Abstract
Objective The adverse effects of selective sodium-glucose co-transporter 2 (SGLT2) inhibitors generally appear within about two or three months after treatment initiation in Japan. Therefore, we investigated the impact of tofogliflozin, a class of SGLT2 inhibitors, on glycemic control and body composition during this period in Japanese patients with type 2 diabetes mellitus.
Methods This single-arm open-label study enrolled 20 patients. Patients received tofogliflozin 20 mg once daily for 8 weeks. At week 8, changes from baseline in body weight, serum metabolic markers, and body composition were evaluated.
Results A total of 17 patients completed the 8-week administration of tofogliflodin. No serious adverse events were noted. Hemoglobin A1c (HbA1c) decreased significantly, from 7.8% to 7.3% with 8-week administration of tofogliflozin. Both the body weight and body mass index (BMI) also decreased. In addition, a decreased renal function of the boundary zone and hemoconcentration were detected. As for body composition, the free fat mass, total body water, extracellular water and intracellular water were all decreased significantly. Interestingly, the amount of fat mass did not change. The degree of improvement in HbA1c was correlated with the baseline fat mass and BMI.
Conclusion An eight-week administration of tofogliflozin improved glycemic control and reduced the body weight and free fat mass in type 2 diabetic patients without affecting the fat mass. In this period, the hematocrit level and renal function should be monitored to guard against hemoconcentration and renal impairment, respectively.
Keywords: tofogliflozin, SGLT2 inhibitor, body composition, hemoconcentration
Introduction
The incidence of type 2 diabetes has been dramatically increasing worldwide in association with the prevalence of obesity and an unhealthy lifestyle (1). This disease is characterized by a decreased insulin action induced by insulin resistance and insulin deficiency. Diet and exercise are regarded as a basic and important strategy for treating type 2 diabetes (2). However, controlling the blood glucose level through lifestyle improvement alone is difficult. Therefore, a number of novel anti-diabetic drugs have been developed. Recently, selective sodium-glucose co-transporter 2 (SGLT2) inhibitors have been developed as a novel potential therapeutic option for the treatment of type 2 diabetes. These drugs reduce the circulating glucose concentrations via a unique mechanism which inhibits the renal reabsorption of glucose and increases urinary glucose excretion (3,4). Given that the mechanism of action of SGLT2 inhibitors is independent of insulin secretion and action, the risk of hypoglycemia is low (3-5).
Existing reports have indicated that the long-term administration of SGLT2 inhibitors improves hyperglycemia and reduces body weight, fat mass, and subcutaneous and visceral adipose tissue (6). SGLT2 inhibitors have been available for clinical use in Japan since 2014. In many cases, various side effects of SGLT2 inhibitors, such as urogenital infection, volume depletion, and skin disorders, appear within about 2-3 months after beginning to use these inhibitors in Japan (7), indicating the importance of close observation of any physiological and metabolic changes during this period. We herein addressed the impact of tofogliflozin, a class of SGLT2 inhibitors, by examining the serum markers and body composition measurements in Japanese type 2 diabetic patients for 8 weeks from the beginning of its administration.
Materials and Methods
Study design and participants
This study was carried out at Chibune General Hospital between August 2014 and March 2015 as a single-arm open-label study in Japanese patients with type 2 diabetes mellitus. All participants continued their pre-study treatment without any change. In addition to any pre-study treatment, the participants received a single 20 mg dose of tofogliflozin daily for 8 weeks. All patients received general diet and exercise advice. The treatment was not changed in any participants throughout the current study.
The inclusion criteria of this study were as follows: age >20 years old, body mass index (BMI) ≥18.5, hemoglobin A1c (HbA1c) levels >6.2%, and estimated glomerular filtration rate (eGFR) >45 mL/min. In addition, C-peptide immunoreactivity index >1.5 was added to the inclusion criteria when the duration of diabetes exceeded 10 years. The exclusion criteria were as follows: using insulin; having severe hepatic, renal, or cardiac diseases; having severe infection; having severe trauma; having cancer; and being pregnant or breastfeeding. The study complied with the Declaration of Helsinki, Good Clinical Practice, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, and Japanese law. This study was approved by the ethics committee at Chibune General Hospital. All patients provided their informed consent prior to being enrolled.
