Abstract
c-Abl and Atm have been implicated in cell responses to DNA damage and oxidative stress. However, the molecular mechanisms by which they regulate oxidative stress response remain unclear. In this report, we show that deficiency of c-Abl and deficiency of ATM differentially altered cell responses to oxidative stress by induction of antioxidant protein peroxiredoxin I (Prx I) via Nrf2 and cell death, both of which required protein kinase C (PKC) δ activation and were mediated by reactive oxygen species. c-abl-/- osteoblasts displayed enhanced Prx I induction, elevated Nrf2 levels, and hypersusceptibility to arsenate, which were reinstated by reconstitution of c-Abl; Atm-/- osteoblasts showed the opposite. These phenotypes correlated with increased PKC δ expression in c-abl-/- osteoblasts and decreased PKC δ expression in Atm-/- cells, respectively. The enhanced responses of c-abl-/- osteoblasts could be mimicked by overexpression of PKC δ in normal cells and impeded by inhibition of PKC δ, and diminished responses of Atm-/- cells could be rescued by PKC δ overexpression, indicating that PKC δ mediated the effects of c-Abl and ATM in oxidative stress response. Hence, our results unveiled a previously unrecognized mechanism by which c-Abl and Atm participate in oxidative stress response.
Keywords: Oxidative stress, Prx I, Nrf2, c-Abl, Atm, osteoblasts
c-Abl is a nonreceptor tyrosine kinase implicated in oxidative stress response: c-Abl is phosphorylated and activated in response to oxidative stress; and oxidative stress-induced apoptosis is attenuated in c-Abl-deficient fibroblasts, supporting a proapoptotic role for c-Abl in oxidative stress response (Van Etten 1999; Sun et al. 2000a). Oxidants are generated under various physiological conditions such as mitochondrial electron transport, peroxisomal fatty acid metabolism, phagocytosis by macrophages, and bone resorption by osteoclasts and are believed to contribute to the etiology of atherosclerosis, cerebellar ischemia, and the process of aging (for reviews, see Halliwell and Gutteridge 1990; Key et al. 1994; Beckman and Ames 1998). Cells are equipped with antioxidant enzymes as a defense mechanism against oxidants. One such protein is peroxiredoxin I (Prx I), originally identified as a stress-induced mouse peritoneal macrophage protein (Ishii et al. 1993). Six peroxiredoxin proteins have been identified in mammalian cells and they are highly conserved from bacteria to humans. The protein sequences show similarity to thioredoxin-dependent peroxide reductase, and purified rat Prx I has a thio-specific antioxidant activity (Ishii et al. 1995). In cultured macrophage cell lines, Prx I is dramatically up-regulated by oxidative stress exemplified by treatment with H2O2, diethyl maleate, and arsenate, with arsenate being the most potent (Prosperi et al. 1998). In an osteoblast cell line, arsenate-induced Prx I expression requires at least two independent signaling pathways on the basis of studies using specific kinase inhibitors. Activation of protein kinase C (PKC) δ is required for the transactivation of prx I, and activation of p38 MAPK is required for its posttranscriptional regulation. Inhibition of either pathway results in attenuation of Prx I induction (Li et al. 2002).
The Prx I gene contains an antioxidant response element (ARE) located in its promoter region. Transactivation of Prx I and some other antioxidant enzymes is mediated by Nrf2, a basic leucine zipper transcription factor (Chan and Kan 1999; Chan et al. 2001; Ishii et al. 2000). Keap1, an actin-binding protein, binds Nrf2 and confines it to the cytoplasm (Itoh et al. 2003). On oxidative stress, Nrf2 dissociates from Keap1 and translocates to the nucleus, where it forms a dimer with Maf and binds to ARE to initiate transcription. Oxidative stress also stabilizes Nrf2 so that its protein levels are dramatically increased (McMahon et al. 2003; Nguyen et al. 2003; Stewart et al. 2003). It has been shown that the PKC family plays an important role in the regulation of Nrf2/ARE. On PMA stimulation, PKC phosphorylates Nrf2 at Ser 40 and subsequently promotes its dissociation from Keap1. Inhibition of PKC with inhibitors decreases Nrf2 phosphorylation (Huang et al. 2002). To date, the PKC isoform involved in this process has not been identified.
Prx I has been implicated in signaling pathways. Its human counterpart PAG has been identified as a c-Abl interacting protein. It binds to the SH3 domain of c-Abl and inhibits the kinase activity and cytostatic function of c-Abl in fibroblasts (Wen and Van Etten 1997), although the physiological significance of the interaction is not well understood. In addition, Prx I is up-regulated in the S phase of the cell cycle and can be phosphorylated by cdc2 during mitosis. Phosphorylation of Prx I leads to its inactivation and accumulation of H2O2 in the cell (Chang et al. 2002). The function for Prx I in regulating signaling pathways or cell cycle progression remains unclear.
c-Abl has been intensely studied in cell response to DNA damage (Van Etten 1999). DNA damage induced by ionizing radiation activates the tyrosine kinase activity of c-Abl in an Atm-dependent manner (Baskaran et al. 1997; Shafman et al. 1997; Shiloh 1997). Atm is a Ser/Thr kinase involved in the development of ataxia and telangiectasia (A-T), characterized by neuron degeneration, immunodeficiency, cancer predisposition, radio-hypersensitivity, and premature aging. Atm knockout mice recapitulate most of these features (Shiloh 1997). Atm constitutively interacts with c-Abl and activates c-Abl via Ser 465 phosphorylation on irradiation (IR). In Atm-deficient cells, the activation of c-Abl is abolished. Mouse embryonic fibroblasts (MEFs) isolated from c-abl-/- mice are more resistant to ionizing radiation-induced apoptosis. Activated c-Abl may promote apoptosis via up-regulation of p73, a proapoptotic protein and a p53 homolog (Gong et al. 1999). Furthermore, in response to DNA damage, Atm interacts with p53 and phosphorylates the latter at Ser 15 and other residues. This modification leads to alteration of its transactivation activity or stability and therefore affects the expression of p53 target genes (Khanna et al. 1998). p53 has also been shown to interact with c-Abl and is known to play a crucial role in DNA damage/oxidative stress-induced apoptosis (Goga et al. 1995). Recent evidence also suggests that Atm plays an important role in the response to oxidative stress, and Atm-deficient cells show increased oxidative stress, but how Atm and c-Abl participate in this response is not known (Rotman and Shiloh 1997; Kamsler et al. 2001).
In this report, we aim to dissect the roles of c-Abl and Atm in cell responses to oxidative stress, antioxidant protein Prx I induction and cell susceptibility to the cytotoxicity of arsenate, a prooxidant reagent and a highly potent Prx I inducer (Li et al. 2002). We first provided evidence that these two events were regulated by PKC δ. In PKC δ-deficient cells, Prx I induction was significantly compromised, probably via the master transcription factor Nrf2, with cell death rates lowered as well. Conversely, cells overexpressing PKC δ showed the opposite. We found that c-Abl-deficient cells displayed enhanced Prx I induction and Nrf2 accumulation, increased susceptibility to ROS, and elevated levels of PKC δ, mimicking cells overexpressing PKC δ. The hypersensitive phenotypes observed in c-Abl-deficient cells can be rescued by reconstitution of c-Abl and could be blocked by inhibition of PKC δ activation. On the contrary, Atm-deficient cells showed reduced Prx I induction and Nrf2 accumulation, reduced cell susceptibility, and down-regulation of PKC δ. These phenotypes can be rescued by ectopic expression of PKC δ in Atm-deficient cells. Furthermore, we found that PKC δ expression was regulated at the posttranscriptional levels by c-Abl and Atm, with c-Abl deficiency stabilizing PKC δ. Taken together, these data suggest that the different roles of c-Abl and Atm were mediated by PKC δ. The compromised accumulation of Nrf2 in Atm-deficient cells, a master transcription factor for antioxidant gene expression, may be the reason that these cells portray increased oxidative stress.
