Abstract
The genus Antirrhinum comprises about 28 species with a center of origin in the Iberian Peninsula. They show an important diversity of growing niches. We have performed a comprehensive analysis of scent profiles in eight wild species, Antirrhinum linkianum, A. tortuosum, A. cirrigherum, A. latifolium, A. meonanthum, A. braun-blanquetii, A. barrelieri, and A. graniticum. We used also two laboratory inbred lines A. majus, 165E and Sippe50. We identified 63 volatile organic compounds (VOCs) belonging to phenylpropanoids, benzenoids, mono- and sesquiterpenes, nitrogen-containing compounds, and aliphatic alcohols previously described in plants. Twenty-four VOCs were produced at levels higher than 2% of total VOC emission, while other VOCs were emitted in trace amounts. The absolute scent emission varied during flower maturation and species. The lowest emitting was A. meonanthum while A. tortuosum had the largest emissions. Species were clustered according to their scent profiles and the resulting dendrogram matched the current species phylogeny. However, two accessions, A. majus Sippe 50 and A. braun-blanquetii, showed development-specific changes in their VOC composition, suggesting a precise control and fine tuning of scent profiles. Cluster analysis of the different scent components failed to identify a specific synthesis pathway, indicating a key role of scent profiles as blends. There is considerable degree of chemodiversity in scent profiles in Antirrhinum. The specific developmental stage plays an important role in scent quantitative emissions. The relative robustness of the bouquets could be an adaptation to local pollinators.
Keywords: floral scent, flower development, anthesis, phylogeny, biodiversity, chemodiversity, Antirrhinum
Introduction
The interaction between plants and other organisms is thought to be mediated by a complex set of traits among which the emission of chemical compounds plays a key role. The so-called plant volatiles are one of the most diverse set of molecules. Plant volatile emission can be classified according to the source of emission, i.e., leaves, flowers, and roots. And it can also be the result of certain reactions such as defense against herbivores or parasites. The emission of scent by flowers is a cue that helps to make floral sexual organs attractive to potential pollinators, but also works in parasite deterrence (Schiestl, 2010). In most flowers, floral scent is emitted by petals and stamens (Dudareva et al., 1996; Verdonk et al., 2003; Scalliet et al., 2006). Although over 1700 volatile organic compounds (VOCs) are described in plants, the actual composition of floral scent is not fully explored in most plant species (Knudsen et al., 2006).
Petal and stamen development in Antirrhinum and many other species is directly controlled by B function organ-identity genes (Egea Gutierrez-Cortines and Davies, 2000; Causier et al., 2010). The B function genes in Antirrhinum are the MADS-Box genes DEFICIENS and GLOBOSA. Their expression is required in a quantitative manner to attain fully developed petals and stamens (Bey et al., 2004; Manchado-Rojo et al., 2012). Floral scent emission is a late process starting shortly before anthesis in a variety of species (Knudsen et al., 2006), but its quantitative levels are regulated upstream by the B-function genes (Manchado-Rojo et al., 2012). Scent production varies after anthesis showing an increase in production till a point when sharp decreases are caused by flower aging and/or pollination (Pichersky et al., 1994; Ruiz-Ramon et al., 2014).
Antirrhinum, a genus native to the western Mediterranean region, comprises a monophyletic group with approx. 28 species (Liberal et al., 2014), traditionally assigned to the three morphological subsections or clades: Kicksiella, Antirrhinum, and Streptosepalum (Rothmaler, 1956; Webb, 1971; Sutton, 1988). The Antirrhinum flower has an occluded corolla (Vargas et al., 2010; Guzmán et al., 2015). It is apparently specialized in bee pollination as bees such as Rhodanthidium sticticum is the main pollinator of A. microphyllum, (Torres et al., 2003), and seven types of bees account for over 90% of the pollination visits in Antirrhinum charidemi, Antirrhinum graniticum, and Antirrhinum braun-blanquetii (Vargas et al., 2010). Despite the diversity the composition of the Antirrhinum genus floral scent, like that of many other plants, is basically unexplored and only A. majus sp pseudomajus and A. striatum have been analyzed with detail (Suchet et al., 2010).
In this work, we present a comprehensive analysis of floral VOCs in eight wild Antirrhinum species: Antirrhinum linkianum, A. tortuosum, A. cirrigherum, A. latifolium, A. meonanthum, A. braun-blanquetii, A. barrelieri, and A. graniticum. We have also used two laboratory inbred lines, A. majus 165E and Sippe50. These lines have been used for genetic studies, development of an Antirrhinum majus genetic map and for genetic transformation (Schwarz-Sommer et al., 2003, 2010; Manchado-Rojo et al., 2012, 2014). We identified at least 63 VOCs produced at one stage after anthesis and before petal senescence. Each species had a unique blend of VOCs, and tended to show a robust profile except for two species. The scent profiles allowed a cluster reconstruction that matched published phylogenies based on molecular markers indicating a uniqueness of scent signature for each species that may have implications for local adaptation.
Materials and Methods
Plant Material and Growth Conditions
We obtained eight wild species of Antirrhinum and two laboratory inbred lines (Table 1). The wild species include species of subsection Antirrhinum, series Majora: A. barrelieri, A. cirrhigerum, A. graniticum, A. latifolium, and A. tortuosum (Mateu-Andres and De Paco, 2005) as well as the two only members of subsection Streptosepalum, A. braun-blanquetii and A. meonanthum (Feng et al., 2009) (Figure 1). We also used two laboratory inbred lines, A. majus Sippe50 isolated at the beginning of the 20th century in Germany (Stubbe, 1966) and A. majus 165E developed at the John Innes Centre (Harrison and Carpenter, 1979; Sommer and Saedler, 1986). The geographical distribution of the species surveyed includes the Pyrenees, northern Spanish coast, Portugal, southern Spanish coast, and northern Africa (Figure 1). Plants were grown under standard greenhouse conditions using large pots of 3–5 l to increase the number of flowers obtained (Weiss et al., 2016). Four to five plants for each species and line were propagated and flowers were sampled randomly from these plants for further analysis.
Table 1.
Name and origin/supply of Antirrhinum species.
| Species name | Origin |
|---|---|
| Antirrhinum barrelieri Boreau | Vendrell, Tarragona Province, and Spain |
| Antirrhinum braun-blanquetii Rothm. | Province of Oviedo, Picos de Europa, and Spain |
| Antirrhinum meonanthum Hoffmanns and Link | Penacova and Portugal |
| Antirrhinum latifolium Mill. | Ville Franche, Pyrenees, and France |
| Antirrhinum graniticum Rothm. | Unknown |
| Antirrhinum. linkianum | Supplied by Bot. Garden, University of Coimbra, Portugal |
| Antirrhinum cirrhigerum | Unknown, Spain |
| Antirrhinum tortuosum | Unknown, Spain |
| Laboratory lines | |
| Antirrhinum majus L. line 165E | Our stocks |
| A. majus L. line Sippe 50 | Supplied by IPK Gatersleben |
FIGURE 1.
