Abstract
Branched chain α-keto acids (BCKAs) are endogenous metabolites of branched-chain amino acids (BCAAs). BCAA and BCKA are significantly elevated in pathologically stressed heart and contribute to chronic pathological remodeling and dysfunction. However, their direct impact on acute cardiac injury is unknown. Here, we demonstrated that elevated BCKAs significantly attenuated ischemia-reperfusion (I/R) injury and preserved post I/R function in isolated mouse hearts. BCKAs protected cardiomyocytes from oxidative stress-induced cell death in vitro. Mechanistically, BCKA protected oxidative stress induced cell death by inhibiting necrosis without affecting apoptosis or autophagy. Furthermore, BCKAs, but not BCAAs, protected mitochondria and energy production from oxidative injury. Finally, administration of BCKAs during reperfusion was sufficient to significantly attenuate cardiac I/R injury. These findings uncover an unexpected role of BCAAs metabolites in cardioprotection against acute ischemia/reperfusion injury, and demonstrate the potential use of BCKAs treatment to preserve ischemic tissue during reperfusion.
Keywords: Branched-chain amino acids, Branched-chain keto acids, necrosis, cell death, mitochondria, myocardial infarction
INTRODUCTION
Branched chain amino acids (BCAA), including leucine, isoleucine and valine, are essential amino acids which can be degraded via shared catabolic pathway. BCAA degradation yields branched chain α-keto acids (BCKAs), including α-ketoisocaproate (KIC), α-keto-β-methylvalerate (KMV), and α-ketoisovalerate (KIV) [1]. BCAA catabolic defects and elevated tissue levels of BCKA or BCAA have been reported in pathologically stressed mouse hearts or human cardiomyopathy [2-4]. Furthermore, abnormal plasma BCAA/BCKA is a common feature in diabetes and insulin resistance [1]. BCKAs inhibit mitochondrial respiration and energy metabolism in neuronal cells [5]. In heart muscle cells, BCKAs inhibit Complex I activity in mitochondria and induce superoxide production [2]. BCAA catabolic defects significantly contribute to heart failure and myocardial remodeling following chronic pressure-overload or myocardial infarction [2, 3]. However, a direct effect of BCAA/BCKA on acute cardiac injury is unknown.
Prolonged ischemia followed by reperfusion (I/R) leads to loss of cardiac muscle cell viability and functional recovery [6, 7]. Oxidative stress due to elevated reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2) is a major contributor to I/R injury and myocyte death [8]. Apoptosis and necrosis are two fundamental types of cell death. Unlike apoptosis, necrosis was previously considered as an uncontrolled and energy independent cell death process characterized by early plasma membrane rupture and swelling of organelles [9]. However, recent studies have revealed that the necrotic process is also mediated through highly regulated process, referred to as programmed necrosis or necropotosis [10]. Pro-death stimuli trigger a series of cellular events involving protein kinases, ATP depletion, and proteolysis, to induced regulated necrosis [11]. In particular, tumor necrosis factor-α (TNF-α) induced Receptor-Interacting Serine/threonine Kinase (RIPK) pathway has been extensively studied and shown to be important for necrotic cell death [12], It is well reported that high level of H2O2 induces necrosis [13-17]. Preventing or reducing myocyte necrosis death is a potentially effective therapeutic strategy to reduce heart attack injury as well as other ischemic/reperfusion induced organ injuries [18].
In the present study, we investigate the direct effect of BCKAs on oxidative stress induced myocyte death and acute I/R injury in heart. Opposite from our expectation based on previously observed detrimental effect of BCKA in cardiac remodeling and dysfunction, we observed a significant cardio-protective effect of BCKA administration against acute oxidative stress and I/R injury. Mechanistically, we demonstrate that BCKA treatment protected cells from H2O2 induced necrosis and mitochondrial damage. Our study uncovered an unexpected cardiac protective property of BCAA downstream metabolites and revealed a potential novel class of reagents as potential treatment for myocardial infarction.
RESULTS
BCKAs protect hearts from I/R Injury
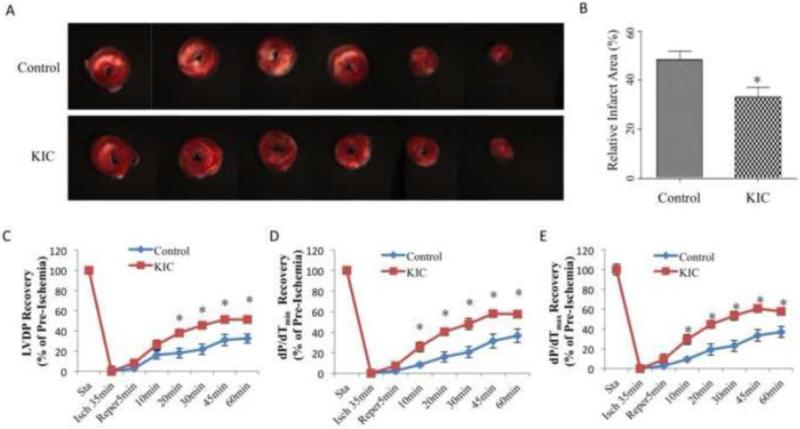
To establish the direct effect of BCKAs on acute I/R injury, we performed studies in the Langendorff murine hearts following 35 min no-flow ischemia and 120 min reperfusion protocol. In addition to saline control, KIC, the branched-chain keto acid from leucine, was perfused throughout the experiment. Pretreatment of KIC significantly attenuated the infarct size after 120min reperfusion (Figure 1A and B). Meanwhile, comparing to the Control group of ~32% recovery of Left Ventricle Developed Pressure (LVDP) after 60min reperfusion, KIC group showed a significant higher functional recovery of ~51% (Figure 1C). The recovery of left ventricular systolic function (dP/dtmax) and diastolic function (dP/dtmin) during reperfusion was also substantially improved by KIC treatment (Figure 1D and E). These data clearly demonstrate an unanticipated protective effect of BCKAs against myocardial I/R injury.