Measurements
At weeks 0 and 8, body weight and serum metabolic markers were measured. The body composition was evaluated by a bioelectrical impedance analysis (BIA) using an In-Body S20Ⓡ body composition analyzer (Biospace, Seoul, Korea). In this analysis, the body weight, total body water, and extracellular body water were actually measured by segmental bioelectric impedance using eight tactile electrodes (8-10). The free fat mass and fat mass were calculated from total body water. The free fat mass consists of total body water and protein and mineral content. The fat mass is obtained by subtracting the free fat mass from the body weight.
All measurements were performed regardless of meals. However, the changes in body composition estimated after food or drink consumption were relatively small and fell within the values of imprecision for the impedance technique, and so are unlikely to be of clinical significance (11). We evaluated any adverse effects during visits every 2 weeks and performed blood examinations every 4 weeks.
Statistical analysis
Since all continuous variables were confirmed to be normally distributed in this study, Student's t-test was used to compare between time points. The values are expressed as the mean ± standard deviation (SD), and p <0.05 was considered to be statistically significant. Correlations among variables were tested using Pearson's correlation coefficient. All calculations were performed using the JMP software program version 8 (SAS Institute, Cary, USA).
Results
The baseline characteristics and medications for the participants are shown in Table 1. The mean age and diabetes duration were 52.1 years old and 6.4 years, respectively. The mean body weight, BMI, HbA1c and casual plasma glucose levels at baseline were 76.6 kg, 28.9 kg/m2, 7.8% and 166 mg/dL, respectively. As anti-diabetic agents, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 receptor agonists, α-glucosidase inhibitors, biguanides, and thiazolidinediones were administered to 25%, 65%, 10%, 15%, 75%, and 20% of participants, respectively. No patients were treated with glinides, and one patient was drug naïve. Of the 20 patients enrolled in this study, 17 completed the study, and we analyzed data from all 17 (men/women =7/10). One patient discontinued administration due to a side effect of staggering. Another patient discontinued due to miliaria of the abdomen. Deteriorated rashes are frequently reported as adverse effects of SGLT2 inhibitors in Japan. After drug discontinuation, the staggering promptly disappeared, but no distinct improvement was noted in the case of miliaria of the abdomen. The other patient stopped visiting the hospital. Of the 17 patients who completed the 8-week administration of tofogliflozine, 2 female patients developed cystitis and were administered antibiotics; their cystitis then improved promptly. No symptomatic hypoglycemic episodes occurred in any participants in the current study, and no other adverse events were observed.
Table 1.
Characteristics of Study Participants.
| n | 20 |
| Age (years) | 52.1 ± 9.8 |
| Sex (male/female) | 9 / 11 |
| Diabetes duration (years) | 6.4 ± 5.8 |
| Waist circumference (cm) | 97.7 ± 10.2 |
| Body weight (kg) | 76.6 ± 15.8 |
| Body mass index (kg/m2) | 28.9 ± 4.6 |
| Casual plasma glucose (mg/dL) | 166 ± 60.0 |
| Hemoglobin A1c (%) | 7.8 ± 1.5 |
| eGFR (mL/min/1.73m2) | 80.7 ± 21.5 |
| Anti-diabetic treatment | |
| Sulfonylurea (%) | 25 % |
| Glinide (%) | 0 % |
| DPP-4 inhibitor (%) | 65 % |
| GLP-1 receptor agonist (%) | 10 % |
| α-glucosidase inhibitor (%) | 15 % |
| Biguanide (%) | 75 % |
| Thiazolidinedione (%) | 20 % |
| Insulin (%) | 0 % |
eGFR: estimated glomerular filtration rate, DPP-4: dipeptidyl peptidase-4, GLP-1: glucagon-like peptide-1. Values are mean ± SD.
As shown in Table 2, 8-week administration of tofogliflozine decreaed HbA1c by 0.5% with statistical significance. The low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL) levels were significantly elevated. eGFR showed a declining trend. Blood urea nitrogen, creatinine (Cr), red blood cell, hematocrit (Hct), total protein, and albumin levels were significantly increased. Uric acid levels did not change significantly. Plasma osmolarity (Posm) did not change markedly, either. As for electrolytes, serum chloride concentration decreased significantly, although neither sodium nor potassium concentrations changed (Table 3). The fractional excretion of both sodium and potassium increased significantly (Table 3).