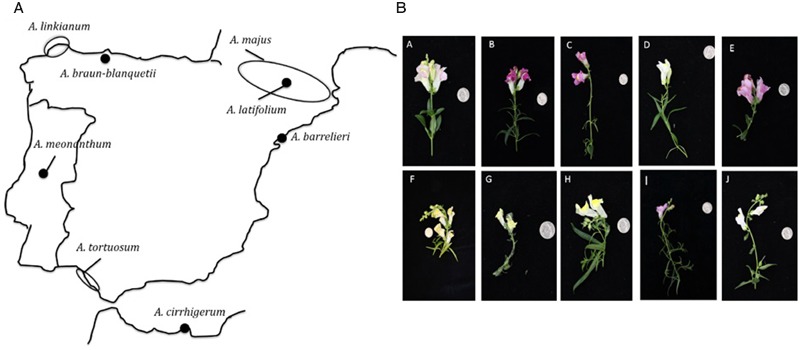
Origin of Antirhinum species used in the study. (A) Origin of seeds (filled circles) used in this study. For seeds of unknown origin, the area of distribution according to Vargas et al. (2009) is indicated (empty circle). A. graniticum is widely spread over central Iberian Penisula and the Western coast area, but absent in the North and East of the Iberian Peninsula (Vargas et al., 2010). (B) Flowers of the studied Antirrhinum species. A 25 cent USA-dollar is photographed for scale: (A) A. majus line 165E, (B) A. majus line Sippe 50, (C) A. linkianum, (D) A. tortuosum, (E) A. cirrigherum, (F) A. latifolium, (G) A. meonanthum, (H) A. braun-blanquetii, (I) A. barrelieri, and (J) A. graniticum.
VOC Collection
Flower samples were taken daily during six days after flower opening and emitted volatiles were analyzed by dynamic headspace analysis (Raguso and Pellmyr, 1998). For each flower developmental stage, three randomly chosen, detached flowers were placed in 5% sucrose solution in transparent glass containers. Volatile sampling was performed over a 24-h period in a growth chamber (model E8; Conviron, Asheville, NC, USA) with a photoperiod of 12:12 light: dark conditions. Scent components were trapped with Porapak Q-filled glass syringes in a closed-loop scent collection system. Trapped volatiles were eluted from the adsorbent with dichloromethane.
Gas-Chromatography Mass Spectrometry
Trapped floral volatiles were analyzed by gas chromatography–mass spectrometry (GC-MS) as described (Dudareva et al., 2003). Data analysis and volatile identification was performed with the MSD ChemStation (Agilent Technologies) software. The compounds were identified by comparing mass spectra and retention time (RT) data with those of authentic standards for benzaldehyde, β-myrcene, 2-ethyl-1 hexanol, β-ocimene, acetophenone, methyl benzoate, linalool, and methyl cinnamate, supplemented with information from the NIST11 spectral library. The relative contribution of volatile compounds was calculated based on the integrated area of particular peaks relative to the total integrated peak area for the flower opening stages I = day 1, II = day 3, and III = day 5. Total volatile amount was calculated based on integrated peak area of a defined amount of the internal standard naphthalene. Total amounts are given as integrated area of peaks normalized to naphthalene/g fresh weight (FW)-1 24 h-1. Supplementary Figure S1 shows one chromatogram of each species at stage III. The different volatiles in percentages can be found Supplementary Table S1.
Cluster Analysis and Principle Component Analysis of Volatiles
For hierarchical cluster analysis, the relative amounts of the 24 most abundant, major volatile compounds were used. We considered as major compounds those that accounted for equal or more than 2% of total amounts in the different flowering stages of the species or subspecies analyzed. The cluster analysis included the volatile profiles of flower opening stages I, II, and III as mentioned above. Clustering of species and developmental stages was achieved using R version 2.13.1, with Pearson correlation and average linkage serving as correlation and agglomeration methods.
Principal component analysis (PCA) was performed with absolute amounts of all VOCs (Table 2). Each sample collected was included in this analysis. To satisfy the assumption of linearity, absolute amounts were log10(n+1)-transformed prior to PCA. PCA with varimax rotation was performed with the prcomp command in the stats package in R version 2.13.1.
Table 2.
List of volatile organic compounds (VOCs) identified in Antirrhinum and known to be biosynthesised by plants.
| Plant emitted volatiles | CAS number | Retention time (RT) | % Probability |
|---|---|---|---|
| Benzenoid – Aldehydes | |||
| Benzeneacetaldehyde | 122-78-1 | 10.925 | 90 |
| Benzaldehyde | 100-52-7 | 9.9076 | 94 |
| Benzaldehyde, 3-ethyl- | 34246-54-3 | 13.316 | 95 |
| Vanillin | 121-33-5 | 17.488 | 90 |
| Benzaldehyde, 4-ethyl- | 4748-78-1 | 13.311 | 90 |
| 3,5-Dimethoxybenzaldehyde | 7311-34-4 | 18.077 | 98 |
| Benzaldehyde, 4-methoxy- | 123-11-5 | 15.016 | 94 |
| Benzenoid – Ketones | |||
| Acetophenone | 98-86-2 | 11.491 | 97 |
| 4-Acetylanisole | 100-06-1 | 16.721 | 94 |
| Ethanone, 1-(4-ethylphenyl)- | 937-30-4 | 15.502 | 97 |
| Benzenoid – Esters | |||
| Benzyl Benzoate | 120-51-4 | 22.924 | 98 |
| Methyl benzoate | 93-58-3 | 11.995 | 94 |
| Benzoic acid, 3,5-dimethoxy-, methyl ester | 2150-37-0 | 20.246 | 98 |
| Methyl salicylate | 119-36-8 | 13.934 | 97 |
| Benzoic acid, 4-methoxy-, methyl ester | 121-98-2 | 17.081 | 81 |
| Benzoic acid, 2-butoxy-, methyl ester | 606-45-1 | 13.934 | 97 |
| Benzenoid – Ethers | |||
| 3,5-Dimethoxytoluene | 4179-19-5 | 15.222 | 98 |
| 1,2,4-Trimethoxybenzene | 135-77-3 | 16.973 | 94 |
| Anisol | 100-66-3 | 8.086 | 91 |
| Benzene, 1,3,5-trimethoxy- | 621-23-8 | 17.625 | 96 |
| Benzenoids – Benzenes | |||
| Benzene, 1,3-diethyl- | 141-93-5 | 1.033 | 97 |
| Benzene, 1,4-diethyl- | 105-05-5 | 11.176 | 97 |
| Benzene, 1,2-diethyl- | 135-01-3 | 11.291 | 96 |
| p-Xylene | 106-42-3 | 6.959 | 95 |
| Ethylbenzene | 100-41-4 | 6.776 | 94 |
| Benzene, 1,2,3-trimethyl- | 526-73-8 | 9.826 | 92 |
| Benzene, 1,4-dimethoxy- | 150-78-7 | 13.294 | 96 |
| Benzene, 1,2-dimethoxy-4-(2-propenyl)- | 93-15-2 | 17.505 | 98 |
| Benzenoids – Alcohols | |||
