Abstract
Candida albicans maintains both commensal and pathogenic states in humans. Both states are dependent on cell surface-expressed adhesins, including those of the Als family. Heterologous expression of Als5p at the surface of Saccharomyces cerevisiae results in Als5p-mediated adhesion to various ligands, followed by formation of multicellular aggregates. Following adhesion of one region of the cell to fibronectin-coated beads, the entire surface of the cells became competent to mediate cell-cell aggregation. Aggregates formed in the presence of metabolic inhibitors or signal transduction inhibitors but were reduced in the presence of 8-anilino-1-naphthalene-sulfonic acid (ANS) or Congo Red (CR), perturbants that inhibit protein structural transitions. These perturbants also inhibited aggregation of C. albicans. An increase in ANS fluorescence, which accompanied Als-dependent cellular adhesion, indicated an increase in cell surface hydrophobicity. In addition, C. albicans and Als5p-expressing S. cerevisiae showed an aggregation-induced birefringence indicative of order on the cell surface. The increase in birefringence did not occur in the presence of the aggregation disruptants ANS and CR. These results suggest a model for Als5p-mediated aggregation in which an adhesion-triggered change in the conformation of Als5p propagates around the cell surface, forming ordered aggregation-competent regions.
Infections due to fungal species are widespread and can lead to death in immunocompromised individuals. Candida albicans is the most common fungal opportunistic pathogen and can exist in a wide range of associations, from a superficial commensal state to systemic infections of a human or animal host. Typical cases of candidiasis in humans include oral and vaginal infections, whereas disseminated Candida infections are a significant cause of morbidity and mortality in immunocompromised patients (3, 21).
Adherence to a host and the subsequent aggregation of the infecting cells serve as initial and critical steps in establishment of C. albicans as a commensal inhabitant or pathogen. The importance of adherence is reflected in the reductions in virulence seen for strains with mutated adhesin genes or with mutations in genes that regulate adhesins (2, 9, 30). Following adherence, C. albicans grows in a colonial state. Indeed, in disseminated disease, microcolonies are present in target organ tissue and are diagnostic for disease (20).
Along with the characterized adhesins Hwp1 and Eap1p (25, 30), the C. albicans genome encodes the Als family of cell surface glycoproteins, which are apparently the most widely expressed adhesins (15, 17, 18). Als1p and Als5p are the best characterized of the Als adhesins (10-13, 16, 20, 27, 31).
Heterologous expression of Als5p in Saccharomyces cerevisiae has been used to identify several ligands, including extracellular matrix proteins, human buccal epithelial cells (11), homopolymers of threonine, serine, and alanine (13), and a tφ+ consensus sequence, which consists of successive turn-like, bulky hydrophobic, and cationic Lys or Arg residues (22). Kinetic analysis of Als5p-mediated adhesion has revealed that there is initial rapid adhesion to a ligand, followed by slower yeast cell-yeast cell binding interactions that form macroscopic aggregates (12). In this paper we refer to binding of cells to ligand-coated beads as adhesion and to cell-to-cell binding as aggregation. Both types of interactions occur over a broad pH range (pH 2 to 9) and are resistant to the shear forces generated by vortexing and to dissolution by nonchaotropic salt or sugar additives (12). Urea (6 M) or 50% formamide reversibly inhibits the adherence and aggregation phenotypes. This binding has been called stable reversible specific adherence (13).
MATERIALS AND METHODS
Cell transformation.
The previously described plasmid pGK114 was used to overexpress Als5p in S. cerevisiae (12). Plasmid pGK114 was transformed into the nonflocculent S. cerevisiae strain W303-1B by the lithium acetate method (14). Transformants were selected for the experiments reported here.
Adherence assays.
