Abstract
Streptococcus pneumoniae is the major pathogen of community-acquired pneumonia and one of the most common causes of death due to infectious diseases in industrialized countries. Lung epithelium lines the airways and constitutes the first line of innate defense against respiratory pathogens. Little is known about the molecular interaction of pneumococci with lung epithelial cells. Apoptosis of lung epithelium is involved in some bacterial lung infections. In this study different pneumococcal strains specifically induced either apoptotic or necrotic death of human alveolar and bronchial epithelial cells. Pneumococcus-induced apoptosis did not depend on the virulence factors pneumolysin and H2O2. Apoptotic cells showed increased activity of caspases 6, 8, and 9 but not increased activity of caspase 3. Moreover, programmed cell death could be strongly reduced by a caspase 6 inhibitor and a pan-caspase inhibitor. Inhibitors of calpain and chymotrypsin- and trypsin-like proteases also reduced pneumococcus-induced apoptosis. Furthermore, pneumococcus-infected human alveolar epithelial cells showed Bid cleavage and reduced levels of Bcl2 and Bax. Overexpression of Bcl2 in these cells reduced apoptosis significantly. Thus, pneumococci induced apoptosis of human alveolar and bronchial epithelial cells. Programmed cell death was executed by caspase 6 and noncaspase proteases, but not by caspase 3, and could be blocked by overexpression of Bcl2.
Pneumonia is the most common cause of death due to infectious diseases in industrialized countries (18). Over 40% of all pneumonia cases are due to Streptococcus pneumoniae, the number of antibiotic-resistant strains is increasing, and mortality rates remain high (13). There is a considerable lack of knowledge about the pathophysiological mechanisms in pneumococcus-host interactions (21).
Pneumococci have an array of important virulence factors that contribute significantly to the disease process. These factors include encapsulation and hydrogen peroxide (H2O2), as well as pneumolysin (7). Recent studies demonstrated that pneumococci and pneumococcal cell wall preparations induced programmed cell death in monocytes (1) and neutrophil granulocytes (54), but no previous studies have addressed apoptosis in lung epithelium.
Apoptosis has been identified as an important pathophysiological event in lung injury in general and in pneumonia in particular (5). The genetically determined and closely regulated process of cell death was found in lung inflammation and involves leukocytes (31), as well as respiratory epithelial cells (30). The time course of apoptosis and the types of dying cells determine the outcome of a bacterial lung infection (27). A plethora of different stimuli activate the proapoptotic machinery, which consists of caspases as the central executioners of programmed cell death. The regulatory protein caspase 8 is directly activated by death receptors, while caspase 9 activation follows mitochondrial stress (for reviews see references 12 and 22). The two pathways merge by activation of the executioner molecule caspase 3 or 6. Different proapoptotic (e.g., Bax and Bid) and antiapoptotic (e.g., Bcl-2 and BclxL) mitochondrial proteins participate in the regulation of apoptosis (12). However, there is limited knowledge about the role of apoptosis in S. pneumoniae-induced pneumonia.
For some other important bacterial pathogens it has been shown that the organisms may either induce (41) or even block (36) epithelial apoptosis. For example, strong proapoptotic effects were seen in infections with Legionella pneumophila (37). Pseudomonas aeruginosa-induced programmed cell death in lung epithelium was considered to be beneficial for clearance of the bacterial infection (19). Interestingly, this process seems to be defective in cystic fibrosis patients (10). On the other hand, the intracellular pathogen Chlamydia pneumoniae inhibits epithelial cell death induced by CD95 ligand to prolong the exploitation of host cells (15).
In the study described here we demonstrated that S. pneumoniae induced apoptosis in human alveolar cells, as well as bronchial epithelial cells. Programmed cell death was executed by caspases 6, 8, and 9 and by different noncaspase proteases, but not by caspase 3. Moreover, expression of Bcl2-like proteins was decreased in infected cells, and overexpression of Bcl2 reduced pneumococcus-induced apoptosis.
(Part of this work will be included in the doctoral thesis of Ralph Gross.)
MATERIALS AND METHODS
Materials.
Dulbecco modified Eagle medium, fetal calf serum, a trypsin-EDTA solution, CA-650, and antibiotics were obtained from Life Technologies (Karlsruhe, Germany). Protease inhibitors, Triton X-100, 4-dichloroisocumarin, and Tween 20 were purchased from Sigma Chemical Co. (Munich, Germany). The pan-caspase inhibitor zVAD, the caspase 6 inhibitor zVEID, carbobenzoxy-valyl-phenylalanial (calpain inhibitor III), Nα-tosyl-Lys chloromethyl ketone (TLCK), and Nα-tosyl-Phe chloromethyl ketone (TPCK) were purchased from Calbiochem (Merck, Darmstadt, Germany), and tumor necrosis factor α (TNF-α) was purchased from R&D Systems (Wiesbaden, Germany). All other chemicals used were of analytical grade and were obtained from commercial sources.
Cell lines.
Bronchial epithelial cell line BEAS-2B was a kindly gift from Curtis Harris, National Institutes of Health, Bethesda, Md. (16, 43). Alveolar epithelial cell line A549 was purchased from the American Type Culture Collection, Rockville, Md. A549 cells stably transfected with pBcl-2/zeo (A549-Bcl2) were a kind gift from Tzipora Goldkorn, Center for Comparative Respiratory Biology and Medicine, Department of Internal Medicine, University of California, Davis (42). A549-Bcl2 cells were grown in medium containing 100 μl of zeocin per ml.
Pneumolysin.
Recombinant pneumolysin was expressed in Escherichia coli and was purified from cell extracts as described elsewhere (47). Protein homogeneity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The stock protein preparation was essentially free (<2 pg/ml) of contaminating bacterial endotoxin. The stock protein concentration was 0.21 mg/ml, which corresponded to 1.3 × 106 hemolytic units/ml (11). The number of hemolytic units was determined by a hemolysis test with sheep erythrocytes. Briefly, erythrocytes were exposed to dilution series of purified pneumolysin, and hemolysis was determined; 1 hemolytic unit resulted in 50% hemolysis.
