8+ T cells to provide “signal 3” to promote proliferation (36–39). In consequence, they usually have a positive impact on CD8 T cell response to vaccination (40, 41). Conversely, for certain single-cycle vaccine vectors, inhibition of viral gene expression by type I IFN can reduce the CD8+ T cell response (42, 43). Type I IFNs can also be directly immunosuppressive. At the point of T cell priming, if IFN signaling precedes TCR engagement, the impact of IFN is suppressive (44). Type I IFNs can promote IL-10-secreting DCs and CD4+ T cells (31, 32, 45, 46). In fact, type I IFN blockade results in clearance of chronic LCMV infection (31, 32), attributable to induction of suppressive DCs that secrete IL-10 and express PD-L1.
Type I IFNs have previously been described to impact DC numbers in several experimental models. In particular, type I IFN has been reported to decrease cDC numbers in the spleen, most likely by increasing their turnover (28) and/or preventing their being replenished from the bone marrow (29). Type I IFNs particularly affect the CD8α+ subset (28), which is required for cross-presentation.
In the study most analogous to our work, Dalod and colleagues studied the CD8+ T cell response to acute MCMV infection in BALB/c mice, focusing on a single epitope (4). They found that higher virus titers, whether due to lack of NK cells or to a drug-sensitive recombinant MCMV resulted in a delayed initial appearance of CD8+ T cells without impacting the peak numbers of CD8+ T cells. They showed that type I IFN reduced splenic CD11c+ DCs during acute MCMV infection (4, 29), and that this was responsible for the delayed appearance of CD8+ T cells in BALB/c mice with higher virus titers. In C57BL/6 mice, we found a similar impact of virus-induced type I IFN on cDCs in the spleen. However, the consequent impact on CD8+ T cells was different. We found no difference in the time of appearance of MCMV-specific T cells, and instead report a later increase in the CD8+ T cell response to that resulted in a substantially higher peak response. As discussed further below, high virus titers impacted responses to only a subset of epitopes. The difference between our results and those of Dalod and colleagues is likely due to the different nature of the epitope-specific CD8+ T cell response in the two mouse strains. The acute CD8+ T cell response in C57BL/6 mice consists of two populations: inflationary and non-inflationary. In contrast, in BALB/c mice, both of the CD8+ T cell responses that are immunodominant during acute infection – including the IE1-specific T cells studied by Dalod and colleagues – also induce memory inflation. It is now known that, after the T cells have been primed, memory inflation is sustained by antigen presentation on non-hematopoietic cells (22, 23). Our results show that type I IFN-induced depletion of DCs results in reduced peak CD8+ T cell responses specifically to non-inflationary immunodominant epitopes while having little impact on inflationary epitopes.
Replicating virus caused a loss of DCs that was most pronounced 2 – 4 days after infection and this could be recapitulated by treatment of CD11c-DTR mice with DT on day 2. In either case, the loss of cDCs after the initial priming of CD8+ T cells resulted in reduced numbers of CD8+ T cells at the peak of infection. These results suggest that a maximal CD8+ T cell response required continued antigen presentation and/or costimulation beyond day 3. Although 24 hours of antigen exposure is sufficient to program the responding T cell proliferate for an initial clonal burst (47), prolonged antigen exposure has been shown in several models to increase the size of the clonal burst (36, 48–52). Thus, the increased response to M45 and M57 that we observed with non-replicating virus is likely caused by prolonged antigen presentation because of preserved cDC populations. Our results reinforce the concept that although initial priming programs a substantial clonal expansion, the continued presence of antigen can give T cells a “second wind” and increase the ultimate size of the response.
In chronic LCMV infection, type I IFN induces a DC population that suppresses CD8+ T cell responses by expressing PD-L1 and secreting IL-10 (31, 32), In our experiments, it is possible that, in addition to impacting CD8+ T cell numbers, type I IFN also modulates the phenotype and perhaps trafficking of cDCs. These factors could be involved in the overall suppressive role for type I IFN in this model. However, blockade of PD-L1 did not alter the immunosuppressive effect of type I IFN in our experiments (Fig 6E). Furthermore, the fact that cDC depletion on day 2 post infection decreased the CD8+ T cell response is evidence for a predominant post-priming immune stimulatory role for cDCs in drug treated mice, rather than a predominant immunosuppressive role in untreated mice.