Adherence assays were performed as previously described (12). Briefly, S. cerevisiae cells containing pGK114 were grown with shaking at 30°C in YPGal medium (10 g of yeast extract per liter, 20 g of peptone per liter, 20 g of galactose per liter) to the late logarithmic phase for Als5p expression. For experiments with C. albicans, strain Ca1 cells was grown in YEPD medium (10 g of yeast extract per liter, 20 g of peptone per liter, 20 g of glucose per liter) to the stationary phase (∼1.50 × 108 cells/ml) (12). Approximately 5 ml of the culture was transferred to fresh YEPD medium, and the cells were incubated for 2 h to maximize ALS expression. Cells were harvested and washed with Tris-EDTA (TE) buffer (pH 7.0) three times and then resuspended in TE buffer. In a glass tube (13 by 100 mm) Als5p-expressing cells were mixed with fibronectin (FN)-coated magnetic beads at a cell-to-bead ratio of 100:1 in TE buffer, briefly vortexed, and incubated at room temperature with gentle shaking for 30 to 45 min. Each tube was vortexed briefly and immediately placed into a magnetic separator (Dynal). Adherent and aggregated cells were gently washed three times with TE buffer while the tube remained within the magnet. The cells were resuspended in TE buffer, and a sample was placed onto a microscope slide for examination. Cells were viewed with a Nikon Optiphot-2 microscope equipped with a Sony DK-5000 camera. For assays in which the effects of additives on adhesion were investigated, additives were added to the cell-bead mixture at the onset of the adherence assays.
Fluorescence of 8-anilino-1-naphthalene-sulfonic acid (ANS).
Adherence assays were performed as described above, except that following cellular incubation the cells were washed once with TE buffer. Aggregates were wet mounted, and photographs were taken after 7 min of exposure to UV light.
Birefringence assays.
Adherence assays were performed as described above. For observation of cell surface birefringence, samples were observed with polarized light under phase with a ×20 or ×40 objective. A second polarizer (analyzer) was inserted between the objective and the eyepiece of the camera and was rotated to give a completely dark background, as described previously (http://www.microscopyu.com/articles/polarized/polarizedintro.html). Micrographs wereexposed for 2 to 4 s at F2.8. For each figure, all micrographs were obtained under identical conditions.
Antibody inhibition and immunofluorescence assays.
Polyclonal Als-specific antibodies were raised in rabbits following injection with the N-terminal region of Als1p. The antibodies were adsorbed twice overnight with heat-killed S. cerevisiae W303-1B before they were used. For adherence assays in which antibodies were used, cells were preincubated with anti-Als antibodies at a ratio of 1:40 for 1 h before the onset of the experiment.
For immunofluorescence assays, S. cerevisiae cells expressing Als5p (cells grown with galactose) and nonexpressing cells (cells grown with glucose or cells without pGK114) were incubated with anti-Als polyclonal antibodies at a ratio of 1:50 for 1 h, and this was followed by three washes in phosphate-buffered saline (pH 7.00). The cells were then incubated with S. cerevisiae-adsorbed fluorescein-labeled goat secondary antibodies at a ratio of 1:100 for 1 h and washed as described above. Cells were examined microscopically.
RESULTS
Effects of additives on adherence and aggregation of ALS5-transformed S. cerevisiae cells.