Bacterial strains.
S. pneumoniae R6x is an unencapsulated derivative of serotype 2 strain D39 (52). A pneumolysin-negative mutant of R6x (R6xply−) was generated by insertion-duplication mutagenesis by using the pJDC9::ply construct (55). After transformation of the plasmid into S. pneumoniae strain R6x, erythromycin-resistant transformants were selected with Luria-Bertani agar supplemented with erythromycin and 5% sheep blood. Insertion of the plasmid into the pneumolysin-encoding gene ply was confirmed by PCR analysis and DNA sequencing. Loss of function was analyzed by the hemolysis assay as described previously (6). Single-colony isolates of R6x and the corresponding mutant R6xply− were maintained at 37°C with 5% CO2 on Columbia agar supplemented with 5% sheep blood at 37°C. For cell culture stimulation studies, single colonies were expanded by resuspension in Todd-Hewitt broth supplemented with 0.5% yeast extract and incubation at 37°C for 3 to 4 h to the mid-log phase (A600, 0.2 to 0.4), harvested by centrifugation, and resuspended in suitable cell culture medium that buffered pneumococcal lactic acid production.
Immunofluorescence.
A total of 1 × 105 cells grown on glass coverslips were stimulated as indicated above, washed twice, incubated in a humidified atmosphere, and stimulated again as indicated above. Briefly, cells were fixed in freshly prepared paraformaldehyde (3% paraformaldehyde in phosphate-buffered saline, pH 7.6), permeabilized, and washed, and DNA stand breaks were labeled by terminal deoxynucleotidyltransferase-mediated fluorescein-dUTP nick end labeling (TUNEL) and analyzed by using a Zeiss Pascal 5 confocal microscope. F-actin was visualized by marking with rhodamine-labeled phalloidin (1.4 μg/ml), as described previously (23).
Cell death detection ELISA.
A commercially available photometric enzyme-linked immunosorbent assay (ELISA) was used for detection of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) in apoptotic epithelial cells (Roche, Mannheim, Germany). A total of 5 × 104 cells cultured in 96-well plates were washed twice, incubated in a humidified atmosphere, and stimulated as indicated above. Microtiter plates were then centrifuged, the medium was removed, the cells were lysed, and the plates were centrifuged again. Twenty microliters of supernatant was transferred into a streptavidin-precoated microtiter plate and incubated with an immunoreagent (anti-histone-biotin, anti-DNA peroxidase) for 2 h at room temperature. After washing, a substrate solution was added, and absorbance at 405 nm was determined (23).
Caspase activity.
A commercially available caspase activity assay (ApoTraget; Biosource, Camarillo, Calif.) based on colorimetric detection of the cleavage of para-nitroaniline-labeled substrates specific for caspases 3 (DEVD), 6 (VEID), 8 (IETD), and 9 (LEHD) was used for analysis of caspase activity according to the manufacturer's instructions. Briefly, A549 cells were grown in 10-cm dishes (5 × 106 cells per dish), stimulated, collected, and lysed on ice. Cleared samples were aligned to determine protein contents, divided into four aliquots, and incubated at 37°C for 2 h in the presence of labeled caspase-specific colorimetric substrate conjugates for caspases 3, 6, 8, and 9. Detection was performed by measuring absorbance at 405 nm.
Western blotting.
A549 cell monolayers grown in 10-cm dishes (5 × 106 cells per dish) were stimulated as indicated above. Cells were then collected after trypsinization, washed, and lysed for 10 min on ice in 20 mM HEPES buffer (pH 7.4) containing 1% Triton X-100, 44 μg of phenylmethylsulfonyl fluoride per ml, 2 μg of leupeptin per ml, and 2 μg of pepstatin per ml. Samples were resuspended in gel-loading buffer as described by Laemmli (28a) and boiled for 5 min. Sixty micrograms of protein per lane was separated on a sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gel and blotted onto a Hybond-ECL membrane (Amersham, Dreiech, Germany). The membranes were blocked, washed, and hybridized with polyclonal antibodies raised against Bid and cleaved caspase 6 (Cell Signaling, Beverly, Mass.), cleaved caspase 3, full-length Bax, Bcl2 (Upstate Biotechnology, Lake Placid, N.Y.), ERK2, cleaved caspase 9 (Santa Cruz Biotechnology, Heidelberg, Germany), or cleaved caspase 8 (BD Biosciences Pharmingen, San Diego, Calif.). Detection was performed by visualization of IRDye 800- or Cy5.5-labeled secondary antibodies (Odyssey infrared imaging system; LI-COR Inc., Lincoln, Nebr.) (24).
Release of LDH.
A549 cell monolayers were exposed to stimuli as indicated above. Lactate dehydrogenase (LDH) activity in the supernatants was determined by colorimetric measurement of the reduction of sodium pyruvate in the presence of NADH (LDH assay; Roche), as described previously (23). Maximum lysis was induced by adding 100 μl of medium containing 0.1% Triton X-100. The level of specific lysis was calculated by using the following formula: percentage of specific LDH release = ([experimental release − minimum release]/[maximal release − minimum release]) × 100 (48).
Statistical methods.
Data are expressed below as means ± standard errors of the means for at least three independent experiments. A one-way analysis of variance was used for the data shown in Fig. 1A (single time points), 2, 3B (single time points), 4A, 4C, 5, 6B, and 7 (single time points). Main effects were then compared by a Newman-Keuls posttest. A P value of <0.05 was considered significant (unless indicated otherwise, the test was performed by comparison with the results for unstimulated control cells for a time point).
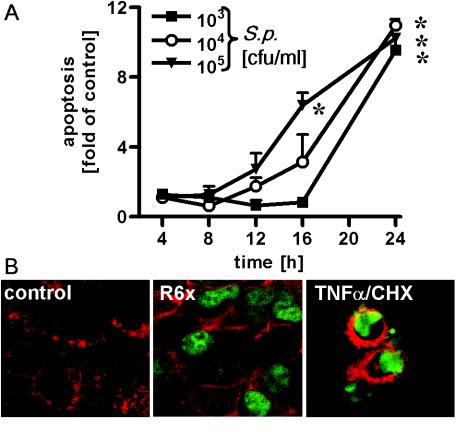
FIG. 1.