Figure 1. BCKAs protected heart from ischemia/reperfusion injury.
A. Representative images of cross-sections of TTC stained ischemic hearts with or without pretreatment of KIC. B, Relative infarct size expressed as a percentage of the total ventricular area was calculated from control group (without KIC treatment) (n=11) and KIC-pretreatment (n=15) groups. C-E, Time courses of functional recovery (percentage of baseline) of ischemic hearts with (n=14) or without (n=11) KIC pre-treatment. Pre-treatment was performed by including KIC in both perfusion and reperfusion buffer. LVDP, Left Ventricular Developed Pressure. *, p<0.05.
BCKAs protect cardiomyocytes against oxidative stress-induced death
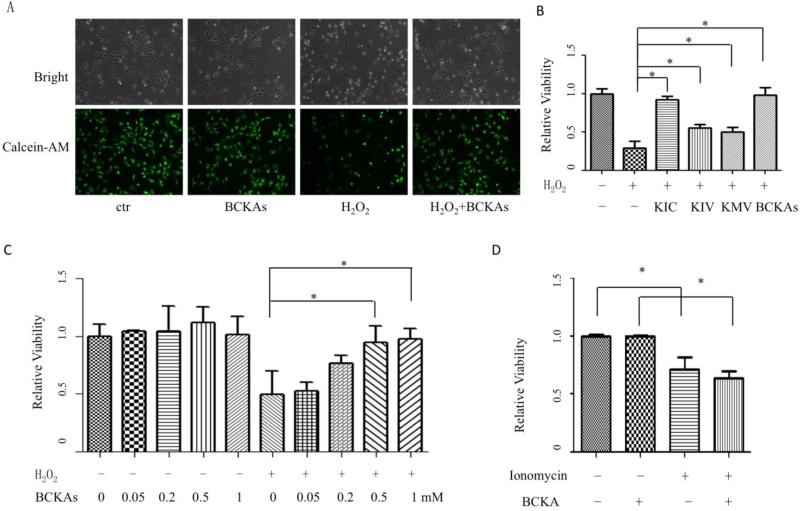
Cardiomyocyte is the functional cell type in myocardium. Oxidative stress is one major contributor of cell death during I/R [8]. We found that H2O2 treatment at a dose of 20 μM induced rapid cell death in primary Neonatal Rat Ventricular Myocytes (NRVM), based on MTT activity, morphological alterations of rounding up and detachment. Treatment of BCKAs significantly reduced H2O2-induced NRVM death (Figure 2A and 2B). Meanwhile, among the three BCKA species, KIC demonstrated the strongest protective activity against NRVM death (Figure 2B). In addition, BCKA-dependent protection against H2O2-induced cell death was also dosage dependent (Figure 2C). In addition to oxidative stress, calcium overload has also been demonstrated as one major contributor for cell death during I/R injury [19]. However, BCKAs failed to protect the calcium overloaded-induced cell death triggered by ionomycin (Figure 2D). Thus BCKAs possess specific cyto-protective activity against oxidative stress- but not calcium overload-induced cell death of cardiomyocytes.
Figure 2. BCKAs protected cardiomyocytes against H2O2-induced death.
A, Representative bright field and green fluorescent images of corresponding NRVM cells stained with calcein-AM. Cells were treated with BCKAs in presence or absence of H2O2 (20 μM). The experiment has been repeated three times with similar result. B, Protective effect of individual BCKA. NRVM cells were treated with individual or mixed BCKAs in presence or absence of H2O2 (20 μM). Cell viability was determined by MTT assay. C, Concentration-dependent protection of BCKAs against H2O2-induced NRVM death. Cells were treated with designated concentrations of BCKAs in presence or absence of H2O2 (20 μM). D, Effect of BCKAs on calcium overload-induced cell death. NRVM cells were treated with BCKAs in presence or absence of ionomycin (1 μM). All results showed relative cell viability determined by MTT assay. The data represented the average values with standard deviation of triplicate samples from one experiment representative of three independent experiments.*, p<0.05.