| Benzyl Alcohol | 100-51-6 | 10.679 | 95 |
| 3-Methoxy-5-methylphenol | 3209-13-0 | 16.120 | 94 |
| Cinnamyl alcohol | 104-54-1 | 15.891 | 98 |
| Benzenepropanol | 122-97-4 | 14.547 | 98 |
| Benzenemethanol, 4-methoxy- | 105-13-5 | 15.502 | 95 |
| Phenol, 4-(1,1-dimethylethyl)-2-methyl- | 98-27-1 | 14.775 | 93 |
| Phenol | 108-95-2 | 9.540 | 74 |
| Isoprenoids–Monoterpenes | |||
| Myrcene | 123-35-3 | 9.786 | 96 |
| Ocimene | 3779-61-1 | 11.002 | 98 |
| Neo-allo-ocimene | 7216-56-0 | 12.618 | 96 |
| Linalool | 78-70-6 | 12.058 | 97 |
| Limonene | 138-86-3 | 10.581 | 99 |
| α-Pinene | 80-56-8 | 8.476 | 95 |
| Terpineol | 98-55-5 | 13.849 | 90 |
| Isoprenoids–Sesquiterpenes | |||
| α-Farnesene | 502-61-4 | 19.164 | 97 |
| Nerolidol | 7212-44-4 | 20.023 | 95 |
| Phenylpropanoids – Alcohols | |||
| Eugenol | 97-53-0 | 16.784 | 98 |
| Phenylpropanoids – Esters | |||
| Methyl cinnamate | 103-26-4 | 17.219 | 97 |
| Cinnamyl formate | 21040-45-9 | 16.692 | 98 |
| Phenylpropanoids –Aldehydes | |||
| Cinnamaldehyde | 104-55-2 | 15.308 | 98 |
| Fatty acid derivatives – Aldehydes | |||
| Decanal | 112-31-2 | 14.066 | 91 |
| Nonanal | 124-19-6 | 12.126 | 87 |
| Hexanal, 2-ethyl- | 123-05-7 | 8.951 | 81 |
| Fatty acid derivatives – Ketones | |||
| 2-Pentadecanone, 6,10,14-trimethyl- | 502-69-2 | 23.833 | 99 |
| 5-Hepten-2-one, 6-methyl- Methylheptenone | 110-93-0 | 9.677 | 94 |
| γ-Hexenol | 928-96-1 | 6.656 | 91 |
| Fatty acid derivatives – Alcohols | |||
| 1-Hexanol, 2-Ethyl, | 104-76-7 | 10.576 | 90 |
| Phenoxyethanol | 122-99-6 | 14.346 | 95 |
| Fatty acid derivatives – Acids | |||
| Dodecanoic acid | 143-07-7 | 19.885 | 91 |
| Amines and other nitrogen containing compounds | |||
| Indolizine | 274-40-8 | 15.714 | 86 |
| Indole | 120-72-9 | 15.708 | 95 |
| Methyl nicotinate | 93-60-7 | 12.807 | 95 |
| Benzyl nitrile | 140-29-4 | 12.836 | 96 |
| Diphenylamine | 122-39-4 | 20.915 | 90 |
| Non-classified | |||
| 1,3,5,7-Cyclooctatetraene | 629-20-9 | 7.474 | 70 |
Retention times are approximated and consistent between all the chromatograms analyzed. % Probability column indicates the existing chances of success in identifying compounds for a given RT, according to the NIST11 database. Internal standard (Naphthalene, CAS no. 91-20-3) probability is over 95%.
Results
Phenotypic Space of Scent Emission
The emission of floral volatiles starts at late stages of petal morphogenesis requiring fully developed petals and anthesis (Manchado-Rojo et al., 2012; Muhlemann et al., 2012). We investigated the production of floral scent over a time span of six days after flower opening and identified a total of 63 based on NIST 11. There were 63 that matched VOCs previously identified in plants (Table 2). They belonged to the following chemical categories: phenylpropanoids, benzenoids, mono- and sesquiterpenes, nitrogen-containing compounds, and aliphatic alcohols (Knudsen et al., 1993). Amongst the compounds identified and found in a variety of plants and in Antirrhinum were benzenoids such as vanillin, o-acetanisole, methyl salicylate, anisole or cuminyl alcohol; isoprenes such as alpha pinene or terpineol. Phenylpropanoids included cinnamyl formate; fatty acid derivatives as aldehydes including octanal, decanal, nonanal or alcohols such as octanol or as acids. Flowers also emitted amines or nitrogen containing compounds such as methyl nicotinate, indole or indolicine 1,3,5,7-cyclooctatetraene, a non-classified compound.
We additionally found nine VOCs that had not been described previously as emitted by plant tissues (Table 3). They could be grouped into the classic set of benzenoids, phenylpropanoids, and fatty acid derivatives VOCs.
Table 3.
List of new VOCs identified in Antirrhinum and previously unidentified in plants.
| New volátiles | CAS number | RT | Quality |
|---|---|---|---|
| Benzenoid – Ketones | |||
| Acetophenone, 2′-hydroxy- | 118-93-4 | 13.311 | 97 |
| Benzenoid – Esters | |||
| Benzenepropanoic acid, methyl ester | 103-25-3 | 15.731 | 92 |
| Benzenoids – Benzenes | |||
| Benzene, 1-(1,1-dimethylethyl)-4-methoxy- | 5396-38-3 | 14.775 | 94 |
| Benzene, 1-ethenyl-3-ethyl- | 7525-62-4 | 11.766 | 96 |
| Benzenoids – Alcohols | |||
| Phenol, p-tert -butyl- | 98-54-4 | 15.645 | 97 |
| Phenol, 2,6-dimethoxy-4-(2-propenyl)- | 6627-88-9 | 20.606 | 97 |
| Benzenethanol | 60-12-8 | 12.338 | 93 |
| Phenylpropanoids – Esters | |||
| Cinnamyl acetate | 103-54-8 | 18.180 | 97 |
| Fatty acid derivatives – Alkanes | |||
| Adamantane, 1,3-dimethyl- | 702-79-4 | 12.378 | 90 |
Retention times are approximated and consistent between all the chromatograms analyzed. % Probability column indicates the existing chances of success in identifying compounds for a given RT, according to the NIST11 database. Internal standard (Naphthalene, CAS no. 91-20-3) probability is over 95%.
From the large dataset presented, there were 24 major compounds comprising more than 2% of the scent emission in the different species (Figure 2 and Supplementary Figure S1). Among these we found benzaldehyde, acetophenone, ocimene, and 2-ethyl 1-hexanol in all the species analyzed, comprising very different percentages of the scent profile. At the other side of the spectrum, 1,4-dimethoxybenzene was present only in A. braun-blanquetii, nerolidol was found in A. braun-blanquetii and A. latifolium and 5,9-dodecadien-2-one, 6,10-dimethyl was found in A. meonanthum and A. graniticum.
FIGURE 2.