It is interesting that famcyclovir primarily impacted the responses to two immunodominant epitopes, M45 and M57, while not increasing the frequency of CD8+ T cell responses to subdominant epitopes or to two other dominant epitopes, m139 and M38. The subdominant epitopes may not be able to compete with the dominant responses when antigen becomes limited, probably explaining both their subdominance and their failure to respond to famcyclovir’s preservation of cDCs. However, within the dominant responses, the selective boosting of only M45- and M57-specific populations, is quite striking. Unlike M45 and M57, dominant responses to m139 and M38 are subject to memory inflation. Memory inflation is the term used to describe the sustained or increased numbers of responses to a subset of MCMV epitopes that occurs during chronic infection. As in other infections, DCs are likely responsible for the very initial priming of both inflationary and non-inflationary responses. Remarkably, however, two recent studies have used elegant bone marrow chimera models to demonstrate that the antigen presentation that sustains memory inflation takes place entirely on cells of non-hematopoietic origin (22, 23). Thus, we propose that, after initial priming by DCs, antigen presented on non-hematopoietic cells is likely sufficient to drive optimal CD8+ T cell responses to M38 and m139 even when DCs were depleted. In contrast, the responses to M45 and M57 do not inflate, and are therefore likely to be dependent on antigen presented by DCs. Several lines of evidence suggest that the acute response to MCMV is driven predominantly or exclusively by cross-presented antigen (25, 26). Indeed, the antigenic peptide from M45 (M45985-993) is dependent on the immunoproteasome for production (53). Hence, depletion of DCs would remove antigen for those responses. Our results are somewhat analogous to a recent study of the primary CD8+ T cell response to influenza virus (52). In that study, depletion of CD11c+DCs at day 6 post-infection reduced the peak (day 8) CD8+ T cell response to the nucleoprotein (NP) antigen, which is strongly cross-presented by DCs at day 6 post-infection. In accordance with those authors, we conclude that antigen cross-presented by DCs beyond day 3 of infection can increase the size of the primary CD8+ T cell response and further bias the response to immunodominant epitopes.
Overall, our data show that modulation of antigen-presenting cDCs after T cell priming can have dramatic effects on the size of the acute CD8+ T cell populations and the numbers of memory cells that are formed. Our work points to type I IFN as a critical mediator of this process, and shows that inflammation can have diverse effects on a developing T cell population.
Acknowledgments
This work was supported by the National Institutes of Health Grants RO1 AI 47206 (to A.B.H), K22A1081866 (to C.M.S), 5T32AI078903-04 (to C.P.L), and 5T32AI007472-17 (to C.P.L).
We thank Dr. Obrahi for supplying Poly(I:C).
Abbreviations used in this article
- APC
antigen presenting cell
- cDC
conventional dendritic cell
- DTR
diphtheria toxin receptor
- HSV
herpes simplex virus
- ICS
intracellular cytokine staining
- IFN
interferon
- LM
listeria monocytogenes
- LCMV
lymphocytic choriomeningitis
- MCMV
murine cytomegalovirus
- pDC
plasmacytoid dendritic cell
- TK
thymidine kinase
- WT
wild-type
References
- 1.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus. PLoS Pathog. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 4.Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadnisky MD, Xie X, Coats ER, Bullock TN, Brown MG. Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood. 2011;117:5133–5141. doi: 10.1182/blood-2010-12-324632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 7.Andrews DM, Andoniou CE, Fleming P, Smyth MJ, Degli-Esposti MA. The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon. J Virol. 2008;82:4931–4937. doi: 10.1128/JVI.02127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitrovic M, Arapovic J, Traven L, Krmpotic A, Jonjic S. Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Med Microbiol Immunol. 2012;201:487–495. doi: 10.1007/s00430-012-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews DM, Andoniou CE, Scalzo AA, van Dommelen SL, Wallace ME, Smyth MJ, Degli-Esposti MA. Cross-talk between dendritic cells and natural killer cells in viral infection. Mol Immunol. 2005;42:547–555. doi: 10.1016/j.molimm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scalzo AA, Manzur M, Forbes CA, Brown MG, Shellam GR. NK gene complex haplotype variability and host resistance alleles to murine cytomegalovirus in wild mouse populations. Immunol Cell Biol. 2005;83:144–149. doi: 10.1111/j.1440-1711.2005.01311.x. [DOI] [PubMed] [Google Scholar]
- 12.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 13.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 14.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 16.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 18.Haring JS, V, Badovinac P, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2006;2:e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 22.Seckert CK, Schader SI, Ebert S, Thomas D, Freitag K, Renzaho A, Podlech J, Reddehase MJ, Holtappels R. Antigen-presenting cells of hematopoietic origin prime cytomegalovirus-specific CD8 T cells but are not sufficient for driving memory inflation during viral latency. J Gen Virol. 2011;92:1994–2005. doi: 10.1099/vir.0.031815-0. [DOI] [PubMed] [Google Scholar]