BCKAs protect different types of cells and inhibit mitochondrial respiration
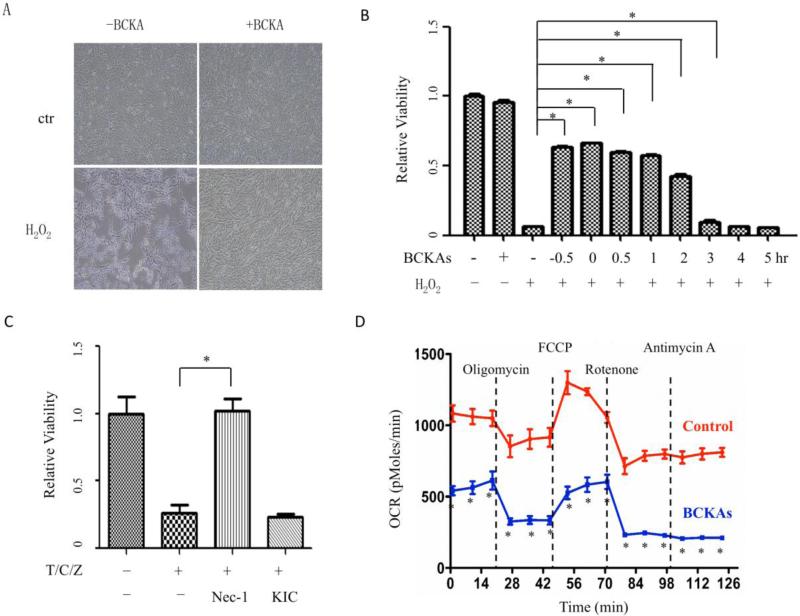
We further tested if the protective effect of BCKAs is cardiomyocyte specific or applicable to other cell types. H2O2 treatment at a dose of 500 μM induced high level of cell death in mouse embryonic fibroblast (MEF) cells (Figure 3A). As shown in Figure 3B, either a 30-minute pre-treatment of BCKAs prior to H2O2 administration (0.5 hr) or concurrent treatment of BCKA and H2O2 (0 hr in Figure 3B) showed significant protective effect in MEFs. Furthermore, adding BCKAs 30 minutes or 1 hour after the initiation of H2O2 treatment still induced similar level of protective effects. In contrast, starting treatment of BCKAs two hours after initial H2O2 treatment or later, the BCKA mediated protective effect diminished. BCKAs also protected Hela cells from oxidative stress induced cell death (Figure S1). In addition, BCKAs failed to protect MEF against the Death Receptor-induced necrotic cell death (Figure 3C). Similar to their inhibitory effect on isolated mitochondria [2], BCKAs suppressed the cellular respiration in cultured MEF (Figure 3D). These data suggest that BCKA mediated cyto-protection against oxidative stress is a conserved effect across different cell lines and species associated with mitochondrial inhibition, although subtle differences do exist in terms of relative strengths of the protection by each of the BCKA species and in different cellular contents.
Figure 3. BCKAs protected MEF cells and inhibited mitochondrial respiration.
A: Representative bright field images showing effects of BCKAs on MEF cell death. MEF cells was incubated with or without H2O2 (500 μM) or BCKAs (500 μM) overnight. The experiment has been repeated three times with similar results. B, Cells were incubated with or without H2O2 (500 μM); BCKAs were added for designated periods of time: 0.5 hr before H2O2 treatment (−0.5), concurrent with H2O2 (0), or 0.5, 1, 2, 3, 4 hr after H2O2 treatment, respectively. *, p<0.05 compared to H2O2 group. C, MEF cells were treated with TNFα(50 ng/ml)/Cycloheximide(1 μg/ml)/z-VAD-fmk (20 μM) (T/C/Z) for overnight in presence or absence of KIC (1000 μM) or Nec-1 (40 μM). D, Oxygen consumption rate (OCR) was measured by Seahorse Bioscience XF-24 analyzer. MEFs (50,000 cells/well) were pretreated with or without BCKAs for 1 hour before analysis. The data represented the average values with standard deviation of triplicate samples from one experiment representative of three independent experiments. *, p<0.05 compared to Control group.
BCKAs inhibit necrosis
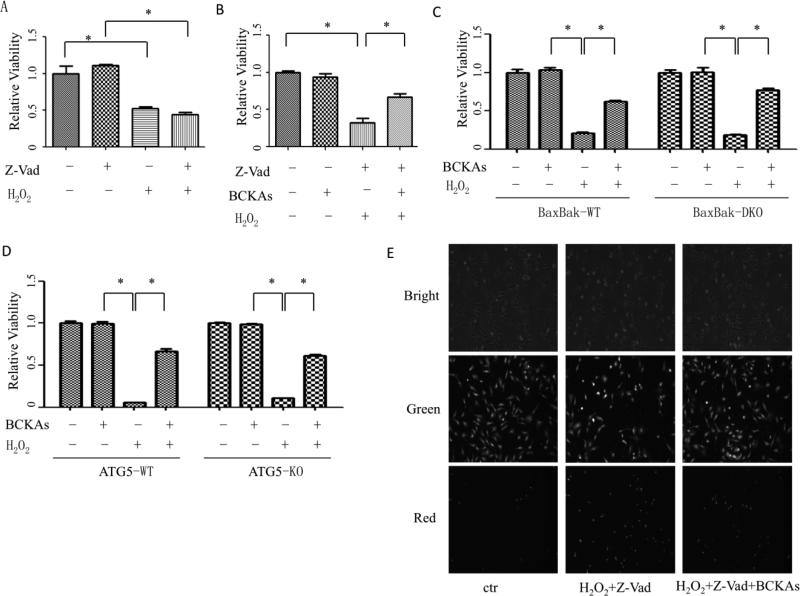
To determine if BCKAs affects oxidative stress induced apoptosis, we then determined the type of cell death under the experimental conditions in the present study. z-VAD-fmk, a pan-caspase inhibitor, failed to block the high dose of H2O2-indcued cell death (Figure 4A). BCKAs still protected cells against oxidative stress even in presence of z-VAD-fmk (Figure 4B). In addition, BCKAs failed to inhibit cells death induced by an apoptosis inducer staurosporine (Figure S2)[20]. Bax and Bak double knockout MEF (BaxBak DKO) is a cell line widely used for studying non-apoptotic cell death [21, 22]. H2O2 induced similar level of cell death in wildtype and the BaxBak DKO MEF, however, they were protected by BCKAs to a similar extent (Figure 4C and Figure S3A). Together, these data indicate that BCKAs treatment affects specifically non-apoptotic cell death induced by H2O2 rather than apoptotic cell death.
Figure 4. BCKAs protected cells from necrotic cell death.