Time- and dose-dependent apoptosis of pneumococcus-infected alveolar epithelial cells. (A) A549 cells were infected for different times with S. pneumoniae (S.p.) (103 to 105 CFU/ml), and DNA fragmentation was measured. An asterisk indicates that the P value was <0.05 for a comparison with the unstimulated control at a single time. (B) A549 cells were incubated with S. pneumoniae (105 CFU/ml) or with TNF-α (10 ng/ml) plus cycloheximide (CHX) (5 μg/ml) for 16 h. DNA fragmentation was detected by the TUNEL assay and was visualized by confocal laser microscopy. Representative results of three different experiments in which similar results were obtained are shown.
RESULTS
S. pneumoniae induces apoptosis in human alveolar epithelial cells.
To study the effect of S. pneumoniae on lung epithelium, human alveolar epithelial A549 cells were infected by unencapsulated S. pneumoniae type 2 strain R6x. DNA fragmentation and microscopic morphology were assessed as measures of apoptotic cell death, while nonspecific necrosis was estimated by the release of LDH into the supernatant. After 16 h of pneumococcal infection, massive DNA fragmentation was observed in alveolar epithelial cells, as shown by nucleosome ELISA (Fig. 1A) and TUNEL staining (confocal laser scanning microscopy) (Fig. 1B). The pneumococcal apoptosis signal was about one-half of the maximal signal obtained by simultaneous exposure of cells to TNF-α and cycloheximide. Cycloheximide blocked TNF-α-related antiapoptotic gene expression, resulting in massive programmed cell death (Fig. 2A). We analyzed the specific numbers of cells by the nucleosome ELISA and the LDH assay and found that the data obtained correlated directly and linearly with the number of apoptotic or necrotic cells (data not shown). In unstimulated control cell preparations, about 2% of the cells showed DNA fragmentation, whereas after 16 h of infection with R6x pneumococci ca. 42% of the cells were apoptotic and there was TNF-α-cycloheximide-induced programmed cell death in about 100% of the cells. In the time frame tested, the LDH activity in cell culture supernatants remained unchanged compared to that in unstimulated cells (data not shown).
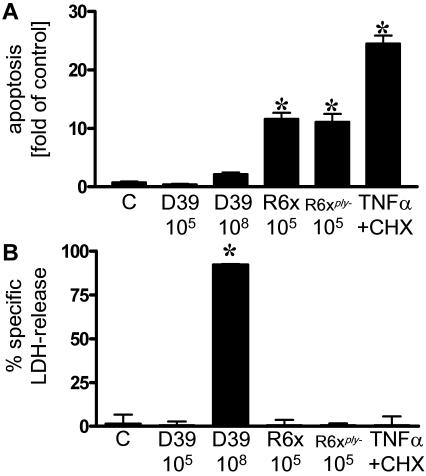
FIG. 2.
Induction of apoptosis or necrosis by different mutants of S. pneumoniae. A549 cells were incubated with S. pneumoniae strain D39 (105 and 108 CFU/ml), R6x (105 CFU/ml), R6xply− (105 CFU/ml), or TNF-α (10 ng/ml) plus cycloheximide (CHX) (5 μg/ml) for 16 h. DNA fragmentation (A) (expressed the fold increase compared with unstimulated control cells [C]) and LDH release (B) (expressed as the percentage of specific LDH release) were measured. An asterisk indicates that the P value was <0.05 for a comparison with the unstimulated control.
Epithelial cell apoptosis is not induced by pneumolysin or H2O2.
By infecting alveolar epithelial cells with encapsulated S. pneumoniae strain D39, its unencapsulated derivate R6x, and the pneumolysin-deficient R6xply− mutant, we analyzed the impact of important pneumococcal factors in more detail. R6x and the pneumolysin-deficient mutant R6xply− (both at a concentration of 105 CFU/ml) induced DNA fragmentation to the same extent after 16 h, and there was no increase in the LDH released in the time frame tested (Fig. 2). In contrast, D39 did not activate epithelial apoptosis at even higher doses (108 CFU/ml), while high levels of LDH release, a sign of necrotic cell death, were observed.
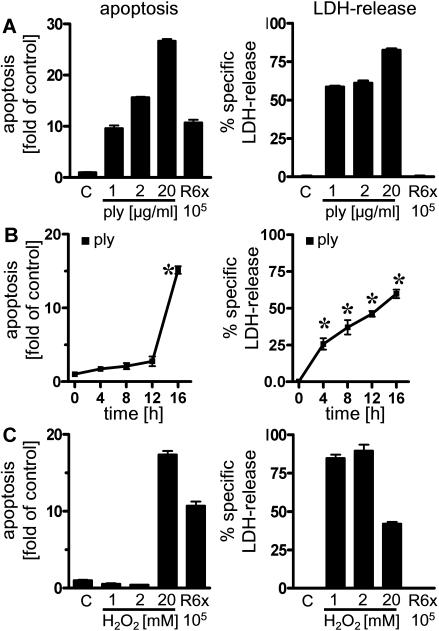
To further analyze the role of pneumolysin, we exposed A549 cells to recombinant-expressed pneumolysin (47). As shown in Fig. 3A and B, in alveolar epithelial cells exposed to pneumolysin DNA fragmentation occurred about 8 h after the onset of LDH release, an observation which suggested that there was rather nonspecific nucleosome formation secondary to pneumolysin-induced necrosis. These cytotoxic effects were observed only when very high concentrations of pneumolysin (1 to 20 μg/ml) were used. Another important virulence factor of pneumococci which has been associated with apoptosis in neuronal cells is H2O2 (7). However, A549 cells exposed to H2O2 exhibited strong LDH release and a necrotic phenotype (data not shown) rather than programmed cell death (Fig. 3C). R6x-induced DNA fragmentation in A549 cells was not reduced by preincubation with the antioxidant N-acetyl-cysteine (10 mM) (data not shown).