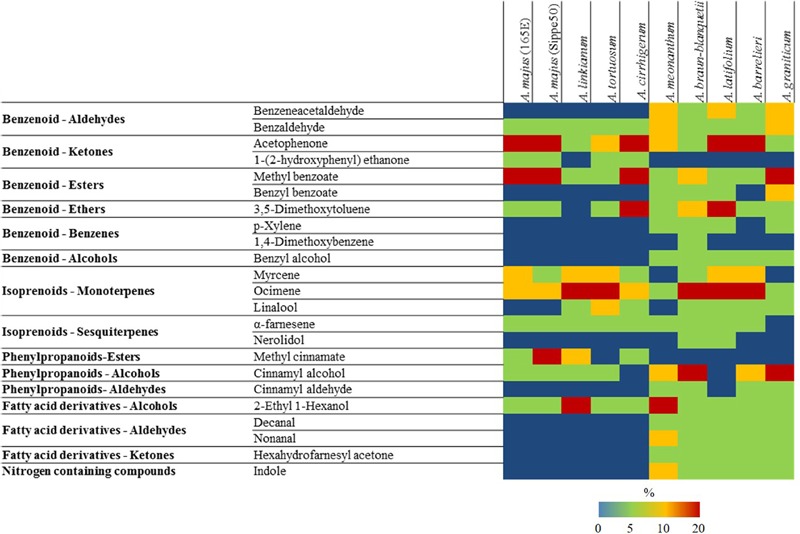
Heat map with major VOCs (above 2% of total volatiles present in chromatograms) emitted by different species of Antirrhinum. Colors reflect the maximum level of emission (%) in stages I–III for each species. Key color emission: blue (0%), green (<5%), yellow (<20%), and red (>20%).
The complexity of the different suggested scent profiles in terms of number of VOCs emitted varied greatly. The most complex profile was exhibited by A. braun-blanquetii comprising 21 VOC compounds above 2% over at least one of the three developmental stages analyzed (Figure 2 and Supplementary Figure S1). In contrast there were five accessions with a much simpler scent profile such as A. linkianum with 10 major VOCs followed by A. cirrhigerum, A. majus Sippe50 and A. tortuosum with 11 VOCs and A. majus 165E with 12.
In summary, the species analyzed appeared to show two distinct suggested profiles as those with relative low scent complexity lack irregular terpenes, fatty acid aldehydes and ketones, and nitrogen containing compounds.
Changes in Total and Relative Emission of VOCs during Flower Development
We analyzed the scent emission during a period of seven days. Flowers of all species produced scent during the entire sampling period. The average emission of scent during the period varied between species (Figure 3). The lowest emitting species was A. meonanthum while the species with larger levels of production corresponded to A. latifolium followed by the two A. majus 165E and Sippe50 inbred lines.
FIGURE 3.
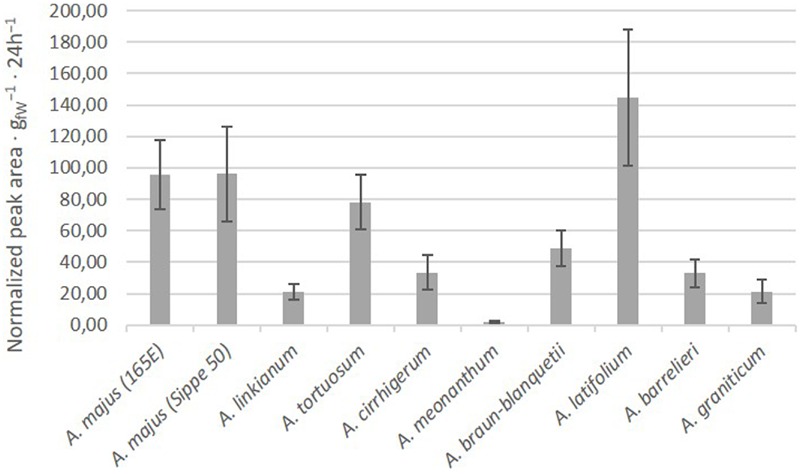
Average emission of VOCs in the different accessions. Quantities are total integrated area normalized to naphthalene/g 24 h-1. Error bars correspond to standard error.
We analyzed the quantitative changes in emission of the different compounds throughout development (Figure 4). The two A. majus inbred lines used for many experiments in plant development and the wild species A. latifolium and A. barrelieri produced acetophenone as major volatile. They also showed comparable levels of methyl benzoate and ocimene emission. However, they differed in the emission of myrcene by A. majus 165E and methyl cinnamate by A. majus S.50. The profile of A. latifolium included ocimene, 3,5-dimethoxytoluene, benzeneacetaldehyde, and myrcene, while A. barrelieri emitted ocimene, cinnamyl alcohol, and myrcene.
FIGURE 4.
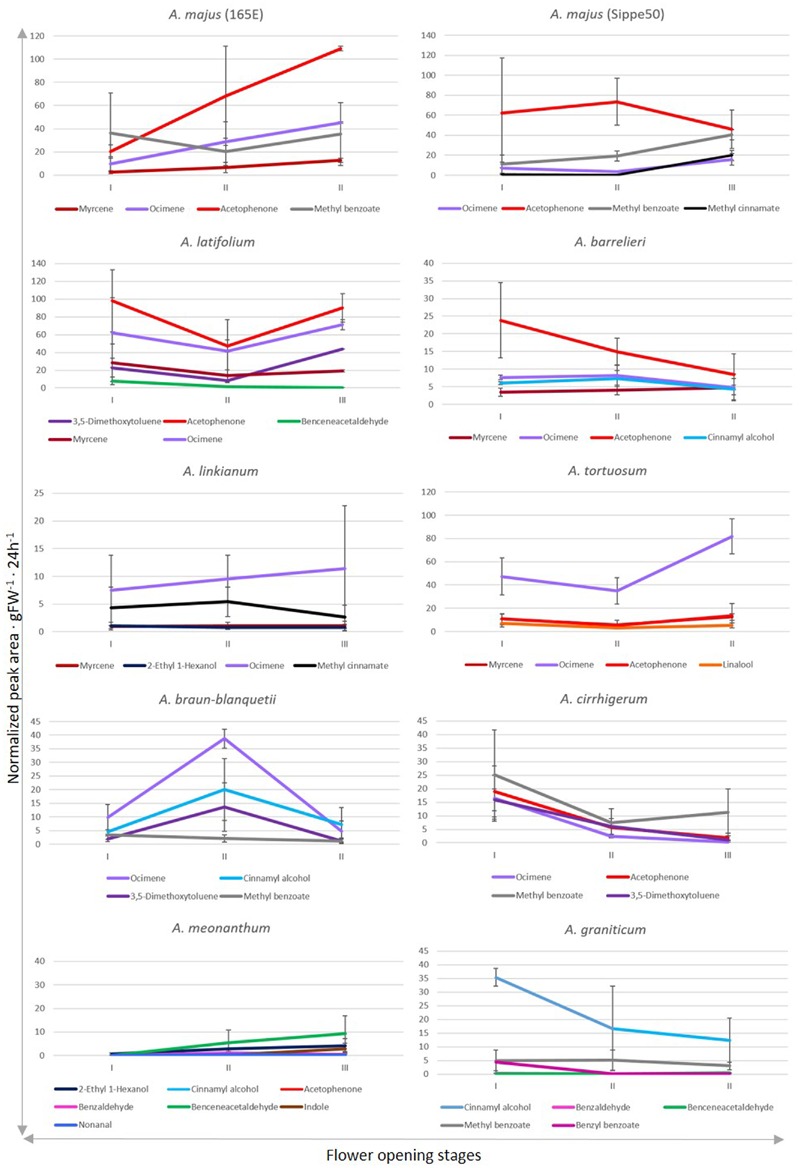
Changes in emission of selected VOCs after anthesis. Stages of development described as stage I, II, and III correspond to 1–2, 3–4, and 5–6 days after anthesis. Quantities are reflected as total peak integrated areas normalized to naphthalene/g 24 h-1. Error bars correspond to standard error. The VOCs represented here have an emission above 5% of the total amount of emitted VOCs.