Results
Genetic evidence that PKC δ plays a key role in Prx I induction
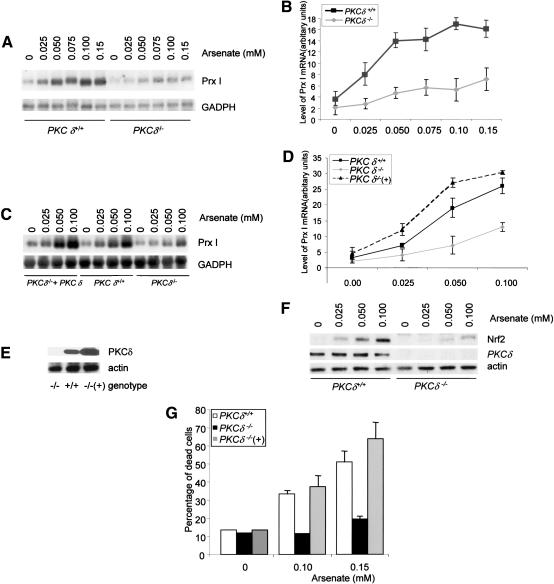
It has been shown that inhibition of PKC δ activation by inhibitors blocked Prx I induction by arsenate in osteoblasts (Li et al. 2002). Arsenate is a prooxidant and is highly potent in the induction of Prx I expression. It also activates PKC δ in osteoblasts (Li et al. 2002). To confirm the conclusion drawn from inhibitor-based studies, we studied Prx I induction using MEFs and calvarial osteoblasts isolated from PKC δ knockout mice. PKC δ-/- and control MEFs were serum starved in order to lower the basal levels of Prx I and subsequently treated with increasing concentrations of sodium arsenate for 6 h. Total RNA was isolated and analyzed by Northern blot to detect expression of Prx I. We found that levels of Prx I mRNA increased in a dosage-dependent manner (Fig. 1A,B). In wild-type cells, we observed a maximal induction at 0.10 mM arsenate. In PKC δ-deficient cells, the basal level of Prx I dropped, the induction was much weaker, and the maximal level of Prx I mRNA induction was dramatically reduced (Fig. 1B).
Figure 1.
Induction of Prx I in PKC δ deficient cells is reduced. (A) PKC δ-deficient and wild-type control MEFs were treated with different concentrations of sodium arsenate for 6 h. Total RNA was isolated from these cells and was analyzed by Northern blot using a radiolabeled Prx I probe. (B) Quantitation data from three experiments. (C) PKC δ-deficient osteoblasts showed compromised induction of Prx I, which was rescued by reconstitution of PKC δ. The experiments were done as described in A. (D) Quantitation data from three experiments. (E) Western blot shows that PKC δ was expressed by a retroviral vector in PKC δ-deficient osteoblasts. (F) Up-regulation of Nrf2 is suppressed in PKC δ-deficient cells. Mutant and wild-type control osteoblasts were treated with different concentrations of arsenate for 6 h and collected. Expression of Nrf2 was analyzed by Western blot using anti-Nrf2 antibodies. Actin is used as a loading control. Expression of PKC δ was detected using a monoclonal antibody. (G) PKC δ-deficient osteoblasts were resistant to the cytotoxic effect of arsenate. Mutant and wild-type osteoblasts were treated with different concentrations of arsenate for 20 h and cell death rates were determined by the trypan blue exclusion method.
We then tested Prx I induction in PKC δ-/- calvarial osteoblasts, as primary osteoblasts were used to study the physiological functions of c-Abl and Atm in the following experiments. Primary osteoblasts were isolated from newborn pups of PKC δ-/- mice and control littermates. To reconstitute PKC δ, we infected PKC δ-/- osteoblasts with a retrovirus expressing PKC δ and selected against hygromycin for 2 d. The infection rates were observed to be above 80% on the basis of green fluorescent protein (GFP) expression. These cells were then challenged with increasing concentrations of arsenate for 10 h and Prx I induction was analyzed by Northern blot. PKC δ-/- osteoblasts showed a severely compromised Prx I induction (Fig. 1C,D), indicating that PKC δ is required for the maximal induction of Prx I in osteoblasts. Arsenate induction of Prx I was restored to levels higher than that of wild-type cells in reconstituted PKC δ-/- osteoblasts. Western blot analysis confirmed that reconstituted cells expressed two- to threefold more PKC δ than wild-type cells (Fig. 1E). These data indicate that PKC δ plays a positive role in the induction of Prx I in both MEFs and osteoblasts. The incomplete suppression of Prx I in cells lacking PKC δ suggests that redundant signaling molecules, probably other PKC members, may also participate in the process.
Nrf2 accumulation is repressed by PKC δ deficiency
Prx I and some other antioxidant proteins have ARE in their promoters. Their induction during oxidative stress requires Nrf2, a transcription factor that binds ARE. In Nrf2-deficient cells, Prx I induction was abolished (Ishii et al. 2000). Nrf2 is regulated in two ways: translocation from the cytoplasm to the nucleus and stabilization of the protein, both of which contribute to transactivation of genes containing ARE (Itoh et al. 2003). Having shown that PKC δ-deficient cells were defective in Prx I induction, we wanted to test whether there were any related defects in Nrf2 translocation or accumulation. PKC δ-/- and control osteoblasts were treated with arsenate for different periods of time and cytosol and nuclear fractions were prepared from cell lysates. Nrf2 protein levels in the two fractions were analyzed by Western blot. No significant translocation of Nrf2 was observed in either mutant or wild-type cells (data not shown), suggesting that PKC δ did not significantly regulate Nrf2 translocation. However, we observed an increase in Nrf2 levels on arsenate treatment in wild-type cells, whereas a compromised increase was seen in PKC δ-deficient cells (Fig. 1F), suggesting that PKC δ is involved in Nrf2 accumulation/stabilization. Expression of Keap1, the cytoplasmic partner of Nrf2, was not affected (data not shown). Aono et al. (2003) recently reported that arsenate could up-regulate the level of Nrf2 and elevation in Nrf2 could activate ARE-containing antioxidant genes (Nguyen et al. 2003). We did find that ectopic expression of Nrf2 could up-regulate the mRNA levels of Prx I in MC3T3-E1, a murine osteoblast cell line (data not shown). The compromised accumulation of Nrf2 may contribute to reduced Prx I induction in PKC δ-deficient cells.
Cells undergo apoptosis or necrosis following prolonged treatment with high dosages of stress-inducing reagents. In order to test whether PKC δ plays any role in arsenate-induced cell death, PKC δ-/- and control osteoblasts were treated with different concentrations of sodium arsenate for 20 h and the percentage of dead cells was determined by the trypan blue exclusion method and by fluorescence-activated cell sorting (FACS) analysis after PI staining. Similar results were obtained from the two methods and only the results obtained from the trypan blue exclusion method are shown. We found that in both wild-type and PKC δ-deficient cells, cell death rates increased in a dose-dependent manner, yet PKC δ-/- cells consistently showed a lowered number of dead cells (Fig. 1G). Reconstitution of PKC δ rendered PKC δ-/- osteoblasts more susceptible to arsenate (Fig. 1G). Similarly, MEFs deficient for PKC δ showed resistance to the cytotoxicity of arsenate (data not shown). The results indicate that cells survive better against arsenate in the absence of PKC δ and that the function of PKC δ is to promote cell death. Similar roles have been obtained from studies on Jurkat cells and smooth muscle cells against other proapoptotic signals (Leitges et al. 2001).
c-abl-/- osteoblasts show enhanced induction of Prx I by sodium arsenate
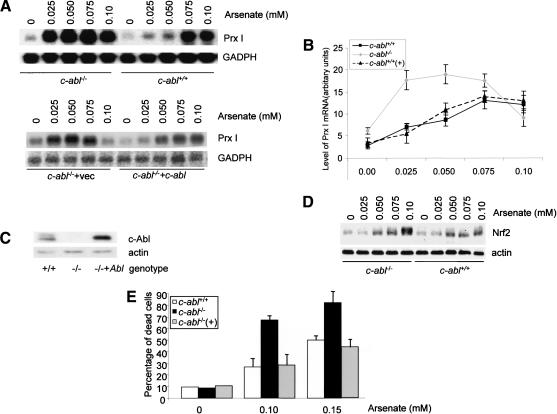
The c-Abl kinase is activated by H2O2, and thus might participate in the response to oxidative stress. We chose osteoblasts to study the function of c-Abl from the findings that it plays an important role in other cellular processes of osteoblasts and that c-Abl-deficient mice show osteoporosis (Li et al. 2000). Furthermore, Prx I interacts with c-Abl and regulates its activation (Wen and Van Etten 1997). We first tested whether c-Abl deficiency alters Prx I induction using primary osteoblasts isolated from the calvaria of the newborn c-abl-/- mice and their control littermates. c-abl-/- and control osteoblasts were treated with different concentrations of sodium arsenate for 10 h and induction of Prx I was assayed by Northern blot (Fig. 2A,B). c-abl-/- osteoblasts showed increased Prx I induction compared with wild-type controls at lower concentrations of arsenate. In wild-type osteoblast cultures, the Prx I mRNA level was only minimal at 0.05 mM of arsenate, whereas c-abl-/- osteoblasts showed strong induction at 0.025 mM of arsenate. When c-abl-/- and normal osteoblasts were challenged with 0.04 mM of arsenate for different periods of time, no Prx I mRNA induction was observed in wild-type cells, whereas induction was significant after 4 h of treatment in c-Abl-deficient cells (data not shown). The c-Abl reconstituted c-abl-/- cells behaved like control wild-type cells, showing Prx I induction comparable to that of normal cells (Fig. 2A [bottom panel], B). Figure 2C shows that retroviral encoded c-Abl expression is slightly higher than that of endogenous c-Abl. These results indicate that osteoblasts deficient for c-Abl are more sensitive to Prx I induction and that c-Abl normally limits or negatively regulates Prx I induction at the level of mRNA.
