Abstract
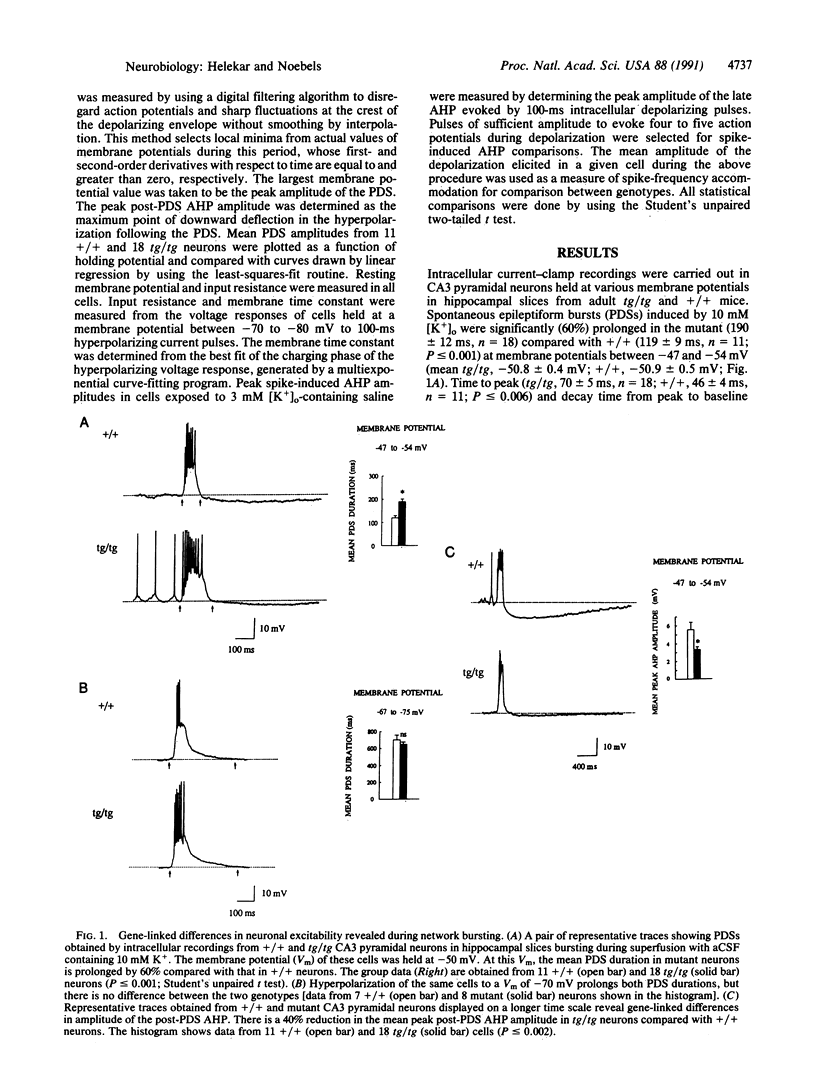
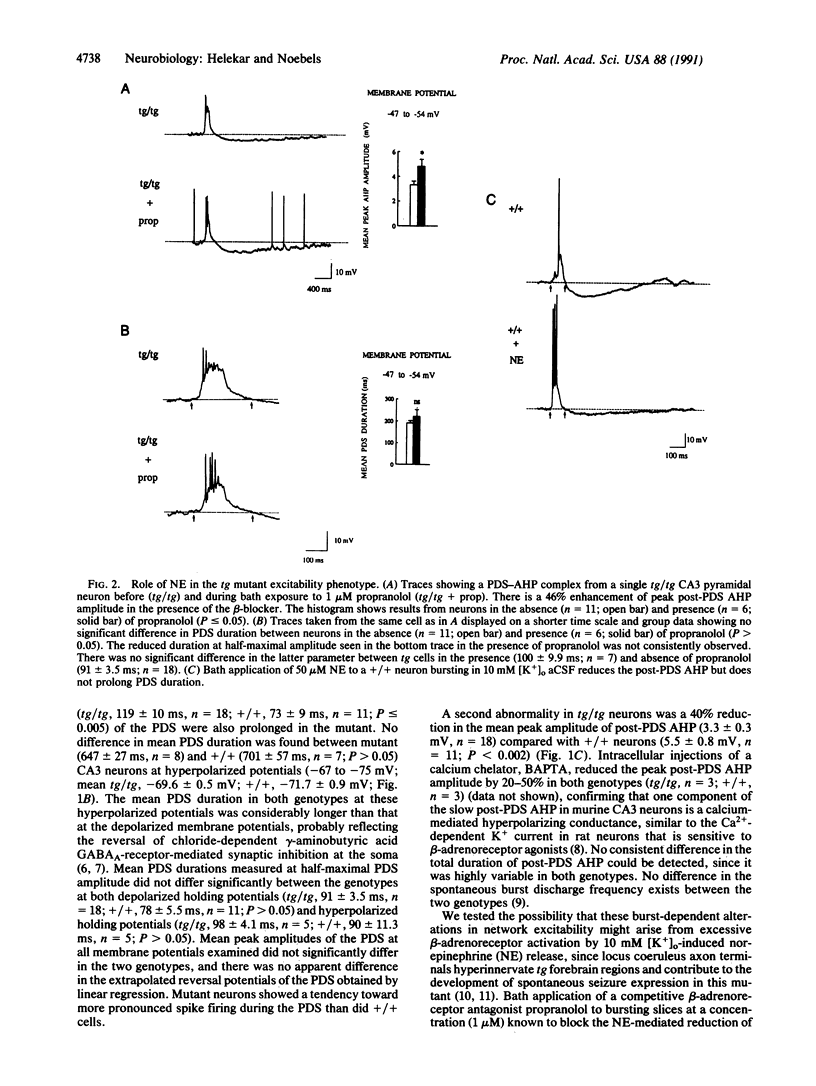
A mutation at the tottering locus (tg, recessive, on chromosome 8) stimulates noradrenergic locus coeruleus axon terminal outgrowth and predisposes the brain to generalized spike-wave epilepsy in the young mouse. In an isolated synaptic circuit studied in vitro, the hyperinnervated mutant hippocampal pyramidal neurons respond normally when individually activated; however, latent neuronal signaling defects emerge during synchronous network bursting, revealing two conditional excitability phenotypes: a voltage-dependent prolongation of a complex synaptic response, the paroxysmal depolarizing shift, and a beta-adrenoreceptor-linked attenuation of the afterhyperpolarization. In this target brain region, the tg locus transforms neuronal excitability without altering measured intrinsic membrane properties, indicating that gene control of inherited epileptic traits may be mediated in part by activity-dependent modulation of network behavior favoring synchronous neuronal firing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Williamson A. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol. 1988 May;399:191–205. doi: 10.1113/jphysiol.1988.sp017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Ayala G. F. Cellular mechanisms of epilepsy: a status report. Science. 1987 Jul 10;237(4811):157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- GREEN M. C., SIDMAN R. L. Tottering--a neuromusclar mutation in the mouse. And its linkage with oligosyndacylism. J Hered. 1962 Sep-Oct;53:233–237. doi: 10.1093/oxfordjournals.jhered.a107180. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Effects of intracellular injections of chloride and EGTA on postepileptiform-burst hyperpolarizations in hippocampal neurons. Neurosci Lett. 1981 Mar 10;22(2):159–163. doi: 10.1016/0304-3940(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984 Oct 19;226(4672):350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981 Jan 16;211(4479):294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G., Psarropoulou C., Haas H. L. Membrane properties, response to amines and to tetanic stimulation of hippocampal neurons in the genetically epileptic mutant mouse tottering. Exp Brain Res. 1988;72(1):45–50. doi: 10.1007/BF00248499. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels in neurons and other cells. Annu Rev Neurosci. 1988;11:119–136. doi: 10.1146/annurev.ne.11.030188.001003. [DOI] [PubMed] [Google Scholar]