There were three species, A. linkianum, A. tortuosum, and A. braun-blanquetii that emitted ocimene as major volatile. However, the rest of the volatiles were not in common as A. linkianum produced methyl cinnamate, myrcene, and 2-ethyl 1-hexanol. The scent profile of A. tortuosum included myrcene, acetophenone, and linalool while A. braun-blanquetii showed high levels of cinnamyl alcohol, 3,5-dimethoxytoluene, and methyl benzoate.
Finally, three species showed a different VOC as major compound. The major VOC in A. cirrhigerum was methyl benzoate, and emitted acetophenone, 3,5-dimethoxytoluene and ocimene. The scent profile of A. meonanthum was complex as its debut was dominated by 2-ethyl-hexanol but was taken over by benzene acetaldehyde. It also emitted acetophenone, cinnamyl alcohol, benzaldehyde, nonanal, and the nitrogen containing indole. The main component emitted by A. graniticum was cinnamyl alcohol, methyl benzoate, benzyl benzoate, benzaldehyde, and benzeneacetaldehyde.
Concerning the quantitative changes in emission during flower maturation, the quantities varied and the variance was high. This is probably due to temperature changes during flower maturation. Thus a general pattern of emission cannot be found for all the species.
Scent-Based Clustering of Antirrhinum Species and Robustness of Scent Profiles
To determine whether differences in scent emission between species are greater than emission differences between developmental stages within species, we collected volatile samples for the developmental stages I–III. Suggested volatile profiles for most of the species presented here, except A. majus line Sippe 50 and A. braun-blanquetii, clustered together for all flower developmental stages (Figure 5), demonstrating that the profile of the 24 major volatiles changed less between developmental stages than between the species. In case of A. majus Sippe 50 and A. braun-blanquetii, suggested scent profiles of different flower ages clustered in different branches, indicating variations in the composition of fragrances during development. This highlights that sampling several developmental stages is a critical factor if the volatile profile is to be used for taxonomic interpretation.
FIGURE 5.
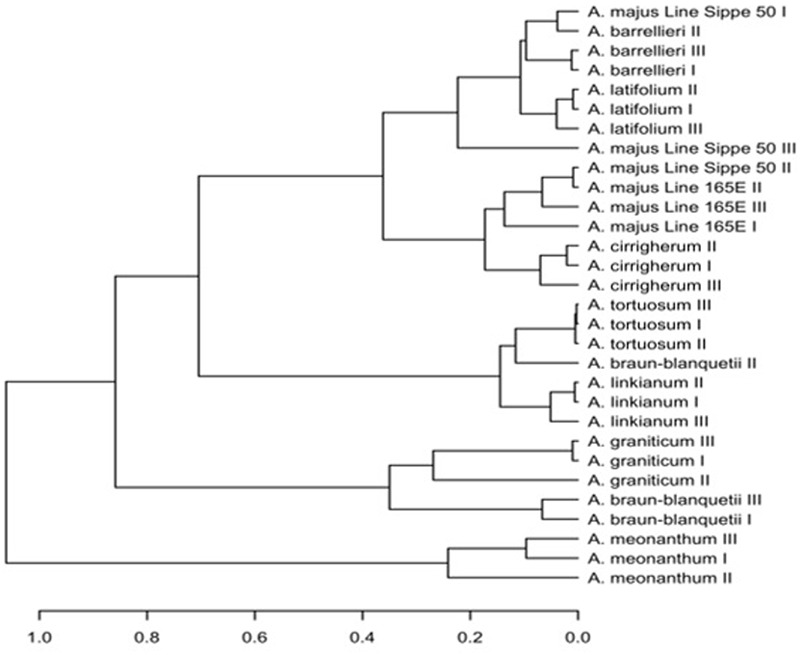
Cluster analysis based on scent profiles of the different Antirrhinum species sampled at different flower developmental stages.
The suggested volatile profiles of A. meonanthum and A. braun-blanquetii, both belonging to subsection Streptosepalum, build separate clusters from members of subsection Antirrhinum, with the exception of A. graniticum, which clustered with A. braun-blanquetii, separate from all other members of subsection Antirrhinum.
Within subsection Antirrhinum, except for A. graniticum, species branched into two main clusters. One of these two branches contained A. linkianum and tortuosum. Within the second major branch, A. majus and cirrigherum on one side and A. barrilieri and latifolium on the other side showed a closer relatedness.
Identification of Associated Odor Descriptors by PCA
To identify scent compounds that contribute to the variation in VOC profiles between species, we performed a PCA. We extracted four components that account for 82% of the variance in the data (Table 4). The first principal component, which explains 58% of the variance observed in scent emission between species, displays negative loadings for acetophenone and ocimene. The two compounds with the highest correlation to the second principal component were cinnamyl alcohol and 2-ethyl-1-hexanol. The third principal component contrasted the presence of cinnamyl alcohol with that of acetophenone, with a positive loading for cinnamyl alcohol and a negative loading for acetophenone. Lastly, the fourth principal component was highly correlated to methyl benzoate and ocimene. These data reveal that variance in volatile profiles between Antirrhinum species is caused by differences in emission levels of VOCs originating from different biosynthetic pathways, rather than by the presence of VOCs derived from a single pathway within a species. This observation suggests a selection for complex profiles rather than for a specific pathway.
Table 4.
Principal component loadings for the four principal components explaining more than 80% of the variance.
| Compound | PC1 (58.32%) | PC2 (10.90%) | PC3 (7.20%) | PC4 (5.89%) |
|---|---|---|---|---|
| Benzaldehyde | -0.158 | -0.245 | -0.118 | 0.064 |
| Benzeneacetaldehyde | -0.113 | -0.227 | -0.156 | 0.079 |
| Methyl benzoate | -0.328 | -0.150 | 0.089 | -0.659 |
| Cinnamaldehyde | -0.043 | -0.151 | 0.153 | -0.062 |
| Cinnamyl alcohol | -0.226 | -0.469 | 0.589 | -0.081 |
| Methyl cinnamate | -0.111 | -0.147 | -0.170 | 0.109 |
| Acetophenone | -0.487 | 0.316 | -0.395 | -0.396 |
| 3,5-Dimethoxytoluene | -0.271 | -0.042 | 0.147 | 0.060 |
| Indole | -0.090 | -0.284 | -0.099 | 0.061 |
| 1-Hexanol, 2-ethyl- | -0.191 | -0.367 | -0.381 | 0.214 |
| Nonanal | -0.059 | -0.169 | -0.176 | 0.063 |
| a,β-Ocimene | -0.537 | 0.277 | 0.235 | 0.423 |
| β-Myrcene | -0.310 | 0.252 | 0.034 | 0.187 |
| α-Farnesene | -0.061 | 0.003 | -0.065 | 0.155 |
The variance explained by each component is indicated in parentheses. Only compounds with a loading ≥| 0.15| in at least one component are shown. Loadings with highest correlation to individual components are in boldface.