FIG. 3.
Epithelial necrosis induced by pneumolysin and H2O2. A549 cells were incubated with different concentrations of pneumolysin (ply) for 16 h (A), for different times with 2 μg of pneumolysin per ml (B), or for 16 h with different concentrations of H2O2 (C). DNA fragmentation and specific LDH release were assessed. An asterisk indicates that the P value was <0.05 for a comparison with the unstimulated control at a single time.
S. pneumoniae-induced epithelial apoptosis depends on caspase 6 and on different noncaspase proteases.
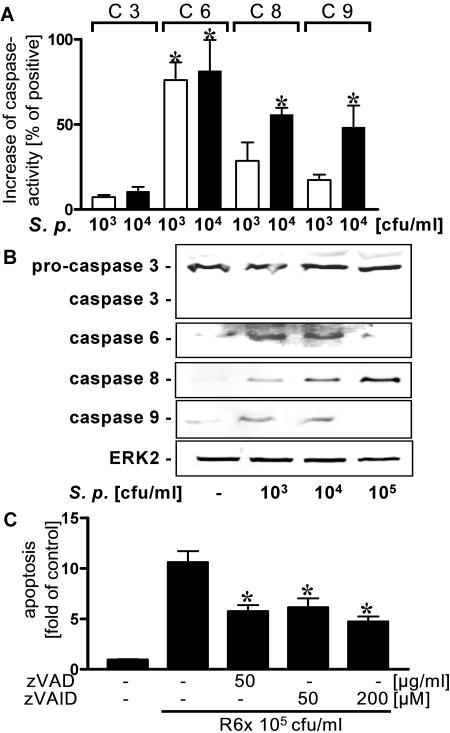
Programmed cell death often is executed by complex hierarchic caspase cascades comprising regulatory caspases (caspases 8 and 9) and executing caspases (caspases 3 and 6). Therefore, we measured the specific activities of caspases 3, 6, 8, and 9 in pneumococcus-infected alveolar epithelial cells by a colorimetric assay. S. pneumoniae activated caspases 6, 8, and 9 in a dose-dependent manner (Fig. 4A). Thus, a Western blot of pneumococcus-exposed epithelial cells showed cleavage products of procaspases 6, 8, and 9 but not caspase 3 (Fig. 4B). In contrast, in TNF-α-cycloheximide-stimulated A549 cells strong cleavage of caspase 3 was noted (data not shown). However, in cells infected with 105 CFU of pneumococci per ml no cleavage products of caspases 6 and 9 were detected, possibly due to further degradation of caspases (50).
FIG. 4.
Activation of caspases 6, 8, and 9 by S. pneumoniae. (A) A549 cells were incubated with strain R6x (103 and 104 CFU/ml), and specific caspase activity was assessed. An asterisk indicates that the P value was <0.05 for a comparison with the control. C 3, caspase 3; C 6, caspase 6; C 8, caspase 8; C 9, caspase 9; S. p., S. pneumoniae. (B) A549 cells were incubated with R6x (103 to 105 CFU/ml) for 4 h, and active forms of caspases were detected by Western blotting. Blots representative of three different experiments in which similar results were obtained are shown. (C) A549 cells were preincubated with the pan-caspase inhibitor (zVAD) or the inhibitor of caspase 6 (zVEID) for 30 min and then stimulated with R6x (105 CFU/ml) for 16 h. DNA fragmentation was analyzed. An asterisk indicates that the P value was <0.05 for a comparison with the unstimulated control.
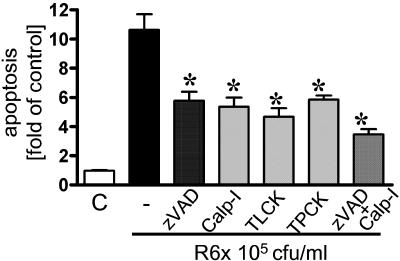
Using a pan-caspase inhibitor (zVAD) and a specific caspase 6 inhibitor (zVEID), we verified the impact of caspase activation for pneumococcus-related apoptosis. Both inhibitors reduced DNA fragmentation significantly, yet some apoptosis remained despite an increase in the dose (Fig. 4C). In recent studies, pneumococcus-induced cell death appeared to depend at least in part on noncaspase proteases (8). We found that inhibition of calpain and trypsin- and chymotrypsin-like serine proteases by carbobenzoxy-valyl-phenylalanial (calpain inhibitor III), TLCK, and TPCK, respectively, reduced apoptosis to a great extent. When an inhibitor of caspases (zVAD) and calpain were added together, the reduction in apoptosis was greater, yet it was not complete (Fig. 5). Thus, pneumococcus-induced epithelial apoptosis was dependent on caspase and noncaspase proteases.
FIG. 5.
Impact of inhibition by different proteases on S. pneumoniae-induced apoptosis. A549 cells were preincubated with the pan-caspase inhibitor (zVAD) (50 μg/ml) and/or inhibitors of calpain (carbobenzoxy-valyl-phenylalanial [Calp-I]) (50 μM), trypsin-like proteases (TLCK) (50 μM), or chymotrypsin-like proteases (TPCK) (10 μM) for 30 min and then stimulated with strain R6x (105 CFU/ml) for 16 h. DNA fragmentation was analyzed. An asterisk indicates that the P value was <0.05 for a comparison with pneumococcus-infected cells without inhibitor pretreatment (control [C]).
Bcl2 blocks S. pneumoniae-induced apoptosis.