- Levitt P., Noebels J. L. Mutant mouse tottering: selective increase of locus ceruleus axons in a defined single-locus mutation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4630–4634. doi: 10.1073/pnas.78.7.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986 Mar;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988 Feb 23;442(1):131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987 Oct 22;329(6141):724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Noebels J. L. A single gene error of noradrenergic axon growth synchronizes central neurones. Nature. 1984 Aug 2;310(5976):409–411. doi: 10.1038/310409a0. [DOI] [PubMed] [Google Scholar]
- Noebels J. L., Rutecki P. A. Altered hippocampal network excitability in the hypernoradrenergic mutant mouse tottering. Brain Res. 1990 Aug 6;524(2):225–230. doi: 10.1016/0006-8993(90)90695-8. [DOI] [PubMed] [Google Scholar]
- Noebels J. L., Sidman R. L. Inherited epilepsy: spike-wave and focal motor seizures in the mutant mouse tottering. Science. 1979 Jun 22;204(4399):1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- Schaad N. C., Schorderet M., Magistretti P. J. Prostaglandins and the synergism between VIP and noradrenaline in the cerebral cortex. Nature. 1987 Aug 13;328(6131):637–640. doi: 10.1038/328637a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978 May 19;147(1):117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Foehring R. C., Chubb M. C., Crill W. E. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988 Feb;59(2):450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Stelzer A., Galvan M. Kindling-like stimulus patterns induce epileptiform discharges in the guinea pig in vitro hippocampus. Neurosci Lett. 1985 Sep 16;60(1):25–31. doi: 10.1016/0304-3940(85)90376-3. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Bragdon A. C., Wilson W. A. Induction of epileptiform activity in hippocampal slices by trains of electrical stimuli. Brain Res. 1985 Oct 7;344(2):296–302. doi: 10.1016/0006-8993(85)90807-8. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N. T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987 Apr 16;326(6114):698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Tehrani M. H., Barnes E. M., Jr Basal and drug-induced cAMP levels in cortical slices from the tottering mouse. Epilepsy Res. 1990 Dec;7(3):205–209. doi: 10.1016/0920-1211(90)90016-o. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. J Neurophysiol. 1983 Feb;49(2):442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]