By plotting the principal component scores of each species (Figure 6), we found that the scores for each species along the first and second principal component (PC1 and PC2) axis most display a considerable spread. A. braun-blanquetii and A. majus Line Sippe 50, for example, have a large variation in scores along PC1 and PC2, reflecting findings from the cluster analysis. Indeed, developmental stages for these species did not cluster as tightly as for other species.
FIGURE 6.
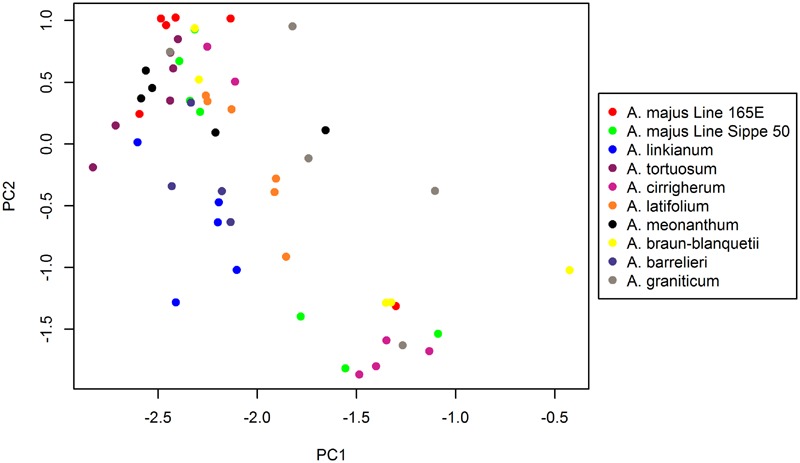
Principal component analysis (PCA) of Antirrhinum species based on emitted volatiles. The two axes represent principal components 1 and 2, which explain 58.32% and 10.90% of the total variance, respectively.
Discussion
In the present study, we have determined the phenotypic space of scent profiles in eight wild species of Antirrhinum and two laboratory inbred lines. The species used in the present study are found in very distant regions of the Iberian Peninsula and have very different ecological niches. Our data show that the complexity of scent profiles in the Antirrhinum genus is remarkable with at least 63 different compounds previously identified, and an additional set of nine that may require further studies to verify their presence in plants. The number of independent major VOCs found is similar to most species described. Species have been identified with as little as one compound emitted by Nicotiana africana to 35 in N. bonariensis during the night (Raguso et al., 2006). Other species such as Petunia (Kondo et al., 2007) have a range of scent components between 10 and 21, similar to the one found in the current study. The diversity of compounds produced in Antirrhinum is large but 500 VOCs have been described in studies in roses demonstrating the possibility of being a highly complex trait (Knudsen et al., 2006).
The most common volatiles found in between 71 and 52% of all plant families are, in decreasing order, limonene, ocimene, myrcene, linalool alpha-pinene, benzaldehyde, ß-pinene, methyl 2-hydroxybenzoate also known as methyl salicylate, benzyl alcohol, 2-phenyl ethanol, caryophyllene, and 6-methyl-5-hepten-2-one (Knudsen et al., 2006). The compounds found in most Antirrhinum species thus fall within the major VOCs found in flowering plants. The main VOCs found in all the Antirrhinum accessions analyzed were benzaldehyde, acetophenone ocimene and the fatty acid derivative 2-ethyl 1-hexanol indicating a common set of VOCs in the species analyzed. Highly ubiquitous compound such as benzyl alcohol was clearly forming a separate group of accessions that either did or did not produce this specific compound (Figure 2). The only common scent compound we did not find was caryophyllene (Knudsen et al., 2006), and others such as the commonly found limonene, or alpha and beta pinene were detected only in trace amounts. Other compounds found less often but still generally found in plants included indole. Altogether the major VOCs found in Antirrhinum are a good representation of the different biosynthetic pathways found for scent VOCs in the plant kingdom. This is in sharp contrast to well established models such as Arabidopsis that produces sesquiterpenes as major VOCs (Tholl et al., 2005), or Petunia producing mainly phenylpropanoids (Hoballah et al., 2007; Bombarely et al., 2016).
The scent profile of flowers of a specific plant can change in response to the physiological stage of the flower, including flower age (Pichersky et al., 1994; Dudareva et al., 1998), pollination status the circadian rhythm or temperature (Sagae et al., 2008; Cna’ani et al., 2014). We found that all the species analyzed except two, A. meonanthum and A. latifolium, displayed an increase in emission followed by a decrease after 5–6 days after anthesis. Moreover, A. majus Sippe 50 and A. graniticum also had a major VOC showing a trend increasing towards the end of the flower lifespan. As the compounds showing this trend were very diverse including acetophenone, ocimene, 2-ethyl hexanol, indole, cinnamyl alcohol, and methyl cinnamate, we cannot conclude that it is a single pathway that is differentially regulated during flower aging. Our results indicate that there must be a common mechanism of control involved in the quantitative control of scent emission linked to flower aging, and this mechanism is subject to changes as found for individual components that differed in the emission kinetics.
The diversity among the major compounds was strong enough to allow a phylogenetic reconstruction. There are several phylogenies described for the genus Antirrhinum, including reconstructions based on chloroplast genes such as combined psbA-trnH/trnT-trnL/ trnK-matK/trnS-trnG sequences (Carrio et al., 2010), trnS-trnG/trnK-matK (Liberal et al., 2014) statistical parsimony networks of plastid haplotypes trnS-trnG and trnK-matK (Vargas et al., 2009), the nuclear CYCLOIDEA gene (Gübitz et al., 2003), and AFLP nuclear markers (Wilson and Hudson, 2011). All the aforementioned studies show A. meonanthum and A. braun-blanquetii are on a single clade while the rest of the species analyzed in the current study cluster together. Our data, show clustering of A. menonanthum and A. braun-blanquetii, while the other species form a different clade. Thus we can conclude that the complex scent profiles and both chloroplast and nuclear markers show a similar separation. A current hypothesis is that a multilocus under selection pressure maybe responsible for the complex phylogeny of Antirrhinum (Wilson and Hudson, 2011). Indeed the major local pollinators have been analyzed for three different Antirrhinum species and they are different (Vargas et al., 2010). Amongst the species studied, two are present in our work, i.e., A. braun-blanquetii and A. graniticum. However, we do not have evidence about a co-evolution or selection of the different scent profiles found in the different species and local pollinators, and they could be the result of a combination of selection and genetic drift. Variation between the different species is not based on single pathways but appears to occur at the aroma level, i.e., at the level of combination of components. As the levels of monoterpenes in A. meonanthum and A. graniticum are nearly absent, it remains to be determined if these changes are the result of single mutations affecting regulatory elements or key enzymes in the biosynthesis pathway.
