Abstract
Infection with Helicobacter pylori is usually asymptomatic but sometimes progresses to peptic ulcer disease or gastric adenocarcinoma. The development of disease involves both host and bacterial factors. In order to better understand host factors in pathogenesis, we studied the gastric transcription profile of H. pylori infection in the rhesus macaque by using DNA microarrays. Significant changes were found in the expression of genes important for innate immunity, chemokines and cytokines, cell growth and differentiation, apoptosis, structural proteins, and signal transduction and transcription factors. This broad transcription profile demonstrated expected up-regulation of cell structural elements and the host inflammatory and immune response, as well as the novel finding of down-regulation of heat shock proteins. These results provide a unique view of acute H. pylori infection in a relevant animal model system and will direct future studies regarding the host response to H. pylori infection.
Helicobacter pylori is a gram-negative spiral bacterium that chronically infects the gastric mucosa of approximately 60% of the world's population. All infected individuals develop histologic gastritis, but most have no clinical disease. However, 10 to 15% of those infected develop peptic ulcer disease or gastric adenocarcinoma (68, 93), which is the second most common cause of cancer mortality worldwide (43). The development of disease associated with H. pylori infection is a multifactorial process that is not well understood (69) but involves both host and bacterial factors. Infection with strains of H. pylori that contain the cag (cytotoxin-associated gene) pathogenicity island (PAI) has been associated with a greater degree of inflammation and with the development of peptic ulcer disease and gastric cancer in developed countries, but not in many developing countries, where nearly all H. pylori strains have the Cag PAI (16). The response to chronic H. pylori infection is also influenced by host genetic factors (30) and probably by environmental factors, such as diet and others (52).
Analysis of host gene expression in response to H. pylori infection is one way to better understand the role of host factors in pathogenesis. Most investigators have exploited gastric cancer cell lines cocultured with H. pylori and subsequent analysis by DNA microarray (5, 17, 21, 40, 48, 54, 57, 65, 81, 98). Although cell culture experiments offer the advantage of a defined cell type, there are some important disadvantages to this strategy. Cell culture experiments are generally limited to 24 to 48 h of infection, while natural infection is typically lifelong. Perhaps more importantly, experiments using cell culture lack the rich microenvironment and cell diversity, including cellular and humoral constituents of the host immune response, that are encountered in the gastric mucosa. Furthermore, cancer cell lines frequently differ in gene expression from normal tissue (13, 51). While some findings from these cell culture experiments have been validated in infected human tissues by reverse transcription-PCR (21), there is little known of global host gene expression in infected humans. Human studies are limited because, in the absence of human challenge, which is generally considered unacceptable, control over the particular H. pylori strain, duration of infection, and other variables is impossible.
Animal models provide a means to study the acute host response to H. pylori infection by comparison of gene expression before and after experimental infection (66). Perhaps the most relevant model to human infection is the rhesus macaque, which in captive populations is naturally infected with strains of H. pylori that are indistinguishable from strains that infect humans (26, 29, 85). Infection is associated with rapid induction of histologic gastritis that mimics what is seen in infected humans (28, 85). Furthermore, some animals go on to develop atrophic gastritis, the histologic precursor to gastric adenocarcinoma (29). Thus, the rhesus macaque model provides a unique opportunity to study acute infection; the time during which the host and bacteria establish an equilibrium and the outcome of the relationship has yet to be determined.
Although nonhuman primate-based DNA microarrays are not currently available, there is sufficient DNA sequence similarity between humans and nonhuman primates that human genome microarrays can be used to analyze samples from rhesus macaques and other nonhuman primates (12, 47, 104). For example, analysis of normal human and rhesus macaque jejunum showed that comparable numbers of expressed genes were identified by microarray, with about 90% overlap (36). Therefore, the purpose of this study was to examine host gene expression during acute H. pylori infection with use of the rhesus macaque model and DNA microarrays of the human genome.
MATERIALS AND METHODS
Animals.
Three male rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All experiments were approved by the Research Advisory Committee of the CNPRC and the Institutional Animal Care and Use Committee of the University of California, Davis, and were conducted by trained staff of the CNPRC. All monkeys were hand raised in the nursery from the day of birth by methods described previously (85). At approximately 6 months of age they were documented to be specific pathogen free for H. pylori by serology, histology, and culture of gastric biopsy specimens. To eliminate “Helicobacter heilmannii” infection, which causes minimal inflammation and does not lead to H. pylori seroconversion (85), monkeys were treated by gavage with omeprazole (0.3 mg/kg of body weight), clarithromycin (11 mg/kg), bismuth subsalicylate (20 mg/kg), and amoxicillin (14 mg/kg) twice daily for 14 days.
Bacterial strains.
H. pylori J166 is a human-derived strain that has previously been shown to effectively colonize rhesus macaques (27, 86). Six low-passage-number H. pylori J166 isolates, each derived from experimentally infected monkeys, were used for inoculation. A mixture of six J166 strains was chosen because inoculation with single-colony isolates colonizes less efficiently in both primates (J. V. Solnick, unpublished observations) and mice (K. A. Eaton, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. D-213, 2004). All strains were determined to contain the Cag PAI by PCR with use of primers and conditions that have been described previously (90). The six strains were analyzed in vitro for induction of interleukin 8 (IL-8) and CagA phosphorylation in AGS cell culture by methods previously described (3, 79). Of the six strains, three induced IL-8 (mean = 1,594 pg/ml; standard deviation = 396 pg/ml) and showed CagA tyrosine phosphorylation and three did not (mean = 482 pg/ml; standard deviation = 81 pg/ml).
Bacterial inoculation.
H. pylori J166 aliquots were subcultured once on brucella agar with 5% newborn calf serum (Gibco Invitrogen, Grand Island, N.Y.) supplemented with TVPA (trimethoprim, 5 mg/liter; vancomycin, 10 mg/liter; polymyxin B, 2.5 IU/liter; amphotericin B, 4 mg/liter; all from Sigma, St. Louis, Mo.) and incubated at 37°C with 5% CO2. The subculture was then used to inoculate brucella broth (Difco Laboratories, Detroit, Mich.) with 5% newborn calf serum and TVPA. The liquid culture was incubated at 37°C with 5% CO2 until the optical density at 600 nm was approximately 0.2 to 0.4 (about 15 h). The bacteria were pelleted and resuspended at a concentration of 105 CFU/2 ml of brucella broth. Prior to inoculation, the culture was examined by Gram stain, wet mount, and rapid urease assay with urea-indole medium. Quantitation of the inoculum was confirmed by plating serial dilutions. Monkeys under ketamine anesthesia (10 mg/kg intramuscularly) were inoculated with a 2-ml bacterial inoculum followed by a 5-ml phosphate-buffered saline flush of the orogastric tube.
Endoscopy and quantitative culture.
Endoscopy was performed under ketamine anesthesia (10 mg/kg intramuscularly) after an overnight fast. Samples were obtained before and 2, 8, and 24 weeks after inoculation with H. pylori. Three biopsy specimens of the gastric antrum were processed for quantitative culture by serial dilution as previously described (86). H. pylori infection was confirmed in the conventional manner by colony morphology (pinhead-sized translucent colonies), microscopy (gram-negative curved organisms), and biochemistry (oxidase, catalase, and urease positive).
RNA isolation.
Ten gastric biopsy specimens, five each from the antrum and corpus, were taken at each time point. The antral and corporal biopsy specimens for each animal were pooled and processed together to provide enough RNA for microarray analysis. Biopsy specimens were ground with a glass pestle in Trizol reagent (Sigma), and RNA was isolated according to protocols provided by the manufacturer. All RNA samples were treated with DNase I (Roche Applied Science, Mannheim, Germany), purified with an RNeasy kit (Qiagen, Valencia, Calif.) according to the RNA cleanup protocol, and resuspended in molecular-biology-grade water (BioWhittaker, Rockland, Maine). The yield of RNA was between 17.7 and 37.3 μg for 10 gastric biopsy specimens. Samples were stored at −80°C prior to analysis.
Microarray methods.
Labeling and hybridization to Affymetrix HumanFL (HuFL) chips were done according to the recommendations of the manufacturer (Affymetrix, Santa Clara, Calif.). Briefly, biotin-labeled RNA was prepared by first reverse transcribing the RNA into double-stranded cDNA (Superscript II; Invitrogen Life Technologies, Carlsbad, Calif.) with an oligo(dT)24 primer containing a T7 RNA polymerase promoter. Then an in vitro transcription reaction was carried out (Enzo High Yield RNA Transcript Labeling kit; Enzo Biochem, Farmingdale, N.Y.) during which biotin-labeled ribonucleotides were incorporated into the cRNA. Following fragmentation by heating to 94°C for 35 min, 10 μg of labeled cRNA was hybridized first to a Test Array (Affymetrix) to verify cRNA quality and then to HuFL chips for 16 h at 45°C. Arrays were washed and stained with streptavidin-phycoerythrin with use of an automated fluidics station. The chips were then scanned with an Agilent GeneArray scanner.