- 23.Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A. Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection. PLoS Pathog. 2011;7:e1002313. doi: 10.1371/journal.ppat.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busche A, Jirmo AC, Welten SP, Zischke J, Noack J, Constabel H, Gatzke AK, Keyser KA, Arens R, Behrens GM, Messerle M. Priming of CD8+ T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J Immunol. 2013;190:2767–2777. doi: 10.4049/jimmunol.1200966. [DOI] [PubMed] [Google Scholar]
- 25.Snyder CM, Allan JE, Bonnett EL, Doom CM, Hill AB. Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One. 2010;5:e9681. doi: 10.1371/journal.pone.0009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torti N, Walton SM, Murphy KM, Oxenius A. Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T cell priming and memory inflation. Eur J Immunol. 2011 doi: 10.1002/eji.201041075. [DOI] [PubMed] [Google Scholar]
- 27.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattei F, Bracci L, Tough DF, Belardelli F, Schiavoni G. Type I IFN regulate DC turnover in vivo. Eur J Immunol. 2009;39:1807–1818. doi: 10.1002/eji.200939233. [DOI] [PubMed] [Google Scholar]
- 29.Baranek T, Manh TP, Alexandre Y, Maqbool MA, Cabeza JZ, Tomasello E, Crozat K, Bessou G, Zucchini N, Robbins SH, Vivier E, Kalinke U, Ferrier P, Dalod M. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 2012;12:571–584. doi: 10.1016/j.chom.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Benedict CA, Banks TA, Senderowicz L, Ko M, Britt WJ, Angulo A, Ghazal P, Ware CF. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus detente. Immunity. 2001;15:617–626. doi: 10.1016/s1074-7613(01)00222-9. [DOI] [PubMed] [Google Scholar]
- 31.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bon A, Montoya M, Edwards MJ, Thompson C, Burke SA, Ashton M, Lo D, Tough DF, Borrow P. A role for the transcription factor RelB in IFN-alpha production and in IFN-alpha-stimulated cross-priming. Eur J Immunol. 2006;36:2085–2093. doi: 10.1002/eji.200535228. [DOI] [PubMed] [Google Scholar]
- 35.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 36.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 37.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 38.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faul EJ, Wanjalla CN, McGettigan JP, Schnell MJ. Interferon-beta expressed by a rabies virus-based HIV-1 vaccine vector serves as a molecular adjuvant and decreases pathogenicity. Virology. 2008;382:226–238. doi: 10.1016/j.virol.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenz T, Waibler Z, Hofmann J, Hamdorf M, Lantermann M, Reizis B, Tovey MG, Aichele P, Sutter G, Kalinke U. Concomitant type I IFN receptor-triggering of T cells and of DC is required to promote maximal modified vaccinia virus Ankara-induced T-cell expansion. Eur J Immunol. 2010;40:2769–2777. doi: 10.1002/eji.201040453. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Lore K, Gall JG, Gostick E, Lefebvre F, Cameron MJ, Price DA, Haddad E, Sekaly RP, Seder RA, Koup RA. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J Immunol. 2012;188:6109–6118. doi: 10.4049/jimmunol.1103717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkelmann ER, Widman DG, Xia J, Ishikawa T, Miller-Kittrell M, Nelson MH, Bourne N, Scholle F, Mason PW, Milligan GN. Intrinsic adjuvanting of a novel single-cycle flavivirus vaccine in the absence of type I interferon receptor signaling. Vaccine. 2012;30:1465–1475. doi: 10.1016/j.vaccine.2011.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 45.Dikopoulos N, Bertoletti A, Kroger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8+ T cell responses through IL-10-producing CD4+ T regulatory 1 cells. J Immunol. 2005;174:99–109. doi: 10.4049/jimmunol.174.1.99. [DOI] [PubMed] [Google Scholar]
- 46.Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, Merkler D, Detje C, Gutcher I, Mages J, Lang R, Martin R, Gold R, Becher B, Bruck W, Kalinke U. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Bevan MJ, Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2:381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]
- 48.Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 49.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storni T, Ruedl C, Renner WA, Bachmann MF. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J Immunol. 2003;171:795–801. doi: 10.4049/jimmunol.171.2.795. [DOI] [PubMed] [Google Scholar]
- 52.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41:127–140. doi: 10.1016/j.immuni.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchinson S, Sims S, O’Hara G, Silk J, Gileadi U, Cerundolo V, Klenerman P. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PLoS One. 2011;6:e14646. doi: 10.1371/journal.pone.0014646. [DOI] [PMC free article] [PubMed] [Google Scholar]