Abstract
The main cause of the high morbidity and mortality of cystic fibrosis (CF) is the progressive lung inflammation associated with Pseudomonas aeruginosa colonization. During the course of chronic CF infections, P. aeruginosa undergoes a conversion to a mucoid phenotype. The emergence of mucoid P. aeruginosa in CF is associated with increased inflammation, respiratory decline, and a poor prognosis. Here we show, by the use of microarray analysis, that upon P. aeruginosa conversion to mucoidy, there is a massive and preferential induction of genes encoding bacterial lipoproteins. Bacterial lipoproteins are potent agonists of Toll-like receptor 2 (TLR2) signaling. The expression of TLR2 in human respiratory epithelial cells was ascertained by Western blot analysis. Human respiratory epithelial cells responded in a TLR2-dependent manner to bacterial lipopeptides derived from Pseudomonas lipoproteins induced in mucoid strains. The TLR2 proinflammatory response was further augmented in CF cells. Thus, the excessive inflammation in CF is the result of a global induction in mucoid P. aeruginosa of lipoproteins that act as proinflammatory toxins (here termed lipotoxins) superimposed on the hyperexcitability of CF cells. Blocking the signaling cascade responding to bacterial lipotoxins may provide therapeutic benefits for CF patients.
Cystic fibrosis (CF) is the most common lethal inheritable disease affecting Caucasians (19). CF is caused by mutations in the gene encoding cystic fibrosis transmembrane conductance regulator (CFTR), resulting in multiorgan malfunctions, particularly within the respiratory, gastrointestinal, hepatobiliary, and reproductive tracts (29, 44). The lung complications in CF include chronic respiratory infections, which are the main cause of CF remaining an incurable lethal disease (15). The predominant CF pathogen is Pseudomonas aeruginosa: the lungs of >90% of all CF patients eventually become colonized with this bacterium (11). A classical feature of P. aeruginosa strains infecting CF patients is that they mutate into a mucoid, exopolysaccharide alginate-overproducing form in a process referred to as the conversion to mucoidy (15). The conversion to mucoidy is concomitant with the establishment of chronic bacterial colonization (20, 27). Infections with mucoid P. aeruginosa are associated with heightened inflammation, tissue destruction, and pulmonary function decline (3). It has been recognized that the establishment of mucoid P. aeruginosa biofilms correlates with a poor prognosis for CF patients (11, 20, 27). Conversion to mucoidy results from mutations that render the P. aeruginosa stress response sigma factor AlgU constitutively active (21, 22). This in turn activates genes of the alginate biosynthesis pathway and additional genes that still need to be fully characterized (9, 10).
However, the overproduction of alginate, an immunologically inert exopolysaccharide associated with mucoid conversion, cannot explain the increased inflammation in CF. This makes it likely that additional, less conspicuous, but potentially more damaging products of P. aeruginosa are produced by mucoid P. aeruginosa in the CF host. Here we describe the previously unappreciated induction of proinflammatory products in mucoid P. aeruginosa and how they affect signaling pathways in host respiratory cells. Using microarray analysis, we found that the most prominently induced genes in mucoid P. aeruginosa encode lipoproteins. We show that these P. aeruginosa products cause the activation of NF-κB in human lung epithelial cells and that this occurs through Toll-like receptor 2 (TLR2).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The mucoid P. aeruginosa strain PAO578II (mucA22 sup-2) and its isogenic nonmucoid PAO6865 (algU::Tcr) derivative have been described previously (7). For RNA isolation, strains were cultured at 37°C overnight in Luria broth. One milliliter of the overnight culture was used to inoculate 100 ml of Luria broth containing 0.3 M NaCl, and the culture was grown for 4 h at 37°C to mid-log phase (optical density at 600 nm of 0.5).
Microarray analysis.
For microarray analysis and primer extension, RNAs were isolated with Trizol (Invitrogen Life Technologies, Carlsbad, Calif.) and an RNeasy kit (Qiagen, Valencia, Calif.) according to the method described by Lory et al. (http://cfgenomics.unc.edu/protocols_rna_prep.htm). Labeled cDNAs were generated according to the protocol for the Affymetrix (Santa Clara, Calif.) Pseudomonas microarray chip. cDNAs were synthesized by annealing random primers (Invitrogen) to purified RNAs and extending them with SuperScript II (Invitrogen). Transcripts corresponding to Bacillus subtilis genes dap, thr, phe, lys, and trp were spiked into the cDNA synthesis reaction mixtures as a control to monitor cDNA synthesis, labeling, hybridization, and staining efficiency (courtesy of Steve Lory, Harvard Medical School). RNAs were removed by the addition of 1 N NaOH and incubation at 65°C for 30 min. The reaction was neutralized with 1 N HCl, and the cDNAs were purified with a Qiaquick PCR purification kit (Qiagen). The yields were quantified, and cDNAs were fragmented with 0.6 U of DNase I (Amersham Pharmacia Biotech) per μg of cDNA for 10 min at 37°C, followed by heat inactivation. Chips were hybridized overnight at 50°C and then washed, stained, and scanned the next day according to the steps of the Affymetrix Microarray Suite software specified for the Pseudomonas chip. The results from three independent experiments were merged for each strain. The merged data were used for comparisons, and statistical significance was assessed with Student's t test.
