Abstract
Reactive arthritis (ReA) induced by infection with several gram-negative bacteria is strongly associated with expression of the major histocompatibility complex class I molecule HLA-B27. It is thought that due to the intracellular lifestyle of ReA-inducing bacteria, bacterial fragments can be presented by HLA-B27. Cytotoxic T cells recognizing such bacterial peptides or other induced host peptides could cross-react with self peptides presented in the joints, giving rise to disease. Studies to analyze the B27 peptide repertoire in relation to infection were severely hampered, as complex peptide profiles obtained from separate infected and noninfected cell preparations had to be compared. For this study, we applied a new approach to examine the effect of Salmonella enterica serovar Typhimurium infection on the B27 peptide repertoire presented by the HLA-B*2704 subtype associated with disease. Firstly, we showed that both host cell and S. enterica serovar Typhimurium proteins can be tagged metabolically with stable-isotope-labeled arginine. We then designed experiments so that either the tagged endogenous or tagged bacterial B*2704-presented peptide repertoires from infected cells could be analyzed by mass spectrometry from single peptide preparations that included uninfected controls. Using this new approach, we found no evidence for significant changes in endogenous B*2704 peptide presentation after infection or for any S. enterica serovar Typhimurium-derived B27-bound peptide. In conclusion, the hypothesis that S. enterica serovar Typhimurium induces changes in B27 peptide presentation could not be supported.
The major histocompatibility complex (MHC) class I molecule HLA-B27 confers increased susceptibility to spondyloarthropathies (SpA) such as ankylosing spondylitis and reactive arthritis (ReA) (7, 8). The HLA-B27 association is so strong that a pathogenetic role for the B27 molecule itself is supposed (4, 6, 27). In addition, HLA-B27 transgenic rats and mice develop diseases resembling human SpA (21, 25, 55).
ReA occurs after gastrointestinal infection with several gram-negative bacteria, including Salmonella, Shigella, and Yersinia species, or upon urogenital infection with Chlamydia trachomatis (9). Among several theories to explain the association of B27 with ReA, the arthritogenic peptide hypothesis (3) focuses on the peptide-presenting function of HLA-B27. In its simplest form, this hypothesis proposes that after infection with one of the aforementioned bacteria, B27 presents an arthritogenic peptide resembling certain self peptides to B27-restricted cytotoxic T lymphocytes (CTLs), thus triggering an autoimmune reaction against these self peptides.
Several findings support this hypothesis. Classical MHC class I molecules like B27 mainly present peptides derived from endogenous sources (17, 18). A general feature of the bacteria inducing ReA is their ability to enter nonprofessional phagocytic cells, suggesting that these microorganisms might be processed for presentation by HLA-B27 (11, 12, 52). Although the relevance of MHC class I-presented Salmonella peptides in immunity against Salmonella is not completely clear, Salmonella-specific MHC class I-restricted CTLs have been detected in experimentally infected mice (28, 33, 34, 40). The strongest support for a role of peptides in the pathogenesis of B27-associated diseases is provided by B27 subtype studies. It has been demonstrated that not all subtypes predispose the host to SpA. While B*2705 and B*2704 are associated with SpA, B*2706 and B*2709 are not or are only weakly associated with SpA (30, 37, 42). The disease association of these subtypes apparently correlates with the peptide-presenting domain, which contains nearly all HLA-B27 subtype polymorphisms (32, 46).
Since B27-restricted Salmonella- and Yersinia-specific CTL clones from the synovium of a ReA patient were isolated (23), efforts were undertaken to identify the peptide(s) presented by B27 to these CTLs (44, 51). Until now, for the elucidation of which bacterial peptides are presented by HLA-B27 upon infection, reversed-phase chromatography-fractionated peptide repertoires isolated from B27-expressing cells with or without prior infection were compared. Such comparative data analysis has yielded very limited information so far, although slight differences have been suggested to occur after Salmonella and Shigella infections (5, 31, 41, 43). The comparative approach poses several problems. Firstly, peptides isolated from classical HLA class I molecules form very complex mixtures, and the arthritogenic bacterially derived peptide(s) might be present at a very low copy number per cell, making it very difficult to detect. Secondly, a comparison of peptide profiles from infected cells and noninfected cells, each obtained in separate experiments, is clearly not an accurate and reliable method to assess quantitative differences related to infection.
For our study, we used another approach which was developed by Meiring et al. (35) to study quantitative changes in SpA-associated B*2704 peptide presentation after Salmonella enterica serovar Typhimurium infection. The setup is based on stable isotope labeling of proteins, which is now commonly used in proteomic studies (20, 38). Peptides from control cells as well as from infected cells were isolated and purified together. By this approach, we eliminated artifacts induced by separate peptide purification and chromatographic procedures, which commonly frustrate the comparative data analysis approach, and were able to detect bacterially derived peptides with greater ease.
MATERIALS AND METHODS
Bacteria, cell lines, and antibodies.
The B*2704 transfectant of the human lymphoid cell line Hmy2.C1R-B*2704 (47, 54) (C1R-B*2704), with low-level expression of its endogenous MHC class I molecules, was described in detail previously (16). C1R-B*2704 cells were cultured in RPMI 1640 (Gibco) supplemented with 5% fetal calf serum (FCS; Boehringer, Mannheim, Germany) and 25 mM HEPES and were expanded to the required amount of cells in 2-liter roller bottles (Becton Dickinson, Grenoble, France). All infection experiments were performed with the virulent S. enterica serovar Typhimurium strain SL1344 (19). The hybridoma W6/32 (2), which produces a monoclonal antibody specific for the trimeric structure of the HLA class I heavy chain, β2-microglobulin, and peptide, was obtained from the American Type Culture Collection.
Infection of C1R-B*2704 cells with S. enterica serovar Typhimurium.