A, H2O2 induced caspase-independent cell death in NRVM. Cells were treated with H2O2 (20 μM) after pretreatment with vehicle or zVAD-fmk (40 μM), the caspase inhibitor. B, BCKAs diminished caspase-independent cell death in MEFs. Cells were left untreated or treated with BCKAs, z-VAD-fmk, and/or H2O2. C and D, BCKAs diminished cell death independent of autophagy and BaxBak. BaxBak double knockout (C) and ATG5 knockout (D) MEFs cells and their wildtype MEFs, respectively, were treated with or without BCKAs in presence or absence of H2O2 (500 μM). A-D, Cell viability was determined by MTT assay. E, Representative images of PI staining showing effects of BCKAs on plasma membrane integrity. NRVM cells were treated with or without H2O2 (20 μM) in presence or absence of BCKAs and/or zVAD-fmk (40 μM), 3hr after H2O2 treatment, Propidium iodide (PI) and/or calcein-AM were loaded and cell images were taken with bright light or different excitation light to visualize PI (red) or calcein-AM (green) fluorescent signal. The experiment has been repeated three times with similar results (E). The data represented the average values with standard deviation of triplicate samples from one experiment representative of three independent experiments (A-D). *, p<0.05.
Autophagy is also known to contribute myocyte death during ischemia and reperfusion injury[23]. Using an autophagy deficient MEF (ATG5-KO) (Figure S3B), we observed no significant differences between ATG5-KO MEFs vs. wildtype control MEFs in H2O2-induced cell death, while BCKAs protected both wildtype and ATG5 deficient MEFs at similar extents (Fig 4D), suggesting autophagy was not involved in cell death protection by BCKAs.
A distinguished character of necrosis is the loss of membrane integrity at the early stage of cell death, which can be detected by propidium iodide (PI) staining of nuclei. Under the present experimental conditions, cell nuclei were readily stained with PI early after H2O2 treatment in presence of caspase inhibitor z-VAD-fmk, strongly supporting necrotic cell death. BCKAs markedly reduced the number of PI-positive cells treated with H2O2, indicating that BCKAs protected cells against oxidative stress-induced necrosis (Figure 4E).
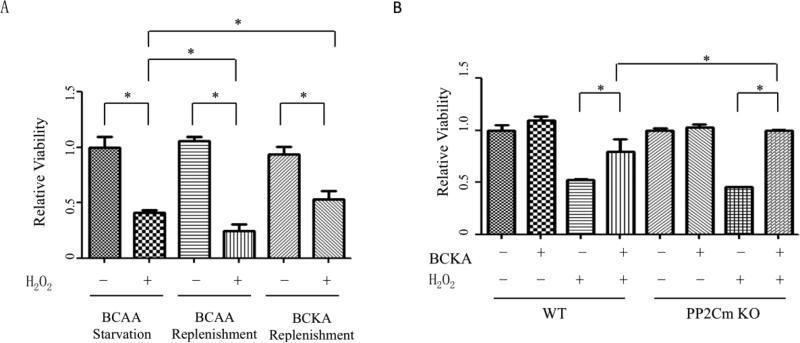
BCKAs, but not BCAAs, inhibit oxidative stress-induced cell death
We then attempted to explore the mechanism underlying BCKAs’ protection against necrotic cell death. BCKAs can be converted into BCAAs in cells with Branched Chain Aminotransferase 2 (BCAT2) expression [1]. We then investigated whether BCKAs’ protective effect is exerted by BCAAs. Without BCAT2 expression, hepatocytes cannot convert BCKAs to BCAAs [24]. In AML12 murine hepatocyte cell line, replenishment of BCAAs did not protect cells but instead enhanced H2O2-induced cell death. Meanwhile, BCKAs protected hepatocytes against H2O2 (Figure 5A). We further explored the role of BCKAs by using PP2Cm deficient MEFs, a cell line with defective BCKAs degradation [25]. Compared to wildtype MEFs, a significantly stronger protection of BCKAs in the PP2Cm deficient MEFs suggested that BCKAs played a critical role in the cellular protection (Figure 5B).
Figure 5. BCKAs, but not BCAAs, protected cells against H2O2-induced death.
A, Cell viability analysis showing the effect of BCAAs- and BCKAs- replenishment on AML-12 murine hepotocyte survial. Cells were treated with BCAAs-free DMEM and then replenished with BCAAs (800 μM) or BCKAs (500 μM) and treated with H2O2 (500 μM). B, Cell viability analysis showing the effect of BCKAs on MEF cell death. Wildtype or PP2Cm deficient MEF cells were treated with BCKAs in presence or absence of H2O2 (500 μM).
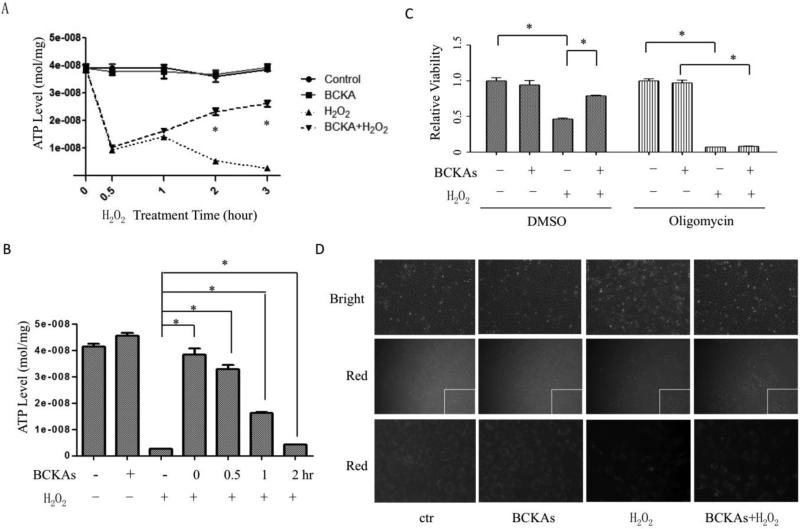
BCKAs protect mitochondria and energy production
A major reason that cells undergo necrosis is the exhaustion of ATP [10]. As shown in Figure 6A, H2O2 dramatically decreased the intracellular ATP level to approximately one fourth of normal abundance after 30 mins of treatment and completely depleted ATP after 3 hour of treatment, consistent with the characteristics of necrosis. Interestingly, although BCKAs failed to maintain the ATP level at the early stage of H2O2 treatment, they reconstituted ATP abundance in cells at later stages, suggesting that avoiding ATP depletion by BCKAs prevented cells from necrosis (Figure 6A). Meanwhile, post-treatment of BCKAs also attenuated ATP depletion by H2O2 (Figure 6B), consistent with delayed protective effect against cell death (Figure 3B). To further verify the role of energy homeostasis in BCKA mediated protection against cell death, we treated the cells with oligomycin, a mitochondrial ATP synthase inhibitor. As expected, the inhibition of ATP production abolished the BCKAs’ protective effect (Figure 6C). Moreover, BCKAs inhibited the loss of mitochondrial membrane potential induced by H2O2 (Figure 6D). Together, these data indicate that BCKAs protect cells from H2O2-induced necrosis through preserving mitochondrial integrity and energy production.