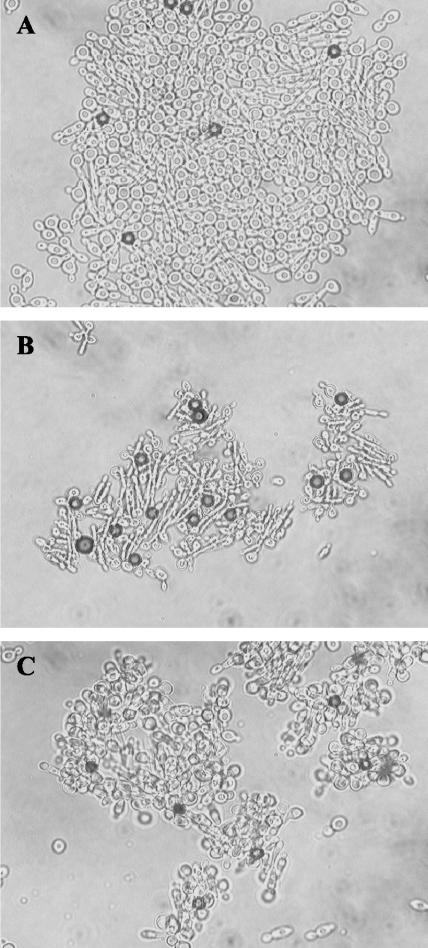
In W303-1B, a nonflocculent strain of S. cerevisiae, galactose-induced expression of Als5p led to adhesion of the cells to FN-coated beads and formation of large aggregates that included cell-to-cell associations (Fig. 1A). The adherence and aggregation were similar to those seen in S. cerevisiae strain YPH499 (11-13). Neither adhesion to beads nor aggregation was seen in nontransformed cells (Fig. 1F) or in transformed cells grown in glucose (data not shown). Adherence to ligand beads and cellular aggregation were similar for nontreated control cells and for cells incubated with cycloheximide (10 μg/ml) (Fig. 1B) or sodium azide (10 mM) (Fig. 1C). The signal transduction inhibitors adenosine-5′(β,γ-imido)triphosphate (AMPNP; 300 μM), staurosporine (30 μM) (4), and okadaic acid (2 μM) also had no effect (data not shown). There was also adherence or aggregation in experiments in which Als5p-expressing cells were heat killed prior to the adherence assay (data not shown). Quantification of adhered and aggregated cells showed similar binding for controls and samples treated with cycloheximide or sodium azide (Fig. 2).
FIG. 1.
Effects of additives on adherence and aggregation of ALS5-transformed S. cerevisiae cells. (A) S. cerevisiae strain W303-1B expressing Als5p binding to FN-coated beads (dark spheres) and adhering to other cells. (B) Cycloheximide (10 μg/ml) added to the cell-bead mixture at the onset of the experiment. (C) Sodium azide (1 mM) added at the onset of the experiment. (D) Adhesion and aggregation in the presence of ANS (100 μM). (E) Cells treated with CR (30 μM). (F) Nontransformed S. cerevisiae W303-1B cells. Bar = 10 μm.
FIG. 2.
Quantitative analysis of adherence and aggregation. Cellular aggregates were disrupted by addition of 0.1 M NaOH, and cells and beads were counted with a hemocytometer. The data are means and standard deviations from three separate experiments.
Effects of antibodies on adherence and aggregation.
When Als5p expression was induced by growth in galactose, the protein was displayed at the surface of intact cells and reacted with a polyclonal antibody raised against Als1p (Fig. 3A and B) (9). The pGK114-transformed cells showed no fluorescence after growth in repressing glucose medium (Fig. 3C and D). Cells not transformed with pGK114 also showed no fluorescence (data not shown). An Als1p-specific monoclonal antibody (9) did not react with Als5p-expressing cells (data not shown).
FIG. 3.
Antibodies raised against Als1p inhibit Als5p-mediated adhesion and aggregation. (A) Cell surface fluorescence of Als5p-expressing S. cerevisiae cells. (B) Bright-field micrograph of the cells in panel A. (C) Nonexpressing S. cerevisiae cells (grown in glucose). (D) Bright-field micrograph of the cells in panel C. (E) Adherence assay with Als5p-expressing S. cerevisiae in the presence of Als1p antibodies. (F) Adherence assay in the absence of antibodies.
The polyclonal antibody also inhibited both adherence to beads and cell-to-cell binding (Fig. 3E and F). This result implied that cell surface Als5p was essential for both the adhesion and the cell-to-cell binding of the Als5p-expressing S. cerevisiae cells.
Als5p-mediated aggregation in the presence of ANS and Congo red (CR).