Table 2.
Laboratory Findings before and after Administration of Tofogliflodine for 8 Weeks.
| n=17 | Week 0 | Week 8 | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | p value | |
| Blood cells count | |||||
| WBC (×104/μL) | 0.68 | 0.18 | 0.69 | 0.20 | 0.90 |
| RBC (×104/μL) | 458 | 37 | 482 | 34 | <0.001 |
| Hct (%) | 40.3 | 3.4 | 42.6 | 3.1 | <0.001 |
| Plt (×104/μL) | 25.4 | 6.3 | 26.0 | 6.0 | 0.45 |
| Biochemisty | |||||
| Total protein (g/dL) | 7.0 | 0.4 | 7.3 | 0.5 | 0.02 |
| Albumin (mg/dL) | 4.2 | 0.4 | 4.4 | 0.3 | 0.01 |
| Triglyceride (mg/dL) | 190 | 101 | 192 | 109 | 0.94 |
| HDL cholesterol(mg/dL) | 52.7 | 12.3 | 56.4 | 12.1 | 0.03 |
| LDL cholesterol (mg/dL) | 111 | 18 | 120 | 26 | 0.04 |
| AST (IU/L) | 22.7 | 6.7 | 22.5 | 9.8 | 0.93 |
| ALT (IU/L) | 32.5 | 19.0 | 29.4 | 16.0 | 0.11 |
| LDH (IU/L) | 175 | 25 | 174 | 40 | 0.90 |
| ALP (IU/L) | 226 | 83 | 232 | 85 | 0.61 |
| γ-GTP (IU/L) | 33.8 | 15.3 | 29.8 | 12.8 | 0.04 |
| Urea nitrogen (mg/dL) | 12.2 | 3.5 | 14.5 | 4.0 | 0.002 |
| Creatinine (mg/dL) | 0.75 | 0.25 | 0.79 | 0.28 | 0.02 |
| eGFR (mL/min/1.73m2) | 78.9 | 20.0 | 75.1 | 19.2 | 0.06 |
| Uric acid (mg/dL) | 5.5 | 1.70 | 5.2 | 1.81 | 0.16 |
| Plasma osmolarity (mOsm/L) | 283.5 | 4.3 | 284.5 | 6.0 | 0.55 |
| Glucose metabolism | |||||
| HbA1c (%) | 7.8 | 1.6 | 7.3 | 1.3 | 0.04 |
| GA (%) | 17.2 | 3.5 | 16.3 | 2.9 | 0.12 |
| Casual plasma glucose (mg/dL) | 161 | 60 | 142 | 36 | 0.11 |
| Total ketone body (μmol/L) | 91.3 | 56.8 | 196.4 | 194.5 | 0.02 |
| CPR index | 2.1 | 0.9 | 2.6 | 1.6 | 0.13 |
WBC: white blood cells, RBC: red blood cells, Hct: hematocrit, Plt: platelets, HbA1c: hemoglobin A1c, GA: glycoalbumin, CPR index: C-peptide immunoreactivity index, HDL: high-density lipoprotein, LDL: low-density lipoprotein, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, eGFR: estimated glomerular filtration rate
Table 3.
Electrolytes and Fractional Excretion before and after Administration of Tofogliflodine for 8 Weeks.
| n=17 | Week 0 | Week 8 | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | p value | |
| Electrolyte | |||||
| Na (mEq/L) | 139.5 | 2.68 | 139.5 | 2.55 | 1.0 |
| K (mEq/L) | 4.05 | 0.33 | 4.11 | 0.35 | 0.47 |
| Cl (mEq/L) | 103.3 | 2.34 | 102.2 | 2.38 | 0.032 |
| Urine | |||||
| Fractional excretion of sodium (%) | 0.91 | 0.48 | 1.36 | 0.77 | 0.025 |
| Fractional excretion of potassium (%) | 8.67 | 2.84 | 13.3 | 6.70 | 0.006 |
Concerning the physical findings, the body weight and BMI both significantly decreased at 8 weeks compared to baseline (Table 4). An examination of the body composition using BIA showed the free fat mass, total body water, intracellular water (ICW), and extracellular water (ECW) to all decrease significantly at 8 weeks compared to baseline, whereas the fat mass had not changed markedly at 8 weeks (Table 4).