Cell culture.
Primary normal human bronchial epithelial cells (NHBEs) (Cambrex Bio Science, Baltimore, Md.) were cultured in bronchial epithelial medium (BEGM; Cambrex Bio Science). IB3-1 (47) is a CF-affected human airway epithelial cell line. Genotypically, IB3-1 is a compound heterozygote containing the ΔF508 mutation and W1282X. The C38 and S9 cell lines, created by correcting IB3-1 cells for chloride conductance by the introduction of functional CFTR (8), were grown in LHC-8 medium (Bio-fluids, Rockville, Md.) supplemented with 10% fetal bovine serum and antibiotics. S9 and C38 cells are both functionally complemented for the major known CFTR effects. They differ in that the CFTR cDNA used to complement IB3-1 cells encodes a complete CFTR molecule in the case of S9 cells, while it lacks the first CFTR extracellular loop in the case of C38 cells (47). NuLi-1 cells, derived from normal human airway epithelial cells by J. Zabner (46), were cultured in collagen-coated plastic dishes (Sigma, St. Louis, Mo.) in serum-free bronchial epithelial cell growth medium with supplements (Clonetics/BioWhittaker, San Diego, Calif.). The 9/HTEo− tracheal epithelial cell line (28), obtained from P. Davis (Case Western Reserve University, Cleveland, Ohio), was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2.5 mM l-glutamine (in the presence of 50 U of hygromycin/ml as a marker of the cell line due to its stable transfection with the pCEP2 vector). All cell lines were grown at 37°C in 5% CO2. For luciferase and cytokine assays, cells were seeded at 5 × 105 or 1 × 105 cells/well in 12-well plates, respectively.
Peptide design and synthesis.
Leader sequences denoting lipopeptide modification (31) were observed for two open reading frames (ORFs) downstream of AlgU-dependent promoters. Two peptides, LPTA(6) (Pam3Cys-DKKEE-OH) and LPTB(6) (Pam3Cys-DSQTN-OH), consisting of a palmitylated cysteine (Pam3Cys) after the cleavage site plus five amino acids from the amino terminus of each lipoprotein, were synthesized (Bio-Synthesis, Inc., Lewisville, Tex.). The proinflammatory synthetic bacterial lipopeptide Pam3Cys-SKKKK-OH was also synthesized (2, 17).
Transfection, luciferase reporter, and cytokine assays.
To monitor transient NF-κB activation, we seeded the primary NHBE, IB3-1, C38, and S9 cell lines at 5 × 105 cells per well in a 12-well plate and transfected them by using the Effectene transfection reagent (Qiagen) with 0.05 μg of a human TLR2 (hTLR2) or dominant-negative TLR2 (DN-TLR2) expression plasmid, 0.15 μg of an NF-κB-responsive luciferase reporter construct, 0.15 μg of a control Renilla luciferase construct, and 0.05 μg of a control β-galactosidase reporter construct for normalization. Eighteen hours after transfection, the cells were incubated with a stimulus (tumor necrosis factor alpha [TNF-α], 20 ng/ml; lipopolysaccharide [LPS], 1 μg/ml; Pam3Cys, 5 μg/ml; or lipopeptides, 5 μg/ml) for 6 h and assayed for luciferase activity by use of a luciferase assay reagent (Promega) or for β-galactosidase activity by use of the Galacto-Star luminescence system (Tropix, Bedford, Mass.). The transfection efficiency was controlled by standardizing the luciferase activity to constitutive β-galactosidase production. For the cytokine assay, NHBE confluent monolayers were incubated with LPS (1 μg/ml), bacterial lipopeptides (1 or 10 μg/ml), or no stimulation for 24 h. Cell culture supernatants were assayed at a 100-fold dilution for secreted interleukin-8 (IL-8) according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn.). All experiments were repeated at least three times, and all errors shown represent experimental errors for at least three independent cultures.
Western blot analysis and antibodies.
Protein samples were prepared by homogenizing lung epithelial cells in lysis buffer (8.5% sucrose and protease inhibitors [10 mg of leupeptin/ml, 1 μM pepstatin, 1.8 mg of E64/ml, and 5 mg of Nα-p-tosyl-l-lysine chloromethyl ketone/ml]) (all chemicals were from Sigma Chemical Co.) on ice. Protein concentrations were determined by the use of a micro BCA protein assay reagent kit (Pierce, Rockford, Ill.). An antibody against TLR2 for Western blotting was obtained from Imgenex (San Diego, Calif.). Immune complexes were detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Bio-Rad, Hercules, Calif.). The signal was visualized by incubating the complexes with Super Signal chemiluminescent substrate (Pierce). Densitometric analyses of Western blots were performed with NIH Image software (http://rsb.info.nih.gov/nih-image). Western blot quantification was performed as previously described (13, 14, 43). A blocking antibody against TLR2 was obtained from Douglas Golenbock.