ANS is fluorescent in hydrophobic environments, so it is commonly used to monitor secondary structure changes during protein folding and unfolding (1, 23, 24, 28, 29). ANS (100 μM) reduced aggregation in assays of Als5p-expressing cells (Fig. 1D and 4A) and resulted in a fivefold decrease in the number of cells per bead (Fig. 2). There was also a marked increase in the fluorescence of aggregating cells compared to that of nonaggregating ALS5-expressing cells or nontransformed S. cerevisiae cells (Fig. 4A to C). Reduced cellular aggregation and increased fluorescence associated with ANS treatment were also observed in C. albicans (Fig. 5A and C and Fig. 4D and E). The surfaces of adherent cells were uniformly fluorescent or not fluorescent rather than fluorescent only in the region next to the ligand bead (Fig. 4A and D). This observation implied that Als5p molecules around the entire surface had changed conformation to show similar exposure of hydrophobic regions.
FIG. 4.
Increased ANS surface fluorescence accompanies Als5p-mediated adhesion and aggregation. Adherence assays were performed with S. cerevisiae in the presence of 100 μM ANS. (A) Als5p-expressing cells binding to FN-coated beads and to other cells. (B) Als5p-expressing cells in the absence of FN-coated beads. (C) Nonexpressing S. cerevisiae cells. Bar = 10 μm. There are similar numbers of cells in panels B and C. (D) Cellular aggregation of C. albicans Ca1 cells incubated with FN-coated beads in the presence of 1 mM ANS. (E) ANS-treated Ca1 cells in the absence of beads.
FIG. 5.
Effects of ANS and CR on C. albicans adherence and aggregation with FN-coated beads. (A) No additive. (B) CR (300 μM) added. (C) ANS (1 mM) added.
The dye CR stains amyloid structures and inhibits their formation (5, 26, 29). Like ANS, CR significantly reduced cellular aggregation in S. cerevisiae expressing Als5p (Fig. 1E and 2) and in C. albicans (Fig. 5A and B). CR had no effect in aggregation assays of cells that did not express Als5p.
Surface birefringence of Als5p aggregates.
When Als5p-mediated cellular aggregates of S. cerevisiae were viewed between crossed polarizers, the cell surface was clearly birefringent, whereas there was less birefringence in nonaggregating cells (Fig. 6A and B). Inspection of Als5p-expressing cells throughout the course of the adherence assay showed that cell surface birefringence increased as the size of the aggregates increased but was most apparent after 30 min of the assay (Fig. 7). The aggregation-induced birefringence was observed in the presence of metabolic inhibitors (data not shown) but was absent from the small adhesive cell groups seen in the presence of ANS or CR (Fig. 6C and D). Cell surface birefringence was also observed in adherence assays with C. albicans and was much greater in the presence of FN-coated beads than in their absence (Fig. 6E and F). To determine whether the surface birefringence mediated by Als5p aggregation was a general property of yeast cell-yeast cell binding, we also observed the cell surface of S. cerevisiae strain YIY345 during Flo11p-mediated flocculation and sexual aggregates of S. cerevisiae formed through sexual agglutinins. In neither case was there surface birefringence that was greater than that in nonaggregated controls (data not shown).
FIG. 6.
Als5p-mediated aggregation increases cell surface birefringence. Cells were viewed between crossed polarizers. (A) Cellular aggregation mediated by S. cerevisiae expressing Als5p following incubation with FN-coated beads. (B) Als5p-expressing cells mixed with uncoated magnetic beads and centrifuged prior to observation. (C) Als5p-expressing cells incubated in the presence of 100 μM ANS. (D) Als5p-expressing cells incubated in the presence of 30 μM CR. (E) Cellular aggregation of C. albicans strain Ca1 in the presence of FN-coated beads. (F) Ca1 cells in the absence of FN-coated beads. Bar = 50 μm.
FIG. 7.
Kinetic analysis of Als5p-mediated cell surface birefringence. Aggregation assays were performed, and cells were monitored after 5 min (A), 15 min (B), and 30 min (C to E) of incubation with FN-coated beads. (C, D, and E) Different fields from the same experiment.
DISCUSSION
Als5p mediates adhesion to FN and cellular aggregation.