Figure 2.
Prx I induction by arsenate is enhanced in c-Abl-deficient osteoblasts. (A) Induction of Prx I under different concentrations of sodium arsenate in abl-/-, c-Abl reconstituted abl-/-, and control calvarial osteoblasts. Equal numbers of osteoblasts were plated and treated with different amounts of sodium arsenate for 10 h, and Prx I mRNA levels were analyzed by Northern blot. (B) Quantitation data from three repeated experiments. (C) Western blot analysis showing that expression of retroviral-driven c-Abl is slightly higher than that of endogenous levels. (D) Up-regulation of Nrf2 was enhanced in c-Abl-deficient osteoblasts. Mutant and control osteoblasts were treated with different concentrations of arsenate for 6 h and collected, and Western blot analysis was carried out to test the expression of Nrf2. (E) c-Abl-deficient osteoblasts are hypersensitive to the cytotoxicity of arsenate. Cell death rates in abl-/-, reconstituted abl-/-, and wild-type control osteoblasts induced by arsenate. The values are mean ± S.D. (n = 4).
We also tested whether the enhanced Prx I induction correlated with Nrf2 up-regulation in c-Abl-deficient cells. Mutant and control cells were treated with increasing concentrations of arsenate and Nrf2 levels were analyzed by Western blot (Fig. 2D). While both cell types showed a dosage-dependent increase in Nrf2, c-Abl-deficient osteoblasts showed enhanced accumulation of Nrf2, consistent with enhanced induction of Prx I observed in c-abl-/- cells. The results suggest that lack of c-Abl sensitized the cells to oxidative stress-induced Nrf2 accumulation.
c-abl-/- osteoblasts are more sensitive to sodium arsenate-induced cell death
To test whether c-Abl plays a role in arsenate-induced cell death, we used different concentrations of sodium arsenate to treat c-abl-/- and control osteoblasts for 20 h and the number of dead cells was determined by the trypan blue exclusion method. Untreated cell populations, as well as cells treated with only 0.05 mM arsenate, contained <10% dead cells. Under these conditions, c-abl-/- and control osteoblasts did not show any difference. At higher concentrations of sodium arsenate, increased cell death was observed in both cell lines, with c-abl-/- cells consistently showing a higher percentage of dead cells than wild-type cells (Fig. 2E). Both mutant and control cells treated with 0.1 mM arsenate showed a dramatic increase in cell death rates. Whereas wild-type cultures showed <30% dead cells, c-abl-/- cultures showed >60% dead cells. Reconstitution of c-Abl rescued the hypersensitive phenotype of c-abl-/- osteoblasts (Fig. 2E). These results indicate that c-abl-/- osteoblasts are more sensitive to killing caused by sodium arsenate, implying that c-Abl may be required for the protection of cells from stress. These results are analogous to findings showing that c-abl-/- pre-B cells were more sensitive to IL-7 withdrawal and dexamethasone treatment (Dorsch and Goff 1996).
Oxidative stress induces cell death by apoptosis and/or necrosis in many cell types. Necrosis may be the more likely mechanism of cell death because >80% of cultured macrophages are killed by arsenate in the form of necrosis (Sakurai et al. 2000). To determine whether cell death in osteoblasts was due to apoptosis or necrosis, we performed TUNEL assays on osteoblasts treated with 0.1 mM arsenate, a concentration sufficient to kill >30% of the normal primary osteoblasts. We observed that very few cells were TUNEL positive under these conditions. The osteoblasts in these cultures retained functional apoptotic pathways, were capable of undergoing apoptosis, and could be scored by TUNEL: when serum-starved by growth in 0.5% serum for 24 h, a high proportion of the cells became TUNEL positive (data not shown). All of these results indicate that the cell death resulting from sodium arsenate treatment was not due to the classical apoptosis.
Up-regulation of PKC δ in c-abl-/- osteoblasts
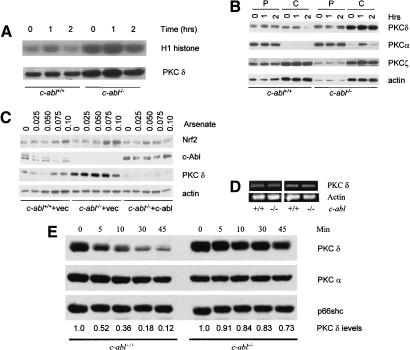
Having shown that activation of PKC δ was required for arsenate-induced Prx I up-regulation and cell death, and that c-Abl-deficient osteoblasts showed enhanced responses in both events, we decided to test whether a link exists between PKC δ and c-Abl. It has been shown that PKC δ interacts with c-Abl and this interaction is promoted by oxidative stress (Sun et al. 2000b). We first tested whether c-Abl deficiency affects the activation of PKC δ. In vitro kinase assays were carried out using immunoprecipitated PKC δ from control and c-abl-/- osteoblasts treated with arsenate. Activation of PKC δ by arsenate is much weaker in primary osteoblasts than in MC3T3-E1, an immortalized murine osteoblast line (Fig. 3A; Li et al. 2002). This could be due to the existence of a higher basal level of PKC δ kinase activity in the former. Nevertheless, we observed that the basal level of PKC δ activity was much stronger in c-abl-/- cells than in the control cells (Fig. 3A). Higher PKC δ activity turned out to be due to its elevated protein levels in c-abl-/- osteoblasts. The expression levels of PKC δ have been analyzed in osteoblasts isolated from different litters, and c-abl-/- cells consistently showed increased levels of PKC δ (data not shown). Increased PKC δ may sensitize the cells to oxidative stress and thus explain why induction of Prx I was enhanced in the absence of c-Abl.
Figure 3.
PKC δ expression was elevated in c-abl-/- osteoblasts. (A) Activation of PKC δ by arsenate. Mutant and control osteoblasts were treated with 0.10 mM arsenate for different periods of time, PKC δ was immunoprecipitated with an anti-PKC δ antibody, and in vitro kinase assay was performed using H1 histone as a substrate. (B) Expression of PKC δ in c-Abl-deficient and normal osteoblasts. Cell lysate was fractionated into cytosol and particulate portions and equal amounts were analyzed by Western blot using a monoclonal anti-PKC δ antibody. The blot was also probed with antibodies against other isoforms of the PKC family. (P) Particulate fraction; (C) cytosol fraction. (C) Reconstitution of c-Abl by a retroviral vector down-regulated the level of PKC δ in c-Abl-deficient cells and restored its normal accumulation of Nrf2. (D) The mRNA levels for PKC δ were normal in c-abl-/- osteoblasts. Total RNA was isolated from mutant and control osteoblast cultures and was used for RT–PCR with actin as a control. (E) Degradation of PKC δ induced by PMA was slower in c-abl-/- osteoblasts. Wild-type and c-abl-/- osteoblasts were treated with 500-nM PMA for different periods of time and the protein levels of PKC δ were determined by Western blot. The blot was stripped and reprobed with anti-PKC α and Shc, respectively, which served as a loading control. The levels of PKC δ were determined by densitometry and compared with the basal levels, which were set at 1.0.