RESULTS
The most highly induced genes in mucoid P. aeruginosa encode lipoproteins.
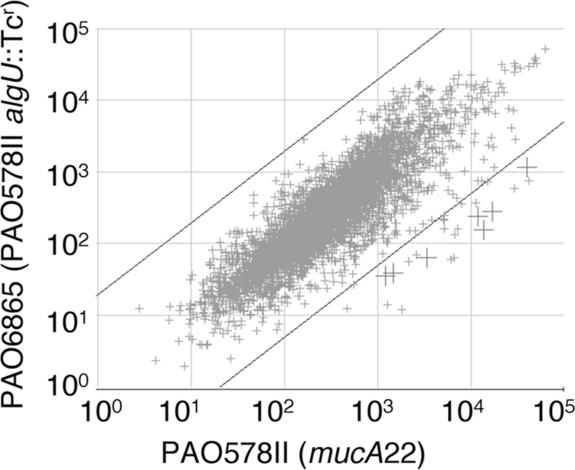
It has been shown that the conversion to mucoidy in P. aeruginosa CF isolates occurs via mutations in the mucA gene (22) that activate the alternative sigma factor AlgU (21, 22). We tested how the activation of AlgU in a mucoid P. aeruginosa strain carrying the most common mucA mutation found in CF isolates affected global gene expression. PAO578II carries the mucA22 mutation and an additional sup-2 mutation, also common among CF isolates, that renders it responsive to growth conditions for the maximal production of alginate (35). The strain PAO6865 is an algU knockout (algU::Tcr) derivative of PAO578II (4). When the microarray expression data (Fig. 1) for PAO578II and PAO6865 were compared, the analysis revealed massive and selective lipoprotein induction in mucoid P. aeruginosa, with high expression ratios (Fig. 1, crosses, and Table 1). We found that 70% of the genes showing induction above 30-fold encoded uncharacterized lipoproteins (Table 1). The highest levels of AlgU-dependent expression were observed with the lipoprotein genes lptE and lptG. Two of the lipoprotein-encoding genes, lptA and osmE, have known AlgU-dependent promoters (10). The majority of the other highly induced genes encoding products with lipoprotein leader sequences contained an AlgU promoter consensus motif (Table 2). Since lipoproteins have been implicated in inflammatory processes and the pathogenesis of several important bacterial infections, including Mycobacterium tuberculosis (5), Treponema pallidum (40), Listeria monocytogenes (12), and Borrelia burgdorferi (32), this observation warranted further analysis.
FIG. 1.
Global gene expression analysis by use of microarrays reveals massive and selective lipoprotein gene induction in mucoid P. aeruginosa. The experimental points represent merged expression data from three independent cultures run on three separate chips for each strain. Mucoid strain PAO578II carries the typical CF mutation mucA22, which causes mucoidy in P. aeruginosa. Gene expression in PAO578II was compared to that in the isogenic, nonmucoid, algU knockout strain PAO6865. Large crosses, lipoprotein genes induced in mucoid P. aeruginosa (P < 0.001). Diagonal lines delimit regions of ≥30-fold induction.
TABLE 1.
Activation of lipoprotein gene expression in mucoid P. aeruginosaa
| Gene no.b | Gene name | Descriptionc | Fold activation | P value |
|---|---|---|---|---|
| PA1323 | Unknown | 103 | 9.8 × 10−6 | |
| PA3691 | lptE | Lipoprotein LptE | 87 | 4.9 × 10−6 |
| PA5526 | lptG | Lipoprotein LptG | 61 | 0.0005 |
| PA3819 | slyB | Possible porin | 56 | 0.0002 |
| PA3692d | lptF | Lipoprotein LptF | 53 | 0.004 |
| PA4876 | osmE | Lipoprotein OsmE | 49 | 6.0 × 10−5 |
| PA0737 | lptD | Lipoprotein LptD | 49 | 0.0002 |
| PA1592 | lptA | Lipoprotein LptA | 35 | 4.8 × 10−6 |
| PA0062 | lptC | Lipoprotein LptC | 34 | 8.8 × 10−5 |
The data shown are for all P. aeruginosa genes with activation exceeding 30-fold in mucoid cells and with P values of <0.001.
Genes are ordered by decreasing levels of induction.
A Lpt designation indicates the presence of a typical lipoprotein signal sequence.
PAO3692 lptF was included based on a genetic linkage to PAO3691 lptE and a statistically significant induction (P < 0.005).