Previous studies showed that Als5p expression in S. cerevisiae results in a rapid cell-to-ligand adhesion step, followed by slower cell-to-cell binding, which we call aggregation (12). The biphasic kinetics suggest that following adhesion to a ligand, Als5p may undergo a modification that mediates cellular aggregation. Such a modification might include covalent modification of the cell surface Als5p, synthesis and display of new adhesin molecules with aggregative properties, or a noncovalent modification of the Als5p structure.
Surface display of Als5p resulted in adhesion to FN-coated beads and cellular aggregation. Both interactions were inhibited by a specific antibody that reacts with Als5p. In addition, indirect immunofluorescence confirmed Als5p surface localization. Together with galactose-induced adhesion and aggregation, these results imply that cell surface Als5p is necessary and sufficient for binding to FN-coated beads and aggregation.
To eliminate the possibility that the Als5p-mediated aggregation phenotype resulted from adherence-induced expression of an altered, aggregate-competent version of Als5p or from induction of another adhesin altogether, aggregation assays were performed in the presence of various inhibitors. Adhesion and aggregation were similar for nontreated Als5p-expressing cells and cells treated with inhibitors of signal transduction, intermediary metabolism, or protein synthesis and indeed for heat-killed cells. These results indicated that aggregation occurs independent of cellular metabolic activity.
ANS fluorescence increases upon aggregation.
On the other hand, there is evidence which supports the hypothesis that there is a ligand-induced change in the three-dimensional structure of Als5p. The fluorescent dye ANS has been extensively used to study protein surface hydrophobicity and changes in exposure of hydrophobic regions after structural transitions (1, 23, 24, 28). Indeed, ANS fluorescence revealed an increase in cell surface hydrophobicity upon aggregation. Primary and secondary amino acid sequence analyses of Als5p indicated that there was a significant amount of residue hydrophobicity and predicted that a conformational shift would correlate with a change in ANS fluorescence (7, 16). The fluorescence was greater for aggregated cells than for nonaggregating Als5p-expressing cells and untransformed control S. cerevisiae cells. The binding and fluorescence of ANS were uniform around the entire cell surface. Additionally, treatment with ANS reduced the Als5p-mediated aggregation. These results of cellular aggregation assays mimicked the effects of ANS in assays of protein unfolding for carbonic anhydrase and other proteins (1, 23, 24, 28). In those studies the dye revealed increased surface hydrophobicity during denaturation. Furthermore, in the presence of ANS, protein aggregation is decreased (1, 23, 24, 28). Thus, the characteristics of dye binding in the cellular aggregation assays imply that a similar structural transition is involved in the conversion to competence for aggregation and is dependent on the presence of both Als5p and its ligand, FN.
Amyloid-binding dye CR inhibits aggregation.
The dye CR stains amyloid proteins (5, 26). CR has a strong affinity for proteins with high β-sheet secondary structure contents, like Als5p, and inhibits formation of amyloid protein aggregates (16, 26). To determine whether CR inhibited Als5p-mediated cellular aggregates, adherence assays were performed in the presence of 30 μM CR. Similar to our results obtained with ANS, CR treatment of Als5p-expressing cells inhibited aggregation. This result is similar to the CR-mediated inhibition of amyloid formation in other systems (26). Thus, aggregation of Als5p-expressing cells was not affected by metabolic poisons but was greatly reduced by two agents that perturb protein secondary structure.
Als5p-mediated aggregation results in increased order in cell surface molecules.