The PKC family has at least 11 isoforms and is divided into three groups: conventional PKCs such as PKC α and PKC β that are sensitive to both diacylglycerol and calcium; novel PKCs such as δ and ε that are only responsive to DAG; and atypical PKCs such as ζ that are irresponsive to either DAG or Ca++ (for review, see Gschwendt 1999). Activation of PKC δ requires its translocation to the plasma membrane (Gschwendt 1999). To determine whether c-Abl deficiency affects the localization of PKC δ, we separated cell lysates into cytosol and particulate fractions, and the levels of PKC δ, along with two other members, PKC α and PKC ζ, were determined by Western blot. No significant translocation was observed for PKC δ, suggesting that the activation of PKC δ here may be mediated by mechanisms other than membrane translocation. Konishi et al. (2001) has reported that H2O2 treatment led to tyrosine phosphorylation and subsequent activation of PKC δ. The expression levels of PKC α and PKC ζ did not show any significant alteration in c-Abl deficient osteoblasts, whereas the level of PKC δ was dramatically increased, especially in the cytosolic fraction (Fig. 3B), indicating an isoform-specific effect. As expected, reconstitution of c-Abl reduced the protein levels of PKC δ and restored the normal Nrf2 accumulation (Fig. 3C). To determine how expression of PKC δ protein is regulated by c-Abl, we first carried out RT–PCR assays using total RNA isolated from c-Abl deficient and wild-type osteoblasts with primers for PKC δ. No significant difference was observed (Fig. 3D), suggesting the regulation occurs at posttranscriptional levels. We then tested whether PKC δ was stabilized in c-Abl-deficient osteoblasts. Western blot analysis following inhibition of translation with cycloheximide revealed that PKC δ protein had a long lifespan that was not significantly affected by deficiency in c-Abl (data not shown). An alternative way to regulate PKC δ stability is mediated by its activation. It is known that PKC δ is destabilized after prolonged activation, especially with PMA (Gschwendt 1999). Therefore, we treated c-Abl-deficient and wild-type osteoblasts with 500 nM of PMA, an agonist for classical and novel PKC members, for varying periods of time. The levels of PKC δ were determined by Western blot using Shc and PKC α as controls. We observed rapid degradation of PKC δ in wild-type cells, but not in c-Abl-deficient cells (Fig. 3E). Degradation of PKC α was much slower and was not significantly affected by c-Abl deficiency. These results suggest that the increase in PKC δ levels in c-Abl-deficient osteoblasts could be attributed to protein stabilization. The levels of Shc, a PKC δ interacting protein, was not affected by PMA and therefore were used as a loading control (Leitges et al. 2001).
PKC δ mediated the hypersensitivity phenotypes of c-abl-/- osteoblasts
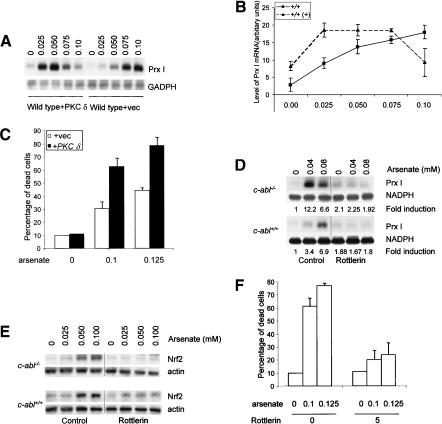
Having shown that c-abl-/- osteoblasts were hypersensitive to arsenate-induced Prx I expression and cell death, correlated with elevated levels of PKC δ, a signaling molecule required in these two cellular events, we wanted to test whether elevated expression of PKC δ mediated the enhanced response of c-abl-/- cells. We first tested whether enforced expression of PKC δ could make wild-type cells behave like c-abl-/- cells with regard to Prx I induction and cell death. We made use of primary wild-type osteoblasts and infected them with retroviruses expressing either GFP or PKC δ and selected against hygromycin for 1 wk. Expression of PKC δ was increased five- to sixfold by the retroviral infection, as confirmed by Western blot analysis (data not shown). These cells were challenged with increasing concentrations of arsenate for 10 h. Induction of Prx I was assessed by Northern blot. We observed that cells overexpressing PKC δ showed enhanced induction of Prx I, with a strong induction even at 0.025 mM of arsenate, whereas cells infected with a control virus required 0.075 mM of arsenate to achieve a similar level of induction (Fig. 4A,B). Also, overexpression of PKC δ rendered the cells more susceptible to the cytotoxicity of arsenate (Fig. 4C). Therefore, normal cells overexpressing PKC δ would mimic c-abl-/- osteoblasts in these responses, suggesting that elevation in PKC δ levels in c-abl-/- cells could contribute to improved Prx I induction and increased cell death.
Figure 4.
PKC δ mediated the enhanced response of c-abl-/- osteoblasts. (A) Enforced expression of PKC δ in normal osteoblasts made the cells more sensitive to arsenate-induced Prx I expression. Normal osteoblasts were infected with retroviruses expressing GFP or PKC δ, selected against hygromycin for 7 d with medium changed every 2 d, and challenged with arsenate, and Prx I induction was analyzed with Northern blot. (B) Quantitation data from three experiments. (C) Overexpression of PKC δ led to increased cell death following arsenate treatment. The experiment was carried out as described in Figure 1G. (D) Induction of Prx I in both abl-/- and wild-type control osteoblasts could be inhibited by Rottlerin treatment. Cells were pretreated with rottlerin for 1 h before the addition of arsenate. Prx I mRNA levels were determined by Northern blot. Fold induction was calculated by comparing to the basal level of Prx I in untreated cells. (E) Arsenate-induced Nrf2 accumulation was blocked by inhibition of PKC δ with rottlerin. (F) Arsenate-induced cell death in abl-/- osteoblasts was rescued by rottlerin. abl-/- osteoblasts were pretreated with 5 μM rottlerin for 1 h before arsenate was added to the final concentrations of 0.10 or 0.125 mM. Cell death rates were determined after 20-h treatments.
We next tested whether elevated PKC δ levels were required for enhanced oxidative stress response of c-abl-/- cells. We used rottlerin, a widely used inhibitor for PKC δ, to inhibit PKC δ activation. When wild-type and c-abl-/- osteoblasts were pretreated with 5 μM rottlerin, a concentration shown to inhibit PKC δ activation in primary osteoblasts and in osteoblast cell line MC3T3-E1 (data not shown; Li et al. 2002), induction of Prx I, as well as accumulation of Nrf2, was severely hindered in both wild-type and mutant cells (Fig. 4D,E). Inhibition of PKC δ activation also protected c-abl-/- cells from arsenate-induced cell death (Fig. 4F). Taken together, these results suggest that elevated PKC δ levels mediated the hypersensitive phenotypes of c-abl-/- cells.
Atm, but not p53, is involved in Prx I up-regulation and cell death
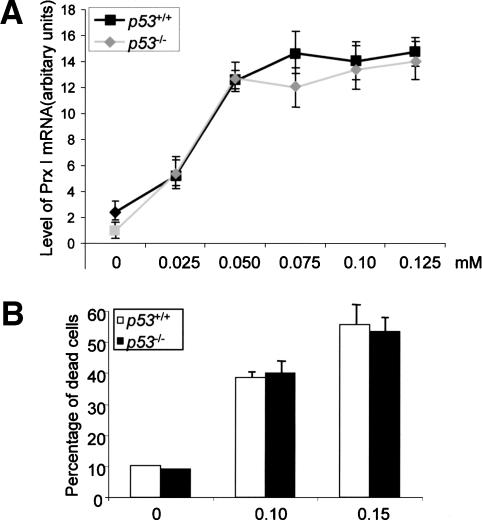
p53 plays important roles in apoptosis induction and in cell cycle control on a variety of stress including oxidative stress (Gudkov and Komarova 2003; Sharpless and DePinho 2002). p53 was also found to interact with c-Abl and can be directly phosphorylated by ATM, an upstream kinase and an interacting protein for c-Abl (Goga et al. 1995). To determine whether p53 plays a role in the response to arsenate treatment, we studied p53-deficient and control cells for both Prx I induction and cell death. Primary osteoblasts isolated from p53-/- mice and their control littermates were cultured in the presence of different concentrations of sodium arsenate for 10 h. Total RNA was isolated from these cells and Northern blot analysis was used to test the induction of Prx I (Fig. 5A). We found that at all concentrations, there was no significant difference between p53-/- and wild-type cells. Western blot analysis of Prx I induction did not reveal any significant difference either (data not shown). These results suggest that p53 is not involved in the induction of Prx I.
Figure 5.
Osteoblasts deficient for p53 showed a normal response to arsenate treatment. (A) Induction of Prx I by arsenate in p53-/- and control osteoblasts. Osteoblasts isolated from p53 knockout mice and their control littermates were stimulated with different concentrations of arsenate for 10 h. Total RNA was isolated from these cells and analyzed by Northern blot to compare the expression levels of Prx I. (B) Cell death rates under arsenate treatment. Osteoblasts deficient for p53 and their control cells were treated with 0.10 or 0.15 mM of arsenate for 16 h and the percentage of dead cells was determined by the trypan blue exclusion method.