S. enterica serovar Typhimurium cells were grown overnight in Luria-Bertani (LB) medium at 37°C without shaking. Next, the bacterial suspension was diluted 1:100 in 500 ml of RPMI 1640 (Gibco) and incubated for 4 h at 37°C with shaking until an optical density at 530 nm of 0.7 was reached. Prior to infection, about 3 × 109 C1R-B*2704 cells grown in roller bottles were collected by centrifugation and resuspended in two 175-cm2 culture flasks with filter caps (Nunc, Roskilde, Denmark) containing 200 ml of RPMI 1640 supplemented with 5% FCS and 25 mM HEPES. The bacterial cells were collected by centrifugation at 1,000 × g for 12 min and were divided between the two flasks containing the C1R-B*2704 cells, resulting in a multiplicity of infection of 50 to 100 bacteria per cell. The C1R-B*2704 cells and the bacteria were incubated at 37°C in an atmosphere containing 5% CO2 for 1 h. The cells were then collected by centrifugation at 500 × g for 12 min. Cell pellets were washed twice with RPMI 1640 supplemented with 5% FCS to remove nonadherent bacteria and were resuspended in four 175-cm2 culture flasks containing 200 ml of RPMI 1640 supplemented with 25 mM HEPES, 5% FCS, and 100 μg of gentamicin/ml to kill extracellular bacteria. After the cells were incubated at 37°C in 5% CO2 for 4 h, they were collected, washed three times with cold phosphate-buffered saline (Gibco, Paisley, Scotland), and finally pelleted by centrifugation at 800 × g for 12 min. At this time point, the number of intracellular bacteria was determined as described previously (43) to check whether a similar number of intracellular bacteria was obtained as before (43, 52). Cell pellets were snap frozen in liquid nitrogen and stored at −70°C until the isolation of B27 molecules. For infections with labeled bacteria, a suspension of S. enterica serovar Typhimurium grown overnight in LB was diluted 100-fold in custom-made RPMI 1640 without arginine (Gibco) supplemented with 250 μg of labeled arginine/ml (two 14N atoms were replaced with two 15N atoms, creating l-arginine-guanido[15N2] · HCl) (Cambridge Isotope Laboratories). This dilution was then incubated at 37°C with shaking until an optical density at 530 nm of 0.7 was reached. The labeled bacteria were mixed with bacteria grown in normal RPMI 1640 (Gibco) at a ratio of 1:1 before use in the infection experiments at a total multiplicity of infection of 50 to 100.
Labeling of C1R-B*2704 cells.
To study changes in endogenous peptide presentation upon S. enterica serovar Typhimurium infection, we cultured C1R-B*2704 cells for 4 days in custom-made RPMI 1640 (Gibco) without arginine to which 250 μg of l-arginine-guanido[15N2] · HCl (Cambridge Isotope Laboratories)/ml as well as 5% FCS and 25 mM HEPES had been added. During the infection of labeled cells, all incubations of the cells were carried out in custom-made RPMI 1640 without arginine (Gibco) to which 250 μg of labeled arginine/ml had been added. Four hours after infection, infected labeled C1R-B*2704 cells were mixed at a ratio of 1:1 with uninfected unlabeled C1R-B*2704 cells before the cells were collected for storage at −70°C. A control labeling experiment was performed exactly as described above, but without bacteria.
Isolation of B*2704-associated peptides.
The isolation of peptides was performed as described previously (43), with the following modifications. Cells (3 × 109) were lysed in lysis buffer containing 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (Roche) instead of NP-40 and including a cocktail of protease inhibitors. B27-peptide complexes were immunoprecipitated with the monoclonal antibody W6/32 coupled to CNBr-activated Sepharose (Pharmacia). After a thorough washing, the peptides were acid eluted from the bound MHC molecules with 10% acetic acid instead of 0.1% trifluoroacetic acid (TFA). The eluate was passed through a 10-kDa-cutoff Centricon Plus-20 PL-10 filter device (Amicon, Beverly, Mass.) to remove high-molecular-weight material. After concentration by freeze-drying, the peptide extracts were further purified in portions equivalent to 5 × 108 cells by reversed-phase chromatography using 0.2-mm by 2-cm reversed-phase columns with a Hypercarb carbon (Thermo Electron) stationary phase (packed in-house). Peptides were eluted with an acetonitrile gradient (0 to 80%) with 0.1% TFA in water and were collected in one fraction that was then dried and dissolved in 0.1% TFA in water for mass spectrometric analysis.
Nanoscale LC-MS.
Purified B27-bound peptide extracts were analyzed by nanoscale reversed-phase high-performance liquid chromatography (HPLC)-electrospray ionization-mass spectrometry (nanoscale LC-ESI-MS) as described previously (36), with some minor modifications. B27-bound peptides were injected in a 10-μl mixture containing 0.1% TFA in water for transfer onto a 3-cm by 100-μm Aqua reversed-phase trapping column (Phenomenex, Torrance, Calif.) (packed in-house) with water containing 0.01% TFA at a flow rate of 3 μl/min for 10 min. After being trapped, the peptides were eluted from the trapping column directly onto a 50-μm by 23-cm Aqua analytical column (packed in-house) by use of a linear gradient from 0.01% TFA in water to 0.1 M acetic acid containing 45% acetonitrile in 112 min, at a flow rate of 125 nl/min. The column effluent was passed directly into a disposable gold-coated 5-μm-long ID nano-ESI emitter (Nanoseparations, Bilthoven, The Netherlands) (36) butt connected to the analytical column for online ESI-MS measurements. Peptide identities were confirmed by comparing LC retention times and MS/MS spectra obtained from synthetic peptide analogues.
ESI-MS.
Mass spectra were obtained with either an LCQ Classic quadrupole ion trap mass spectrometer (Finnigan MAT, San Jose, Calif.) or a Micromass LCT orthogonal acceleration time of flight mass spectrometer (Waters, Milford, Mass.). Positive ion mass spectra were acquired in the range from m/z 350 to m/z 1,500. Ionization for the LCQ instrument was performed at an electrospray voltage of 1.7 kV and a capillary temperature of 150°C. Ionization for the LCT instrument was performed at an electrospray voltage of 2.4 kV and a source temperature of 90°C. The scan time for the LCT was 0.5 s with an interscan delay of 0.1 s.
Collision-induced dissociation (CID) mass spectra were obtained with the LCQ instrument at a relative collision energy of 35%. The maximum injection time was 150 ms. The isolation width of the parent ion was 3 Da.
MALDI-TOF.
For matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis, a few microliters of several peptide solutions were concentrated on a C18 ZipTip (Millipore) and eluted in 5 μl of 60% acetonitrile-1% HCOOH. Next, 0.5 μl of this solution was mixed with 0.5 μl of a solution of 52 mM α-cyano-4-hydroxycinnamic acid in 49% ethanol-49% acetonitrile-2% TFA and 1 mM ammonium acetate. Before it dissolved, the α-cyano-4-hydroxycinnamic acid (Sigma) was washed briefly with acetone. The mixture was spotted onto a target plate and allowed to dry at room temperature. Reflectron MALDI-TOF spectra were acquired with a Micromass MALDI instrument.
Tryptic digests.
To check the efficiency of protein labeling of the S. enterica serovar Typhimurium bacteria with l-arginine-guanido[15N2], whole-protein isolates from the bacteria were digested with trypsin and peptide fragments were analyzed by MS. Roughly 2 × 109 bacteria (labeled, unlabeled, or a 1:1 mixture of both) were washed three times in 10 mM Tris, pH 8.0, resuspended in 1.5 ml of 10 mM Tris, pH 8.0, and sonicated three times for 30 s each at the maximum intensity in a B18 cell disrupter (Branson). The lysate was cooled on ice and treated with a solution containing 100 μl of 10 mM Tris (pH 8.0), 1 mM β-mercaptoethanol, and 1% sodium dodecyl sulfate at 96°C for 10 min. After the addition of 1 ml of H2O, cooling on ice, and spinning at 14,000 rpm for 1 min, 500 μl of the supernatant was mixed with 1.5 ml of methanol and incubated for 30 min at room temperature, followed by centrifugation at 16,000 × g for 10 min to collect precipitated proteins. The pellet was dried, and 100 μl of 50 mM NH4HCO3 (pH 8.0) with 20 μl of 0.1-μg/μl unmodified sequencing-grade trypsin (Roche, Mannheim, Germany) in 1 mM HCl was added. Digestion was carried out overnight at 37°C and stopped with 13.3 μl of 1% TFA in H2O.
The retentate, obtained after the filtration of B27 peptide eluates with 10-kDa-cutoff filter units (Amicon), was used as a source of B27 heavy-chain proteins to check for the correct 1:1 mixing of labeled and unlabeled C1R-B*2704 cells. About 20 μg of protein from the retentate was dried, and 100 μl of 12.5-ng/μl sequencing-grade modified trypsin (Promega, Madison, Wis.) in 100 mM NH4HCO3, pH 8.0, was added. Digestion was carried out at 37°C overnight. Seven microliters of formic acid was added, and the digest was incubated with 10 mM dithiothreitol, dried, and dissolved in 100 μl of 5% dimethyl sulfoxide-5% formic acid.
RESULTS
Efficiency of stable isotope labeling of endogenous B*2704-presented peptides.
Our aim was to compare the endogenous B*2704-presented peptide repertoires from infected and uninfected cell populations in a single peptide preparation. Therefore, we first examined whether it was possible to tag the peptide repertoire of one of two cell populations by metabolic labeling. Stable-isotope-labeled arginine was used, since arginine is a required anchor residue for peptide binding to B*2704. The l-arginine-guanido[15N2]-labeled cells were mixed at a ratio of 1:1 with unlabeled C1R-B*2704 cells, and subsequently the B27-bound peptides were isolated and purified from this mixture.
We expected that each B27-presented peptide species isolated from the cell mixture would be represented by two masses of equal intensities, representing unlabeled and 15N2-labeled peptides derived from their respective cell populations. The labeled peptides were expected to be 2, 4, or 6 Da heavier than the unlabeled peptides, representing peptides containing one, two, or three arginines, respectively. Because the presence of two 15N atoms in labeled arginines has no effect on peptide retention times in reversed-phase chromatography, unlabeled and labeled peptides will coelute for LC-MS. These peptides will thus be seen in mass spectra as mass doublets consisting of two masses at equal intensities, with a mass difference of 2 Da for each arginine.
Upon LC-MS analysis of the B*2704-bound peptides isolated from the 1:1 mixture of unlabeled and labeled cells with the LCT mass spectrometer, the B27-bound peptides were indeed found to be present as doublets with mass differences of 2 to 6 Da. Thus, peptides were presented in their unlabeled and labeled forms (Fig. 1).
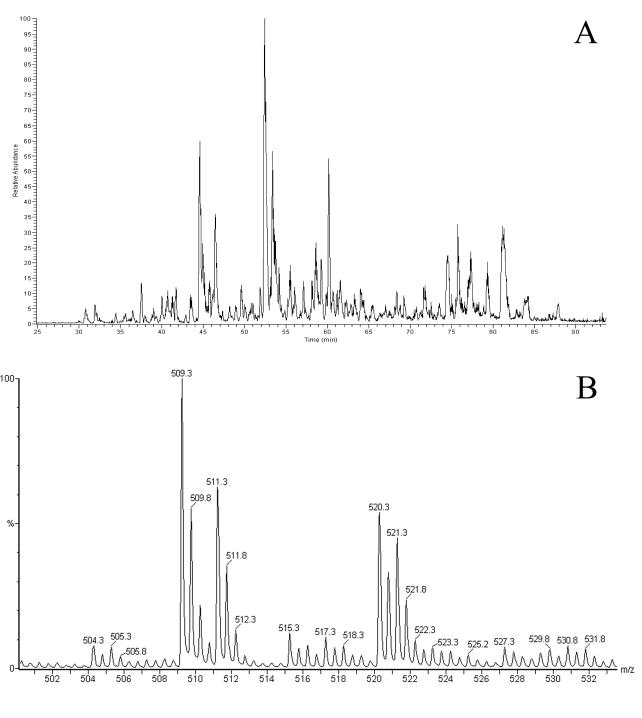
FIG. 1.
B27-bound peptides isolated from a 50% mixture of labeled and unlabeled C1R-B*2704 cells. A base peak chromatogram from an LC-MS experiment (A) and a typical MS spectrum (B) are shown, revealing two doubly positive charged ions representing a peptide containing two arginines (m/z 509.3 and 511.3) and a peptide containing one arginine (m/z 520.3 and 521.3).