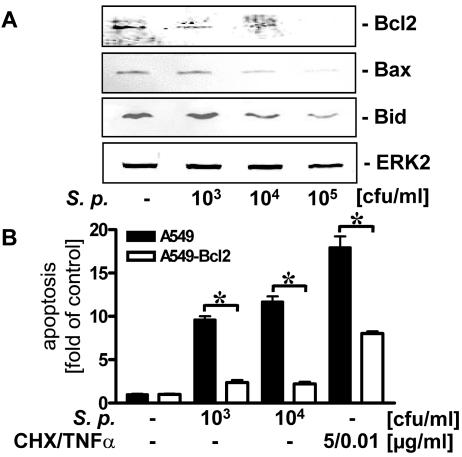
Recent studies suggested that antiapoptotic Bcl2 proteins play an important role in regulation of bacterium-induced cell death and apoptosis (33). Proapoptotic Bax has been found to be cleaved by calpain and to integrate in mitochondrial membranes to initiate programmed cell death (3), whereas proapoptotic Bid could be cleaved by caspase 8 and inhibit Bcl2 function (12). To test pneumococcal influence on the equilibrium of these proteins, we analyzed the levels of Bcl2, Bax, and Bid in S. pneumoniae-infected alveolar epithelial cells by Western blotting. As shown in Fig. 6A, increasing the concentration of R6x decreased the levels of Bcl2, Bax, and Bid.
FIG. 6.
Bcl2-dependent inhibition of S. pneumoniae-induced epithelial apoptosis. (A) A549 cells were incubated with strain R6x (103 to 105 CFU/ml) for 4 h, and Bcl2, Bax, Bid, and ERK2 (loading control) were detected by Western blotting. Blots representative of three different experiments in which similar results were obtained are shown. (B) A549 wild-type cells or A549 cells stably expressing Bcl2 (A549-Bcl2) were infected with R6x (103 and 104 CFU/ml) or treated with TNF-α (10 ng/ml) plus cycloheximide (CHX) (5 μg/ml) for 16 h, and DNA fragmentation was analyzed. An asterisk indicates that the P value was <0.05 for a comparison of A549 and A549-Bcl2. S. p., S. pneumoniae.
Due to the important role of Bcl2 in apoptosis regulation, we used A549 cells overexpressing Bcl2 (A549-Bcl2). Comparing DNA fragmentation in R6x-exposed A549-Bcl2 and wild-type cells, we found that there was a strong reduction in pneumococcus-related apoptosis in A549-Bcl2 cells. In contrast, protection against cycloheximide- and TNF-α-induced cell death was incomplete in these cells (Fig. 6B).
S. pneumoniae induces apoptosis in human bronchial epithelial cells.
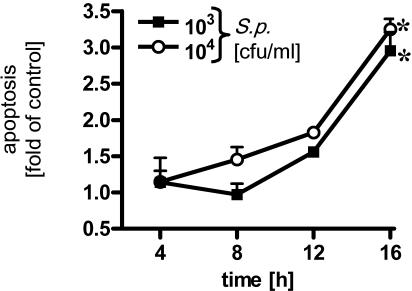
We extended our observations to tracheobronchial epithelial cells by using the human bronchial epithelial cell line BEAS-2B. Bronchial cells were infected with R6x for different times, and apoptosis and LDH release were assessed. BEAS-2B cells displayed pneumococcus-induced DNA fragmentation kinetics which were comparable to those of alveolar cells (Fig. 7). In addition, no increase in LDH activity in the supernatant was detected during the time studied (data not shown).
FIG. 7.
Time- and dose-dependent apoptosis of pneumococcus-infected human bronchial epithelial cells. BEAS-2B cells were infected for different times with S. pneumoniae (S.p.) (103 and 104 CFU/ml), and DNA fragmentation was measured. An asterisk indicates that the P value was <0.05 for a comparison with the unstimulated control at a single time.
DISCUSSION
In the study described here S. pneumoniae induced apoptotic as well as necrotic cell death in human alveolar and bronchial epithelium. Unencapsulated pneumococcus strain R6x induced programmed cell death in a time- and dose-dependent manner, whereas encapsulated D39 and high concentrations of exogenously added recombinant pneumolysin or H2O2 caused necrosis in alveolar epithelium. Pneumococcal infection activated regulatory caspases 8 and 9 and executive caspase 6, while caspase 3 activity was not significantly increased. Also, pan-caspase inhibitor or specific caspase 6 inhibition strongly reduced pneumococcus-induced programmed cell death. In addition, we found that apoptosis could also be reduced by blocking calpain and trypsin- and chymotrypsin-like proteases. Moreover, cleavage of Bid and Bax, as well as decreasing the level of Bcl2, contributed to an apoptotic phenotype. Overexpression of antiapoptotic Bcl2 significantly reduced pneumococcus-related apoptosis of human alveolar epithelial cells.
The tracheobronchial and alveolar epithelium functions as an important barrier against invading pathogens. It represents the first line of innate immune response, and therefore studies of direct interactions of lung epithelium with pneumococci, the major pathogen of community-acquired pneumonia (13), are warranted. Pneumococcus-induced host defense in the lung may cause severe inflammation, resulting in respiratory distress (4). Recently, programmed cell death has been implicated as an important phenomenon in lung inflammation (14). In particular, massive epithelial apoptosis may pave the way for further pneumococcal invasion and systemic host infection. Hakonsson et al. described a loss of viability and an increase in oligonuclosomes in pneumococcus-exposed lung epithelial cells (20), implicating apoptotic or necrotic cell death. Therefore, we studied apoptosis and necrosis of lung epithelial cells exposed to S. pneumoniae in more detail.
We found different patterns of apoptotic and necrotic cell death in S. pneumoniae-exposed alveolar and bronchial epithelial cells. Unencapsulated strain R6x induced programmed epithelial cell death, whereas the encapsulated D39 strain caused massive LDH release in the absence of DNA fragmentation, indicating the predominance of necrosis. Similarly, in macrophages unencapsulated pneumococci are more potent inducers of apoptosis than encapsulated pneumococci (1), possibly because encapsulation masks or reduces liberation of apoptosis-inducing factors. In a different model, Braun et al. found severe neuronal apoptosis in the hippocampus region induced by unencapsulated as well as encapsulated pneumococci (7, 8).
Other important respiratory pathogens, like L. pneumophila and P. aeruginosa, have been identified as inducers of lung epithelial apoptosis (41, 51). However, some other bacteria, like Chlamydia pneumoniae, had the opposite effect (40).