Molecular birefringence is a qualitative indicator of ordered and regular structure. When viewed under polarized light, depending on the molecular orientation of the material in the light path, anisotropic substances refract light so that there are variations in the brightness and/or color of an image (6, 8, 19). If the formation of Als5p-mediated cellular aggregates is dependent on the formation of an ordered array of Als5p molecules, then we would expect an increase in cell surface birefringence for aggregating cells compared to that for nonaggregating cells. Such birefringence would reflect a change from a more isotropic state to a more anisotropic state. Our results show that Als5p-mediated aggregates of S. cerevisiae are more birefringent than nonaggregating cells expressing Als5p or cells aggregated by other adhesions. Similar birefringent aggregates were observed in C. albicans. (Note that birefringence of C. albicans cannot be attributed to a function of Als5p activity exclusively, since C. albicans may also express other Als proteins, as well as other surface adhesions [9, 15].) CR or ANS prevented the development of increased cell surface birefringence. These results are also consistent with the idea that Als5p undergoes a conformational shift to form ordered domains on the cell surface in cellular aggregates. Thus, development of birefringence in S. cerevisiae depended on expression Als5p and the presence of beads coated with the Als5p ligand. In C. albicans, the presence FN-coated beads in aggregates also increased birefringence (Fig. 6E and F).
All of the results suggest a model in which Als proteins bind to a few bead-bound peptide ligands (22) and then undergo a structural change to an aggregative state. This transition propagates around the entire cell surface, and the kinetics of aggregation imply that the change takes place over 5 to 30 min (12). The aggregative state is characterized by the formation of birefringent and hydrophobic domains on the entire surface of the cell. The increases in both ANS fluorescence and birefringence show long-range order around the entire surface of individual cells. Thus, the ordered state appears to encompass the surface of each cell following ligand binding at a single region of the cell surface.
The development of birefringence was Als5p specific in S. cerevisiae, dependent on binding to ligand-coated beads, and independent of cellular metabolism, and it was inhibited by ANS and CR, agents that perturb secondary structure. Furthermore, although ANS and CR compete for aggregative cell-to-cell binding, the increase in ANS fluorescence documents that there are ligand-induced structural changes in Als5p that accompany the transition to the aggregative state. This change is coupled with a global increase in cell surface hydrophobicity for the aggregative state. The similarity in the behaviors of Als5p-expressing S. cerevisiae and C. albicans, which expresses many Als proteins, implies that similar molecular properties mediate adhesion and aggregation in the pathogenic organism. The structural and functional similarities in the Als proteins support this idea (9, 13, 15, 22).
Acknowledgments
We thank Anne Dranginis (St. Johns University) for the Flo11p-expressing strains. We also thank Anne Dranginis, Laurel Eckhardt, Shirley Raps, and Rafael Ovalle for their thoughtful comments on the manuscript.
Support for this work was provided by the NIGMS-MBRS/SCORE Program under grant S06 60654 to Hunter College, NSF Magnet-AGEP and NIH Ruth Kirschstein fellowships to J.M.R., and the Southern Arizona Veterans Administration. J.E.E. is supported by grant R01 AI19990 from the National Institute of Allergy and Infectious Diseases. Core facilities at Hunter College are supported by NIH RCMI Program grant RR 03037.
Editor: T. R. Kozel
REFERENCES
- 1.Bailey, R. W., A. K. Dunker, C. J. Brown, E. C. Garner, and M. D. Griswold. 2001. Clusterin, a binding protein with a molten globule-like region. Biochemistry 40:11828-11840. [DOI] [PubMed] [Google Scholar]
- 2.Buurman, E. T., C. Westwater, B. Hube, A. J. Brown, F. C. Odds, and N. A. Gow. 1998. Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 95:7670-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderone, R. A. (ed.). 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 4.Chai, B., J. M. Hsu, J. Du, and B. C. Laurent. 2002. Yeast RSC function is required for organization of the cellular cytoskeleton via an alternative PKC1 pathway. Genetics 161:575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman, M. R., L. S. Robinson, J. S. Pinkner, R. Roth, J. Heuser, M. Hammar, S. Normark, and S. J. Hultgren. 2002. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Campos Vidal, B., and H. F. de Carvalho. 1990. Aggregational state and molecular order of tendons as a function of age. Matrix 10:48-57. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg, D., E. Schwarz, M. Komaromy, and R. Wall. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125-142. [DOI] [PubMed] [Google Scholar]