TABLE 2.
P. aeruginosa AlgU (σE) promoter consensus sequence in front of the lpt genes
| Gene category and no. | Gene name | AlgU consensusa | Distance (bp) from initiation codon |
|---|---|---|---|
| Lpt-encoding genes with AlgU promoter consensus sequence | |||
| PA0737 | lptD | TCGAACTGGATCCGTTCGACGATGGCTACTACGGCT | 155 |
| PA1323 | Unknown | CTGAACTTTTTCACTGCGGCGCCTATCAACTCCTTT | 30 |
| PA5526 | lptG | AAGAATTTCCCTCGATTGCGACGGTCACAAGGGCAA | 55 |
| PA1592 | lptA | TGGAACTTCACGCCAGCGCAAATGTTCAAAGGGCTA | 35 |
| PA3262 | lptB | TTGAACTTATCCGCGCGCACCTGTTCCTATTGCCCA | 82 |
| PA3819 | slyB | TGGAACTTGGTGGTTTTTGCCCAGTCCTAGGCAAGG | 60 |
| PA4876 | osmE | GCGAACTCTGCGCGAGGGCCTGCGTTCCAATGTTCG | 37 |
| Lpt-encoding genes with no discernible AlgU promoter consensus sequence | |||
| PA0062 | lptC | ||
| PA3691 | lptE |
The AlgU consensus sequence was GAACTT-(16 or 17 bp)-TCCAA-(5 or 6 bp). The consensus matches are shown in bold; underscored residues map mRNA 5′ ends.
P. aeruginosa lipopeptides stimulate NF-κB activation in human lung epithelial cells.
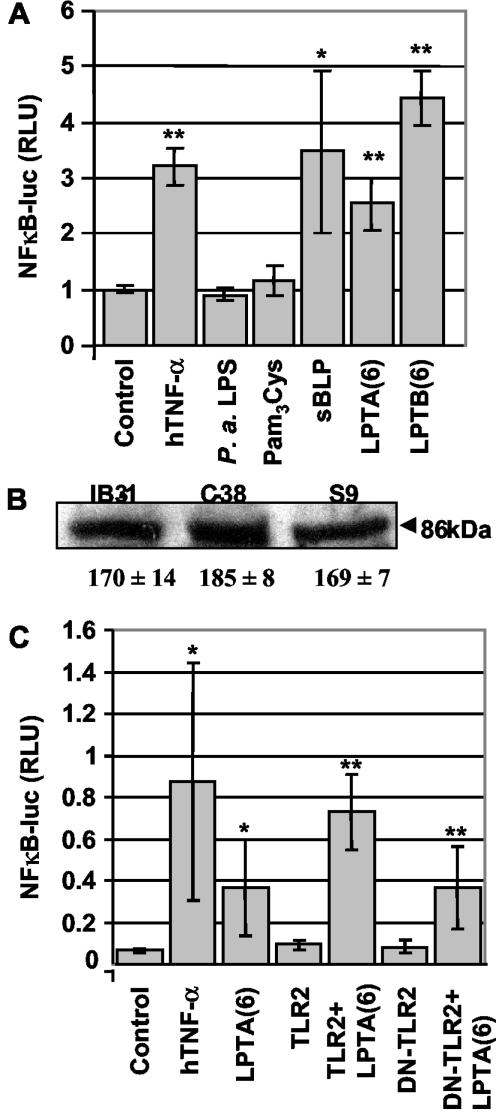
Considering the preponderance of lipoprotein genes induced in mucoid P. aeruginosa, we next investigated whether lipoproteins and/or lipopeptides could induce inflammation in clinically relevant host cells. P. aeruginosa colonization is largely limited to the lower airways, and the bronchioles of the CF lung are where respiratory epithelial cells are exposed to Pseudomonas products. To determine whether P. aeruginosa lipopeptides activate NF-κB in human respiratory epithelial cells, we first measured NF-κB-dependent promoter activity by use of a luciferase reporter plasmid in primary human bronchial epithelial cells (NHBEs). We exposed NHBEs to the lipopeptides LPTA(6) and LPTB(6) (9), derived from Pseudomonas lipoproteins induced in mucoid cells. Responses to LPTA(6) and LPTB(6) were detected (Fig. 2A) that were comparable to the response to the standard bacterial lipoprotein (sBLP) (2, 17). NHBEs did not respond to P. aeruginosa LPS. As a negative control, we used a palmitylated cysteine residue (Pam3Cys) that does not stimulate TLR2 (2, 17). Pam3Cys did not cause NF-κB activation, consistent with a requirement for both the acyl groups and a peptide moiety for lipopeptide recognition by TLRs (2). These results show that lipopeptides derived from lipoproteins induced in mucoid P. aeruginosa activate NF-κB in human respiratory epithelial cells.
FIG. 2.