We next tested whether p53 deficiency affects cell death caused by arsenate. Primary osteoblasts were treated with 0.05, 0.10, and 015 mM of arsenate for 20 h and the cell death rates were determined (Fig. 5B). We did not detect any significant difference between p53-/- and wild-type cells, implying that p53 is not involved in arsenate-induced cell death.
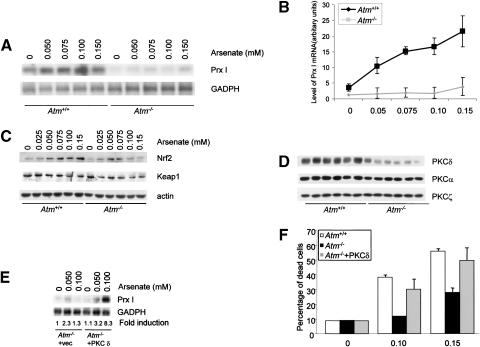
Atm interacts with c-Abl and is required for c-Abl activation in response to ionizing radiation (Shafman et al. 1997). It has been suggested that Atm is a sensor for oxidative stress (Rotman and Shiloh 1997). To determine whether Atm plays similar roles as c-Abl in Prx I induction and cell death in response to arsenate treatment, we isolated primary osteoblasts from Atm knockout mice and their control littermates. These cells (only up to passage 2), having a similar doubling time and similar cell cycle profiles, were treated with different concentrations of arsenate for 10 h and Northern blot analysis was carried out to detect the levels of Prx I mRNA (Fig. 6A,B). We found that induction of Prx I was severely reduced in Atm-/- osteoblasts. Osteoblasts deficient for Atm showed lower basal levels of Prx I and the induction was much weaker, hinting that Atm played a positive role in Prx I induction. As expected, we found that Nrf2 induction was reduced in Atm-/- cells (Fig. 6C). These data, taken together, indicate that cells do not respond to arsenate as well in the absence of Atm.
Figure 6.
Osteoblasts deficient for Atm showed a diminished response to arsenate treatment. (A) Induction of Prx I by arsenate in Atm-/- and control osteoblasts. Osteoblasts isolated from Atm knockout mice and their control littermates were stimulated with different concentrations of arsenate for 10 h. Total RNA was isolated from these cells and analyzed by Northern blot to compare Prx I levels. (B) Quantitation data from repeated experiments. (C) Up-regulation of Nrf2 is diminished in Atm-/- osteoblasts. (D) Expression of PKC δ is down-regulated in the absence of Atm at the protein level. The blot of Figure 3C was reprobed with anti-PKC δ, anti-PKC α, and anti-PKC ζ antibodies, respectively. (E) Ectopic expression of PKC δ in Atm-/- osteoblasts rescued defective Prx I induction. (F) Atm-/- osteoblasts were resistant to the cytotoxic effects of arsenate. Osteoblasts deficient for Atm and their control cells were treated with 0.10 or 0.15 mM of arsenate for 20 h and the percentage of dead cells was determined by the trypan blue exclusion method.
In order to determine whether expression of PKC δ was affected by the deficiency of Atm, the blot used for Nrf2 analysis (Fig. 6C) was probed with anti-PKC δ antibody and Atm-/- osteoblasts were found to express much less PKC δ than were wild-type cells (Fig. 6D). Expression of PKC α and PKC ζ was not altered. These results suggest that down-regulated PKC δ may contribute to the difference observed between Atm-/- and control osteoblasts. To prove this hypothesis, PKC δ was ectopically expressed by a retroviral vector in Atm-/- osteoblasts, as described in Figure 4. These cells were challenged with arsenate at 0.04 and 0.08 μM for 10 h and the induction of Prx I was determined by Northern blot analysis. We observed a drastic induction in Atm-/- osteoblasts expressing PKC δ, whereas Atm-/- osteoblasts expressing GFP did not show much induction (Fig. 6E, cf. 6A). These data suggest that down-regulation of PKC δ contributes to the compromised Prx I induction of Atm-/- osteoblasts. To determine whether the reduction of PKC δ occurred at the levels of mRNA, we carried out RT–PCR assays and no significant difference was observed between Atm-/- and wild-type osteoblasts (data not shown), suggesting that Atm deficiency, like c-Abl deficiency, modulated PKC δ expression posttranscriptionally.
We next tested the cell death rates on arsenate treatment in Atm-/- and control osteoblasts. We found that Atm-/- cells were resistant to the cytotoxic effect of arsenate (Fig. 6F). At a concentration of 0.10 mM, whereas ∼38% wild-type cells were dead, only ∼12% of mutant cells were dead, suggesting that Atm is required for this stress-induced cell death. This is consistent with the findings showing that murine central nervous systems and thymocytes are resistant to IR-induced cell death (Xu et al. 1996; Westphal et al. 1997; Herzog et al. 1998). Furthermore, overexpression of PKC δ rendered Atm-/- osteoblasts more sensitive to the cytotoxic effect of arsenate (Fig. 6F). Taken together, the results indicate that reduced expression of PKC δ mediated the compromised response of Atm-/- osteoblasts to oxidative stress.
Arsenate-induced Prx I up-regulation and cell death were mediated by reactive oxygen species
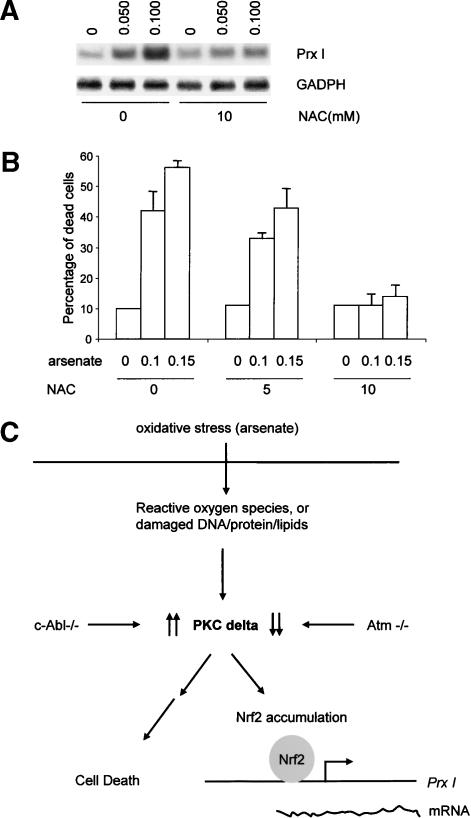
We have shown that c-Abl and Atm play distinct roles in arsenate-induced Prx I up-regulation and cell death. Arsenate has been demonstrated to increase levels of reactive oxygen species (ROS) in both yeast and mammalian cells. To ascertain whether the effect of arsenate is mediated by oxygen-free radical production, we pretreated osteoblasts with a ROS blocker, N′ acetylcysteine (NAC) prior to addition of arsenate. We found that 5 mM NAC did not affect arsenate-induced Prx I up-regulation (data not shown), but NAC at 10 mM significantly suppressed the induction (Fig. 7A). Similarly, 10 mM of NAC dramatically blocked the cytotoxic effect of arsenate (Fig. 7B). These results indicate that the effect of arsenate on Prx I induction and cell death is dependent on ROS production. Therefore c-Abl and Atm are involved in the response to oxidative stress caused by arsenate treatment.
Figure 7.
Effects of NAC on Prx I induction (A) and cell death (B) in response to arsenate. Primary osteoblasts isolated from wild-type mice were pretreated with 5 or 10 mM NAC for 1 h and then arsenate was added to the cultures to a final concentration of 0.05, 0.10, or 0.15 mM. Some cell cultures were collected after 10 h of treatment for total RNA isolation and the rest were collected after 20 h of treatment for cell death measurement. (C) Regulation of Prx I induction and cell death by c-Abl and Atm via PKC δ.