The number and type of VOCs found in the Antirrhinum species analyzed indicated that there are several biosynthetic pathways that in parallel give rise to the scent blends identified. An important question raised is if the different profiles identified are the result of differences in biosynthetic pathways or rather result from the combination of components. As compounds belonging to a single pathway maybe correlated they would obscure statistics. Our data show that this is not the case. The first two compounds accounting for 58% of the variance correspond to the benzenoid acetophenone and the terpenoid ocimene, indicating that the major compounds do not belong to single pathways. This was corroborated with the other compounds that showed significant effects shared by cinnamyl alcohol, a benzenoid and 1-hexanol-2-ethyl, a fatty acid derivative. Altogether the PCA analysis indicates that the different scent profiles identified are not the result of changes in regulatory pathways or changes in one specific type of scent compound, suggesting a scent structure based on blends in Antirrhinum. This is not always the case as scent profiles with major components belonging to distinct pathways have been identified (Majetic et al., 2007). Our results do not exclude the possibility of finding other species where changes in regulatory genes or key enzymes will cause changes in complete VOC biosynthesis pathways. Indeed the only Antirrhinum wild species described so far A. majus. Ssp pseudomajus and A. majus ssp striatum differ in the emission of three benzenoids (Suchet et al., 2010), indicating a complex scenario in terms of scent profiles and differences between species.
Our data show that in general the Antirrhinum genus tends to have a robust scent profile. The fact that A. braun-blanquetii and A. majus Sippe 50 display modified scent profiles with aging indicates a genetic component establishing the complete scent profile. In this case it is not the effect of a single master activator as scent was produced by both species. As a pathway of regulatory genes plays a key role in control of scent production in Petunia (Klahre et al., 2011; Van Moerkercke et al., 2011; Spitzer-Rimon et al., 2012), activation of floral scent production in Antirrhinum at anthesis may be controlled by several non-redundant genes. Our results also suggest that the use of scent profiles for phylogenetic analysis may require sampling at different ages or developmental stages in order to define profiles that may or may not resolve distances. The richness of volatiles and the marked differences between the different species open the possibility to study the genetic structure of scent as a trait, and its use in evolutionary studies. The robustness of scent profiles may be seen as a signature and it may help in creating fidelity to pollinators.
Author Contributions
JW, JM, ND, and ME-C designed experiments; JW, obtained data; JW, JM, VR-H, and ME-C analyzed data; JW, JM, VR-H, ND, and ME-C wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Ministerio de Ciencia e Innovación – Fondo de Desarrollo Regional (BFU2013-45148-R) to MEC, Ministerio de Educación, Cultura y Deporte (FPU13/03606) to VRH and Grant Salvador de Madariaga (PR2010-0592) to JW.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01903/full#supplementary-material
References
- Bey M., Stuber K., Fellenberg K., Schwarz-Sommera Z., Sommer H., Saedler H., et al. (2004). Characterization of Antirrhinum petal development and identification of target genes of the class B MADS box gene DEFICIENS. Plant Cell 16 3197–3215. 10.1105/tpc.104.026724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombarely A., Moser M., Amrad A., Bao M., Bapaume L., Barry C., et al. (2016). Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2 1–9. 10.1038/nplants.2016.74 [DOI] [PubMed] [Google Scholar]
- Carrio E., Forrest A. D., Güemes J., Vargas P. (2010). Evaluating species nonmonophyly as a trait affecting genetic diversity: a case study of three endangered species of Antirrhinum L. (Scrophulariaceae). Plant Syst. Evol. 288 43–58. 10.1007/s00606-010-0311-4 [DOI] [Google Scholar]
- Causier B., Schwarz-Sommer Z., Davies B. (2010). Floral organ identity: 20 years of ABCs. Semin. Cell Dev. Biol. 21 73–79. 10.1016/j.semcdb.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Cna’ani A., Mühlemann J. K., Ravid J., Masci T., Klempien A., Nguyen T. T. H., et al. (2014). Petunia x hybrida floral scent production is negatively affected by high-temperature growth conditions. Plant. Cell Environ. 38 1333–1346. 10.1111/pce.12486 [DOI] [PubMed] [Google Scholar]
- Dudareva N., Cseke L., Blanc V. M., Pichersky E. (1996). Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C-breweri flower. Plant Cell 8 1137–1148. 10.1105/tpc.8.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N., Martin D., Kish C. M., Kolosova N., Gorenstein N., Fäldt J., et al. (2003). (E)-beta-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15 1227–1241. 10.1105/Tpc.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N., Raguso R. A., Wang J. H., Ross E. J., Pichersky E. (1998). Floral scent production in Clarkia breweri - III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol. 116 599–604. 10.1104/pp.116.2.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea Gutierrez-Cortines M., Davies B. (2000). Beyond the ABCs: ternary complex formation in the control of floral organ identity. Trends Plant Sci. 5 473–478. [DOI] [PubMed] [Google Scholar]
- Feng X., Wilson Y., Bowers J., Kennaway R., Bangham A., Hannah A., et al. (2009). Evolution of allometry in Antirrhinum. Plant Cell 21 2999–3007. 10.1105/tpc.109.069054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gübitz T., Caldwell A., Hudson A. (2003). Rapid molecular evolution of CYCLOIDEA-like genes in Antirrhinum and its relatives. Mol. Biol. Evol. 20 1537–1544. 10.1093/molbev/msg166 [DOI] [PubMed] [Google Scholar]
- Guzmán B., Gómez J. M. M., Vargas P. (2015). Bees and evolution of occluded corollas in snapdragons and relatives (Antirrhineae). Perspect. Plant Ecol. Evol. Syst. 17 467–475. 10.1016/j.ppees.2015.07.003 [DOI] [Google Scholar]
- Harrison B. J., Carpenter R. (1979). Resurgence of genetic instability in Antirrhinum-Majus. Mutat. Res. 63 47–66. 10.1016/0027-5107(79)90103-9 [DOI] [Google Scholar]
- Hoballah M. E., Gubitz T., Stuurman J., Broger L., Barone M., Mandel T., et al. (2007). Single gene-mediated shift in pollinator attraction in Petunia. Plant Cell 19 779–790. 10.1105/tpc.106.048694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U., Gurba A., Hermann K., Saxenhofer M., Bossolini E., Guerin P. M., et al. (2011). Pollinator choice in Petunia depends on two major genetic Loci for floral scent production. Curr. Biol. 21 730–739. 10.1016/j.cub.2011.03.059 [DOI] [PubMed] [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J., Ståhl B., Stahl B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72:1 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2 [DOI] [Google Scholar]