Table 4.
Physical Findings and Body Composition before and after Administration of Tofogliflozin.
| n=17 | Week 0 | Week 8 | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | p value | |
| Physical findings | |||||
| Body weight (kg) | 75.6 | 13.8 | 74.5 | 12.3 | <0.001 |
| Body mass index (kg/m2) | 28.8 | 4.0 | 28.3 | 4.1 | <0.001 |
| Systolic blood pressure (mmHg) | 130.0 | 18.7 | 125.8 | 13.4 | 0.25 |
| Diastolic blood pressure (mmHg) | 79.6 | 15.0 | 78.5 | 13.3 | 0.66 |
| Body composiiton | |||||
| Waist to hip ratio | 0.96 | 0.04 | 0.96 | 0.04 | 0.52 |
| Body fat percentage (%) | 37.5 | 6.1 | 38.1 | 6.1 | 0.16 |
| Fat mass (kg) | 28.3 | 6.7 | 28.1 | 7.2 | 0.78 |
| Free fat mass (kg) | 47.3 | 10.3 | 45.9 | 10.0 | 0.01 |
| Total body water (L) | 34.9 | 7.6 | 33.9 | 7.4 | 0.007 |
| Extracellular water (L) | 13.6 | 2.8 | 13.2 | 2.8 | 0.001 |
| Intracellular water (L) | 21.3 | 4.8 | 20.7 | 4.6 | 0.02 |
The results of a bivariate correlation analysis revealed that a strong correlation between BMI of baseline and the alteration of HbA1c from baseline to week 8 (ΔHbA1c) (Figure A). The fat mass at baseline also had a relatively strong correlation with ΔHbA1c (Figure B). With respect to the renal function, a correlation was noted in the alterations of Cr (ΔCr) and Hct (ΔHct) between baseline and week 8 (Figure C). However, no correlation was observed between ΔCr and ΔECW, the alteration of ECW from baseline to week 8 (r=0.16, p=0.53). The observed correlation between ΔCr and ΔHct may therefore reflect a decrease in the renal function due to a reduction in the circulating plasma volume induced by hemoconcentration. Furthermore, we detected a significant correlation in the alterations of serum potassium (ΔK) and ICW (ΔICW) between baseline and week 8 (Figure D). However, no significant correlations were noted between the alteration of serum sodium (ΔNa) and ΔICW or between the alteration of Posm (ΔPosm) and ΔICW (data not shown). In addition, no significant correlations were noted between ΔNa, ΔK, or ΔPosm and ΔECW, either (data not shown). A correlating trend was observed between ΔECW and ΔLDL, the alteration of LDL from baseline to week 8 (r=0.42, p=0.097). In addition, the alteration of HDL from baseline to week 8 (ΔHDL) tended to correlate with ΔHct (r=0.42, p=0.096).
Figure.