Lipopeptides induce inflammatory response in human respiratory epithelial cells. (A) Lipopeptides induce NF-κB activation in primary human bronchial epithelial cells. NHBEs were transfected with an NF-κB-responsive luciferase construct and incubated for 6 h in the presence of 1 μg of LPS/ml, 20 ng of TNF-α/ml, or 5 μg of lipopeptide/ml. The data were normalized to a cotransfected β-galactosidase construct. sBLP, synthetic bacterial lipopeptide (Pam3Cys-SKKKK-OH); LPTA(6), N terminus of lptA gene product (Pam3Cys-DKKEE-OH); LPTB(6), N terminus of lptB gene product (Pam3Cys-DSQTN-OH). (B) Western blot analysis of TLR2 protein expression in CF (IB3-1) and CFTR-corrected (C38) cells with an anti-hTLR2 monoclonal antibody (IMG-319), detected as an 86-kDa band. Equal amounts of proteins were loaded. A densitometry analysis of the TLR2 protein was performed for three independent experiments with NIH Image 1.62 software. The numbers underneath the gel represent the means ± standard errors (arbitrary density units). (C) NF-κB luciferase assay with primary NHBEs showing that TLR2 activates the LPTA(6)-induced response in epithelial cells by employing DN-TLR2. *, P < 0.05; **, P < 0.01.
TLR2 is expressed in human lung epithelial cells and responds to P. aeruginosa lipopeptides.
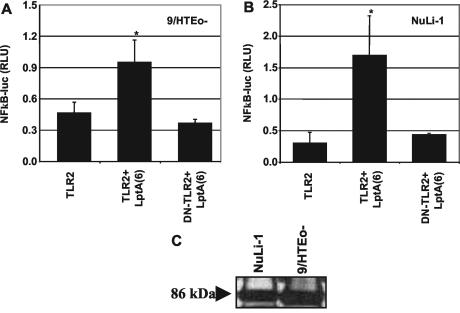
In contrast to its relatively high expression in lymphoid tissues, TLR2 is believed to be expressed only at a low level in epithelial cells, although TLR2 has been detected in human epithelial HeLa cells (36). For this study, we extended these investigations to verify whether TLR2 is expressed in human respiratory epithelial cells. TLR2 protein expression was detected by Western blots in the bronchial epithelial cell line IB3-1, derived from a CF patient, by using a monoclonal antibody against human TLR2 (Fig. 2B). Equal amounts of TLR2 were observed in IB3-1 cells and in their CFTR-corrected, genetically matched derivatives C38 and S9 cells (Fig. 2B). Similar results were obtained with primary NHBEs. Since TLR2 is known to be the receptor for bacterial lipoproteins, we next tested whether TLR2 is involved in P. aeruginosa lipopeptide-stimulated NF-κB activation in NHBEs (37, 41, 45). In cotransfection experiments with hTLR2 and DN-TLR2 cDNAs, a dependence on TLR2 for LPTA(6) stimulation was observed, as detected by an NF-κB-dependent luciferase reporter assay (Fig. 2C). In addition to NHBEs, two human respiratory epithelial cell lines, 9HTEo− (28) and NuLi-1 (46), were tested. Both the dependence on TLR for stimulation with LPTA(6) (Fig. 3A and B) and the presence of TLR2 (Fig. 3C) were demonstrated in experiments with 9HTEo− and NuLi-1 cells.
FIG. 3.
TLR-dependent induction of NF-κB-mediated transcription in bronchial epithelial cells stimulated with P. aeruginosa lipopeptide. 9/HTEo− (A) and NuLi-1 (B) cells (both derived from normal human lung cells) were transiently cotransfected with an NF-κB-responsive luciferase reporter plasmid and TLR2 or DN-TLR2 and then incubated for 6 h in the presence of 10 μg of lipopeptide (LptA)/ml. The data were normalized to a cotransfected β-galactosidase construct and are means ± standard deviations. (C) Western blot analysis of TLR2 protein expression in NuLi and 9/HTEo− cells with an anti-hTLR2 monoclonal antibody (IMG-319). Equal amounts of proteins were loaded. *, P < 0.05.
TLR2 is involved in Pseudomonas lipopeptide-induced NF-κB activation in CF cells.