Data analysis.
Three independent analyses were performed. All data were first collated and scaled (scaling factor between 10 and 30) in Microarray Suite 5.0 (MAS 5.0; Affymetrix). Initial analysis was performed by inspection with use of Microsoft Excel and cross comparisons imported from MAS 5.0. For this analysis each animal's preinoculation time point (baseline) was compared to its own postinoculation (p.i.) time point as well as the p.i. time point of the other two animals, yielding nine total comparisons per time point. Genes were considered significantly changed if at least seven of nine comparisons indicated increased or decreased expression and had a P value of ≤0.05. P values for this analysis were determined in MAS 5.0 and were based on the comparisons at the probe level (n = 20 probes/probe set). We next used dChip (version 1.2) for comparison of baseline to each p.i. time point with use of fluorescence intensity values (.CEL files) from MAS 5.0 (www.dchip.org) (53). Finally, BRB ArrayTools (version 3.1), developed by Richard Simon and Amy Lam of the Biometric Research Branch of the National Cancer Institute, was used for microarray analysis (http://linus.nci.nih.gov/BRB-ArrayTools.html). Baseline and p.i. data were compared with the class comparison tool, which uses a t test to compare the log signal intensity between two classes (i.e., baseline and one p.i. time point). The t test uses a pooled variance (pooled across all genes) to estimate the variability of the log signal for each gene (97). However, it uses a separate estimate of variability for each gene rather than assuming the same variability for all genes. Genes with at least 2.0-fold change averaged over three animals and a P value of ≤0.05 were considered changed over preinfection values. Genes were annotated using DRAGON View (http://pevsnerlab.kennedykrieger.org/dragon.htm) (14) and individual gene queries of the National Center for Biotechnology Information-PubMed (http://www.ncbi.nlm.nih.gov).
RESULTS AND DISCUSSION
Quantitative culture.
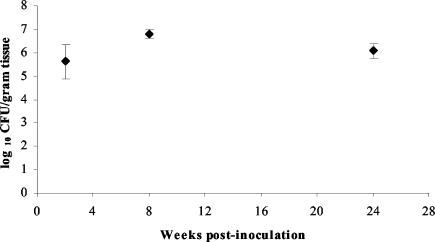
Three juvenile rhesus macaques specific pathogen free for Helicobacter species were inoculated with a mixture that contained 105 CFU of six rhesus-passaged H. pylori J166 isolates. Quantitative cultures of three biopsy specimens from the gastric antrum at 2, 8, and 24 weeks p.i. showed that all animals were chronically infected with 105 to 107 CFU/g of gastric tissue (Fig. 1). This degree of colonization resembles that seen in natural human infection (4).
FIG. 1.
Mean quantitative H. pylori cultures from three biopsy specimens of the gastric antrum taken from three monkeys 2, 8, and 24 weeks p.i. with 105 CFU of H. pylori J166, with error bars showing standard deviations. All animals were culture negative for H. pylori prior to inoculation (0 weeks).
Host gene expression.
Microarray analysis was performed on samples of the gastric antrum and corpus for each time point with use of the Affymetrix HuFL DNA GeneChip, which contains oligonucleotides representing over 7,000 cDNAs. Three independent methods of analysis were employed as described above. Although the fold change varied somewhat depending upon the method used, a core group of genes with consistently altered expression was identified with each analysis. Here we present the results from BRB ArrayTools primarily because of its ease of use and strong statistical methods.
Approximately one-third of the genes represented had a positive signal intensity on the microarray, which is similar to previous studies with Affymetrix human arrays with nonhuman primate samples (18). Comparison of each p.i. transcription profile to the baseline profile identified 148 genes (84 up, 64 down) at 2 weeks p.i., 129 genes (66 up, 63 down) at 8 weeks p.i., and 206 genes (105 up, 101 down) at 24 weeks p.i. that were significantly changed. A subset of the genes whose expression changed significantly were grouped into functional categories, which included the innate immune-inflammatory response, chemokines and cytokines, cell growth and differentiation, apoptosis, structural proteins, signal transduction, and transcription factors (Tables 1 to 3). A full data set that includes all genes with significantly altered expression is available at http://solnicklab.compmed.ucdavis.edu/.
TABLE 1.
Changes in expression 2 weeks p.i.a
| Category | Description | Accession no. | Fold change | P value |
|---|---|---|---|---|
| Innate immune-inflammatory response | Antithrombin III | M21642 | −5.35 | 0.046 |
| MMP-3 | X05232 | −3.70 | 0.002 | |
| Serine protease inhibitor, Kazal type 1 | M20530 | −3.23 | 0.003 | |
| Alpha-1-antitrypsin | X05826 | −3.21 | 0.035 | |
| Squamous cell carcinoma antigen 2 | U19557 | −2.86 | 0.012 | |
| Leukocyte immunoglobulin-like receptor | U82275 | −2.49 | 0.040 | |
| Peroxisomal farnesylated protein | X75535 | −2.26 | 0.040 | |
| Human epididymis-specific 3 alpha | X76383 | −2.16 | 0.038 | |
| Cysteine-rich secretory protein 3 (CRISP 3) | X95240 | −2.07 | 0.011 | |
| Sialidase 1 (lysosomal sialidase) | X78687 | 2.12 | 0.046 | |
| MMP-15 | Z48482 | 2.51 | 0.027 | |
| Eosinophil cationic-related protein | X55989 | 2.63 | 0.015 | |
| Cathepsin O | X77383 | 2.85 | 0.041 | |
| Fc fragment of immunoglobulin G binding protein | D84239 | 2.86 | 0.026 | |
| Polymeric immunoglobulin receptor | X73079 | 2.87 | 0.008 | |
| Prostaglandin E receptor EP3 subtype | D86096 | 3.04 | 0.001 | |
| CD44 glycoprotein | L05424 | 3.17 | 0.030 | |
| ICAM-5 | U72671 | 3.28 | 0.035 | |
| Pregnancy specific beta-1-glycoprotein 5 | M25384 | 3.88 | 0.018 | |
| Neutrophil cytosolic factor 4 | X77094 | 4.41 | 0.003 | |
| Glutaredoxin (thioltransferase) | X76648 | 4.62 | 0.022 | |
| Tibrinogen | HG2730-HT2828 | 4.78 | 0.026 | |
| Neutrophil gelatinase-associated lipocalin | X99133 | 4.80 | 0.013 | |
| Neutrophil gelatinase-associated lipocalin | S75256 | 5.10 | 0.011 | |
| Defensin, beta 2 | Z71389 | 11.88 | 0.003 | |
| Protease inhibitor 3 (elafin) | L10343 | 41.53 | 0.002 | |
| Chemokine-cytokine | Small inducible cytokine B11 (CXCL11) | U59286 | −2.98 | 0.044 |
| CCR6 | U68031 | −2.34 | 0.048 | |
| IL-2-inducible T-cell kinase | L10717 | 2.76 | 0.049 | |
| Leptin receptor | U66497 | 2.88 | 0.048 | |
| CCR2 | U95626 | 3.07 | 0.048 | |
| Leukemia inhibitory factor receptor | X61615 | 3.51 | 0.020 | |
| Heat shock | DnaJ (Hsp40) B4 | U40992 | −2.19 | 0.031 |
| Heat shock 70-kDa protein 1B | M59830 | −2.26 | 0.029 | |
| Heat shock 70-kDa protein 1A | M11717 | −2.46 | 0.026 | |
| Cell growth and differentiation | Fibroblast activation protein, alpha | U09278 | −8.62 | 0.000 |
| Ret proto-oncogene | M57464 | −3.42 | 0.033 | |
| Platelet-derived growth factor receptor-like protein | D37965 | −3.05 | 0.040 | |
| Retinoblastoma binding protein 1 | S57153 | 2.63 | 0.028 | |
| Ras-related protein Rab-3B | M28214 | 3.15 | 0.003 | |
| Latent TGF-β binding protein 2 | Z37976 | 3.62 | 0.005 | |
| Receptor protein-tyrosine kinase erB-4 | L07868 | 3.86 | 0.009 | |
| Synaptotagmin I | M55047 | 5.34 | 0.026 | |
| Apoptosis | TNF receptor-associated factor 3 | U15637 | 2.17 | 0.030 |
| Prothymosin, alpha | M14483 | 2.27 | 0.017 | |
| Death-associated protein | X76105 | 2.97 | 0.037 | |
| Structural | Tropomodulin | M77016 | −3.61 | 0.040 |
| Actin-related protein 1B | X82207 | −2.06 | 0.020 | |
| Collagen, type XIX, alpha 1 | D38163 | 2.38 | 0.030 | |
| Cytokeratin 20 | X73501 | 2.88 | 0.010 | |
| Myosin IF | X98411 | 2.92 | 0.050 | |
| Elastin | S57887 | 3.56 | 0.039 | |
| Dynamin 1-like | AF000430 | 4.36 | 0.020 | |
| F-actin capping protein, alpha 2 | U03851 | 6.05 | 0.040 | |
| Signal transduction | Protein tyrosine phosphatase type 2 | M25393 | −4.72 | 0.036 |
| G protein-coupled receptor 21 | U66580 | −2.79 | 0.024 | |
| G protein-coupled receptor 22 | U66581 | −2.13 | 0.038 | |
| TYRO3 protein tyrosine kinase | U02566 | −2.10 | 0.012 | |
| Guanylate cyclase 2C | M73489 | 2.14 | 0.036 | |
| MAPK kinase 1 (MAP2K1) | L05624 | 2.19 | 0.045 | |
| MAPK10 | U07620 | 3.03 | 0.001 | |
| Guanine nucleotide binding protein, alpha 13 | L22075 | 3.14 | 0.008 | |
| Phospholipase C, gamma 1 | M34667 | 3.21 | 0.045 | |
| Calcium, calmodulin-dependent protein kinase II gamma | U50360 | 3.57 | 0.012 | |
| Protein tyrosine phosphatase, receptor type, J | D37781 | 4.39 | 0.031 | |
| Transcription factor | Zinc finger protein 77 | X65230 | −3.61 | 0.008 |
| Hepatocyte nuclear factor 1-beta | X71348 | −2.01 | 0.047 | |
| Glucocorticoid receptor DNA binding factor 1 | M73077 | 2.23 | 0.036 | |
| Homeobox D3 | Y09980 | 2.55 | 0.039 | |
| Signal transducer and activator of transcription (STAT1) | M97935 | 2.97 | 0.019 | |
| Homeotic protein Hpx-2 | X74861 | 3.36 | 0.002 |
Data for genes with altered expression at more than one time point are shown in boldface.