- Knudsen J. T., Tollsten L., Bergström L. G. (1993). Floral scents–a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33 253–280. 10.1016/0031-9422(93)85502-I [DOI] [Google Scholar]
- Kondo M., Oyama-Okubo N., Sagae M., Ando T., Marchesi E., Nakayama M. (2007). Metabolic regulation of floral scent in Petunia axillaris lines: biosynthetic relationship between dihydroconiferyl acetate and iso-eugenol. Biosci. Biotechnol. Biochem. 71 458–463. 10.1271/bbb.60507 [DOI] [PubMed] [Google Scholar]
- Liberal I. M., Burrus M., Suchet C., Thébaud C., Vargas P. (2014). The evolutionary history of Antirrhinum in the Pyrenees inferred from phylogeographic analyses. BMC Evol. Biol. 14:146 10.1186/1471-2148-14-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majetic C. J., Raguso R. A., Tonsor S. J., Ashman T. L. (2007). Flower color-flower scent associations in polymorphic Hesperis matronalis (Brassicaceae). Phytochemistry 68 865–874. 10.1016/J.Phytochem.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Manchado-Rojo M., Delgado-Benarroch L., Roca M. J., Weiss J., Egea-Cortines M. (2012). Quantitative levels of Deficiens and Globosa during late petal development show a complex transcriptional network topology of Bfunction. Plant J. 72 294–307. 10.1111/j.1365-313X.2012.05080.x [DOI] [PubMed] [Google Scholar]
- Manchado-Rojo M., Weiss J., Egea-Cortines M. (2014). Validation of Aintegumenta as a gene to modify floral size in ornamental plants. Plant Biotechnol. J. 12 1053–1065. 10.1111/pbi.12212 [DOI] [PubMed] [Google Scholar]
- Mateu-Andres I., De Paco L. (2005). Allozymic differentiation of the Antirrhinum majus and A. siculum species groups. Ann. Bot. 95 465–473. 10.1093/aob/mci055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlemann J. K., Maeda H., Chang C. Y., Miguel P. S., Baxter I., Cooper B., et al. (2012). Developmental changes in the metabolic network of snapdragon flowers. PLoS ONE 7:e40381 10.1371/journal.pone.0040381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Raguso R. A., Lewinsohn E., Croteau R. (1994). Floral scent production in Clarkia (Onagraceae).1. Localization and Developmental Modulation of Monoterpene Emission and Linalool Synthase Activity. Plant Physiol. 106 1533–1540. 10.1104/pp.106.4.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso R. A., Pellmyr O. (1998). Dynamic headspace analysis of floral volatiles: a comparison of methods. Oikos 81 238–254. 10.2307/3547045 [DOI] [Google Scholar]
- Raguso R. A., Schlumpberger B. O., Kaczorowski R. L., Holtsford T. P. (2006). Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry 67 1931–1942. 10.1016/j.phytochem.2006.05.038 [DOI] [PubMed] [Google Scholar]
- Rothmaler W. (1956). Taxonomische Monographie der Gattung Antirrhinum. Feddes Rep. Berlin: Akademie Verlag. [Google Scholar]
- Ruiz-Ramon F., Águila D. D. J., Egea-Cortines M., Weiss J., Ruíz-Ramón F., Águila D. D. J., et al. (2014). Optimization of fragrance extraction: daytime and flower age affect scent emission in simple and double narcissi. Ind. Crops Prod. 52 671–678. 10.1016/j.indcrop.2013.11.034 [DOI] [Google Scholar]
- Sagae M., Oyama-Okubo N., Ando T., Marchesi E., Nakayama M. (2008). Effect of temperature on the floral scent emission and endogenous volatile profile of Petunia axillaris. Biosci. Biotechnol. Biochem. 72 110–115. 10.1271/bbb.70490 [DOI] [PubMed] [Google Scholar]
- Scalliet G., Lionnet C., Le Bechec M., Dutron L., Magnard J. L., Baudino S., et al. (2006). Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol. 140 18–29. 10.1104/pp.105.070961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl F. P. (2010). The evolution of floral scent and insect chemical communication. Ecol. Lett. 13 643–656. 10.1111/j.1461-0248.2010.01451.x [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Davies B., Hudson A. (2003). An everlasting pioneer: the story of Antirrhinum research. Nat. Rev. Genet. 4 657–666. 10.1038/nrg1127 [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gübitz T., Weiss J., Gómez-di-Marco P., Delgado-Benarroch L., Hudson A., et al. (2010). A molecular recombination map of Antirrhinum majus. BMC Plant Biol. 10:275 10.1186/1471-2229-10-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H., Saedler H. (1986). Structure of the chalcone synthase gene of Antirrhinum majus. Mol. Gen. Genet. 202 429–434. 10.1007/BF00333273 [DOI] [PubMed] [Google Scholar]
- Spitzer-Rimon B., Farhi M., Albo B., Cna’ani A., Ben Zvi M. M., Masci T., et al. (2012). The R2R3-MYB-like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural scent-related genes in petunia. Plant Cell 24 5089–5105. 10.1105/tpc.112.105247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe H. (1966). Genetik und Zytologie von Antirrhinum L. Sect. Antirrhinum. Jena: Veb Gustav Fischer Verlag. [Google Scholar]
- Suchet C., Dormont L., Schatz B., Giurfa M., Simon V., Raynaud C., et al. (2010). Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 65 1015–1027. 10.1007/s00265-010-1106-x [DOI] [Google Scholar]
- Sutton D. A. (1988). A Revision of the Tribe Antirrhineae. London: Oxford University Press. [Google Scholar]
- Tholl D., Chen F., Petri J., Gershenzon J., Pichersky E. (2005). Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 42 757–771. 10.1111/J.1365-313x.2005.02417.X [DOI] [PubMed] [Google Scholar]
- Torres E., Iriondo J. M., Escudero A., Perez C. (2003). Analysis of within-population spatial genetic structure in Antirrhinum microphyllum (Scrophulariaceae). Am. J. Bot. 90 1688–1695. 10.3732/ajb.90.12.1688 [DOI] [PubMed] [Google Scholar]
- Van Moerkercke A., Haring M. A., Schuurink R. C. (2011). The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant Petunias. Plant J. 67 917–928. 10.1111/j.1365-313X.2011.04644.x [DOI] [PubMed] [Google Scholar]
- Vargas P., Carrió E., Guzmán B., Amat E., Güemes J. (2009). A geographical pattern of Antirrhinum (Scrophulariaceae) speciation since the Pliocene based on plastid and nuclear DNA polymorphisms. J. Biogeogr. 36 1297–1312. 10.1111/j.1365-2699.2008.02059.x [DOI] [Google Scholar]
- Vargas P., Ornosa C., Ortiz-Sánchez F. J., Arroyo J. (2010). Is the occluded corolla of Antirrhinum bee-specialized? J. Nat. Hist. 44 1427–1443. 10.1080/00222930903383552 [DOI] [Google Scholar]
- Verdonk J. C., Ric de Vos C. H., Verhoeven H. A., Haring M. A., van Tunen A. J., Schuurink R. C., et al. (2003). Regulation of floral scent production in Petunia revealed by targeted metabolomics. Phytochemistry 62 997–1008. 10.1016/S0031-9422(02)00707-0 [DOI] [PubMed] [Google Scholar]
- Webb D. A. (1971). Taxonomic notes on Antirrhinum L. Bot. J. Linn. Soc. 64 271–275. [Google Scholar]
- Weiss J., Alcantud-Rodriguez R., Toksöz T., Egea-Cortines M. (2016). Meristem maintenance, auxin, jasmonic and abscisic acid pathways as a mechanism for phenotypic plasticity in Antirrhinum majus. Sci. Rep. 6 2–11. 10.1038/srep19807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Y., Hudson A. (2011). The evolutionary history of Antirrhinum suggests that ancestral phenotype combinations survived repeated hybridizations. Plant J. 66 1032–1043. 10.1111/j.1365-313X.2011.04563.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