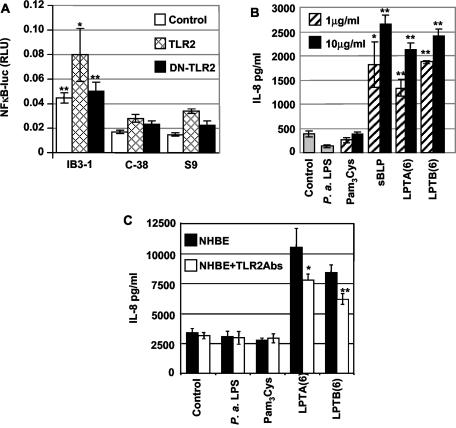
We next assessed the role of Pseudomonas lipoproteins and TLR2 in the stimulation of NF-κB in IB3-1 cells, derived from a compound heterozygote CF patient carrying the ΔF508 CFTR and W1282X CFTR alleles. In addition to the CFTR mutant cell line IB3-1, its CFTR-corrected derivatives C38 and S9 were included in the study. IB3-1, C38, and S9 cells were transfected with TLR2 or DN-TLR2, exposed to the LPTA lipopeptide, and tested for the ability to activate the NF-κB reporter gene. The CF (IB3-1) cells showed a higher reactivity to P. aeruginosa lipopeptides than the CFTR-corrected, genetically matched C38 and S9 cells, in a TLR2-dependent manner (Fig. 4A). Transfection with TLR2 further enhanced LPTA-induced NF-κB activation in the mutant IB3-1 cells. Taken together, these data indicate that the TLR2-dependent activation of NF-κB in response to Pseudomonas lipopeptides is augmented in CFTR mutant epithelial cells.
FIG. 4.
Proinflammatory action of LptA lipopeptide in the CF bronchial epithelial cell line IB3-1 and its CFTR-corrected derivatives C38 and S9 and TLR2 dependence of the response. (A) Induction of NF-κB-mediated transcription in vitro. For a luciferase assay, IB3-1, C38, and S9 cells were transiently transfected with an NF-κB-responsive luciferase reporter plasmid and TLR2 or DN-TLR2 and then incubated for 6 h in the presence of 5 μg of lipopeptide/ml. The data were normalized to a cotransfected β-galactosidase construct. (B) P. aeruginosa-based lipopeptides induce inflammatory IL-8 chemokine production by NHBEs. Confluent monolayers of NHBEs were left unstimulated (in medium) or were incubated for 24 h with P. aeruginosa LPS, 1 or 10 μg of lipopeptide/ml [sBLP, LPTA(6), or LPTB(6)], or a palmitylated cysteine control. (C) NHBEs were stimulated with LPTA(6) and LPTB(6) in the absence or presence of 20 μg of TLR2-blocking antibody (TL2.1)/ml. *, P < 0.05; **, P < 0.01.
Mucoid P. aeruginosa lipopeptides induce IL-8 production.
IL-8 is a potent chemoattractant for neutrophils that has been implicated in a neutrophil infiltration and inflammatory cascade in CF (26). Elevated levels of the chemokine IL-8 represent one of the hallmarks of excessive inflammation in CF (3, 15, 19). Thus, we tested whether lipopeptides corresponding to the highly induced lipoproteins in mucoid P. aeruginosa could induce IL-8 production in human lung epithelial cells. To measure IL-8 production, we stimulated confluent NHBE monolayers for 24 h with P. aeruginosa LPS, with 1 or 10 μg of lipopeptide/ml, or a palmitylated cysteine control or left the cells unstimulated. Both lipopeptides, LPTA(6) and LPTB(6), induced detectable IL-8 levels in culture supernatants (Fig. 4B). Furthermore, the induction of IL-8 production was at least partially suppressed with TLR2-blocking antibodies (Fig. 4C). IL-8 was not induced in response to either LPS or palmitylated cysteine. Thus, primary human respiratory epithelial cells have the machinery to recognize and respond to bacterial lipoproteins and produce IL-8 upon stimulation with Pseudomonas lipopeptides.
DISCUSSION
The results of this study show that P. aeruginosa causes excessive inflammation in CF due to a massive and selective induction of lipoprotein genes upon conversion to mucoidy. The lipopeptides derived from such proteins show a strong proinflammatory potential in primary lung epithelial cells. In our view, these products are toxic to the CF host and thus should be considered bacterial toxins. Due to their significance in CF and their implications in pathogenesis in other infections (1, 24, 33), lipoproteins should be viewed as a double-edged sword in host-pathogen interactions: they can serve both as signals recognized by the host to activate its defenses and as agents causing excessive host damage by the pathogen in some situations, such as in CF. We have consequently designated the highest expressing lipoprotein genes in mucoid P. aeruginosa as genes encoding lipotoxins (LPTs): lptA, lptC, lptD, lptE, lptF, and lptG (Table 1).
Increased levels of IL-8 have been noted in the sputum and bronchoalveolar lavage of patients with CF (3, 34). IL-8 is known to play a major role in inflammatory pathogenesis in the airways of CF patients (3, 23, 34, 39). Synthetic lipopeptides corresponding to the N termini of the mature, processed LPTs caused IL-8 production in primary human epithelial cells, as shown here, and in human macrophages derived from peripheral blood monocytes (9). Previous studies have shown IL-8 secretion in response to P. aeruginosa in human bronchial epithelial cells (6, 7, 42), although the factors responsible have not been identified. Our results show that the primary human respiratory epithelial cells have the machinery to recognize and respond to bacterial lipoproteins and that the production of IL-8 in CF may be due to stimulation with Pseudomonas lipopeptides.