Discussion
We demonstrated that both c-Abl and Atm play important roles in response to arsenate-induced oxidative stress. In all aspects analyzed, from Prx I induction, Nrf2 accumulation, and cell death, to expression levels of PKC δ, c-abl-/- osteoblasts and Atm-/- osteoblasts showed opposite results, providing genetic evidence to suggest that Atm and c-Abl may play different roles in cell response to oxidative stress in mammalian cells. This conclusion is not in agreement with the hypothesis that Atm and c-Abl are in the linear pathway in response to ionizing irradiation (Baskaran et al. 1997), but was supported by our findings that DNA damage-induced nuclear foci formation was differentially regulated by Atm and c-Abl in murine MEFs (L. Zeng, Y. Hu, and B. Li, unpubl.). Our conclusion is further supported by studies using chicken B-cell lines. DT40 cells deficient for Atm show hypersensitivity to ionizing radiation and increased radiation-induced chromosomal aberrations, whereas DT40 cells deficient for c-Abl showed resistance to radiation (Takao et al. 2000). Therefore, it is possible that c-Abl and Atm, two kinases that physically interact, regulate cell responses to oxidative stress and DNA damage in different ways.
The results are also consistent with the notion that Atm may function as a sensor for oxidative stress (Rotman and Shiloh 1997). Without Atm, osteoblasts were not able to detect the damage caused by arsenate and do not respond as well as wild-type cells. Compromised upregulation of Nrf2 in Atm-deficient cells may be why Atm-deficient cells and Atm-deficient mice display more damage caused by ROS (Barlow et al. 1999; Kamsler et al. 2001). Furthermore, cancer chemopreventive agents have been reported to up-regulate Nrf2, and mice deficient for Nrf2 were hypersensitive to carcinogenesis. We speculate that deregulation of Nrf2 may be one of the reasons that ATM patients and Atm-deficient mice are highly predisposed to cancer development (Ramos-Gomez et al. 2001).
The results presented here also confirmed that c-Abl has cell type-specific effects. In fibroblasts, c-Abl functions to promote cell death in response to stress. Thus, c-Abl deficient MEFs were resistant to the cytotoxic effects of both H2O2 and ionizing radiation (Van Etten 1999; Wang 2000). We found that c-abl-/- MEFs were slightly resistant to arsenate compared with control MEFs and that they did not show altered expression of PKC δ (data not shown). In contrast, osteoblasts deficient for c-Abl were more susceptible to the cytotoxic effect of arsenate, which was shown to be mediated by elevated expression of PKC δ. Similarly, Atm appears to have cell type-specific effects. Atm is required for ionizing radiation-induced cell death (mainly apoptosis) in mouse CNS and thymocytes, consistent with our observation that Atm is required for oxidative stress-induced cell death in osteoblasts. In these cell types, the function of Atm is to promote cell death on stress or IR. On the other hand, Atm has been reported to possess antiapoptotic function. Cultured A-T fibroblasts and lymphoblasts, as well as Atm-deficient DT-40 cells, were more sensitive to IR-induced apoptosis (Takao et al. 2000). The underlying molecular mechanisms whereby c-Abl and Atm exert different functions in different cell types remain unclear.
We also provided genetic evidence to support the involvement for PKC δ in the signaling pathway that regulates expression of Prx I, a gene containing antioxidant responsive element, and a role for PKC δ in the signaling pathway that controls cell death in response to arsenate treatment. We also provided evidence that altered PKC δ levels in c-abl and Atm knockout osteoblasts contribute to their abnormal behavior in response to oxidative stress. In the absence of PKC δ, the two cellular events were greatly compromised. Furthermore, cells overexpressing PKC δ showed enhanced Prx I induction and more sensitivity to the cytotoxic effect of arsenate. These data indicate that PKC δ plays a positive role in cell response to oxidative stress (Fig. 7C). This conclusion is further supported by studies on c-abl and Atm knockout osteoblasts. c-Abl deficiency leads to enhanced expression of Prx I and hypersensitivity to the cytotoxic effect of arsenate, which were mediated by elevated levels of PKC δ. Atm deficiency leads to reduced Prx I expression and resistance to the toxic effect of arsenate, which were mediated by decreased levels of PKC δ (Fig. 7C). Because PKC δ deficiency, as well as inhibition of PKC δ activation, compromised the stabilization of Nrf2, a master transcription factor for genes containing ARE in their promoter regions, we believe that PKC δ may play a general role in cell responses to oxidative stress, as proposed by Gopalakrishna and Jaken (2000). Further experiments will be needed to study how PKC δ controls cell death and Nrf2 accumulation in response to oxidative stress.
Prx I is a member of the peroxiredoxin family. These proteins have antioxidant activities, and elevated expression of Prx I is believed to protect the cells (Wood et al. 2003). Similarly, Nrf2 activation (stabilization and translocation to the nucleus) would up-regulate proteins that have antioxidant properties and this is thought to aid cell survival. Thus, it is predicted that cells with higher expression of Prx I and other antioxidant proteins would survive better against oxidative stress. In contrast, we found that PKC δ-deficient cells are resistant to oxidative stress even though they express less Prx I. Atm-deficient osteoblasts express less Prx I but are resistant to cell death, whereas c-Abl deficient osteoblasts express more Prx I but are more sensitive to cell death. Hence, it appears that activation of Nrf2 and up-regulation of Prx I was not able to protect these cells. One possible explanation could be that PKC δ is acting upstream of the two pathways controlling Prx I induction and cell death, for example, a sensor for ROS, as suggested by Gopalakrishna and Jaken (2000), or a very early signaling molecule in the pathway. Without it, the signals to trigger cellular responses such as Prx I induction or cell death may not be passed on to the downstream signaling molecules. It is also quite likely that ROS-activated signals other than PKC δ may be participating in decision making about whether to survive or die. Defined coordination among different pathways is crucial for normal cell function in vivo.
How does c-Abl or Atm regulate the protein level of PKC δ? c-Abl or Atm may affect the transcription of PKC δ gene, the stability of PKC δ mRNA or protein, or the translation efficiency of PKC δ mRNA. RT–PCR assays did not reveal any significant difference in the levels of PKC δ mRNA among wild-type and c-Abl-deficient osteoblasts, suggesting that c-Abl regulates PKC δ expression posttranscriptionally. We did find that c-Abl deficiency inhibited activation-induced degradation of PKC δ, but the molecular mechanism behind this warrants further investigation. Studies have indicated that in cells expressing activated Src (Y527F), PKC δ was down-regulated (Blake et al. 1999). This down-regulation is a result of phosphorylation-mediated degradation. We speculate that c-Abl, a member of the Src family, may have a similar function in regulating the level of PKC δ. It has also been shown that c-Abl interacts with PKC δ in response to oxidative stress. c-Abl was able to phosphorylate PKC δ in fibroblasts. Unfortunately, PKC δ immunoprecipitated from c-Abl-deficient and control osteoblasts did not show significant difference in phosphorylation at tyrosine residues (data not shown). One possible explanation is that PKC δ might have multiple sites for tyrosine phosphorylation that are carried out by several kinases. Hence, c-Abl deficiency would not make a detectable difference. The role for Atm in the regulation of PKC δ expression is even less clear. RT–PCR analysis revealed no significant difference in the levels of PKC δ mRNA, suggesting that the regulation, like that of c-Abl, occurred at posttranscriptional levels. To our surprise, degradation of PKC δ was similar in Atm-/- osteoblasts and wild-type cells. One likely explanation is that the portion of degraded PKC δ molecules in Atm-/- osteoblasts may have a shortened lifespan, whereas the rest have a normal lifespan. We did find that treatment of Atm-/- osteoblasts with MG132, a proteosome inhibitor, appeared to increase the PKC δ levels to that of control osteoblasts (data not shown). The molecular mechanisms by which Atm regulates PKC δ protein levels need further investigation. Because Atm interacts with c-Abl and can activate it, it is possible that there exists a tertiary complex composed of PKC δ, c-Abl, and Atm in the cells, and that c-Abl may mediate the function of Atm in controlling PKC δ expression.
Another layer of complexity is that Prx I/PAG is also a c-Abl interacting protein (Wen and Van Etten 1997). We demonstrated here that c-Abl, a nonreceptor tyrosine kinase, plays a negative role in Prx I induction. Without c-Abl, osteoblasts showed an enhanced induction of Prx I. On the basis of these facts, we propose that in normal osteoblasts, the induction of Prx I is suppressed, facilitating the activation of c-Abl. When c-Abl is deficient, the suppression is lifted and more Prx I is expressed. Therefore, a feedback circuit may exist that controls the activity of c-Abl in response to stress. Alternatively, interaction between c-Abl and Prx I may be involved in regulating the antioxidant activity of Prx I, for example, phosphorylation of Prx I by c-Abl. One such example is that Prx I could be phosphorylated by cdc2 and this phosphorylation reduces the activity of Prx I (Chang et al. 2002).