Figure 6. BCKAs protected mitochondria and energy production.
A, ATP concentrations were measured in cultured cells that were pre-treated with BCKAs and then treated with or without H2O2 for the designated time. *, p<0.05 compared to H2O2 group. B, ATP concentrations in MEFs cells treated with H2O2. BCKAs were added at the designated time (0, 0.5,1,2 hours) after H2O2 treatment started. C, Cell viability assay result. Cells were pretreated with or without Oligomycin (0.5 μM), followed by H2O2 treatment in presence or absence of BCKAs. D, Mitochondrial membrane potential measurement. Cells were incubated in absence or presence of BCKAs and treated with H2O2, then stained with TMRM (0.2 μM). Fluorescent pictures were taken after 20 min staining. Representative bright field (top row) and red fluorescent (middle row) images of corresponding cells were shown. The images in bottom rows were amplified corner sections (white squares) of the corresponding images in the middle row. The experiment has been repeated three times with similar results (D). The data represented the average values with standard deviation of triplicate samples from one experiment representative of three independent experiments (A-C). *, p<0.05.
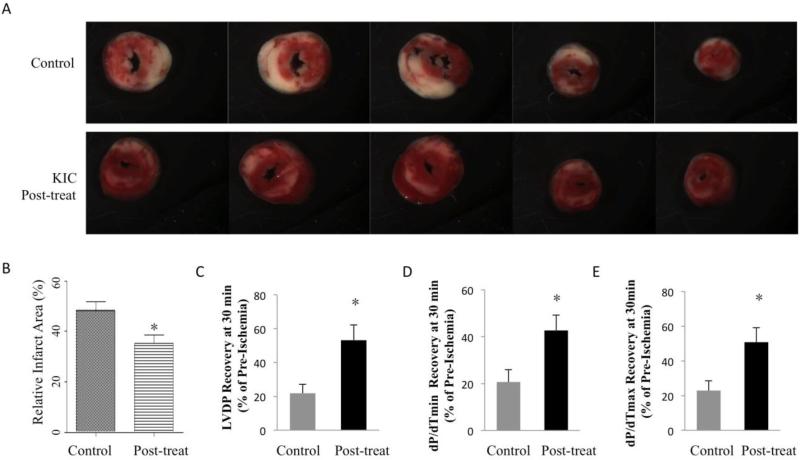
Post-treatment of BCKAs attenuates myocardial I/R injury
In clinical setting when a heart attack has already occurred, a protective treatment after the ischemia or at the time of reperfusion should be beneficial to reduce I/R injury. We then examined the effect of BCKAs on intact hearts when administrated at reperfusion. The Langendorff murine heart was perfused and underwent 35 min no-flow ischemia, followed by 120 min reperfusion with (post-treatment) or without KIC (Control). KIC post-treatment significantly attenuated infarct size (Figure 7A and 7B), in agreement with their cellular protective effect (Figure 3B and 6B). LVDP, left ventricular systolic function (dP/dtmax), and diastolic function (dP/dtmin) were also substantially improved by KIC post-treatment after 30min reperfusion (Figure 7C-E). These ex-vivo data support the potential clinical benefits of BCKAs administration during reperfusion in ischemic hearts.
Figure 7. Post-treatment of BCKA protected heart from ischemia/reperfusion injury.
A. Representative images of cross-sections of TTC stained ischemic hearts with or without post-treatment of KIC. B, Relative infarct size expressed as a percentage of the total ventricular area was calculated from control group (without KIC treatment) (n=11), and KIC post-treatment group (n=7). C-E, Functional recovery (percentage of baseline) of ischemic hearts at 30 minutes after reperfusion with (n=7) or without (n=11) KIC post-treatment. Post-treatment was performed by including KIC (600 μM)in reperfusion buffer. *, p<0.05.
DISCUSSION
In the present study, we found that BCKAs attenuated acute I/R injury in hearts. BCKAs, but not BCAAs, protected cells from oxidative stress induced cell death by inhibiting necrosis without affecting apoptosis or autophagy. Mechanistic study revealed that mitochondria and energy production were protected by BCKAs, contributing to cell survival. Importantly, BCKAs’ protective effect was observed in both cultured cells and intact hearts with either pre- or post-treatment. These results identified a novel anti-necrotic function of BCAAs’ metabolites and a potential application of BCKAs-like compounds in the treatment of ischemic diseases.