However, it was apparent that the ratio of labeled to unlabeled peptide was less than one. Therefore, the 25 most abundant masses, containing at least two arginines in order to rule out adulteration of the signal intensity of the labeled peptide by natural 13C isotopes of the unlabeled peptide, were analyzed. We found that the mean intensity of labeled peptides was 60.5% ± 7.7% of the intensity of unlabeled peptides.
The underrepresentation of the labeled peptides may have been caused by the mixing of nonidentical numbers of viable unlabeled and labeled C1R-B*2704 cells. To verify this assumption, we assessed the ratio of unlabeled to labeled proteins that were coisolated during the peptide isolation procedure. We performed tryptic digests with the protein mixture and used MALDI-TOF to quantify the ratio of unlabeled to labeled peptides in the digests. Thus, we deduced the ratio of B27 molecules derived from unlabeled cells to those derived from labeled cells in the cell pellet used for the isolation of B27-bound peptides. In this experiment, we found that the amount of labeled proteins was 67% ± 13% that of unlabeled proteins (data not shown), which is in accordance with the ratio of unlabeled to labeled B27-bound peptides found with the first experiment (see above).
With this proof of principle for our experimental setup, our method could be used to compare the B27-presented peptide repertoires of two different cell populations by using unlabeled peptides derived from an unlabeled cell population as an internal experimental control.
Analysis of endogenous presented peptide repertoire after S. enterica serovar Typhimurium infection.
To study the influence of S. enterica serovar Typhimurium infection on the B27-presented peptide repertoire, we compared the peptide repertoire of unlabeled control cells with that of infected labeled cells. The l-Arginine-guanido[15N2]-labeled C1R-B*2704 cell population was infected with S. enterica serovar Typhimurium prior to mixing of the labeled cells with unlabeled, uninfected C1R-B*2704 control cells at a ratio of 1:1. Subsequently, the B27-bound peptides were isolated and analyzed.
As in the previous control experiment, we studied the peptide mass doublets derived from the cell mixture of unlabeled and infected labeled cells by using LC-MS and found labeled peptide masses at lower intensities than those of unlabeled peptides (Fig. 2).
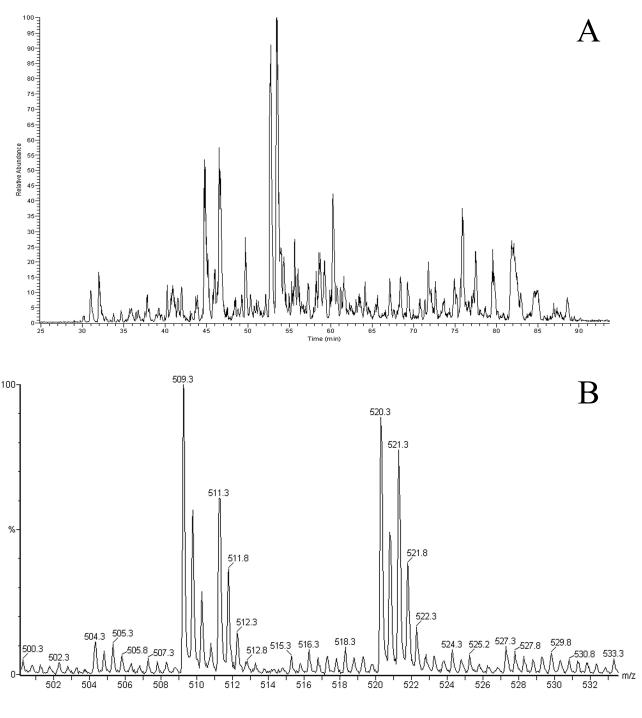
FIG. 2.
B27-bound peptides isolated from a 50% mixture of labeled C1R-B*2704 cells infected with S. enterica serovar Typhimurium and of unlabeled, uninfected cells. A base peak chromatogram from an LC-MS experiment (A) and a typical MS spectrum (B) are shown, revealing two doubly positive charged ions representing a peptide containing two arginines (m/z 509.3 and 511.3) and a peptide containing one arginine (m/z 520.3 and 521.3).
In this experiment, for the same 25 most intense peptide mass doublets studied in the previous control experiment, the average intensity of labeled peptides was 69% ± 9.1% of the intensity of unlabeled peptides. In all other peptide mass doublets, containing at least two arginines in order to rule out adulteration of the signal intensity of the labeled peptide by natural 13C isotopes of the unlabeled peptide, we found no deviations from this percentage of more than three times the standard deviation. As we determined previously in a control experiment by quantifying the relative amount of labeled protein obtained during B27 isolation procedures with tryptic digests and MALDI-TOF, this finding reflects the unsuccessful mixing of equal amounts of unlabeled and infected labeled C1R-B*2704 cells.
For peptides containing only one arginine, the signal intensity of the second natural 13C isotope of the unlabeled peptide coincided with the signal for the labeled peptide. We estimated that the intensity of the second natural isotope peak of all peptides was 30% of the intensity of the unlabeled peptide masses based on an average length of B27-presented peptides between 9 and 13 residues. After correction of the signal of the labeled peptide mass for this contribution, no significant deviations from the previously observed ratio were found. Moreover, a comparison of the ratios of labeled to unlabeled peptide signals in these one-arginine-containing doublets with the ratios found for the same peptides (based on identical masses and retention times) in the control experiment showed that they were the same if they were corrected for the mean ratios of the experiments.
Thus, S. enterica serovar Typhimurium infection induced no significant increase or decrease in certain B27-presented peptides in C1R-B*2704 cells compared to normal uninfected C1R-B*2704 cells. This also applies to a histone H3-derived peptide (RRYQKSTEL) (Fig. 3), for which it was previously reported that presentation by B27 increased upon S. enterica serovar Typhimurium infection (43).
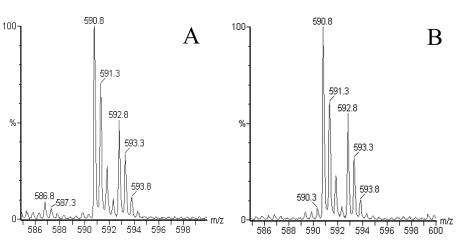
FIG. 3.