TABLE 3.
Changes in expression 24 weeks p.i.a
| Category | Description | Accession no. | Fold change | P value |
|---|---|---|---|---|
| Innate immune-inflammatory response | CEA-related cell adhesion molecule 5 | M29540 | −8.77 | 0.004 |
| Fatty acid binding protein 1, liver | M10050 | −5.15 | 0.013 | |
| Placental protein 14, glycodelin | HG721-HT4827 | −4.55 | 0.039 | |
| CD44 glycoprotein | M83328 | −4.26 | 0.046 | |
| Histatin 3 | L05514 | −3.89 | 0.036 | |
| Integrin, alpha 3 | M59911 | −3.76 | 0.028 | |
| Tissue plasminogen activator | K03021 | −3.75 | 0.042 | |
| Hyaluronidase PH-20 | S67798 | −3.70 | 0.035 | |
| Serine protease inhibitor, Kazal type 1 | M20530 | −3.45 | 0.007 | |
| Integrin, alpha L (CD11A) | Y00796 | −3.18 | 0.013 | |
| Chitinase 1 | U29615 | −2.85 | 0.028 | |
| Lymphocyte antigen 9 | L42621 | −2.84 | 0.032 | |
| Annexin II | D28364 | −2.26 | 0.048 | |
| Peroxisome receptor 1 | U35407 | 2.28 | 0.009 | |
| Heparin cofactor | M58600 | 2.30 | 0.008 | |
| Mucin 1 | X83412 | 2.37 | 0.022 | |
| Mucin 6 | L07517 | 2.44 | 0.010 | |
| Surfactant protein Sp-A2 delta | S69683 | 2.90 | 0.006 | |
| Sucrase-isomaltase | X63597 | 3.14 | 0.011 | |
| Carboxypeptidase B2 | M75106 | 3.19 | 0.034 | |
| Peroxisomal biogenesis factor 12 | U91521 | 3.29 | 0.038 | |
| Ceruloplasmin | M13699 | 3.44 | 0.008 | |
| Galectin 8 | L78132 | 3.82 | 0.048 | |
| 2′-5′-Oligoadenylate synthetase 2 | M87284 | 4.21 | 0.002 | |
| Defensin, beta 2 | Z71389 | 4.39 | 0.045 | |
| Protease inhibitor 3 (elafin) | L10343 | 10.97 | 0.007 | |
| Chemokine-cytokine | Small inducible cytokine B10 (CXCL10) | X02530 | −2.26 | 0.038 |
| CCR2 | AF014958 | −2.16 | 0.028 | |
| IL-2 receptor, beta | M26062 | 2.94 | 0.049 | |
| CCR2 | U95626 | 3.05 | 0.012 | |
| IL-10 receptor, alpha | U00672 | 4.76 | 0.008 | |
| Chemokine-like receptor 1 | U79526 | 5.40 | 0.021 | |
| Heat shock | Heat shock 70-kDa protein 2 | L26336 | −3.41 | 0.044 |
| Heat shock 70-kDa protein 1B | M59830 | −2.68 | 0.013 | |
| Heat shock 70-kDa protein 1A | M11717 | −2.65 | 0.015 | |
| Cell growth and differentiation | Fibroblast activation protein, alpha | U09278 | −5.24 | 0.002 |
| Cyclin-dependent kinase inhibitor | L25876 | −4.74 | 0.026 | |
| RAB5B, member RAS oncogene family | X54871 | −2.35 | 0.036 | |
| Platelet-derived growth factor alpha polypeptide | M19989 | −2.07 | 0.024 | |
| Peptidyl-prolyl isomerase G (cyclophilin G) | U40763 | 2.15 | 0.015 | |
| M-phase phosphoprotein 9 | X98258 | 2.23 | 0.038 | |
| Proto-oncogene tyrosine-protein kinase ABL1 | U07563 | 2.41 | 0.029 | |
| Lethal giant larva homolog 1 | D50550 | 2.45 | 0.031 | |
| Mast/stem cell growth factor receptor | X06182 | 2.66 | 0.041 | |
| Glypican 3 | Z37987 | 3.11 | 0.007 | |
| Latent TGF-β binding protein 2 | Z37976 | 3.62 | 0.005 | |
| BRCA1-associated RING domain 1 | U76638 | 3.81 | 0.049 | |
| Synaptotagmin I | M55047 | 5.14 | 0.040 | |
| Apoptosis | TNF-α | X02910 | −3.83 | 0.049 |
| Glucagon | J04040 | −3.56 | 0.038 | |
| FK506 binding protein 8 (Bcl-2 interacting) | L37033 | −2.79 | 0.006 | |
| Prothymosin, alpha | M14483 | −2.27 | 0.019 | |
| Milk fat globule-EGF 8 protein | U58516 | 2.38 | 0.003 | |
| Bcl-2 homolog | S82185 | 2.57 | 0.038 | |
| TNF receptor superfamily, member 6 | X89101 | 3.36 | 0.001 | |
| Structural | Myosin, nonmuscle | M69180 | −4.24 | 0.022 |
| Desmocollin 3 | X83929 | −2.68 | 0.044 | |
| 2′,3′-Cyclic nucleotide 3′ phosphodiesterase | M19650 | −2.36 | 0.031 | |
| Procollagen C-endopeptidase enhancer | L33799 | −2.15 | 0.027 | |
| Elastin | X52896 | 2.68 | 0.023 | |
| F-actin capping protein, alpha 2 | U03851 | 9.00 | 0.022 | |
| Signal transduction | Tyrosine kinase, nonreceptor, 1 | U43408 | −4.44 | 0.010 |
| Protein tyrosine kinase | U07794 | −4.17 | 0.020 | |
| G protein-coupled receptor 21 | U66580 | −3.09 | 0.032 | |
| Tyrosine kinase 2 | X54637 | −2.69 | 0.014 | |
| Serine/threonine kinase 4 | U18297 | −2.49 | 0.011 | |
| Protein tyrosine phosphatase, nonreceptor type 9 | M83738 | 2.42 | 0.014 | |
| Protein tyrosine phosphatase, nonreceptor type substrate 1 | Y10375 | 2.76 | 0.020 | |
| Bone morphogenetic protein 5 | S81957 | 3.01 | 0.037 | |
| PCTAIRE protein kinase 3 | X66362 | 3.41 | 0.010 | |
| Protein kinase C, beta 1 | X07109 | 3.50 | 0.025 | |
| MAP-microtubule affinity-regulating kinase 3 | M80359 | 3.94 | 0.012 | |
| Cadherin 3, type 1, P cadherin | X63629 | 4.23 | 0.018 | |
| Cadherin 12, type 2 | L33477 | 4.49 | 0.010 | |
| MAPK kinase kinase 10 (MAP3K10) | X90846 | 5.89 | 0.004 | |
| Transcription factor | Transcription factor 8 | D15050 | −4.67 | 0.010 |
| ZFP-36 for a zinc finger protein | X51760 | 3.00 | 0.042 | |
| Homeotic protein Hpx-2 | X74861 | 3.33 | 0.049 |
Data for genes with altered expression at more than one time point are shown in boldface.