Materials and methods
Isolation and culture of calvarial osteoblasts
In all experiments, gene targeted mice and their control littermates were used to isolate calvarial osteoblasts. c-abl-/- mice (abl2) were used for the experiments. The PKC δ-deficient mice were generated in the laboratory K.I. Nakayama (Miyamoto et al. 2002). Atm and p53 knockout mice were obtained from the Jackson Labs. To make osteoblasts, we followed a protocol described in Li et al. (2000). Briefly, calvaria from newborn mice or 20-d fetus were cut from the skull, washed in PBS, and digested in α medium containing 0.1% collagenase (Type IV, Sigma) and 0.2% dispase (Roche) for 10 min at 37°C four times. The supernatant of the first digestion was discarded and the supernatant from the last three digestions were pooled. These cells were washed and plated onto 60-mm plates and grown until confluency. The osteoblast cultures were passaged three times and frozen. Cells within passage five were used in these experiments. MEFs were isolated from 13-d embryos as described (Cong et al. 2000), and were used after no more than five passages. The primary osteoblasts and the osteoblast line MC3T3-E1 were cultured in α DMEM (Gibco) containing 10% fetal calf serum (Research Sera).
Osteoblasts were subcultured the day before and then treated with sodium arsenate for various durations of time. For the analysis of mRNA, cells were treated for10 h. Total RNA was isolated and analyzed by Northern blot. For cell death, cells were treated for 20 h. To test the effects of kinase inhibitors and NAC on cell death rates, we added various concentrations of the compounds to the cell cultures 1 h prior to the addition of sodium arsenate. Cells were further treated in α DMEM containing sodium arsenate and rottlerin for 20 more hours. The cells were then harvested for Western blot analysis or cell death measurement.
Western blot analysis
Cells were washed with PBS and lysed in a buffer containing 50 mM Tris (pH 7.5), 100 mM KCl, 1 mM EDTA, 0.5% NP-40, 1 mM PMSF, 1 mM sodium orthorvanadate, 10 mM sodium fluoride, 1 mM β-glycerolphosphate, and 10 μg/mL each of aprotonin, pepstatin, and leupeptin. Protein concentrations were determined by the Bio-Rad protein quantitation assay. The same amounts of protein (20 μg) were fractionated by electrophoresis on a 10% SDS-PAGE gel, transferred to a nitrocellulose membrane (Immobilon-p), probed with polyclonal anti-Prx I antibodies (developed by Dr. T. Ishii), and visualized using an ECL kit (Amersham). Anti-PKC δ, ζ, and α antibodies and anti-Abl antibodies were purchased from BD Transduction Labs; anti-Nrf2 and anti-Keap1 were purchased from Santa Cruz Biotechnology.
Northern blot analysis
Calvarial osteoblasts (1 × 106) were plated onto 100-mm plates and were treated the next day with sodium arsenate at various concentrations. After 10 h, cells were collected and total RNA was isolated using RNAzol (TelTest), fractionated on a 1.5% formaldehyde agarose gel, transferred to Nytran membrane (S&S), and probed with a random primer labeled Prx I cDNA (the open reading frame).
Cell death measurement
Cells were treated with sodium arsenate for various durations of time and then collected by pooling cells from the culture medium as well as the trypsinized adherent cells. Dead cells were counted by the trypan blue exclusion method and by FACS analysis of propidium iodide-positive cells (Gong et al. 1999).
Kinase Assay for PKC δ and cell fractionation
Cells were challenged with arsenate for various durations of time and then lysed with phosphorylation lysis buffer (50 mM HEPES, 150 mM NaCl, 200 μM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 1 mM EDTA, 1.5 mM magnesium chloride, 10% glycerol, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of aprotonin/mL). Cell lysates were immunoprecipitated with an antibody against PKC δ using protein G-Sepharose beads (Amersham Pharmacia Biotech). The immune complexes were washed three times with phosphorylation lysis buffer and two times with kinase buffer (25 mM Tris-HCl at pH 7.4, 5 mM MgCl2, 0.5mM EGTA, 1 mM DTT, 20 μg of phosphatidylserine, and 20 μM ATP) and were resuspended in 30 μL of kinase buffer containing 5 μg of histone H1, after which 20 μCi of 32P-ATP was added. The reaction was incubated for 30 min at room temperature and was terminated by the addition of SDS-sample buffer. Proteins were analyzed by SDS-PAGE, and the phosphorylated form of histone H1 was detected by autoradiography.
For cell fractionation, cells were lysed with phosphorylation lysis buffer (50 mM HEPES, 150 mM NaCl, 200 μM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 1 mM EDTA, 1.5 mM magnesium chloride, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of aprotonin/mL). The cell lysate was separated to cytosolic and particulate fractions by centrifugation for 30 min at 100,000g and the particulate fractions were dissolved in the same buffer with Triton X-100 added to it.
RT–PCR
Total RNA (1.5 μg) was used to synthesize the cDNA using the Retroscript kit (Ambion, Inc.) and oligo(dT) primers. One microliter of this reaction mixture (20 μL in total) was used for the PCR using PKC δ primers: forward, 5′-GAAGACTATCAAC TGGTCC-3′; reverse, 5′-AATGTCCAGGAATTGCTC-3′. The primers for β-actin are forward, 5′-AGATGTGGATCAGCA AGCAG-3′; reverse, 5′-GCGCAAGTTAGGTTTTGTCA-3′. Standard protocol was followed for these PCR reactions.
Retrovirus infection
Retroviral constructs encoding PKC δ, c-Abl, or GFP were used to transfect Plat E packaging cell line using FUGENE (Roche; Morita et al. 2000). After 2 d, media containing the transfected cells were collected and directly used for infection of primary osteoblasts at early passages. Osteoblasts were then selected against hygromycin (PKC δ and GFP) or puromycin (c-Abl) for 2 or 7 d. Expression of the virus-encoded proteins was confirmed by Western blot analysis. These cells were then challenged with arsenate to test Prx I induction, cell death, or Nrf2 accumulation.
Acknowledgments
We thank H-I. Ian, D-Y. Cai, C-P. Tan, H-Y. Kua, and K. de los Santos for technical assistance; Drs. J.W. Soh and Alan Porter for discussion; Drs. J.Y. Wang and M. B. Kastan for c-Abl and Atm construct, respectively; and Dr. T Kitamura for Plat E cells. S.P.G. is an Investigator of the Howard Hughes Medical Institute. B.L. is supported by the Agency for Science and Technology of the Republic of Singapore.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1223504.