CF cells showed increased responses to lipopeptide stimulation relative to genetically matched CFTR-corrected cell lines. This indicates that CF cells can be primed for TLR2 signaling to levels that are higher than normal. Thus, both the pathogen and the host conspire to bring about excessive inflammation in CF. The differences in TLR2 responsiveness between CF and CFTR-corrected cells cannot be explained, however, by differences in the expression levels of TLR2 in CF and CFTR-corrected cells, as we did not observe any dissimilarity between IB3-1, C38, and S9 cells (Fig. 2B). Thus, it is likely that other parts of the signaling pathway downstream of or parallel to the TLR2 function differ between CF and CFTR-corrected cells. For example, recent data that were published while this study was under review suggest that TLR2 may be distributed slightly differently within plasma membrane domains in CF and normal cells (25). Furthermore, TLR2 activity may be additionally amplified in CF by asialoganglioside gangliotetraosylceramide (aGM1) (38), as aGM1 is increased in CF respiratory epithelial cells (18) due to hyperacidification of the trans-Golgi network in CF cells (29, 30). Also, note that in our experiments, transfection with DN-TLR2 did not eliminate the endogenous TLR2 response (Fig. 2C). One explanation for this observation is that the endogenous, preassembled signaling complexes responding to lipopeptides [Fig. 2C, control and LPTA(6)] may be resistant [Fig. 2C, LPTA(6) versus DN-TLR2+LPTA(6)] in respiratory epithelial cells to the superimposed expression of DN-TLR2 (defined as dominant negative in myeloid cells).
While in most challenges the TLR response may serve to limit infections, in other situations it can result in tissue destruction, as in CF and other diseases (2). The pattern recognition receptors of the host play a beneficial role in inducing innate clearance mechanisms. However, when preexisting conditions preclude smooth clearance of the invading pathogens, the same receptors may become targets for the destructive action of bacterial toxins such as LPTs, as in the case of P. aeruginosa in CF.
The microarray data and follow-up experiments presented here help to explain critical aspects of the runaway inflammatory processes in the CF lung. Other factors linked to the genetic lesion in CFTR and other modifier genes in CF patients certainly contribute to the colonization and establishment of P. aeruginosa in the lung (16). Superimposed on such preconditioned milieu, the previously unrecognized massive induction of genes encoding proinflammatory lipoproteins is likely responsible for the tissue destruction that causes high morbidity and mortality of CF patients. We envision that a simultaneous induction of a large number of lipoprotein genes may result in an additive action, although additional more complex synergisms cannot be excluded.
The knowledge of the molecular basis for the excessive inflammation in CF via bacterial lipoproteins and TLR2 may provide targets for blocking or dampening the signaling cascade, including potential applications of neutralizing or blocking antibodies, thus eliminating the factors that are ultimately responsible for respiratory failure in CF patients.
Acknowledgments
We thank P. Zaitlin for IB3-1, C38, and S9 cells, J. Zabner for NuLi-1 cells, P. Davis for 9HTEo− cells, and D. Golenbock for TLR2-blocking antibodies.
Microarray instrumentation was supported by the Keck-UNM Genomics Resource core facility at the University of New Mexico. The cost of P. aeruginosa microarray chips was defrayed in part by subsidy grant 012 from the Cystic Fibrosis Foundation Therapeutics Inc. This work was supported by Philip Morris Incorporated and by NIH grants AI31139 and AI050825.