Innate immune-inflammatory response. (i) Antimicrobial proteins and peptides.
At each time point p.i., there was up-regulation of antimicrobial proteins or peptides (Tables 1 to 3), including human beta-defensin 2 (hBD-2), protease inhibitor 3 (elafin), neutrophil gelatinase-associated lipocalin (NGAL), and surfactant. The best studied of these molecules are the defensins, which are a family of antimicrobial peptides that are expressed in neutrophils and on mucosal surfaces, where they are thought to play an important role in innate host defense (11). Recent evidence from cell culture experiments and gastric biopsies of chronically infected humans supports our finding that hBD-2 is induced by H. pylori and can inhibit its growth at concentrations as low as 10−5 μmol/μl (6, 41, 92, 95). Protease inhibitor 3 (elafin) is a defensin-like protein with a clear antimicrobial role in innate immune defense in the lung (78). Similarly, NGAL has direct antimicrobial effects as well as the ability to scavenge bacterial products (56) and is expressed in normal gastric mucosa by parietal cells (33). During H. pylori infection NGAL may also be expressed from neutrophils or even Paneth cells, which may be present in the setting of intestinal metaplasia after chronic infection (56). Finally, surfactant protein A2 delta expression may also contribute antimicrobial effects similar to those of surfactant protein D, which binds bacteria, causing aggregation and phagocytosis, and is up-regulated during H. pylori infection (64). Taken together, these results extend previous findings of H. pylori-induced expression of hBD-2 to an experimental model system and suggest that this is part of a family of antimicrobial peptides whose expression in the stomach is increased by H. pylori infection.
(ii) Mucin.
The gastric mucous layer also provides a protective barrier against infection. Mucins 1 and 6, which are key components of the mucous layer, showed increased expression at 24 weeks p.i. (Table 3). Mucin 1 can limit cell-cell adhesion and H. pylori binding to the gastric epithelium and may have the additional function of signal transduction to alert the cell to changing extracellular conditions (35). Increased expression of mucin 1 fits with decreased expression of transcription factor 8 (ZEB1) (Table 3), which is known to repress mucin 1 in epithelial cells (39). H. pylori infection has been associated with an increase in the expression of mucin 6 and expansion of mucin 6 expression from mucous glands to surface mucous cells (15, 59). It is not known what effect up-regulation of mucins 1 and 6 have on the H. pylori adhesion in vivo. Mucin 5AC was not significantly changed in this study. This may at first seem surprising, since mucin 5AC has recently been identified as the primary source for Lewisb binding of H. pylori BabA adhesin (94), and its expression in humans is induced by H. pylori infection (15). However, passage of H. pylori J166 through rhesus macaques results in deletion of babA and duplication of babB (84). This gene conversion event results in loss of adhesion to Lewisb, and it may explain the failure of H. pylori J166 to induce expression of mucin 5AC in rhesus macaques. Alternatively, since the mucin 5AC DNA sequence is not known for rhesus macaques, a lack of up-regulation could be due to sequence differences.
(iii) Extracellular matrix remodeling.
Expression of collagen VIII and IX was increased following H. pylori infection (Tables 1 to 3). Altered expression was found for several protease inhibitors, such as antithrombin III, serine protease inhibitor (Kazal type I), alpha-1-antitrypsin, and matrix metalloproteinase 3 (MMP-3) (all decreased) and MMP-15 (increased) (Tables 1 to 3). These results differ somewhat from recent studies using cultured gastric epithelial cells that demonstrated increased expression of proteases and protease inhibitors, such as MMP-1, -2, -3, -7, and -9 and ADAM (a disintegrin and metalloproteinase)-10 and -17 in gastric epithelial cells (9, 10, 24, 38, 48, 63, 99). Our results may emphasize differences between gene expression in cultured cells and an experimental animal model associated with H. pylori infection. Overall there is a growing appreciation that remodeling of the extracellular matrix is a key event in H. pylori infection. Up-regulation of some of these proteins may contribute to the carcinogenic potential of H. pylori, as up-regulation of MMP-7 has been associated with both gastric adenocarcinoma and premalignant lesions in the stomach and other tissues of the gastrointestinal tract (24).
(iv) Cell adhesion molecules.
Changes in cell adhesion molecules were also present at all time points p.i. CD44 and intercellular adhesion molecule 5 (ICAM-5) were increased in expression 2 weeks p.i. ICAM-5 has previously been associated with expression in neuronal tissue (55). CD44 and ICAM-1 were up-regulated in H. pylori-infected AGS cell cultures (32), and CD44 expression was found in gastric epithelial cells of H. pylori-infected patients (31). CD44 expression was decreased at 24 weeks postinfection as were the integrins α3 and αL (CD11A). These changes generally reflect alterations in cell-cell interactions and cell migration. CD44, a hyaluronin receptor, provides a docking station for MMP-9 (100). MMP-9 and MMP-2 cleave latent transforming growth factor β (TGF-β) to form active TGF-β. Both CD44 and a TGF-binding protein were up-regulated 2 weeks p.i., and MMP-9 is increased in expression in H. pylori-infected human patients (10). These changes reflect alterations in cell-cell interactions and cell migration that support and extend previous findings from cell culture and clinical samples (31, 32). Together they suggest participation by and interaction among tissue remodeling enzymes, cell surface receptors, and growth factors.
Also observed was the increased expression of galectin 8 (24 weeks p.i., Table 3), which regulated inflammatory cell adhesion (67, 103) and is a member of a family of highly conserved β-galactoside binding lectins that mediate cell-cell and cell-matrix interactions. Galectins 3 and 4 also had increased expression in our study but did not meet the significance criteria. Up-regulation of galectins 1 and 3 has been observed in AGS cells (54) but has not been studied in vivo. Galectins 1 and 3 are important mediators of the inflammatory cascade via neutrophil recruitment and induction of the respiratory burst (1). Galectin expression is typically altered in neoplastic tissue, including gastric adenocarcinoma (61). Although early changes in galectin expression could represent preneoplastic changes in gastric epithelial cells as has been suggested previously (54), the functional role of the galectins and the acute timing in these animals suggest that they are part of the innate immune response to infection. It is also possible that binding between galectins and H. pylori could occur. β-Galactoside carbohydrates are common components of bacterial membranes, and interactions between host galectins and other microorganisms such as Leishmania spp. and Neisseria gonorrhoeae have been described previously (45, 74), as have associations between H. pylori and host glycoproteins (44).
(v) Hsp.
The expression of several heat shock proteins (Hsp) was decreased at all time points p.i. (Tables 1 to 3). Although best described as protein chaperones during cellular stress (42), recent evidence suggests that Hsp are also important for activation of the innate immune response (8). Release of the inducible Hsp, such as Hsp70, from necrotic cells provides a danger signal to antigen-presenting cells, which induces cellular activation and cytokine production (88, 91). In addition, peptides bound to Hsp70 serve as a source of antigen and Hsp70 itself is a maturation signal for dendritic cells (60, 88). Up-regulation of Hsp is the general response to all types of cellular stress, including infection (50). We propose that down-regulation of host Hsp70 by H. pylori is a mechanism to limit the host inflammatory response and facilitate chronic infection. This immunomodulation can be viewed in the larger context of emerging evidence that H. pylori has multiple strategies to promote chronic infection and avoid host immunity, such as synthesis of a lipopolysaccharide with low biological activity (62), down-regulation of IL-2 signaling (34), and the presence of flagellar proteins that do not activate toll-like receptor 5 (37). These results suggest that the role of Hsp70 expression in H. pylori infection, which to date has been little studied (49, 89), may be a fruitful area of investigation to better understand bacterial persistence in the face of an active humoral and cellular immune response.
Cytokines-chemokines.