References
- Aono J., Yanagawa, T., Itoh, K., Li, B., Yoshida, H., Kumagai, Y., Yamamoto, M., and Ishii, T. 2003. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem. Biophys. Res. Commun. 305: 271-277. [DOI] [PubMed] [Google Scholar]
- Barlow C., Dennery, P.A., Shigenaga, M.K., Smith, M.A., Morrow, J.D., Roberts, L.J., Wynshaw-Boris, A., and Levine, R.L. 1999. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc. Natl. Acad. Sci. 96: 9915-9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran R., Wood, L.D., Whitaker, L.L., Canman, C.E., Morgan, S.E., Xu, Y., Barlow, C., Baltimore, D., Wynshaw-Boris, A., Kastan, M.B., et al. 1997. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387: 516-519. [DOI] [PubMed] [Google Scholar]
- Beckman K.B. and Ames, B.N. 1998. The free radical theory of aging matures. Physiol. Rev. 78: 547-581. [DOI] [PubMed] [Google Scholar]
- Blake R.A., Garcia-Paramio, P., Parker, P.J., and Courtneidge, S.A. 1999. Src promotes PKCdelta degradation. Cell Growth Differ. 10: 231-241. [PubMed] [Google Scholar]
- Chan K. and Kan, Y.W. 1999. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. 96: 12731-12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Han, X.D., and Kan, Y.W. 2001. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. 98: 4611-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.S., Jeong, W., Choi, S.Y., Yu, S., Kang, S.W., and Rhee, S.G. 2002. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J. Biol. Chem. 277: 25370-25376. [DOI] [PubMed] [Google Scholar]
- Cong F., Spencer, S., Cote, J.F., Wu, Y., Tremblay, M.L., Lasky, L.A., and Goff, S.P. 2000. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol. Cell 6: 1413-1423. [DOI] [PubMed] [Google Scholar]
- Dorsch M. and Goff, S.P. 1996. Increased sensitivity to apoptotic stimuli in c-abl-deficient progenitor B-cell lines. Proc. Natl. Acad. Sci. 93: 13131-13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga A., Liu, X., Hambuch, T.M., Senechal, K., Major, E., Berk, A.J., Witte, O.N., and Sawyers, C.L. 1995. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene 11: 791-799. [PubMed] [Google Scholar]
- Gong J.G., Costanzo, A., Yang, H.Q., Melino, G., Kaelin Jr., W.G., Levrero, M., and Wang, J.Y. 1999. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399: 806-809. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R. and Jaken, S. 2000. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 28: 1349-1361. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. 1999. Protein kinase C δ. Eur. J. Biochem. 259: 555-564. [DOI] [PubMed] [Google Scholar]
- Gudkov A.V. and Komarova, E.A. 2003. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 3: 117-129. [DOI] [PubMed] [Google Scholar]
- Halliwell B. and Gutteridge, J.M. 1990. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 186: 1-85. [DOI] [PubMed] [Google Scholar]
- Herzog K.H., Chong, M.J., Kapsetaki, M., Morgan, J.I., and McKinnon, P.J. 1998. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280: 1089-1091. [DOI] [PubMed] [Google Scholar]
- Huang H.C., Nguyen, T., and Pickett, C.B. 2002. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 277: 42769-42774. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yamada, M., Sato, H., Matsue, M., Taketani, S., Nakayama, K., Sugita, Y., and Bannai, S. 1993. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J. Biol. Chem. 268: 18633-18636. [PubMed] [Google Scholar]
- Ishii T., Kawane, T., Taketani, S., and Bannai, S. 1995. Inhibition of the thiol-specific antioxidant activity of rat liver MSP23 protein by hemin. Biochem. Biophys. Res. Commun. 216: 970-975. [DOI] [PubMed] [Google Scholar]
- Ishii T., Itoh, K., Takahashi, S., Sato, H., Yanagawa, T., Katoh, Y., Bannai, S., and Yamamoto, M. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275: 16023-16029. [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi, N., Katoh, Y., Ishii, T., O'Connor, T., and Yamamoto, M. 2003. Keap1 regulates both cytoplasmicnuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8: 379-391. [DOI] [PubMed] [Google Scholar]
- Kamsler A., Daily, D., Hochman, A., Stern, N., Shiloh, Y., Rotman, G., and Barzilai, A. 2001. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 61: 1849-1854. [PubMed] [Google Scholar]
- Key L.L., Wolf, W.C., Gundberg, C.M., and Ries, W.L. 1994. Superoxide and bone resorption. Bone 15: 431-436. [DOI] [PubMed] [Google Scholar]
- Khanna K.K., Keating, K.E., Kozlov, S., Scott, S., Gatei, M., Hobson, K., Taya, Y., Gabrielli, B., Chan, D., Lees-Miller, S.P., et al. 1998. ATM associates with and phosphorylates p53: Mapping the region of interaction. Nat. Genet. 20: 398-400. [DOI] [PubMed] [Google Scholar]
- Konishi H., Yamauchi, E., Taniguchi, H., Yamamoto, T., Matsuzaki, H., Takemura, Y., Ohmae, K., Kikkawa, U., and Nishizuka, Y. 2001. Phosphorylation sites of protein kinase C δ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. 98: 6587-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M., Mayr, M., Braun, U., Mayr, U., Li, C., Pfister, G., Ghaffari-Tabrizi, N., Baier, G., Hu, Y., and Xu, Q. 2001. Exacerbated vein graft arteriosclerosis in protein kinase Cdeltanull mice. J. Clin. Invest. 108: 1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Boast, S., de los, S.K., Schieren, I., Quiroz, M., Teitelbaum, S.L., Tondravi, M.M., and Goff, S.P. 2000. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat. Genet. 24: 304-308. [DOI] [PubMed] [Google Scholar]
- Li B., Ishii, T., Tan, C.P., Soh, J.W., and Goff, S.P. 2002. Pathways of induction of peroxiredoxin I expression in osteoblasts: Roles of p38 mitogen-activated protein kinase and protein kinase C. J. Biol. Chem. 277: 12418-12422. [DOI] [PubMed] [Google Scholar]
- McMahon M., Itoh, K., Yamamoto, M., and Hayes, J.D. 2003. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 278: 21592-21600. [DOI] [PubMed] [Google Scholar]
- Miyamoto A., Nakayama, K., Imaki, H., Hirose, S., Jiang, Y., Abe, M., Tsukiyama, T., Nagahama, H., Ohno, S., Hatakeyama, S., et al. 2002. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase C δ. Nature 416: 865-869. [DOI] [PubMed] [Google Scholar]
- Morita S., Kojima, T., and Kitamura, T. 2000. Plat-E: An efficient and stable system for transient packaging of retroviruses. Gene Ther. 7: 1063-1066. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Sherratt, P.J., Huang, H.C., Yang, C.S., and Pickett, C.B. 2003. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 278: 4536-4541. [DOI] [PubMed] [Google Scholar]
- Prosperi M.T., Ferbus, D., Rouillard, D., and Goubin, G. 1998. The pag gene product, a physiological inhibitor of c-abl tyrosine kinase, is overexpressed in cells entering S phase and by contact with agents inducing oxidative stress. FEBS Lett. 423: 39-44. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M., Kwak, M.K., Dolan, P.M., Itoh, K., Yamamoto, M., Talalay, P., and Kensler, T.W. 2001. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. 98: 3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G. and Shiloh, Y. 1997. Ataxia-telangiectasia: Is ATM a sensor of oxidative damage and stress? Bioessays 19: 911-917. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Oba, M., Matsumoto, K., Tokura, Y., Furukawa, F., and Takigawa, M. 2000. Acute infectious urticaria: Clinical and laboratory analysis in nineteen patients. J. Dermatol. 27: 87-93. [DOI] [PubMed] [Google Scholar]
- Shafman T., Khanna, K.K., Kedar, P., Spring, K., Kozlov, S., Yen, T., Hobson, K., Gatei, M., Zhang, N., Watters, D., et al. 1997. Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387: 520-523. [DOI] [PubMed] [Google Scholar]
- Sharpless N.E. and DePinho, R.A. 2002. p53: Good cop/bad cop. Cell 110: 9-12. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. 1997. Ataxia-telangiectasia and the Nijmegen breakage syndrome: Related disorders but genes apart. Annu. Rev. Genet. 31: 635-662. [DOI] [PubMed] [Google Scholar]
- Stewart D., Killeen, E., Naquin, R., Alam, S., and Alam, J. 2003. Degradation of transcription factor Nrf2 via the ubiquitin–proteasome pathway and stabilization by cadmium. J. Biol. Chem. 278: 2396-2402. [DOI] [PubMed] [Google Scholar]
- Sun X., Majumder, P., Shioya, H., Wu, F., Kumar, S., Weichselbaum, R., Kharbanda, S., and Kufe, D. 2000a. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J. Biol. Chem. 275: 17237-17240. [DOI] [PubMed] [Google Scholar]
- Sun X., Wu, F., Datta, R., Kharbanda, S., and Kufe, D. 2000b. Interaction between protein kinase C δ and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J. Biol. Chem. 275: 7470-7473. [DOI] [PubMed] [Google Scholar]
- Takao N., Mori, R., Kato, H., Shinohara, A., and Yamamoto, K. 2000. c-Abl tyrosine kinase is not essential for ataxia telangiectasia mutated functions in chromosomal maintenance. J. Biol. Chem. 275: 725-728. [DOI] [PubMed] [Google Scholar]
- Van Etten R.A. 1999. Cycling, stressed-out and nervous: Cellular functions of c-Abl. Trends Cell Biol. 9: 179-186. [DOI] [PubMed] [Google Scholar]
- Wang J.Y. 2000. Regulation of cell death by the Abl tyrosine kinase. Oncogene 19: 5643-5650. [DOI] [PubMed] [Google Scholar]
- Wen S.T. and Van Etten, R.A. 1997. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes & Dev. 11: 2456-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C.H., Rowan, S., Schmaltz, C., Elson, A., Fisher, D.E., and Leder, P. 1997. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat. Genet. 16: 397-401. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schroder, E., Robin, H.J., and Poole, L.B. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28: 32-40. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ashley, T., Brainerd, E.E., Bronson, R.T., Meyn, M.S., and Baltimore, D. 1996. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 10: 2411-2422. [DOI] [PubMed] [Google Scholar]