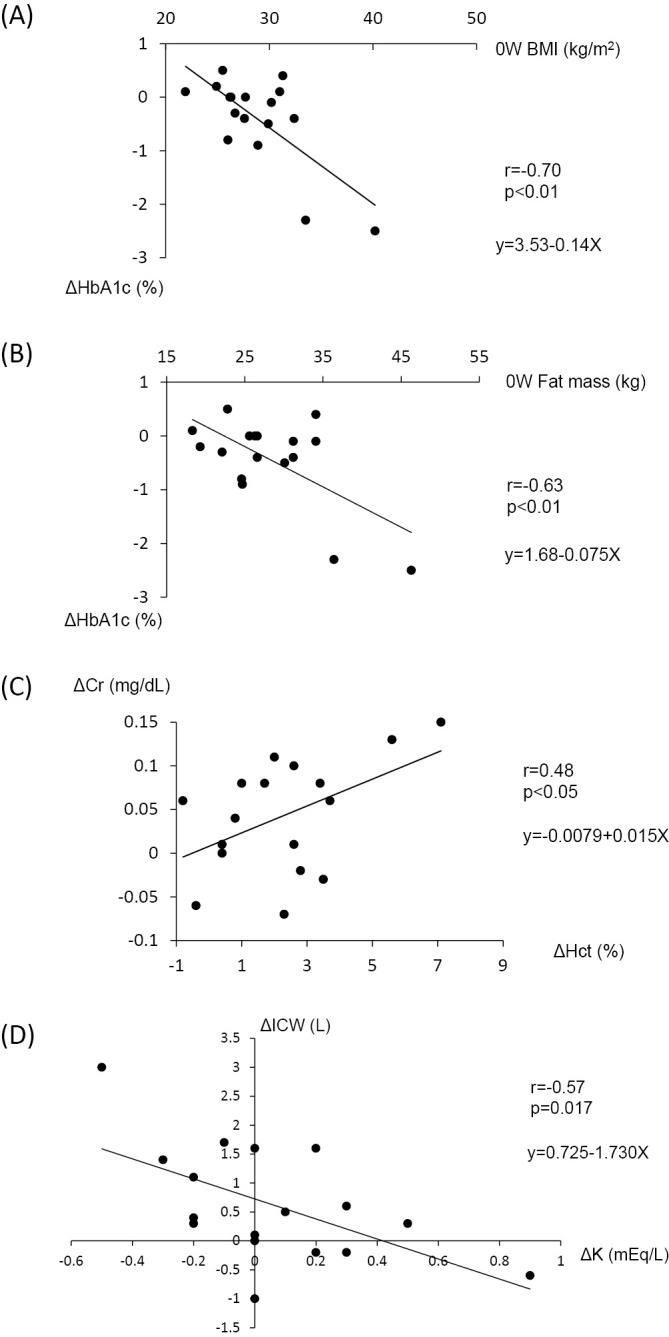
Scatter plots of the correlation analysis. Correlation between ΔHbA1c and 0W BMI (r=-0.70, p=0.002) (A), ΔHbA1c and 0W fat mass (r=-0.63, p=0.006) (B), ΔCr and ΔHct (r=0.48, p=0.049) (C), and ΔK and ΔICW (r=-0.57, p=0.017) (D). ΔHbA1c, ΔCr, ΔHct, ΔK, and ΔICW refer to the differences in HbA1c, Cr, Hct, K, and ICW, respectively, obtained by subtracting the values at week 8 from those at baseline.
Discussion
In the current study, tofogliflozin administration for 8 weeks had the favorable effects of lowering HbA1c and reducing total body weight without inducing any severe side effects. Interestingly, the observed decrease in body weight was due to a decrease in the free fat mass including total body water, but not the fat mass. This is the first report using BIA to directly demonstrate that the decrease in body weight observed during the initial phase of administration of an SGLT2 inhibitor is derived from a reduction in the free fat mass. In addition, the degree of improvement in glycemic control was correlated with the baseline fat mass value as well as baseline BMI. This implies that SGLT2 inhibitors may be suitable for use in obese patients.
Our findings in the present study using tofogliflodin were consistent with those of previous reports that showed SGLT2 inhibitors to be effective in glycemic control without major adverse effects, leading to weight loss in Japanese patients with type 2 diabetes mellitus (12-14). In the present study, the mean body weight loss was 1.1 kg. This degree of body weight loss was comparable to that achieved using placebo-corrected reductions (1.0-2.6 kg) in dapagliflozin treatment studies (6,15-18). The fat mass did not decrease in the present study. In general, osmotic diuresis is responsible for the relatively fast initial decline in body weight, while caloric loss to urine and any subsequent loss in fat mass are responsible for the long-term gradual decrease in body weight in the process of prescribing SGLT2 inhibitors (19). Our observation of no significant decrease in the fat mass with short-term administration in the present study is therefore not very surprising. In fact, a report showed that the administration of SGLT2 inhibitors, including dapagliflozin, ipragliflozin, and tofogliflozin, for 3 months did not affect the body fat mass (20). On the other hand, two studies on the effects of ipragliflozin administration for 1-3 months showed a significant reduction in body fat (21,22). The reason for the difference in fat mass reduction after the short-term administration of SGLT2 inhibitors remains to be elucidated. The general consensus holds that the long-term weight loss associated with SGLT2 inhibitors is due to energy loss through urinary glucose excretion (UGE) (23-26). However, the complete inhibition of SGLT2 forces SGLT1 to reabsorb glucose in full capacity, leading to a decrease in UGE to <50% of the filtered glucose load (27). Therefore, this may be the second reason for the prevention of fat mass loss in the present study. Another possible explanation for the lack of any change in the fat mass is incomplete dietetic therapy. Numerous placebo-controlled randomised clinical trials have demonstrated that body weight decreases only negligibly over the course of the study period (26,28-32). Such a plateau in body weight may be due to sustained increases in food intake to compensate for the caloric loss achieved through UGE (33). In fact, several reports have shown that SGLT2 inhibitors stimulate the energy intake to compensate for the caloric loss due to glycosuria, which may be sufficient to offset the weight loss induced by calorie loss to urine (24-26). An important strategy for treatment with SGLT2 inhibitors is to restrict caloric intake and concurrently curb the appetite. Combining the administration of SGLT2 inhibitors with dietetic therapy to limit energy intake is expected to be associated with a greater weight loss than by either strategy alone (33,34).