Pneumococci express an array of different virulence factors (26). Pneumococcus-induced epithelial apoptosis appeared to be independent of at least pneumolysin and H2O2 because necrosis was observed several hours before DNA fragmentation could be detected, indicating that there was secondary DNA damage. Rather high concentrations of pneumolysin (1 to 20 μg/ml) had to be used to see cytotoxic effects. For example, pneumococci (105 CFU/ml) produced about 20 ng of pneumolysin per ml in 12 h (49). This stresses the importance of synergism in the action of bacterial virulence factors. In accordance with these observations, Hirst et al. found membrane damage and loss of viability in pneumolysin-exposed A549 cells, as well as in human monocytes; apoptosis, however, was not addressed in this study (25).
In experimental meningitis both pneumolysin and H2O2 were found to cause neuronal and microglial apoptosis (7). In other studies, however, pneumococcus-induced hippocampal apoptosis was not blocked by free radical scavengers (29). These findings highlight the different contributions that these factors may make to the pathogenesis of pneumonia and meningitis. Therefore, cellular effects of pneumococcus infection have to be seen in an organ- and cell type-specific context. Apoptosis-inducing factors other than pneumolysin and H2O2 must also be considered in S. pneumoniae infection. In addition, we used a tumor-derived alveolar cell line (A549 cells) and simian virus 40-transformed bronchial epithelial cells in this study, which may have eo ispo modified cell death mechanisms. Further studies, including studies with freshly isolated lung epithelial cells, as well as analysis of apoptosis in infected lungs, are recommended to confirm the results obtained.
Caspases 6, 8, and 9 were activated in S. pneumoniae-exposed respiratory epithelial cells. In addition, cleavage of Bid, which is known to be processed by caspase 8 (12), was observed in pneumococcus-exposed epithelial cells. Thus, von Mehring et al. found increased expression of caspases, including caspase 6, in a mouse model of pneumococcal meningitis and sepsis (53). In another study, hippocampal apoptosis in experimental pneumococcal meningitis could be blocked by caspase inhibition (9). In contrast, pneumococcus-infected apoptotic neuronal cells did not show caspase activity, and cell death could not be blocked by caspase inhibitors (8).
An interesting finding in our study was the activation of executing caspase 6 but not caspase 3. Caspase 6 has been found to play an essential role in apoptosis in lamin A cleavage in apoptotic condensation (46) and apoptosis of primary neuronal cells (45). Caspase 6 was also activated in epithelial cells by P. aeruginosa (10) and Helicobacter pylori (39), but the lack of caspase 3 activity seems to be unique in bacterial infection. There might be cross talk between executing caspases, because in some experimental systems caspase 6 activated caspase 3 (2). However, in two studies the workers described activation of caspase 6 without caspase 3 activity, which is similar to the findings obtained for pneumococcus-exposed epithelial cells in this study (32, 38). As Kottke et al. have shown, caspase activity assays may sometimes underestimate some caspase activities, possibly due to enzyme sequestration (28). However, in agreement with the data obtained with the caspase activity assay, we did not find cleavage of procaspase 3 in a Western blot analysis of pneumococcus-exposed epithelial cells, although TNF-α-cycloheximide-induced caspase 3 activation and procaspase cleavage were found. Therefore, we concluded that there was pneumococcus-induced caspase 3-independent programmed cell death in A549 cells.
To evaluate the physiological impact of caspase activation in pneumococcus-induced epithelial apoptosis, we used specific enzyme inhibitors. Preexposure of human alveolar epithelial cells to the pancaspase inhibitor zVAD or the caspase 6 inhibitor zVEID strongly reduced pneumococcus-induced apoptosis but could not completely rescue alveolar epithelial cells. In this context, recent reports suggesting the existence of noncaspase proteases in apoptotic pathways are of interest (35, 44). Therefore, we tested inhibitors of different possibly proapoptotic, non-caspase-like proteases. Epithelial apoptosis could be reduced by inhibition of calpain, tryspin-like, and chymotrypsin-like proteases. Accordingly, the observed decrease in Bax in S. pneumoniae-stimulated epithelial cells might be executed by calpain activation, as Bax cleavage was found to be the central event of calpain-induced mitochondrion-dependent apoptosis (3, 17). Caspase-dependent epithelial apoptosis and calpain-dependent epithelial apoptosis were also identified in Neisseria gonorrhoeae infections (34). Overall, a complex apoptosis regulatory system to which bacteria, as well as host-cell-specific factors, contribute has to be considered.
Several reports have provided evidence of mitochondrial involvement in bacterium-induced apoptosis (8). Therefore, we analyzed the levels of expression of apoptosis-modifying mitochondrial proteins and found that the levels of Bcl2 and Bax were reduced in pneumococcus-infected alveolar epithelial cells. Both of these proteins direct cells to apoptosis, because Bcl2 is well characterized as an antiapoptotic factor (12) and cleavage of Bax was recently identified as a central event in calpain-induced apoptosis (3, 17). To verify the physiological relevance of these findings, we exposed Bcl2-overexpressing alveolar epithelial cells to S. pneumoniae. In these cells, programmed cell death was greatly reduced, suggesting that Bcl2 proteins play an important role in pneumococcus-dependent epithelial cell apoptosis. The incomplete blocking of apoptosis in TNF-α-cycloheximide-stimulated cells may have been due to inhibition of protein translation of transfected Bcl2 by cycloheximide. Interestingly, mycobacteria persisting in human macrophages upregulate Bcl2 to prolong survival of their host cells (33).
In summery, unencapsulated S. pneumoniae induced apoptosis of human alveolar and bronchial epithelial cells, whereas exposure to the pneumococcal virulence factors pneumolysin and H2O2 led to necrotic cell death. Pneumococcus-induced epithelial cell apoptosis was dependent on caspases 6, 8, and 9, as well as noncaspase proteases, and was accompanied by reduced levels of Bcl2-like proteins. Overexpression of Bcl2 desensitized human alveolar epithelia against S. pneumoniae-induced apoptosis. Therefore, these studies indicate that pneumococcus-related apoptosis of pulmonary epithelium has an important role in pneumococcal pneumonia.