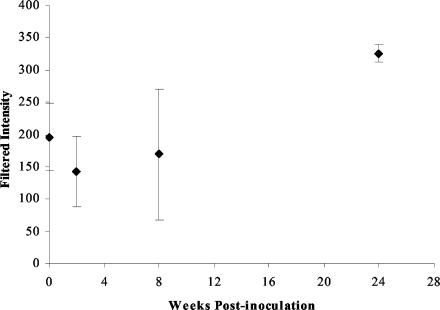
One of the hallmarks of infection with Cag PAI-positive strains of H. pylori is the up-regulation of IL-8 expression by gastric epithelial cells (23). It was therefore surprising that we and others (72, 96) did not find IL-8 expression increased according to the data analysis criteria that were established. One possible explanation for this observation is sequence differences between rhesus macaques and humans for IL-8. Since the monkeys were inoculated with a mixture of strains of H. pylori J166, only some of which induced IL-8 in cell culture, there was a possibility that only non-IL-8-inducing strains colonized the monkeys or that colonizing strains subsequently lost the ability to induce IL-8. This latter observation has been reported previously in a mouse model of H. pylori (75). However, assays of IL-8 induction from multiple bacterial colonies at each time point p.i. showed that IL-8-inducing strains were recovered from each monkey at all time points p.i. (data not shown). Analysis of the fluorescence intensity values for the IL-8 expression indicated increased expression at 24 weeks with a fold change that was just below the twofold cutoff (Fig. 2). In addition to IL-8, other proinflammatory chemokines such as IL-1β, epithelial neutrophil-activating peptide 78, growth-related oncogene α, and monocyte chemotactic protein 1 are also up-regulated by H. pylori infection (22, 82), which serves to recruit neutrophils and monocytes and promote the T-cell response to infection. While no changes in these chemokines were noted, increased expression of chemokine receptor 2, the receptor for monocyte chemotactic protein 1, was present at 2 and 24 weeks p.i.
FIG. 2.
Plot of mean filtered intensity for IL-8 (accession no. M28130) on Affymetrix HuFL chip. Intensity values were filtered in BRB ArrayTools (Materials and Methods). Each point represents the mean filtered intensity for three monkeys, with error bars showing standard deviations. At 24 weeks p.i. mean filtered intensity was above the preinoculation value (P = 0.026), but it failed to meet the twofold change cutoff.
Evidence of an anti-inflammatory response was also found p.i., such as decreased expression of T-cell chemotactic factors (CXCL11 and CXCL10) (19) at 2 and 24 weeks p.i. Similarly, expression of glycodelin (placental protein 14) was decreased at 8 and 24 weeks p.i. This extensively glycosylated protein primarily has a role in pregnancy, where it appears to inhibit T-cell proliferation (77), and it may have similar immunomodulatory effects in H. pylori infection. These findings are consistent with recent observations that H. pylori can interfere with the normal host immune response and can alter the degree of inflammation in the epithelium, thus permitting chronic infection. For example, a recent study has shown that VacA, the H. pylori vacuolating cytotoxin, directly interferes with T-cell receptor and IL-2 signaling (34). Increased expression of IL-2-inducible T-cell kinase (2 weeks p.i.) and the IL-2 receptor (24 weeks p.i.) may be compensatory changes in response to VacA-induced down-regulation of the IL-2 signaling pathway. These results emphasize that the response to H. pylori infection must be viewed in a systems manner, with attention to both inflammatory and anti-inflammatory circuits, which likely represent a mechanism of immune evasion.
Structural elements.
Structural changes to gastric epithelial cells are a key feature of H. pylori infection. Attachment to the host epithelial cell allows Cag PAI-positive H. pylori to translocate CagA into the host epithelial cell by a type IV secretion system, which leads to signaling events that cause cell elongation (80). Changes in actin, elastin, and cytoskeletal gene expression were apparent at all three time points p.i. (Tables 1 to 3). Tropomodulin was decreased in expression 2 weeks p.i., while F-actin capping protein was increased in expression at 2 and 24 weeks p.i., indicating changes in the actin structure. Other actin-associated genes were changed at 2 weeks, such as myosin IF (increased) and actin-related protein 1B (decreased), both of which are involved in intracellular transport and indicate alterations in this mechanism in the gastric mucosa. Decreased expression of coronin 2A, which interacts with the actin cytoskeleton in epithelial cells (25), was noted 8 weeks p.i. Recently VacA of H. pylori was shown to interrupt phagosome maturation in macrophages by retaining coronin 1 in the phagosome, preventing phagosome-lysosome fusion (101). It is not known whether VacA could interact with coronin 2A in epithelial cells. Finally, at 8 and 24 weeks p.i. there was a decrease in desmocollin 3 expression, indicating a change in desmosomal junction structure. At 24 weeks p.i., just below the twofold change cutoff, decreases in tight junction protein 1 (zona occludens 1) and gap junction protein beta 1 expression were noted (data not shown). These changes are intriguing in light of recent evidence that CagA directly interacts with zona occludens 1 of the apical-junctional complex of epithelial cells (2).
Cell growth and differentiation.
In the face of gastritis, there is an expectation to find promotion of cell growth to replace dying cells. However, the gene expression pattern suggests that cell growth was suppressed at all time points p.i. Numerous growth factors associated with wound healing were decreased in expression, such as fibroblast activation protein α (all time points), fibroblast growth factor (8 weeks p.i.), hepatocyte growth factor (24 weeks p.i.), and platelet-derived growth factor α (24 weeks p.i.). Glypican 3 was increased in expression 24 weeks p.i. Glypican 3 is a heparin sulfate proteoglycan that suppresses cell growth (87), and it is markedly decreased in gastric cancer (102). Suppression of cell growth may favor H. pylori infection by limiting repair and allowing nutrient leakage through damaged epithelium.
Apoptosis.
Both pro- and antiapoptotic gene expression were present p.i., which is consistent with previous findings in vivo (70, 73). Both the Fas-tumor necrosis factor (TNF) receptor-mediated pathway and the mitochondrially based pathway involving cytochrome c have been implicated (46, 58). Evidence for the Fas-TNF apoptotic pathway was present at each time point. At 8 and 24 weeks p.i., members of the TNF receptor superfamily showed increased expression. These receptors interact with the death domain-containing protein TRADD to induce apoptosis (7). Daxx, which mediates the apoptotic signal from Fas to JNK, was increased in expression 2 weeks p.i. Conversely, while the TNF receptor was up-regulated, TNF-α expression was decreased (24 weeks p.i.), as was that of Fas ligand (8 weeks p.i.). The mitochondrially based apoptotic pathway may not be active early in infection (2 weeks p.i.) due to the increased expression of prothymosin α, which acts as a negative regulator of caspase 9, inhibiting formation of the apoptosome (76). Subsequently, prothymosin α was decreased in expression (24 weeks p.i.), indicating a change in apoptosis at this time. In conflict with the apparent proapoptotic state at 24 weeks p.i. was increased expression of two Bcl-2-interacting proteins. These results again emphasize the complexity and reciprocity of host gene expression in response to H. pylori infection.
Signal transduction pathways and transcription factors.
There were changes noted in transcription factor and signal transduction pathway components present at all time points p.i. Most of these genes are not well defined and have not been associated specifically with H. pylori infection. Of the pathways and factors that have been associated with H. pylori infection, the extracellular signal-regulated kinase-mitogen-activated protein kinase (MAPK) pathway was best represented (71, 83). At both 2 and 24 weeks p.i., elements of the MAPK pathway were increased in expression (Tables 1 and 3). Additional changes in the expression of tyrosine kinases and phosphatases were present at each time point; however, there were no consistencies in the direction of change. No specific changes in the NF-κB pathway were noted, although this pathway is known to be active in H. pylori infection (71).
Conclusions.
High-throughput gene expression technology, together with the rhesus macaque model, provides unprecedented opportunities to examine the H. pylori host-pathogen interaction in a model system that is relevant to human infection. The results from this initial effort partially confirm and in some cases extend previous results drawn largely from cell culture experiments or observational studies of infected human tissue. Our results only partially agree with a recent study that focused on the expression of inflammatory genes in human patients infected with H. pylori, which could stem from true biological differences or from technical differences. The complexity of the results should not be discouraging. It probably reflects in part technical limitations, such as the use of human rather than rhesus DNA sequences, and the failure to analyze expression of individual cell types, a problem that can be solved in future studies through the use of laser capture microdissection. However, we would argue that the complexity also reflects a biological system that is not simply proinflammatory or anti-inflammatory, proapoptotic or antiapoptotic, but is rather a complex host response composed of circuits and compensatory responses. Like any screening analysis, the strength of these data lies in the uncovering of potential novel mechanisms of pathobiology that may provide clues for future studies using more conventional methods of hypothesis-driven biological research (20). For example, the unexpected, but consistent, finding that H. pylori down-regulates Hsp provides an impetus to study their role in immunomodulation by using knockout and transgenic mice infected with H. pylori. We are currently beginning these and other studies that derive from this data set, as well as systematic studies to analyze the effects of well-defined knockout strains of H. pylori on host gene expression.
TABLE 2.