MS spectra showing doubly charged ions from the H3-derived peptide RRYQKSTEL isolated from uninfected C1R-B*2704 cells (A) and from a 50% mixture of labeled C1R-B*2704 cells infected with S. enterica serovar Typhimurium and of unlabeled, uninfected cells (B).
Labeling of S. enterica serovar Typhimurium proteins.
In the previous experiments, we studied overall changes in peptide presentation induced by S. enterica serovar Typhimurium infection. We next attempted to identify S. enterica serovar Typhimurium-derived peptides presented by infected C1R-B*2704 cells. For this approach we used unlabeled C1R-B*2704 cells infected with S. enterica serovar Typhimurium bacterial cells, 50% of which were labeled with l-arginine-guanido[15N2]. Thus, B27-presented peptides derived from host cell proteins would be detected as single unlabeled peptide masses with LC-MS, whereas peptides derived from bacteria would be present as mass doublets consisting of labeled and unlabeled peptide masses.
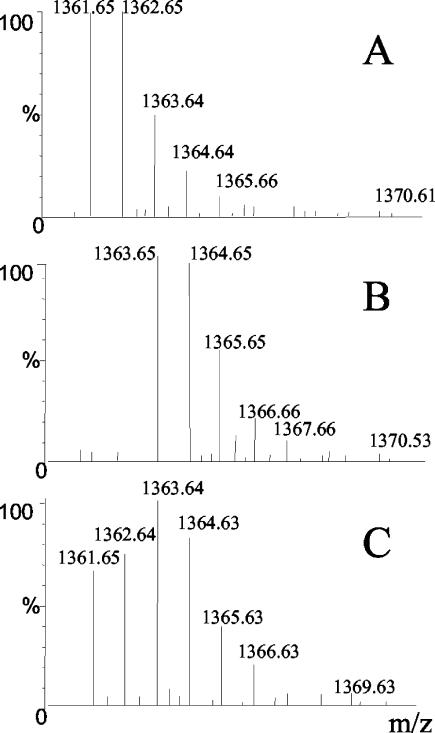
We first tested the labeling of S. enterica serovar Typhimurium proteins with l-arginine-guanido[15N2] and assessed the ratio of labeled to unlabeled bacteria used to infect C1R-B*2704 cells. We set up tryptic digests of the labeled and unlabeled S. enterica serovar Typhimurium proteins and used a 1:1 mixture of them for this experiment. Using MALDI-TOF and peptide mass fingerprinting, we found that the major peptide fragments detected in the tryptic digests of isolated bacterial proteins were derived from the S. enterica serovar Typhimurium murein lipoprotein, as confirmed by actual MS/MS sequencing of two of these peptides, SDVQAAKDDAAR and VDQLSNDVNAMR. Arginine-containing peptide fragments derived from the labeled bacteria were 2 Da heavier per arginine than those from unlabeled bacteria. No masses corresponding to unlabeled peptides were detected among the fragments derived from the labeled bacteria. Therefore, we concluded that our labeling protocol resulted in the complete labeling of S. enterica serovar Typhimurium bacteria with l-arginine-guanido[15N2]. The mixing of equal numbers of labeled and unlabeled bacteria indeed resulted in a 1:1 ratio of labeled and unlabeled peptide signals in the tryptic digests of the mixture. Figure 4 shows MS spectra containing one of the murein lipoprotein fragments with a mass of 1,361.65, corresponding to unlabeled VDQLSNDVNAMR, and a mass of 1,363.65, corresponding to VDQLSNDVNAMR labeled with l-arginine-guanido[15N2]. A suspension of S. enterica serovar Typhimurium containing 50% labeled and 50% unlabeled bacteria was used to infect unlabeled C1R-B*2704 cells.
FIG. 4.
MALDI-TOF spectra showing an S. enterica serovar Typhimurium peptide present in tryptic digests of precipitated proteins from unlabeled S. enterica serovar Typhimurium (A), labeled S. enterica serovar Typhimurium (B), and a 50% mixture of unlabeled and labeled S. enterica serovar Typhimurium (C).
Detection of B*2704-bound S. enterica serovar Typhimurium-derived peptides from C1R-B*2704 cells upon infection with 50% labeled bacteria.
We isolated B27-bound peptides from C1R-B*2704 cells after infection with an S. enterica serovar Typhimurium suspension in which half of the bacteria were labeled with l-arginine-guanido[15N2], as confirmed by MALDI-TOF analysis. Any peptide presented by B27 derived from S. enterica serovar Typhimurium was detected as a spectral doublet consisting of two masses in a 1:1 ratio representing the unlabeled and labeled bacterial peptides.
All MS scans from the LC-MS experiment were analyzed for spectral doublets containing a mass difference of 2, 4, or 6 Da. Subsequently, if in a single scan such spectral doublets were present, we checked whether the two masses indeed represented identical peptides with identical retention times. To this end, we compared the chromatograms of the two masses forming the doublet by tracing their ion signals in the adjacent LC-MS scans. No spectral doublets representing peptides derived from S. enterica serovar Typhimurium were found.
Another approach to searching for bacterially derived MHC class I-presented peptides is to predict in silico which peptides will bind to a particular MHC molecule by combining the sequence of a known bacterial antigen with the peptide binding motif of a particular MHC allele. Protein tyrosine phosphatase (SptP) is one of the S. enterica serovar Typhimurium proteins that are expected to gain access to the MHC class I presentation pathway (even in nonprofessional phagocytes) because it is injected into the cytosol of eukaryotic cells (45). Therefore, after LC-MS analysis of the isolated B27-bound peptides, we looked for the presence of masses corresponding to the calculated masses of all theoretical 9- to 12-mer peptides derived from SptP that contain arginine at amino acid position 2 (the major B27 peptide binding motif). If they were derived from the bacterial SptP protein, such peptides should be present as spectral doublets in our experiment.
Although we found mass values corresponding to those of predicted epitopes from SptP, none of these were present as spectral doublets. Moreover, these single masses were also present in peptide eluates from noninfected cells. Thus, we did not identify S. enterica serovar Typhimurium-derived peptides presented by B27 on infected C1R-B*2704 cells.