Acknowledgments
The excellent technical assistance of Kerstin Möhr and Stefanie Moderer is greatly appreciated.
This work was supported in part by grants from the Bundesministerium für Bildung und Forschung to B. Schmeck (grant BMBF-CAPNETZ C15), S. Hippenstiel (grants BMBF-NBL-III 3.3.1.A and BMBF-CAPNETZ C15), S. Hammerschmidt (grant BMBF-CAPNETZ C8), and N. Suttorp and S. Rosseau (grant BMBF-CAPNETZ C4).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ali, F., M. E. Lee, F. Iannelli, G. Pozzi, T. J. Mitchell, R. C. Read, and D. H. Dockrell. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119-1131. [DOI] [PubMed] [Google Scholar]
- 2.Allsopp, T. E., J. McLuckie, L. E. Kerr, M. Macleod, J. Sharkey, and J. S. Kelly. 2000. Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ. 7:984-993. [DOI] [PubMed] [Google Scholar]
- 3.Altznauer, F., S. Conus, A. Cavalli, G. Folkers, and H. U. Simon. 2004. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J. Biol. Chem. 279:5947-5957. [DOI] [PubMed] [Google Scholar]
- 4.Bardales, R. H., S. S. Xie, R. F. Schaefer, and S. M. Hsu. 1996. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am. J. Pathol. 149:845-852. [PMC free article] [PubMed] [Google Scholar]
- 5.Behnia, M., K. A. Robertson, and W. J. Martin. 2000. Lung infections: role of apoptosis in host defense and pathogenesis of disease. Chest 117:1771-1777. [DOI] [PubMed] [Google Scholar]
- 6.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 7.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, J. S., R. Novak, P. J. Murray, C. M. Eischen, S. A. Susin, G. Kroemer, A. Halle, J. R. Weber, E. I. Tuomanen, and J. L. Cleveland. 2001. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J. Infect. Dis. 184:1300-1309. [DOI] [PubMed] [Google Scholar]
- 9.Braun, J. S., R. Novak, K. H. Herzog, S. M. Bodner, J. L. Cleveland, and E. I. Tuomanen. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5:298-302. [DOI] [PubMed] [Google Scholar]
- 10.Cannon, C. L., M. P. Kowalski, K. S. Stopak, and G. B. Pier. 2003. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 29:188-197. [DOI] [PubMed] [Google Scholar]
- 11.Cockeran, R., C. Durandt, C. Feldman, T. J. Mitchell, and R. Anderson. 2002. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 186:562-565. [DOI] [PubMed] [Google Scholar]
- 12.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 13.Finch, R. 2001. Community-acquired pneumonia: the evolving challenge. Clin. Microbiol. Infect. 7(Suppl. 3):30-38. [PubMed] [Google Scholar]
- 14.Fine, A., Y. Janssen-Heininger, R. P. Soultanakis, S. G. Swisher, and B. D. Uhal. 2000. Apoptosis in lung pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L423-L427. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, S. F., C. Schwarz, J. Vier, and G. Hacker. 2001. Characterization of antiapoptotic activities of Chlamydia pneumoniae in human cells. Infect. Immun. 69:7121-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuhrmann, M., H. U. Jahn, J. Seybold, C. Neurohr, P. J. Barnes, S. Hippenstiel, H. J. Kraemer, and N. Suttorp. 1999. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 20:292-302. [DOI] [PubMed] [Google Scholar]
- 17.Gao, G., and Q. P. Dou. 2000. N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J. Cell Biochem. 80:53-72. [DOI] [PubMed] [Google Scholar]
- 18.Garibaldi, R. A. 1985. Epidemiology of community-acquired respiratory tract infections in adults. Incidence, etiology, and impact. Am. J. Med. 78:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassme, H., S. Kirschnek, J. Riethmueller, A. Riehle, G. von Kurthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 20.Hakansson, A., I. Carlstedt, J. Davies, A. K. Mossberg, H. Sabharwal, and C. Svanborg. 1996. Aspects on the interaction of Streptococcus pneumoniae and Haemophilus influenzae with human respiratory tract mucosa. Am. J. Respir. Crit. Care Med. 154:S187-S191. [DOI] [PubMed] [Google Scholar]
- 21.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 22.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 23.Hippenstiel, S., B. Schmeck, P. D. N′Guessan, J. Seybold, M. Krull, K. Preissner, C. V. Eichel-Streiber, and N. Suttorp. 2002. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L830-L838. [DOI] [PubMed] [Google Scholar]
- 24.Hippenstiel, S., M. Witzenrath, B. Schmeck, A. Hocke, M. Krisp, M. Krull, J. Seybold, W. Seeger, W. Rascher, H. Schutte, and N. Suttorp. 2002. Adrenomedullin reduces endothelial hyperpermeability. Circ. Res. 91:618-625. [DOI] [PubMed] [Google Scholar]
- 25.Hirst, R. A., H. Yesilkaya, E. Clitheroe, A. Rutman, N. Dufty, T. J. Mitchell, C. O'Callaghan, and P. W. Andrew. 2002. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infect. Immun. 70:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazzaz, J. A., S. Horowitz, J. Xu, P. Khullar, M. S. Niederman, A. M. Fein, Z. Zakeri, L. Lin, and G. C. Rhodes. 2000. Differential patterns of apoptosis in resolving and nonresolving bacterial pneumonia. Am. J. Respir. Crit. Care Med. 161:2043-2050. [DOI] [PubMed] [Google Scholar]
- 28.Kottke, T. J., A. L. Blajeski, X. W. Meng, P. A. Svingen, S. Ruchaud, P. W. Mesner, Jr., S. A. Boerner, K. Samejima, N. V. Henriquez, T. J. Chilcote, J. Lord, M. Salmon, W. C. Earnshaw, and S. H. Kaufmann. 2002. Lack of correlation between caspase activation and caspase activity assays in paclitaxel-treated MCF-7 breast cancer cells. J. Biol. Chem. 277:804-815. [DOI] [PubMed] [Google Scholar]
- 28a.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophgae T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Loeffler, J. M., R. Ringer, M. Hablutzel, M. G. Tauber, and S. L. Leib. 2001. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J. Infect. Dis. 183:247-252. [DOI] [PubMed] [Google Scholar]