The BCKAs’ protection against I/R injury is unexpected. Previously, we showed that BCKAs inhibited mitochondrial respiration and induced oxidative stress, promoting heart failure [2]. A recent report also suggested that BCKAs increased the susceptibility to apoptosis in cardiomyocytes [4]. In the current study, a cytotoxic effect of BCKAs was expected at the beginning and the beneficial effect of BCKAs against oxidative stress and I/R injury was unanticipated. However, there are a few major differences between these two situations. The exposure to BCKAs was chronic in the pressure overload-stressed heart where the cell death wasn't a major consequence of BCKAs exposure [2]. In the current study, the BCKAs treatment and I/R injury or oxidative stress-induced cell death were all acute. Part of the discrepancy may be due to the manner of BCKAs exposure and the nature of injury/stress. On the other side, it has been well known that respiratory inhibitors protect hearts from I/R injury [26]. Blockade of electron transport of mitochondria at different Complex sites reduces cardiac I/R injury [27-31]. BCKAs inhibit Complex I activity in cardiac mitochondria[2]. Thus BCKAs may function as mitochondrial inhibitors to protect hearts.
The underlying mechanism of BCKAs’ protection against oxidative stress remains to be further investigated. BCKAs protect cells from H2O2-induced necrosis through preserving mitochondrial function (Figure 6), offering a compelling mechanistic basis. However, the underlying molecular/biochemical mechanism for BCKA's protection on mitochondria remains unclear. It has been shown that oxidative stress-induced necrosis is mediated by mitochondria-dependent burst of ROS. Mitochondrial complex I inhibitor blocked H2O2-induced ROS burst and subsequent neuronal cell death [32]. BCKAs can inhibit cardiac mitochondrial Complex I [2]. It is plausible that BCKAs protect mitochondria and thus cells via inhibiting mitochondria-dependent ROS burst. Meanwhile, mitochondrial suppression by BCKAs may lead to “gradual wake-up of metabolism”, a mechanism implicated in the protective effect of synthetic mitochondrial inhibitors on heart against I/R injury [26]. On the other hand, like the synthetic mitochondrial inhibitors, BCKAs induce oxidative stress [2]. This apparent paradox remains to be resolved. Nevertheless, future investigations are warranted to address how BCKAs protect mitochondria and cells from acute oxidative stress.
It has been reported that JNK signaling pathway is a key modulator in cell death induced by reactive oxygen and nitrogen species [33, 34]. It has also been shown that activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death [35]. Protein kinases of the receptor interacting protein (RIP) family are key mediators of apoptotic and necrotic cell death induced by death receptor proteins [36] and oxidative stress [15]. To test the involvement of these pathways in H2O2-induced cell death, we used pharmacological inhibitors to block these pathways. JNK inhibitor did not significantly block H2O2-induced cell death, suggesting a JNK-independent cell death (data not shown). Inhibition of AKT or mTOR signaling pathways also failed to prevent cell death (Figure S4). Under the present experimental conditions, Rip1 inhibitor Nec-1 [37] did inhibit Death Receptor-induced (Figure 3D) but not H2O2-induced cell death (Figure S5). Meanwhile, BCKAs failed to reduce Death Receptor-induced cell death. These data suggested that BCKAs protected cell against oxidative stress via RIP-, JNK- and AKT-independent mechanisms.
Cell death is one major consequence of I/R injury. Intense investigation has generated significant insights into the pathophysiology of I/R injury. Yet finding pharmacological strategies that ultimately protect cells and reduce the infarct size still remains as a major challenge [6]. The protection of synthetic mitochondrial inhibitors against I/R injury comes with potential toxicity and side effects [26]. BCKAs’ inhibition on respiration is less potent [2] and thus may function as less-toxic mitochondrial respiratory inhibitors to attenuate I/R injury. Meanwhile, the BCKAs’ protection against I/R injury in vivo and the potential neurotoxicity of high level of BCKAs need to be investigated in intact animals [38, 39]. The BCKA derivatives and chemicals with similar structure may provide candidates with lower toxicity. Nevertheless, the common protective effects of pre- and post-treatment of BCKAs on numerous types of cells point to the intriguing property of this class of molecules as novel and potentially potent agents against tissue I/R injury.
MATERIALS AND METHODS
Animals
Wildtype C57BL/6 mice were housed at 22°C with a 12-hour light, 12-hour dark cycle with free access to water and standard chow. Studies were performed with male mice. All animal procedures (including the one used for neonatal rat ventricular myocytes isolation) were carried out in accordance with the guidelines and protocols approved by the University of California at Los Angeles Institutional Animal Care and Use Committee (IACUC). All animals in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Cell Culture
Mouse embryonic fibroblast (MEF) , Hela, and other cell lines were maintained in Dulbecco's minimal essential medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100U/mL penicillin, and 100 μg/ml streptomycin. Neonatal rat ventricular myocytes (NRVM) from 1-2 day old Sprague Dawley rat were prepared and used as described previously [40]. Neonates were euthanized by decapitation and the hearts were excised and both atria were removed. NRVM were prepared by enzymatic digestion with collagenase (Worthington) and pancreatin (Sigma) in 1xADS buffer at 37°C. NRVM were cultured in serum-free DMEM supplemented with 100U/mL Pen/Strep (Invitrogen) and 0.5% ITS (w/v) (BD Biosciences). Murine immortalized hepatocyte cell line AML12 was obtained from ATCC and cells were maintained in DMEM/F-12 media (1:1) containing supplements as instructed. Cells were cultured at 37°C in humidified 5% CO2–95% air. NRVM cells were seeded into 6 well plates at a density of 2×105 cells per well the day before experiments. Cell lines were seeded the day before treatment and reached confluency when treated. BCAAs-free DMEM was customized from Invitrogen. Branched-chain alpha keto acids (BCKAs) stock solution was prepared by dissolving alpha-ketoisocaproate-Na (KIC), alpha-keto-beta-methylvalerate-Na (KMV), and alpha-ketoisovalerate-Na (KIV) into water. The stock concentration of each of BCKAs was 500 mM and used at 500 μM concentration. H2O2, BCAAs, BCKAs, and other chemicals were purchased from Sigma.