DISCUSSION
ReA is induced after infection by certain gram-negative bacteria or C. trachomatis, especially in HLA-B27-positive individuals (26, 39, 49). The arthritogenic peptide hypothesis proposes a role for a peptide(s) presented by B27 molecules in the pathogenesis of ReA (3). It has indeed been shown that the infection of B27-positive C1R cells with Salmonella or Yersinia makes them susceptible to lysis by B27-restricted CTL clones isolated from synovia of ReA patients (23). For the present study, we used a newly developed approach (35) to identify any possible changes induced by S. enterica serovar Typhimurium infection in the peptide repertoire presented by HLA-B*2704, a disease-associated subtype.
Several groups have attempted to identify changes in the B27-bound peptide repertoire after infection with bacteria (5, 31, 41, 43). Most of these studies compared HPLC profiles generated from peptide pools isolated from infected cells with those from uninfected control cells. With this approach, very few significant differences were observed and characterized. Boisgerault et al. (5) identified some peptides, presented only by cells infected with Shigella, that lacked the B27 binding motif. Ringrose et al. concluded from their comparative data analysis that the presentation of certain host cell peptides may be increased upon bacterial infection (43). A more advanced study was performed by Ramos et al., who used MS analysis to study the peptide contents of all peptide fractions generated by reversed-phase HPLC (41). They found no significant differences between peptide profiles from cells infected with S. enterica serovar Typhimurium and those from control cells, although an as yet uncharacterized low-abundance mass was specifically present in isolates from infected cells.
The major difficulty in such comparative data analyses is poor reproducibility. Peptides from infected and control cells are isolated and purified in separate experiments. Results from such experiments are affected by various variables, with a considerable impact on the HPLC profiles obtained. In addition, the sensitivity of every mass spectrometer varies over time, and slight differences in the compositions of the fractions to be analyzed influence the spectra obtained.
In view of the problems and limitations often encountered in studies using comparative data analysis, we used a new approach to study the effect of S. enterica serovar Typhimurium infection on B27 peptide presentation. In addition, we used a sensitive nanoscale HPLC technique connected online to the ESI source of a mass spectrometer (LC-MS) (36). Proteins or peptides from different cell populations, with one population being labeled with stable isotopes, were mixed prior to separation and detection, giving the essential relative abundance of the stable isotope labels. In our approach, the cells themselves are mixed before the isolation procedures so that the peptide samples include their own internal (unlabeled) controls.
Because arginine is the major anchor residue at position 2 of the B27 peptide binding motif (24, 46), we used l-arginine-guanido[15N2] as a label in our experiments. After mixing labeled cells and unlabeled cells, we found that the peptides presented by B*2704 on these cells were present in mass spectra as spectral doublets consisting of two masses, with mass differences of 2 Da for each arginine within the peptide. This finding showed that it is possible to label cells completely with a stable-isotope-labeled anchor residue, resulting in the presentation of labeled peptides by MHC class I molecules. The ratio of unlabeled to labeled B27-bound peptides was found to reflect the ratio of labeled to unlabeled B27 molecules present in the mixture of C1R-B*2704 cells.
By infecting only labeled cells with S. enterica serovar Typhimurium before mixing them with unlabeled control cells, we could study changes in peptide presentation that were possibly induced by S. enterica serovar Typhimurium infection by looking for changes in the ratios of unlabeled to labeled peptide masses. Although previous studies had suggested the existence of such changes (5, 31, 43), we did not find any change in peptide presentation patterns due to S. enterica serovar Typhimurium infection by using this intrinsically more reliable approach. We also clearly demonstrated that the presentation of a histone-derived peptide, which had been suggested to be up regulated, was unaltered by S. enterica serovar Typhimurium infection. Apparently, the increase in presentation of the H3 peptide that was reported previously (43) is a typical artifact of the comparative data approach.
We also specifically searched for S. enterica serovar Typhimurium-derived peptides presented by HLA-B*2704 on infected cells. For this study, we used a 1:1 mixture of l-arginine-guanido[15N2]-labeled and unlabeled S. enterica serovar Typhimurium bacteria to infect C1R-B*2704 cells. We found that S. enterica serovar Typhimurium bacteria were highly efficiently labeled with the arginine. However, we did not find spectral doublets representing S. enterica serovar Typhimurium-derived peptides among the B27-bound peptides isolated from infected C1R-B*2704 cells. This finding is consistent with data reported by Lopez de Castro et al., who used a comparative data approach (41).
Although we failed to detect any peptides derived from S. enterica serovar Typhimurium that were presented by B*2704, there are several clear indications in the literature that Salmonella proteins can be presented by MHC class I molecules. Using foreign epitopes expressed in Salmonella, several studies have shown that Salmonella can induce an MHC class I-restricted CTL response against such foreign epitopes in mice (1, 13, 45, 50). Salmonella-specific MHC class I-restricted responses can also be induced, although it seems that the majority of the anti-Salmonella response is restricted by the nonclassical MHC class I molecule Qa-1b (28, 40). The only characterized Salmonella MHC class I epitope is a Salmonella peptide presented by Qa-1b (29).
However, a distinction should be made between professional phagocytic cells, such as macrophages and dendritic cells, and nonphagocytic cells. Foreign epitopes expressed in Salmonella can be processed and presented by classical MHC class I molecules on professional phagocytic cells (22), while Salmonella is protected from recognition by CTLs in nonphagocytic cells such as epithelial cells (15, 22). This indicates that Salmonella bacteria can gain access to the processing pathway for presentation by classical MHC class I molecules in professional phagocytic cells only. Nevertheless, mechanisms for processing Salmonella for presentation by classical MHC class I molecules might also exist in nonphagocytic cells. One possibility is the presentation by MHC class I molecules of peptides derived from certain Salmonella proteins found in the cytosol. The Salmonella type III secretion system can translocate certain proteins into the cytosol of nonphagocytic cells (14, 53). Indeed, this system has been used to present a viral epitope expressed in the Salmonella SptP protein to MHC class I-restricted CTLs by nonphagocytic cells (45), but we were unable to detect such peptides derived from the SptP protein.
It is unknown which MHC class I peptide presentation mechanisms exist in C1R-B*2704 cells, but Hermann et al. (23) have shown that infection of C1R cells renders them susceptible to lysis by B27-restricted CTL clones. The apparent discrepancy between their results and our observations might be explained by the difference in sensitivity between their bioassay and our methods for detecting B27-presented peptides.