- 30.Matute-Bello, G., R. K. Winn, M. Jonas, E. Y. Chi, T. R. Martin, and W. C. Liles. 2001. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am. J. Pathol. 158:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medan, D., L. Wang, X. Yang, S. Dokka, V. Castranova, and Y. Rojanasakul. 2002. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J. Cell Physiol. 191:320-326. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita, T., K. Nagao, S. Krajewski, G. S. Salvesen, J. C. Reed, T. Inoue, and M. Yamada. 1998. Investigation of glucocorticoid-induced apoptotic pathway: processing of caspase-6 but not caspase-3. Cell Death Differ. 5:1034-1041. [DOI] [PubMed] [Google Scholar]
- 33.Mogga, S. J., T. Mustafa, L. Sviland, and R. Nilsen. 2002. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand. J. Immunol. 56:383-391. [DOI] [PubMed] [Google Scholar]
- 34.Muller, A., D. Gunther, F. Dux, M. Naumann, T. F. Meyer, and T. Rudel. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO. J. 18:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama, N., S. T. Eichhorst, M. Muller, and P. H. Krammer. 2001. Ethanol-induced apoptosis in hepatoma cells proceeds via intracellular Ca2+ elevation, activation of TLCK-sensitive proteases, and cytochrome c release. Exp. Cell Res. 269:202-213. [DOI] [PubMed] [Google Scholar]
- 36.Nakhjiri, S. F., Y. Park, O. Yilmaz, W. O. Chung, K. Watanabe, A. El Sabaeny, K. Park, and R. J. Lamont. 2001. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS. Microbiol. Lett. 200:145-149. [DOI] [PubMed] [Google Scholar]
- 37.Neumeister, B., M. Faigle, K. Lauber, H. Northoff, and S. Wesselborg. 2002. Legionella pneumophila induces apoptosis via the mitochondrial death pathway. Microbiology 148:3639-3650. [DOI] [PubMed] [Google Scholar]
- 38.Olson, N. E., J. D. Graves, G. L. Shu, E. J. Ryan, and E. A. Clark. 2003. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J. Immunol. 170:6065-6072. [DOI] [PubMed] [Google Scholar]
- 39.Potthoff, A., S. Ledig, J. Martin, O. Jandl, M. Cornberg, B. Obst, W. Beil, M. P. Manns, and S. Wagner. 2002. Significance of the caspase family in Helicobacter pylori induced gastric epithelial apoptosis. Helicobacter 7:367-377. [DOI] [PubMed] [Google Scholar]
- 40.Rajalingam, K., H. Al Younes, A. Muller, T. F. Meyer, A. J. Szczepek, and T. Rudel. 2001. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 69:7880-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajan, S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23:304-312. [DOI] [PubMed] [Google Scholar]
- 42.Ravid, T., A. Tsaba, P. Gee, R. Rasooly, E. A. Medina, and T. Goldkorn. 2003. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L1082-L1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddel, R. R., Y. Ke, B. I. Gerwin, M. G. McMenamin, J. F. Lechner, R. T. Su, D. E. Brash, J. B. Park, J. S. Rhim, and C. C. Harris. 1988. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region gene. Cancer Res. 48:1904-1909. [PubMed] [Google Scholar]
- 44.Rideout, H. J., E. Zang, M. Yeasmin, R. Gordon, O. Jabado, D. S. Park, and L. Stefanis. 2001. Inhibitors of trypsin-like serine proteases prevent DNA damage-induced neuronal death by acting upstream of the mitochondrial checkpoint and of p53 induction. Neuroscience 107:339-352. [DOI] [PubMed] [Google Scholar]
- 45.Rouaux, C., N. Jokic, C. Mbebi, S. Boutillier, J. P. Loeffler, and A. L. Boutillier. 2003. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 22:6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruchaud, S., N. Korfali, P. Villa, T. J. Kottke, C. Dingwall, S. H. Kaufmann, and W. C. Earnshaw. 2002. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 21:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders, F. K., T. J. Mitchell, J. A. Walker, P. W. Andrew, and G. J. Boulnois. 1989. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect. Immun. 57:2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedger, L. M., D. M. Shows, R. A. Blanton, J. J. Peschon, R. G. Goodwin, D. Cosman, and S. R. Wiley. 1999. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 163:920-926. [PubMed] [Google Scholar]
- 49.Spreer, A., H. Kerstan, T. Bottcher, J. Gerber, A. Siemer, G. Zysk, T. J. Mitchell, H. Eiffert, and R. Nau. 2003. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob. Agents Chemother. 47:2649-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki, Y., Y. Nakabayashi, and R. Takahashi. 2001. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA 98:8662-8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tateda, K., J. C. Deng, T. A. Moore, M. W. Newstead, R. Paine III, N. Kobayashi, K. Yamaguchi, and T. J. Standiford. 2003. Hyperoxia mediates acute lung injury and increased lethality in murine Legionella pneumonia: the role of apoptosis. J. Immunol. 170:4209-4216. [DOI] [PubMed] [Google Scholar]
- 52.Tiraby, J. G., and M. S. Fox. 1973. Marker discrimination in transformation and mutation of pneumococcus. Proc. Natl. Acad. Sci. USA 70:3541-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Mering, M., A. Wellmer, U. Michel, S. Bunkowski, A. Tlustochowska, W. Bruck, U. Kuhnt, and R. Nau. 2001. Transcriptional regulation of caspases in experimental pneumococcal meningitis. Brain Pathol. 11:282-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zysk, G., L. Bejo, B. K. Schneider-Wald, R. Nau, and H. Heinz. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin. Exp. Immunol. 122:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zysk, G., B. K. Schneider-Wald, J. H. Hwang, L. Bejo, K. S. Kim, T. J. Mitchell, R. Hakenbeck, and H. P. Heinz. 2001. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 69:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]