Measurement of Cell Death
To induce cell death, 20 μM H2O2 diluted in culture medium was used for NRVM and 500-1000 μM H2O2 was used to treat MEFs and other cell lines unless otherwise specified [13, 15]. Treatment usually lasted for 16-24 hours for viability assay. Cell viability was determined by MTT assay, trypan blue staining, or calcein-AM staining. For MTT assay, cells were incubated with 0.5 g/L MTT for 0.5 h. After formazan crystals formed, the culture medium was removed, and DMSO was added to dissolve the formazan crystals. The absorbance of the solution at 570 nm was measured. For calcein-AM staining, after treatments, cells were incubated with calcein-AM (2 μM) for 15 min. For Propidium Iodide (PI) staining, PI (2 μM) was incubated with cells for 20 min at room temperature. The stained cells were then observed under an OLYMPUS fluorescent microscope for imaging. To induce the death receptor-mediated cell death, cells were treated with TNFalpha (50 ng/ml), cycloheximide(10 μg/ml), and Z-vad (40 μM).
ATP Measurement
The ATP content in cells was determined using a Luciferase-based Bioluminescence Assay Kit (Beyotime, Haimen, China) following the manufacturer's instruction. Protein concentration of cell lysate was determined using the BCA protein assay. ATP abundance was normalized to the protein content.
Metabolic assays with a Seahorse XF24 analyzer
Cellular oxygen consumption was measured using a Seahorse XF24 extracellular flux analyzer according to manufacturer's instructions. Briefly, MEF cells were seeded on 24-well XF24 well plates in DMEM medium. Before assay, the medium was changed to KHB XF Assay media (2.5 mM glucose, 0.5 mM carnitine, 111 mM NaCl, 4.7 mM KCl, 2 mM MgSO4 , 1.2 mM Na2HPO4, 150 μM BSA) with or without BCKAs (500 μM) and incubated in a non-CO2 incubator at 37°C for 1 hour for pH stabilization. Analyses were performed both at basal conditions and after injection of oligomycin (1 μM), FCCP (3 μM), Rotenone (1 μM), Antimycin A (1 μM).
Real-time RT-PCR analysis
Total RNA was extracted from cells using Trizol Reagent (Invitrogen) according to the manufacturer's instructions. Total RNA was reverse-transcribed into the first-strand cDNA using the Superscript First-Strand Synthesis Kit (Invitrogen). Then cDNA transcripts were quantified by the Step-One Plus Real-Time PCR System (ABI) using SYBR Green (ABI). 18sRNA were used as control. The PCR primers used were as follows: Bak forward primer, 5’-ggaatgcctacgaactcttca-3’; reverse primer, 5’-ccagctgatgccactcttaaa-3’; Bax forward primer, 5’-gtgagcggctgcttgtct-3’; reverse primer, 5’-ggtcccgaagtaggagagga-3’. The PCR products were electrophoresed on 1% agarose gel and visualized under UV light.
Western Blot Analysis
Proteins from cells were harvested in buffer (50 mM HEPES [pH7.4], 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM EGTA, 1 mM glycerophosphate, 2.5 mM sodium pyrophosphate 1 mM Na3VO4, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL of aprotinin, leupeptin, and pepstatin). Samples were separated on 4-12% Bis-Tris gels (Invitrogen), and transferred onto a nitrocellulose blot (Amersham). The blot was probed with the indicated primary antibodies. Protein signals were detected using HRP conjugated secondary antibodies and enhanced chemiluminescence (ECL) western blotting detection regents (Pierce). The primary antibodies were purchased from Cell Signaling Technology.
Murine Hearts for Langendorff Perfusion
The isolated perfusion hearts were prepared and used as described previously [41]. Briefly, male mice (8–10 weeks of age) were anticoagulated with heparin (1000 IU/kg i.p.) and euthanized through cervical dislocation. Hearts were excised and Langendorff perfusion was performed retrogradely via the aorta with modified Krebs-Henseleit buffer oxygenated with 95% O2/5% CO2. In the control group, hearts were perfused and stabilized for 30 min, followed by 35 min of zero-perfusion global ischemia and then 120 min of reperfusion. In the BCKA pre-treatment group, hearts followed the same perfusion protocol as Control group except that KIC (0.6 mM) was included in Krebs-Henseleit buffer throughout the experiments. In the BCKA post-treatment group, KIC was only included in the reperfusion buffer. Cardiac hemodynamic parameters including left ventricular developed pressure (LVDP), dP/dTmax, dP/dTmin was measured with a balloon inserted into the left ventricle [41]. To determine the infarct size, after the perfusion protocol was completed, the heart was perfused with 1% 2,3,5,-triphenyltetrazolium chloride (TTC) for 2 min at 37°C and then frozen at −20°C. The frozen hearts were then sectioned into ~1-mm-thick slices along the long axis of left ventricle and stored in 10% formalin. Images were recorded digitally using a camera mounted on a dissecting scope. The infarct area was measured using SPOT image analysis software by counting the red viable myocardium and pale white infarct area. The relative infarction was calculated as a percentage infracted area over the total ventricular area.
Statistics
Unless otherwise specified, statistical analyses were performed with Student's t-test (2 groups) or one-way ANOVA using Prism5 program (> 2groups) where appropriate. Data are calculated as the mean±STDEV or mean±SEM (standard error of the mean) unless otherwise indicated. Statistical significance is represented in figures by *, p < 0.05.
Supplementary Material
Highlights.