The amount of B27 molecules on the cells used in our experiment can be estimated to be approximately 105 to 106 copies per cell (10). We calculated that considering the femtomole sensitivity of the mass spectrometer, the amount of cell material used in our experiment (5 × 108 cells), infection efficiencies, and estimated losses of 80% during the isolation procedures, Salmonella-derived peptides present at a level of 3 × 102 copies or more per cell should be easily detected by our method. There are important caveats, however. Ionization and detection efficiencies are strongly influenced by the presence of other ions, either contaminants or other abundant peptides. Thus, we observed large variations in the sensitivity of the LC-MS method if model peptides such as oxytocin and angiotensin III spiked in LC-ESI runs of peptide mixtures were used (results not shown). This illustrates that all quantitative methods based on isotope labeling and MS are ideally suited for comparative studies but that they provide only very rough indications of the absolute amounts of peptides present. It has been reported that the sensitivity of CTLs is at the level of a few copies per cell (48). Thus, it is possible that peptides from Salmonella are presented by B27 on C1R cells at immunologically relevant levels but that they are very difficult to detect biochemically. Clearly, in this case biochemical detection cannot yet compete with nature.
In conclusion, although our approach to detecting any B27-bound peptides derived from S. enterica serovar Typhimurium by using stable isotope labeling and MS failed, we have convincingly shown that there is no increase in the presentation of an H3-derived peptide after Salmonella infection, as suggested previously (43). The method used in our study clearly has major advantages compared to the widely used comparative data analysis approach. This method can be used for detecting unknown naturally MHC-presented epitopes from other pathogens as well as for studying changes in peptide presentation resulting from certain stimuli.
Acknowledgments
We thank Jose A. Lopez de Castro, Centro de Biologia Molecular Severo Ochoa, Universidad Autonoma de Madrid, Madrid, Spain, for generously providing us with the C1R-B*2704 transfectant cell line.
This work was supported by grant 534 from Het Nationaal Reumafonds of The Netherlands.
Editor: F. C. Fang
REFERENCES
- 1.Aggarwal, A., S. Kumar, R. Jaffe, D. Hone, M. Gross, and J. Sadoff. 1990. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J. Exp. Med. 172:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnstable, C. J., W. F. Bodmer, G. Brown, G. Galfre, C. Milstein, A. F. Williams, and A. Ziegler. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens—new tools for genetic analysis. Cell 14:9-20. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin, R., and P. Parham. 1990. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol. Today 11:137-142. [DOI] [PubMed] [Google Scholar]
- 4.Berthelot, J. M., J. Glemarec, P. Guillot, Y. Laborie, and Y. Maugars. 2002. New pathogenic hypotheses for spondyloarthropathies. Joint Bone Spine 69:114-122. [DOI] [PubMed] [Google Scholar]
- 5.Boisgerault, F., J. Mounier, V. Tieng, M. C. Stolzenberg, I. Khalil-Daher, M. Schmid, P. Sansonetti, D. Charron, and A. Toubert. 1998. Alteration of HLA-B27 peptide presentation after infection of transfected murine L cells by Shigella flexneri. Infect. Immun. 66:4484-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowness, P., N. Zaccai, L. Bird, and E. Y. Jones. 26 October 1999, posting date. HLA-B27 and disease pathogenesis: new structural and functional insights. Exp. Rev. Mol. Med. [Online.] http://www-ermm.cbcu.cam.ac.uk/99001118h.htm. [DOI] [PubMed]
- 7.Brewerton, D. A., M. Caffrey, A. Nicholls, D. Walters, J. K. Oates, and D. C. James. 1973. Reiter's disease and HL-A 27. Lancet ii:996-998. [DOI] [PubMed] [Google Scholar]
- 8.Brewerton, D. A., F. D. Hart, A. Nicholls, M. Caffrey, D. C. James, and R. D. Sturrock. 1973. Ankylosing spondylitis and HLA-27. Lancet i:904-907. [DOI] [PubMed] [Google Scholar]
- 9.Burmester, G. R., A. Daser, T. Kamradt, A. Krause, N. A. Mitchison, J. Sieper, and N. Wolf. 1995. Immunology of reactive arthritides. Annu. Rev. Immunol. 13:229-250. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard, V. H. 1994. Structure of peptides associated with MHC class I molecules. Curr. Opin. Immunol. 6:13-23. [DOI] [PubMed] [Google Scholar]
- 11.Falkow, S., R. R. Isberg, and D. A. Portnoy. 1992. The interaction of bacteria with mammalian cells. Annu. Rev. Cell Biol. 8:333-363. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., W. R. Weiss, K. A. Norris, H. S. Seifert, S. Kumar, and M. So. 1990. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol. Microbiol. 4:2111-2118. [DOI] [PubMed] [Google Scholar]
- 14.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 15.Gao, X. M., J. P. Tite, M. Lipscombe, S. Rowland-Jones, D. J. Ferguson, and A. J. McMichael. 1992. Recombinant Salmonella typhimurium strains that invade nonphagocytic cells are resistant to recognition by antigen-specific cytotoxic T lymphocytes. Infect. Immun. 60:3780-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, F., A. Marina, and J. A. Lopez de Castro. 1997. Lack of carboxyl-terminal tyrosine distinguishes the B*2706-bound peptide repertoire from those of B*2704 and other HLA-B27 subtypes associated with ankylosing spondylitis. Tissue Antigens 49:215-221. [DOI] [PubMed] [Google Scholar]
- 17.Germain, R. N. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 76:287-299. [DOI] [PubMed] [Google Scholar]
- 18.Gromme, M., and J. Neefjes. 2002. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol. Immunol. 39:181-202. [DOI] [PubMed] [Google Scholar]