Changes in expression 8 weeks p.i.a
| Category | Description | Accession no. | Fold change | P value |
|---|---|---|---|---|
| Innate immune-inflammatory response | Chitinase 1 | U29615 | −6.25 | 0.007 |
| Fatty acid binding protein 1, liver | M10050 | −6.10 | 0.007 | |
| Placental protein 14, glycodelin | HG721-HT4827 | −4.88 | 0.018 | |
| Hemochromatosis | U60319 | −4.72 | 0.010 | |
| CD1B antigen, b polypeptide | M28826 | −3.26 | 0.006 | |
| Leukocyte immunoglobulin-like receptor | U82275 | −3.22 | 0.009 | |
| Antithrombin III | X00237 | −3.04 | 0.031 | |
| Transferrin receptor | X01060 | −2.95 | 0.037 | |
| Killer cell lectin-like receptor | X54867 | −2.50 | 0.038 | |
| Adhesion glycoprotein | U56102 | 2.11 | 0.002 | |
| Eosinophil peroxidase | X14346 | 2.40 | 0.008 | |
| Prostaglandin E receptor EP3 subtype | D86096 | 2.51 | 0.028 | |
| Pancreatitis-associated protein (HIP/PAP) | D30715 | 2.70 | 0.035 | |
| Eosinophil cationic-related protein | X55989 | 2.93 | 0.023 | |
| Interferon, alpha-inducible protein 27 (IFI27) | X67325 | 2.97 | 0.029 | |
| Histidine-rich glycoprotein | M13149 | 3.02 | 0.008 | |
| Tenascin XB | U24488 | 3.39 | 0.013 | |
| Calcitonin-related polypeptide, beta | X02404 | 3.74 | 0.046 | |
| Thrombospondin 1 | X14787 | 4.56 | 0.015 | |
| Chemokine-cytokine | Cytokine receptor common beta chain | M59941 | 2.02 | 0.019 |
| Heat shock | DnaJ (Hsp40) B4 | U40992 | −3.12 | 0.025 |
| Heat shock 70-kDa protein 1B | M59830 | −3.07 | 0.005 | |
| DnaJ (Hsp40) A1 | L08069 | −2.05 | 0.040 | |
| Cell growth and differentiation | Hepatocyte growth factor | X16323 | −4.63 | 0.046 |
| Fibroblast activation protein, alpha | U09278 | −4.52 | 0.043 | |
| Fibroblast growth factor 5 | M37825 | −3.85 | 0.033 | |
| c-myc proto-oncogene | L00058 | −3.64 | 0.011 | |
| c-myc binding protein | D50692 | −2.29 | 0.036 | |
| Basic fibroblast growth factor receptor 1 | X66945 | 2.47 | 0.009 | |
| Cyclin-dependent kinase inhibitor 1C | U22398 | 2.91 | 0.000 | |
| Teratocarcinoma-derived growth factor 1 | X14253 | 4.30 | 0.019 | |
| Fibroblast growth factor 8 | U47011 | 5.60 | 0.008 | |
| Apoptosis | Glucagon | J04040 | −3.82 | 0.049 |
| Fas ligand | D38122 | −3.17 | 0.021 | |
| TNF receptor superfamily, member 25 | U83598 | 2.39 | 0.045 | |
| Structural | Matrilin 3 | AJ001047 | −4.12 | 0.038 |
| Villin 1 | X12901 | −3.98 | 0.005 | |
| Desmocollin 3 | X83929 | −3.66 | 0.024 | |
| Coronin, actin binding protein, 2A | U57057 | −2.56 | 0.007 | |
| Myosin, nonmuscle | M69180 | −2.30 | 0.040 | |
| Utrophin | X69086 | 2.11 | 0.008 | |
| Elastin | X52896 | 2.31 | 0.048 | |
| Collagen, type VIII, alpha 2 | M60832 | 2.67 | 0.026 | |
| Signal transduction | T-cell lymphoma invasion and metastasis 1 | U16296 | −3.92 | 0.012 |
| Protein tyrosine phosphatase, type 11 | D13540 | −3.69 | 0.032 | |
| RAB3 GTPase-activating protein | D31886 | −2.67 | 0.048 | |
| Wiskott-Aldrich syndrome | U12707 | −2.31 | 0.026 | |
| Guanine nucleotide binding protein, alpha 13 | L22075 | 2.42 | 0.028 | |
| Guanylate cyclase 2C | M73489 | 2.51 | 0.030 | |
| G protein-coupled receptor 18 | L42324 | 2.79 | 0.049 | |
| Protein tyrosine phosphatase, nonreceptor type 9 | M83738 | 5.59 | 0.002 | |
| Protein tyrosine phosphatase, receptor type, M | X58288 | 6.19 | 0.001 | |
| Transcription factor | Zinc finger protein 77 | X65230 | −2.01 | 0.022 |
| Runt-related transcription factor 3 | Z35278 | 2.81 | 0.024 | |
| Transcription factor 4 | M74720 | 3.92 | 0.044 | |
| DNA binding protein for surfactant protein B | L10403 | 4.05 | 0.003 | |
| Zinc finger protein ZFP-36 | X51760 | 5.52 | 0.003 |
Data for genes with altered expression at more than one time point are in boldface.
Acknowledgments
This work was supported in part by Public Health Service grants AI42081 and RR15293 from the National Institutes of Health and by a postdoctoral fellowship through the Giannini Family Foundation (J.L.H.).
We thank Michael D. George for advice on microarray data analysis.
Editor: V. J. DiRita
REFERENCES
- 1.Almkvist, J., and A. Karlsson. 2004. Galectins as inflammatory mediators. Glycoconj. J. 19:575-581. [DOI] [PubMed] [Google Scholar]
- 2.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando, T., R. M. Peek, Jr., Y. C. Lee, U. Krishna, K. Kusugami, and M. J. Blaser. 2002. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin. Diagn. Lab. Immunol. 9:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton, J. C., K. T. Tham, R. M. Peek, Jr., T. L. Cover, and M. J. Blaser. 1996. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J. Infect. Dis. 174:552-556. [DOI] [PubMed] [Google Scholar]
- 5.Bach, S., A. Makristathis, M. Rotter, and A. M. Hirschl. 2002. Gene expression profiling in AGS cells stimulated with Helicobacter pylori isogenic strains (cagA positive or cagA negative). Infect. Immun. 70:988-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj-Elliott, M., P. Fedeli, G. V. Smith, P. Domizio, L. Maher, R. S. Ali, A. G. Quinn, and M. J. Farthing. 2002. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut 51:356-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, S. J., and E. P. Reddy. 1998. Modulation of life and death by the TNF receptor superfamily. Oncogene 17:3261-3270. [DOI] [PubMed] [Google Scholar]
- 8.Basu, S., and P. K. Srivastava. 2000. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones 5:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bebb, J. R., D. P. Letley, R. J. Thomas, F. Aviles, H. M. Collins, S. A. Watson, N. M. Hand, A. Zaitoun, and J. C. Atherton. 2003. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut 52:1408-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergin, P. J., E. Anders, W. Sicheng, J. Erik, A. Jennie, L. Hans, M. Pierre, P. H. Qiang, and Q. J. Marianne. 2004. Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter 9:201-210. [DOI] [PubMed] [Google Scholar]
- 11.Bevins, C. L., E. Martin-Porter, and T. Ganz. 1999. Defensins and innate host defence of the gastrointestinal tract. Gut 45:911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussioutas, A., H. Li, J. Liu, P. Waring, S. Lade, A. J. Holloway, D. Taupin, K. Gorringe, I. Haviv, P. V. Desmond, and D. D. Bowtell. 2003. Distinctive patterns of gene expression in premalignant gastric mucosa and gastric cancer. Cancer Res. 63:2569-2577. [PubMed] [Google Scholar]
- 14.Bouton, C. M., and J. Pevsner. 2002. DRAGON View: information visualization for annotated microarray data. Bioinformatics 18:323-324. [DOI] [PubMed] [Google Scholar]
- 15.Byrd, J. C., and R. S. Bresalier. 2000. Alterations in gastric mucin synthesis by Helicobacter pylori. World J. Gastroenterol. 6:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chattopadhyay, S., S. Datta, A. Chowdhury, S. Chowdhury, A. K. Mukhopadhyay, K. Rajendran, S. K. Bhattacharya, D. E. Berg, and G. B. Nair. 2002. Virulence genes in Helicobacter pylori strains from West Bengal residents with overt H. pylori-associated disease and healthy volunteers. J. Clin. Microbiol. 40:2622-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiou, C. C., C. C. Chan, D. L. Sheu, K. T. Chen, Y. S. Li, and E. C. Chan. 2001. Helicobacter pylori infection induced alteration of gene expression in human gastric cells. Gut 48:598-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chismar, J. D., T. Mondala, H. S. Fox, E. Roberts, D. Langford, E. Masliah, D. R. Salomon, and S. R. Head. 2002. Analysis of result variability from high-density oligonucleotide arrays comparing same-species and cross-species hybridizations. BioTechniques 33:516-518, 520, 522. [DOI] [PubMed] [Google Scholar]
- 19.Cole, A. M., T. Ganz, A. M. Liese, M. D. Burdick, L. Liu, and R. M. Strieter. 2001. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167:623-627. [DOI] [PubMed] [Google Scholar]
- 20.Covacci, A., and R. Rappuoli. 2003. Helicobacter pylori: after the genomes, back to biology. J. Exp. Med. 197:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox, J. M., C. L. Clayton, T. Tomita, D. M. Wallace, P. A. Robinson, and J. E. Crabtree. 2001. cDNA array analysis of cag pathogenicity island-associated Helicobacter pylori epithelial cell response genes. Infect. Immun. 69:6970-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabtree, J. E. 1998. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig. Dis. Sci. 43:46S-55S. [PubMed] [Google Scholar]