Editor: J. N. Weiser
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hillard, J. B. Hillard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 4.Boucher, J. C., M. J. Schurr, and V. Deretic. 2000. Dual regulation of mucoidy in Pseudomonas aeruginosa and sigma factor antagonism. Mol. Microbiol. 36:341-351. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 6.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan, M., T. Flotte, S. Afione, R. Solow, P. L. Zeitlin, B. J. Carter, and W. B. Guggino. 1992. Defective regulation of outwardly rectifying Cl− channels by protein kinase A corrected by insertion of CFTR. Nature 358:581-584. [DOI] [PubMed] [Google Scholar]
- 9.Firoved, A. M., J. C. Boucher, and V. Deretic. 2002. Global genomic analysis of AlgU (σE)-dependent promoters (sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J. Bacteriol. 184:1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firoved, A. M., and V. Deretic. 2003. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J. Bacteriol. 185:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FitzSimmons, S. C. 1993. The changing epidemiology of cystic fibrosis. J. Pediatr. 122:1-9. [DOI] [PubMed] [Google Scholar]
- 12.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 13.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratti, R. A., J. Chua, and V. Deretic. 2002. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 277:17320-17326. [DOI] [PubMed] [Google Scholar]
- 15.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guggino, W. B. 1999. Cystic fibrosis and the salt controversy. Cell 96:607-610. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, P., S. Heinle, U. F. Schade, H. Loppnow, A. J. Ulmer, H. D. Flad, G. Jung, and W. G. Bessler. 1988. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology 177:158-170. [DOI] [PubMed] [Google Scholar]
- 18.Imundo, L., J. Barasch, A. Prince, and Q. Al-Awqati. 1995. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc. Natl. Acad. Sci. USA 92:3019-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowles, M. R., and P. R. Durie. 2002. What is cystic fibrosis? N. Engl. J. Med. 347:439-442. [DOI] [PubMed] [Google Scholar]
- 20.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 21.Martin, D. W., B. W. Holloway, and V. Deretic. 1993. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J. Bacteriol. 175:1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, D. W., M. J. Schurr, M. H. Mudd, J. R. Govan, B. W. Holloway, and V. Deretic. 1993. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 90:8377-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massion, P. P., H. Inoue, J. Y. Richman-Eisenstat, D. Grunberger, P. G. Jorens, B. Housset, J. P. Pittet, J. P. Weiner-Konish, and J. A. Nadel. 1994. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J. Clin. Investig. 93:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 25.Muir, A., G. Soong, S. Sokol, B. Reddy, M. I. Gomez, A. Van Heeckeren, and A. Prince. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 30:777-783. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, H., K. Yoshimura, N. G. McElvaney, and R. G. Crystal. 1992. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Investig. 89:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen, S. S. 1992. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS 100(Suppl. 28):1-79. [PubMed] [Google Scholar]
- 28.Perez, A., K. A. Risma, E. A. Eckman, and P. B. Davis. 1996. Overexpression of R domain eliminates cAMP-stimulated Cl− secretion in 9/HTEo− cells in culture. Am. J. Physiol. 271:L85-L92. [DOI] [PubMed] [Google Scholar]
- 29.Poschet, J., E. Perkett, and V. Deretic. 2002. Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mol. Med. 8:512-519. [DOI] [PubMed] [Google Scholar]
- 30.Poschet, J. F., J. C. Boucher, L. Tatterson, J. Skidmore, R. W. Van Dyke, and V. Deretic. 2001. Molecular basis for defective glycosylation and Pseudomonas pathogenesis in cystic fibrosis lung. Proc. Natl. Acad. Sci. USA 98:13972-13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 33.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salva, P. S., N. A. Doyle, L. Graham, H. Eigen, and C. M. Doerschuk. 1996. TNF-alpha, IL-8, soluble ICAM-1, and neutrophils in sputum of cystic fibrosis patients. Pediatr. Pulmonol. 21:11-19. [DOI] [PubMed] [Google Scholar]
- 35.Schurr, M. J., D. W. Martin, M. H. Mudd, and V. Deretic. 1994. Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J. Bacteriol. 176:3375-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuto, T., A. Imasato, H. Jono, A. Sakai, H. Xu, T. Watanabe, D. D. Rixter, H. Kai, A. Andalibi, F. Linthicum, Y. L. Guan, J. Han, A. C. Cato, D. J. Lim, S. Akira, and J. D. Li. 2002. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J. Biol. Chem. 277:17263-17270. [DOI] [PubMed] [Google Scholar]
- 37.Shuto, T., H. Xu, B. Wang, J. Han, H. Kai, X. X. Gu, T. Murphy, D. J. Lim, and J. D. Li. 2001. Activation of NF-kappa B by nontypeable Haemophilus influenzae is mediated by Toll-like receptor 2-TAK1-dependent NIK-IKK alpha/beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. USA 98:8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soong, G., B. Reddy, S. Sokol, R. Adamo, and A. Prince. 2004. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J. Clin. Investig. 113:1482-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standiford, T. J., S. L. Kunkel, M. A. Basha, S. W. Chensue, J. P. Lynch III, G. B. Toews, J. Westwick, and R. M. Strieter. 1990. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J. Clin. Investig. 86:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 41.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkatakrishnan, A., A. Stecenko, G. King, T. R. Blackwell, K. L. Brigham, J. W. Christman, and T. S. Blackwell. 2000. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 23:396-403. [DOI] [PubMed] [Google Scholar]
- 43.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 44.Welsh, M. J., and A. E. Smith. 1993. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251-1254. [DOI] [PubMed] [Google Scholar]
- 45.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 46.Zabner, J., P. Karp, M. Seiler, S. L. Phillips, C. J. Mitchell, M. Saavedra, M. Welsh, and A. J. Klingelhutz. 2003. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L844-L854. [DOI] [PubMed] [Google Scholar]
- 47.Zeitlin, P. L., L. Lu, J. Rhim, G. Cutting, G. Stetten, K. A. Kieffer, R. Craig, and W. B. Guggino. 1991. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell. Mol. Biol. 4:313-319. [DOI] [PubMed] [Google Scholar]