In the present study, tofogliflodin induced an increase in hematocrit, a finding which is compatible with a previous report that dapagliflozin caused an increase in hematocrit, attributed to hemoconcentration due to osmotic diuresis (35). In addition, the observed significant increases in total protein, urea nitrogen, and Cr may have also been due to hemoconcentration. In general, diuretic therapy elevates hemoconcentration by reducing circulating plasma volume. A similar phenomenon might be caused by osmotic diuresis with SGLT2 inhibitors. The correlation between ΔCr and ΔHct in our results suggests that initial renal impairment with SGLT2 inhibitor may result from a decrease in the circulating plasma volume. However, no significant correlation was recognized between ΔCr and ΔECW. This lack of any observed correlation may be due to a shift in ECW from interstitial spaces to plasma to compensate for SGLT2 inhibitor-induced hypovolemia and the subsequent alteration of the component ratio in ECW between interstitial fluid and plasma. The increased fractional excretion of potassium may be due to a decrease in potassium re-absorption in the proximal tubule caused by natriuresis and increased secretion of potassium accompanied by the elevation of sodium re-absorption in the collecting duct. Furthermore, given that there were no significant changes in the serum sodium or potassium concentrations, a variety of mechanisms might be involved in compensating for the increased urinary secretion of these electrolytes. Potassium is abundant in intracellular spaces; thus, the mobilization of potassium from intracellular spaces to extracellular ones can help compensate for the increased urinary secretion of potassium. Given that potassium mainly maintains intracellular osmotic pressure, this mobilization of potassium may lead to the decrease in ICW observed in this study. This mechanism may also explain the significant association observed between ΔK and ΔICW, at least in part.
As with previous reports, LDL and HDL increased significantly in the current study (12,36). However, the details regarding the mechanism remain unclear. According to an existing report, hemoconcentration may also be responsible for the observed significant increase in LDL and HDL (19). The correlating trend observed between ΔLDL and ΔECW and between ΔHDL and ΔHct further suggests that hemoconcentration may have been induced by an SGLT2 inhibitor. Patients administered SGLT2 inhibitors may need to be monitored for increases in LDL and a potentially elevated cardiovascular risk (19), although the fact that multiple meta-analyses have shown no increase in the incidence of cardiovascular events among the patients given SGLT2 inhibitors is therefore reassuring (36,37).
The estimated incidence of all serious adverse events was 26.0 per 10,000 patients receiving ipraglifrozin, dapaglifrozin, tofoglifrozin or luseoglifrozin during the first 3 months after starting the administration of each of these drugs in Japan, a value approximately 6.2-fold higher than that with DPP-4 inhibitors (sitagliptin, vildagliptin and alogliptin) in the corresponding period (7). In the present study, two patients discontinued administration of tofoglifrozin and two female patients developed cystitis. During this period, we observed hemoconcentration and decreased total body water in our study, phenomena which may be related to the development of adverse events, given their timing. Regardless, since side effects of SGLT2 inhibitors are common, particularly during the first three months, it is necessary to exercise caution in the initial stage of administration of SGLT2 inhibitors.