- 23.Crabtree, J. E., A. Covacci, S. M. Farmery, Z. Xiang, D. S. Tompkins, S. Perry, I. J. Lindley, and R. Rappuoli. 1995. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J. Clin. Pathol. 48:41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford, H. C., U. S. Krishna, D. A. Israel, L. M. Matrisian, M. K. Washington, and R. M. Peek, Jr. 2003. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 125:1125-1136. [DOI] [PubMed] [Google Scholar]
- 25.de Hostos, E. L. 1999. The coronin family of actin-associated proteins. Trends Cell Biol. 9:345-350. [DOI] [PubMed] [Google Scholar]
- 26.Drazek, E. S., A. Dubois, and R. K. Holmes. 1994. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J. Clin. Microbiol. 32:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, J. Del Valle, M. Yang, H. P. Wirth, G. I. Perez-Perez, and M. J. Blaser. 1999. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116:90-96. [DOI] [PubMed] [Google Scholar]
- 28.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, G. I. Perez-Perez, and M. J. Blaser. 1996. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect. Immun. 64:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois, A., N. Fiala, L. M. Heman-Ackah, E. S. Drazek, A. Tarnawski, W. N. Fishbein, G. I. Perez-Perez, and M. J. Blaser. 1994. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology 106:1405-1417. [DOI] [PubMed] [Google Scholar]
- 30.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 31.Fan, X., A. Long, M. Goggins, P. W. Keeling, and D. Kelleher. 1996. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut 38:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan, X. G., X. J. Fan, H. X. Xia, P. W. Keeling, and D. Kelleher. 1995. Up-regulation of CD44 and ICAM-1 expression on gastric epithelial cells by H. pylori. APMIS 103:744-748. [DOI] [PubMed] [Google Scholar]
- 33.Friedl, A., S. P. Stoesz, P. Buckley, and M. N. Gould. 1999. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem. J. 31:433-441. [DOI] [PubMed] [Google Scholar]
- 34.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 35.Gendler, S. J. 2001. MUC1, the renaissance molecule. J. Mammary Gland Biol. Neoplasia 6:339-353. [DOI] [PubMed] [Google Scholar]
- 36.George, M. D., S. Sankaran, E. Reay, A. C. Gelli, and S. Dandekar. 2003. High-throughput gene expression profiling indicates dysregulation of intestinal cell cycle mediators and growth factors during primary simian immunodeficiency virus infection. Virology 312:84-94. [DOI] [PubMed] [Google Scholar]
- 37.Gewirtz, A., Y. Yu, U. Krishna, D. Israel, S. Lyons, and R. Peek, Jr. 2003. Mechanisms through which Helicobacter pylori evades recognition by the host innate immune response. Int. J. Med. Microbiol. 293:118. [Google Scholar]
- 38.Gooz, M., M. Shaker, P. Gooz, and A. J. Smolka. 2003. Interleukin 1β induces gastric epithelial cell matrix metalloproteinase secretion and activation during Helicobacter pylori infection. Gut 52:1250-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guaita, S., I. Puig, C. Franci, M. Garrido, D. Dominguez, E. Batlle, E. Sancho, S. Dedhar, A. G. De Herreros, and J. Baulida. 2002. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J. Biol. Chem. 277:39209-39216. [DOI] [PubMed] [Google Scholar]
- 40.Guillemin, K., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 99:15136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamanaka, Y., M. Nakashima, A. Wada, M. Ito, H. Kurazono, H. Hojo, Y. Nakahara, S. Kohno, T. Hirayama, and I. Sekine. 2001. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 49:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 43.Houghton, J., J. G. Fox, and T. C. Wang. 2002. Gastric cancer: laboratory bench to clinic. J. Gastroenterol. Hepatol. 17:495-502. [DOI] [PubMed] [Google Scholar]
- 44.Hynes, S. O., S. Teneberg, N. Roche, and T. Wadstrom. 2003. Glycoconjugate binding of gastric and enterohepatic Helicobacter spp. Infect. Immun. 71:2976-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John, C. M., G. A. Jarvis, K. V. Swanson, H. Leffler, M. D. Cooper, M. E. Huflejt, and J. M. Griffiss. 2002. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell. Microbiol. 4:649-662. [DOI] [PubMed] [Google Scholar]
- 46.Jones, N. L., A. S. Day, H. A. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kayo, T., D. B. Allison, R. Weindruch, and T. A. Prolla. 2001. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc. Natl. Acad. Sci. USA 98:5093-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitadai, Y., A. Sasaki, M. Ito, S. Tanaka, N. Oue, W. Yasui, M. Aihara, K. Imagawa, K. Haruma, and K. Chayama. 2003. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem. Biophys. Res. Commun. 311:809-814. [DOI] [PubMed] [Google Scholar]
- 49.Konturek, J. W., H. Fischer, P. C. Konturek, V. Huber, P. Boknik, H. Luess, J. Neumann, T. Brzozowski, W. Schmitz, E. G. Hahn, W. Domschke, and S. J. Konturek. 2001. Heat shock protein 70 (HSP70) in gastric adaptation to aspirin in Helicobacter pylori infection. J. Physiol. Pharmacol. 52:153-164. [PubMed] [Google Scholar]
- 50.Kregel, K. C. 2002. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92:2177-2186. [DOI] [PubMed] [Google Scholar]
- 51.Lee, J. Y., E. M. Eom, D. S. Kim, Y. M. Ha-Lee, and D. H. Lee. 2003. Analysis of gene expression profiles of gastric normal and cancer tissues by SAGE. Genomics 82:78-85. [DOI] [PubMed] [Google Scholar]
- 52.Lee, S. A., D. Kang, K. N. Shim, J. W. Choe, W. S. Hong, and H. Choi. 2003. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J. Epidemiol. 13:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, C., and W. H. Wong. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim, J. W., H. Kim, and K. H. Kim. 2003. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int. J. Biochem. Cell Biol. 35:1284-1296. [DOI] [PubMed] [Google Scholar]
- 55.Lindsberg, P. J., J. Launes, L. Tian, H. Valimaa, V. Subramanian, J. Siren, L. Hokkanen, T. Hyypia, O. Carpen, and C. G. Gahmberg. 2002. Release of soluble ICAM-5, a neuronal adhesion molecule, in acute encephalitis. Neurology 58:446-451. [DOI] [PubMed] [Google Scholar]
- 56.Logdberg, L., and L. Wester. 2000. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim. Biophys. Acta 1482:284-297. [DOI] [PubMed] [Google Scholar]
- 57.Maeda, S., M. Otsuka, Y. Hirata, Y. Mitsuno, H. Yoshida, Y. Shiratori, Y. Masuho, M. Muramatsu, N. Seki, and M. Omata. 2001. cDNA microarray analysis of Helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem. Biophys. Res. Commun. 284:443-449. [DOI] [PubMed] [Google Scholar]
- 58.Maeda, S., H. Yoshida, Y. Mitsuno, Y. Hirata, K. Ogura, Y. Shiratori, and M. Omata. 2002. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Mol. Pathol. 55:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuzwa, M., H. Ota, M. Hayama, M. X. Zhang, K. Sano, T. Honda, I. Ueno, T. Akamatsu, and J. Nakayama. 2003. Helicobacter pylori infection up-regulates gland mucous cell-type mucins in gastric pyloric mucosa. Helicobacter 8:594-600. [DOI] [PubMed] [Google Scholar]
- 60.Milani, V., E. Noessner, S. Ghose, M. Kuppner, B. Ahrens, A. Scharner, R. Gastpar, and R. D. Issels. 2002. Heat shock protein 70: role in antigen presentation and immune stimulation. Int. J. Hyperthermia 18:563-575. [DOI] [PubMed] [Google Scholar]
- 61.Miyazaki, J., R. Hokari, S. Kato, Y. Tsuzuki, A. Kawaguchi, S. Nagao, K. Itoh, and S. Miura. 2002. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol. Rep. 9:1307-1312. [PubMed] [Google Scholar]
- 62.Moran, A. P. 1999. Helicobacter pylori lipopolysaccharide-mediated gastric and extragastric pathology. J. Physiol. Pharmacol. 50:787-805. [PubMed] [Google Scholar]
- 63.Mori, N., H. Sato, T. Hayashibara, M. Senba, R. Geleziunas, A. Wada, T. Hirayama, and N. Yamamoto. 2003. Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor κB. Gastroenterology 124:983-992. [DOI] [PubMed] [Google Scholar]