- 8.Feitosa, V., B. C. Vidal, and E. R. Pimentel. 2002. Optical anisotropy of a pig tendon under compression. J. Anat. 200:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y. C. Chen, S. W. French, J. E. Cutler, S. G. Filler, and J. E. Edwards, Jr. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed] [Google Scholar]
- 10.Fu, Y., G. Rieg, W. A. Fonzi, P. H. Belanger, J. E. Edwards, Jr., and S. G. Filler. 1998. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect. Immun. 66:1783-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaur, N. K., and S. A. Klotz. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaur, N. K., S. A. Klotz, and R. L. Henderson. 1999. Overexpression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect. Immun. 67:6040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur, N. K., R. L. Smith, and S. A. Klotz. 2002. Candida albicans and Saccharomyces cerevisiae expressing ALA1/ALS5 adhere to accessible threonine, serine, or alanine patches. Cell Commun. Adhes. 9:45-57. [DOI] [PubMed] [Google Scholar]
- 14.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 15.Hoyer, L. L. 2001. The ALS gene family of Candida albicans. Trends Microbiol. 9:176-180. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer, L. L., and J. E. Hecht. 2001. The ALS5 gene of Candida albicans and analysis of the Als5p N-terminal domain. Yeast 18:49-60. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer, L. L., and J. E. Hecht. 2000. The ALS6 and ALS7 genes of Candida albicans. Yeast 16:847-855. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer, L. L., T. L. Payne, and J. E. Hecht. 1998. Identification of Candida albicans ALS2 and ALS4 and localization of Als proteins to the fungal cell surface. J. Bacteriol. 180:5334-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue, S. 1987. Video microscopy. Plenum Press, New York, N.Y.
- 20.Kamai, Y., M. Kubota, T. Hosokawa, T. Fukuoka, and S. G. Filler. 2002. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect. Immun. 70:5256-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klepser, M. E., R. E. Lewis, and M. A. Pfaller. 1998. Therapy of Candida infections: susceptibility testing, resistance, and therapeutic options. Ann. Pharmacother. 32:1353-1361. [DOI] [PubMed] [Google Scholar]
- 22.Klotz, S. A., N. K. Gaur, D. F. Lake, V. Chan, J. Rauceo, and P. N. Lipke. 2004. Degenerate peptide recognition by the Candida albicans adhesins Als5p and Als1p. Infect. Immun. 72:2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu, B., and P. Guptasarma. 1999. Hydrophobic dye inhibits aggregation of molten carbonic anhydrase during thermal unfolding and refolding. Proteins 37:321-324. [DOI] [PubMed] [Google Scholar]
- 24.Kundu, B., and P. Guptasarma. 2002. Use of a hydrophobic dye to indirectly probe the structural organization and conformational plasticity of molecules in amorphous aggregates of carbonic anhydrase. Biochem. Biophys. Res. Commun. 293:572-577. [DOI] [PubMed] [Google Scholar]
- 25.Li, F., and S. P. Palecek. 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell 2:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenzo, A., and B. A. Yankner. 1994. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. USA 91:12243-12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loza, L., Y. Fu, A. S. Ibrahim, D. C. Sheppard, S. G. Filler, and J. E. Edwards, Jr. 2004. Functional analysis of the Candida albicans ALS1 gene product. Yeast 21:473-482. [DOI] [PubMed] [Google Scholar]
- 28.Maiti, N. R., and W. K. Surewicz. 2001. The role of disulfide bridge in the folding and stability of the recombinant human prion protein. J. Biol. Chem. 276:2427-2431. [DOI] [PubMed] [Google Scholar]
- 29.Plakoutsi, G., N. Taddei, M. Stefani, and F. Chiti. 2004. Aggregation of the acylphosphatase from S. solfataricus: the folded and partially unfolded states can be both precursors for amyloid formation. J. Biol. Chem. 279:14111-14119. [DOI] [PubMed] [Google Scholar]
- 30.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstrom. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]
- 31.Zhao, X., C. Pujol, D. R. Soll, and L. L. Hoyer. 2003. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9. Microbiology 149:2947-2960. [DOI] [PubMed] [Google Scholar]