➢ BCKAs attenuate ischemia-reperfusion injury and preserve heart function
➢ BCKAs protect cardiomyocytes from oxidative stress-induced necrosis
➢ BCKAs protect mitochondria and energy production against oxidative injury
➢ BCKAs administration during reperfusion significantly attenuates cardiac I/R injury
ACKNOWLEDGEMENT
This work is supported in part by Ministry of Science and Technology of China Grant 2012BAI02B05 and 2013YQ030923; National Institute of Health grants HL108186, HL103205, HL098954, HL080111, the Laubisch Fund (UCLA); American Heart Association (AHA) Science Development Grant; AHA Established Investigator Award. This work is also supported by National Natural Science Foundation of China (NSFC30971094 and NSFC81270317), Science and Technology Commission of Shanghai Municipality (13ZR1423300 and 16JC1404400).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation. 2016;133:2038–49. doi: 10.1161/CIRCULATIONAHA.115.020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acids catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016:ajpheart 00114 2016. doi: 10.1152/ajpheart.00114.2016. [DOI] [PubMed] [Google Scholar]
- 4.Guo X, Huang C, Lian K, Wang S, Zhao H, Yan F, et al. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem Biophys Res Commun. 2016;480:106–13. doi: 10.1016/j.bbrc.2016.09.162. [DOI] [PubMed] [Google Scholar]
- 5.Sgaravatti AM, Rosa RB, Schuck PF, Ribeiro CAJ, Wannmacher CMD, Wyse ATS, et al. Inhibition of brain energy metabolism by the [alpha]-keto acids accumulating in maple syrup urine disease. BBA-Mol Basis Dis. 2003;1639:232–8. doi: 10.1016/j.bbadis.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–5. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCall K. Genetic control of necrosis—another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–8. doi: 10.1016/j.ceb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kung G, Konstantinidis K, Kitsis RN. Programmed Necrosis, Not Apoptosis, in the Heart. Circ Res. 2011;108:1017–36. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 11.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 12.Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–66. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Lin Y, Kim Y-S, Hande MP, Liu Z-G, Shen H-M. c-Jun N-terminal kinase mediates hydrogen peroxide-induced cell death via sustained poly(ADP-ribose) polymerase-1 activation. Cell Death Differ. 2007;14:1001–10. doi: 10.1038/sj.cdd.4402088. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci USA. 2006;103:1727–31. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen H-M, Lin Y, Choksi S, Tran J, Jin T, Chang L, et al. Essential Roles of Receptor-Interacting Protein and TRAF2 in Oxidative Stress-Induced Cell Death. Mol Cell Biol. 2004;24:5914–22. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, et al. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–30. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 18.Oerlemans MIFJ, Koudstaal S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JPG. Targeting cell death in the reperfused heart: Pharmacological approaches for cardioprotection. Int J Cardiol. 2013;165:410–22. doi: 10.1016/j.ijcard.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Yellon DM, Hausenloy DJ. Myocardial Reperfusion Injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 20.Belmokhtar CA, Hillion J, Segal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–62. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 21.Zong W-X, Ditsworth D, Bauer DE, Wang Z-Q, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–82. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin C, Salloum FN, Kukreja RC. A Novel Role of MicroRNA in Late Preconditioning. Upregulation of Endothelial Nitric Oxide Synthase and Heat Shock Protein 70. Circ Res. 2009;104:572–5. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct Roles of Autophagy in the Heart During Ischemia and Reperfusion: Roles of AMP-Activated Protein Kinase and Beclin 1 in Mediating Autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 24.Brosnan JT, Brosnan ME. Branched-Chain Amino Acids: Enzyme and Substrate Regulation. J Nutr. 2006;136:207S–11. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 25.Lu G, Sun H, She P, Youn J-Y, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–87. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burwell LS, Nadtochiy SM, Brookes PS. REVIEW: Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–10. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: Effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia–reperfusion injury. J Mol Cell Cardiol. 2007;42:812–25. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–9. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of Electron Transport during Ischemia Protects Cardiac Mitochondria. J Biol Chem. 2004;279:47961–7. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Camara AKS, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C47. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible Blockade of Electron Transport during Ischemia Protects Mitochondria and Decreases Myocardial Injury following Reperfusion. J Pharmacol Exp Ther. 2006;319:1405–12. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 32.Choi K, Kim J, Kim GW, Choi C. Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr Neurovasc Res. 2009;6:213–22. doi: 10.2174/156720209789630375. [DOI] [PubMed] [Google Scholar]
- 33.Shen H-M, Liu Z-g. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–39. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y-T, Zhang S, Kim Y-S, Tan H-L, Whiteman M, Ong C-N, et al. Signaling pathways from membrane lipid rafts to JNK1 activation in reactive nitrogen species-induced non-apoptotic cell death. Cell Death Differ. 2007;15:386–97. doi: 10.1038/sj.cdd.4402273. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y-T, Ong C-N, Shen H-M. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5:824–34. doi: 10.4161/auto.9099. [DOI] [PubMed] [Google Scholar]
- 36.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 37.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kand'ar R, Zakova P, Jirosova J, Sladka M. Determination of branched chain amino acids, methionine, phenylalanine, tyrosine and alpha-keto acids in plasma and dried blood samples using HPLC with fluorescence detection. Clin Chem Lab Med. 2009;47:565–72. doi: 10.1515/CCLM.2009.123. [DOI] [PubMed] [Google Scholar]
- 39.Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet. 2014;23:R1–8. doi: 10.1093/hmg/ddu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao C, Ren S, Lee J-H, Qiu J, Chapski DJ, Rau CD, et al. RBFox1-mediated RNA splicing regulates cardiac hypertrophy and heart failure. J Clin Invest. 2015;126:0. doi: 10.1172/JCI84015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan H, Li J, Ren S, Gao J, Li G, Kim R, et al. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.