In conclusion, the 8-week administration of tofogliflozin not only ameliorated glycemic control, but it also reduced the body weight and free fat mass in type 2 diabetic patients without affecting the fat mass. In addition, tofogliflozin caused hemoconcentration and a reduced renal function in the the boundary zone, as well as a decrease in the ECW. These results may reflect the side effects frequently observed during this period. The renal function should therefore be monitored carefully to avoid any risk of renal impairment due to the decrease in circulating plasma volume experienced with SGLT2 inhibitors, especially during the initial phase of usage and in patients whose renal reserve functions are decreased. Furthermore, obese patients thus far seem suitable for the administration of SGLT2 inhibitor. Some limitations associated with the present study are the small number of patients involved, the single-arm clinical design, the absence of a control for food and fluid intake, and the casual blood glucose data. In addition, we were unable to exclude the possibility that the findings for the two obese patients may have skewed our data, resulting in the strong correlation observed between ΔHbA1c and BMI/fat mass at baseline, due to the small number of patient participating in our correlation analysis.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94: 311-321, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. . American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364-1379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 14: 5-14, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol 1: 140-151, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 32: 515-531, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Jan B, Östen L, Joel K, et al. . Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97: 1020-1031, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Yabe D, Nishikino R, Kaneko M, Iwasaki M, Seino Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf 14: 795-800, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Sartorio A, Malavolti M, Agosti F, et al. . Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur J Clin Nutr 59: 155-160, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Ling CH, de Craen AJ, Slagboom PE, et al. . Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 30: 610-615, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int 15: 1013-1022, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Androutsos O, Gerasimidis K, Karanikolou A, Reilly JJ, Edwards CA. Impact of eating and drinking on body composition measurements by bioelectrical impedance. J Hum Nutr Diet 28: 165-171, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Seino Y, Inagaki N, Haneda M, et al. . Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 6: 443-453, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab 15: 1136-1145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Invest 5: 382-391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33: 2217-2224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223-2233, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomised, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13: 928-938, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32: 1656-1662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 5: 124-136, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumiyoshi S, Shimono D, Futada T. Effects of SGLT2 inhibitors to body composition. Diabetes Frontier 26: 240-246, 2015. (in Japanese). [Google Scholar]
- 21.Matsuhashi Y, Chikazawa S, Matsui J, Daimon M. Administration of SGLT-2 inhibitor Ipraglifrozin improves Glycemic control and reduces the body weight and fat mass. Prog Med 34: 1867-1871, 2014. (in Japanese, Abstract in English). [Google Scholar]
- 22.Takagi S, Takagi M, Kiyama A, Nakamura N. Study of clinical effects of Ipragliflozin (SGLT2 inhibitor) and body composition changes by BIA (bioelectrocal impedance analysis). Prog Med 34: 2229-2235, 2014. (in Japanese, Abstract in English). [Google Scholar]
- 23.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 75: 33-59, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124: 499-508, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins BA, Cherney DZ, Partridge H, et al. . Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 37: 1480-1483, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano A, Miller S, Murgatroyd PR, et al. . Exploring glycosuria as a mechanism for weight and fat mass reduction. A pilot study with remogliflozin etabonate and sergliflozin etabonate in healthy obese subjects. J Clin Transl Endocrinol 1: e3-e8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 62: 3324-3328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223-2233, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Kaku K, Watada H, Iwamoto Y, et al. . Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 13: 65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolinder J, Ljunggren Ö, Johansson L, et al. . Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 16: 159-169, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPA-REG H2H-SU trial investigators. . Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2: 691-700, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Leiter LA, Yoon KH, Arias P, et al. . Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, Phase 3 study. Diabetes Care 38: 355-364, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38: 1730-1735, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol 2: 911-922, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Shah N, Deeb W, Choksi R, Epstein B. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy 32: 80-94, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Foote C, Perkovic V, Neal B. Effects of SGLT2 inhibitors on cardiovascular outcomes. Diab Vasc Dis Res 9: 117-123, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Go AS, Mozaffarian D, Roger VL, et al. . Executive summary: heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation 127: 143-152, 2013. [DOI] [PubMed] [Google Scholar]