- 64.Murray, E., W. Khamri, M. M. Walker, P. Eggleton, A. P. Moran, J. A. Ferris, S. Knapp, Q. N. Karim, M. Worku, P. Strong, K. B. Reid, and M. R. Thursz. 2002. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect. Immun. 70:1481-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagasako, T., T. Sugiyama, T. Mizushima, Y. Miura, M. Kato, and M. Asaka. 2003. Up-regulated Smad5 mediates apoptosis of gastric epithelial cells induced by Helicobacter pylori infection. J. Biol. Chem. 278:4821-4825. [DOI] [PubMed] [Google Scholar]
- 66.Nedrud, J. G. 1999. Animal models for gastric Helicobacter immunology and vaccine studies. FEMS Immunol. Med. Microbiol. 24:243-250. [DOI] [PubMed] [Google Scholar]
- 67.Nishi, N., H. Shoji, M. Seki, A. Itoh, H. Miyanaka, K. Yuube, M. Hirashima, and T. Nakamura. 2003. Galectin-8 modulates neutrophil function via interaction with integrin αM. Glycobiology 13:755-763. [DOI] [PubMed] [Google Scholar]
- 68.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 69.Passaro, D. J., E. J. Chosy, and J. Parsonnet. 2002. Helicobacter pylori: consensus and controversy. Clin. Infect. Dis. 35:298-304. [DOI] [PubMed] [Google Scholar]
- 70.Peek, R. M., Jr. 2002. Helicobacter pylori strain-specific modulation of gastric mucosal cellular turnover: implications for carcinogenesis. J. Gastroenterol. 37(Suppl. 13):10-16. [DOI] [PubMed] [Google Scholar]
- 71.Peek, R. M., Jr. 2001. Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G525-G530. [DOI] [PubMed] [Google Scholar]
- 72.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 73.Peek, R. M., Jr., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863-868. [DOI] [PubMed] [Google Scholar]
- 74.Pelletier, I., T. Hashidata, T. Urashima, N. Nishi, T. Nakamura, M. Futai, Y. Arata, K. I. Kasai, M. Hirashima, J. Hirabayashi, and S. Sato. 2003. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 278:22223-22230. [DOI] [PubMed] [Google Scholar]
- 75.Philpott, D. J., D. Belaid, P. Troubadour, J. M. Thiberge, J. Tankovic, A. Labigne, and R. L. Ferrero. 2002. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell. Microbiol. 4:285-296. [DOI] [PubMed] [Google Scholar]
- 76.Piacentini, M., C. Evangelisti, P. G. Mastroberardino, R. Nardacci, and G. Kroemer. 2003. Does prothymosin-alpha act as molecular switch between apoptosis and autophagy? Cell Death Differ. 10:937-939. [DOI] [PubMed] [Google Scholar]
- 77.Rachmilewitz, J., G. J. Riely, and M. L. Tykocinski. 1999. Placental protein 14 functions as a direct T-cell inhibitor. Cell. Immunol. 191:26-33. [DOI] [PubMed] [Google Scholar]
- 78.Sallenave, J. M. 2002. Antimicrobial activity of antiproteinases. Biochem. Soc. Trans. 30:111-115. [DOI] [PubMed] [Google Scholar]
- 79.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Segal, E. D., S. Falkow, and L. S. Tompkins. 1996. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc. Natl. Acad. Sci. USA 93:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sepulveda, A. R., H. Tao, E. Carloni, J. Sepulveda, D. Y. Graham, and L. E. Peterson. 2002. Screening of gene expression profiles in gastric epithelial cells induced by Helicobacter pylori using microarray analysis. Aliment. Pharmacol. Ther. 16(Suppl. 2):145-157. [DOI] [PubMed] [Google Scholar]
- 82.Shimoyama, T., S. Fukuda, M. Tanaka, T. Mikami, A. Munakata, and J. E. Crabtree. 1998. CagA seropositivity associated with development of gastric cancer in a Japanese population. J. Clin. Pathol. 51:225-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slomiany, B. L., and A. Slomiany. 2001. Role of ERK and p38 mitogen-activated protein kinase cascades in gastric mucosal inflammatory responses to Helicobacter pylori lipopolysaccharide. IUBMB Life 51:315-320. [DOI] [PubMed] [Google Scholar]
- 84.Solnick, J. V., L. M. Hansen, N. R. Salama, J. K. Boonjakuakul, and M. Syvanen. 2004. Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. USA 101:2106-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solnick, J. V., D. R. Canfield, S. Yang, and J. Parsonnet. 1999. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Lab. Anim. Sci. 49:197-201. [PubMed] [Google Scholar]
- 86.Solnick, J. V., L. M. Hansen, D. R. Canfield, and J. Parsonnet. 2001. Determination of the infectious dose of Helicobacter pylori during primary and secondary infection in rhesus monkeys (Macaca mulatta). Infect. Immun. 69:6887-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song, H. H., and J. Filmus. 2002. The role of glypicans in mammalian development. Biochim. Biophys. Acta 1573:241-246. [DOI] [PubMed] [Google Scholar]
- 88.Srivastava, P. 2002. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2:185-194. [DOI] [PubMed] [Google Scholar]
- 89.Syder, A. J., J. D. Oh, J. L. Guruge, D. O'Donnell, M. Karlsson, J. C. Mills, B. M. Bjorkholm, and J. I. Gordon. 2003. The impact of parietal cells on Helicobacter pylori tropism and host pathology: an analysis using gnotobiotic normal and transgenic mice. Proc. Natl. Acad. Sci. USA 100:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Telford, J. L., P. Ghiara, M. Dell'Orco, M. Comanducci, D. Burroni, M. Bugnoli, M. F. Tecce, S. Censini, A. Covacci, Z. Xiang, et al. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 179:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todryk, S. M., M. J. Gough, and A. G. Pockley. 2003. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology 110:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uehara, N., A. Yagihashi, K. Kondoh, N. Tsuji, T. Fujita, H. Hamada, and N. Watanabe. 2003. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J. Med. Microbiol. 52:41-45. [DOI] [PubMed] [Google Scholar]
- 93.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 94.Van De Bovenkamp, J. H., J. Mahdavi, A. M. Korteland-Van Male, H. A. Buller, A. W. Einerhand, T. Boren, and J. Dekker. 2003. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter 8:521-532. [DOI] [PubMed] [Google Scholar]
- 95.Wehkamp, J., K. Schmidt, K. R. Herrlinger, S. Baxmann, S. Behling, C. Wohlschlager, A. C. Feller, E. F. Stange, and K. Fellermann. 2003. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J. Clin. Pathol. 56:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wen, S., C. P. Felley, H. Bouzourene, M. Reimers, P. Michetti, and Q. Pan-Hammarstrom. 2004. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. J. Immunol. 172:2595-2606. [DOI] [PubMed] [Google Scholar]
- 97.Wright, G. W., and R. M. Simon. 2003. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 19:2448-2455. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida, N., T. Ishikawa, E. Ichiishi, Y. Yoshida, K. Hanashiro, M. Kuchide, K. Uchiyama, S. Kokura, H. Ichikawa, Y. Naito, Y. Yamamura, T. Okanoue, and T. Yoshikawa. 2003. The effect of rebamipide on Helicobacter pylori extract-mediated changes of gene expression in gastric epithelial cells. Aliment. Pharmacol. Ther. 18(Suppl. 1):63-75. [DOI] [PubMed] [Google Scholar]
- 99.Yoshimura, T., T. Tomita, M. F. Dixon, A. T. Axon, P. A. Robinson, and J. E. Crabtree. 2002. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J. Infect. Dis. 185:332-340. [DOI] [PubMed] [Google Scholar]
- 100.Yu, Q., and I. Stamenkovic. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14:163-176. [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng, P. Y., and N. L. Jones. 2003. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 5:25-40. [DOI] [PubMed] [Google Scholar]
- 102.Zhu, Z., H. Friess, J. Kleeff, L. Wang, M. Wirtz, A. Zimmermann, M. Korc, and M. W. Buchler. 2002. Glypican-3 expression is markedly decreased in human gastric cancer but not in esophageal cancer. Am. J. Surg. 184:78-83. [DOI] [PubMed] [Google Scholar]
- 103.Zick, Y., M. Eisenstein, R. A. Goren, Y. R. Hadari, Y. Levy, and D. Ronen. 2004. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj. J. 19:517-526. [DOI] [PubMed] [Google Scholar]
- 104.Zou, J., S. Young, F. Zhu, F. Gheyas, S. Skeans, Y. Wan, L. Wang, W. Ding, M. Billah, T. McClanahan, R. L. Coffman, R. Egan, and S. Umland. 2002. Microarray profile of differentially expressed genes in a monkey model of allergic asthma. Genome Biol. 3:research0020. [DOI] [PMC free article] [PubMed] [Google Scholar]