- 19.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamdan, M., and P. G. Righetti. 2002. Modern strategies for protein quantification in proteome analysis: advantages and limitations. Mass Spectrom. Rev. 21:287-302. [DOI] [PubMed] [Google Scholar]
- 21.Hammer, R. E., S. D. Maika, J. A. Richardson, T. P. Tang, and J. D. Taurog. 1990. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell 63:1099-1112. [DOI] [PubMed] [Google Scholar]
- 22.Harding, C. V., and R. Song. 1994. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J. Immunol. 153:4925-4933. [PubMed] [Google Scholar]
- 23.Hermann, E., D. T. Yu, K. H. Meyer zum Buschenfelde, and B. Fleischer. 1993. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet 342:646-650. [DOI] [PubMed] [Google Scholar]
- 24.Jardetzky, T. S., W. S. Lane, R. A. Robinson, D. R. Madden, and D. C. Wiley. 1991. Identification of self peptides bound to purified HLA-B27. Nature 353:326-329. [DOI] [PubMed] [Google Scholar]
- 25.Khare, S. D., H. S. Luthra, and C. S. David. 1995. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: a model of human spondyloarthropathies. J. Exp. Med. 182:1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingsley, G., and G. Panayi. 1992. Antigenic responses in reactive arthritis. Rheum. Dis. Clin. N. Am. 18:49-66. [PubMed] [Google Scholar]
- 27.Kingsley, G., and J. Sieper. 1993. Current perspectives in reactive arthritis. Immunol. Today 14:387-391. [DOI] [PubMed] [Google Scholar]
- 28.Lo, W. F., H. Ong, E. S. Metcalf, and M. J. Soloski. 1999. T cell responses to gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J. Immunol. 162:5398-5406. [PubMed] [Google Scholar]
- 29.Lo, W. F., A. S. Woods, A. DeCloux, R. J. Cotter, E. S. Metcalf, and M. J. Soloski. 2000. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat. Med. 6:215-218. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Larrea, C., K. Sujirachato, N. K. Mehra, P. Chiewsilp, D. Isarangkura, U. Kanga, O. Dominguez, E. Coto, M. Pena, F. Setien, and S. Gonzales-Roces. 1995. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens 45:169-176. [DOI] [PubMed] [Google Scholar]
- 31.Maksymowych, W. P., T. Ikawa, A. Yamaguchi, M. Ikeda, D. McDonald, L. Laouar, R. Lahesmaa, N. Tamura, A. Khuong, D. T. Yu, and K. P. Kane. 1998. Invasion by Salmonella typhimurium induces increased expression of the LMP, MECL, and PA28 proteasome genes and changes in the peptide repertoire of HLA-B27. Infect. Immun. 66:4624-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marti, M., I. Alvarez, and J. A. Lopez de Castro. 1999. A molecular insight on the association of HLA-B27 with spondyloarthropathies. Curr. Rheumatol. Rep. 1:78-85. [DOI] [PubMed] [Google Scholar]
- 33.Mastroeni, P., B. Villarreal-Ramos, and C. E. Hormaeche. 1992. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro− Salmonella vaccines. Microb. Pathog. 13:477-491. [DOI] [PubMed] [Google Scholar]
- 34.Mastroeni, P., B. Villarreal-Ramos, and C. E. Hormaeche. 1993. Adoptive transfer of immunity to oral challenge with virulent Salmonella in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 61:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meiring, H. D., C. A. Herberts, E. van der Heeft, G. J. ten Hove, C. A. C. M. van Els, and A. P. J. M. de Jong. 2000. Specific isotope labeling and column switching nanoLC-MS/MS with wideband parent ion isolation: new strategies in MHC associated peptide identification. In Proceedings of the 48th Annual Conference. American Society for Mass Spectrometry, Long Beach, Calif.
- 36.Meiring, H. D., E. van der Heeft, G. J. ten Hove, and A. P. J. M. de Jong. 2002. Nanoscale LC-MS(n): technical design and applications to peptide and protein analysis. J. Separation Sci. 25:557-568. [Google Scholar]
- 37.Nasution, A. R., A. Mardjuadi, S. Kunmartini, N. G. Suryadhana, B. Setyohadi, D. Sudarsono, N. M. Lardy, and T. E. Feltkamp. 1997. HLA-B27 subtypes positively and negatively associated with spondyloarthropathy. J. Rheumatol. 24:1111-1114. [PubMed] [Google Scholar]
- 38.Oda, Y., K. Huang, F. R. Cross, D. Cowburn, and B. T. Chait. 1999. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl. Acad. Sci. USA 96:6591-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penttinen, M. A., Y. Liu, and K. Granfors. 2002. The role of infection in the pathogenesis of spondyloarthropathies with special reference to human leukocyte antigen-B27. Curr. Rheumatol. Rep. 4:518-524. [DOI] [PubMed] [Google Scholar]
- 40.Pope, M., I. Kotlarski, and K. Doherty. 1994. Induction of Lyt-2+ cytotoxic T lymphocytes following primary and secondary Salmonella infection. Immunology 81:177-182. [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos, M., I. Alvarez, F. Garcia-del-Portillo, and J. A. Lopez de Castro. 2001. Minimal alterations in the HLA-B27-bound peptide repertoire induced upon infection of lymphoid cells with Salmonella typhimurium. Arthritis Rheum. 44:1677-1688. [DOI] [PubMed] [Google Scholar]
- 42.Ren, E. C., W. H. Koh, D. Sim, M. L. Boey, G. B. Wee, and S. H. Chan. 1997. Possible protective role of HLA-B*2706 for ankylosing spondylitis. Tissue Antigens 49:67-69. [DOI] [PubMed] [Google Scholar]
- 43.Ringrose, J. H., A. O. Muijsers, Y. Pannekoek, B. A. Yard, C. J. Boog, L. van Alphen, J. Dankert, and T. E. Feltkamp. 2001. Influence of infection of cells with bacteria associated with reactive arthritis on the peptide repertoire presented by HLA-B27. J. Med. Microbiol. 50:385-389. [DOI] [PubMed] [Google Scholar]
- 44.Ringrose, J. H., B. A. Yard, A. Muijsers, C. J. Boog, and T. E. Feltkamp. 1996. Comparison of peptides eluted from the groove of HLA-B27 from Salmonella infected and non-infected cells. Clin. Rheumatol. 15(Suppl. 1):74-78. [DOI] [PubMed] [Google Scholar]
- 45.Russmann, H., H. Shams, F. Poblete, Y. Fu, J. E. Galan, and R. O. Donis. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565-568. [DOI] [PubMed] [Google Scholar]
- 46.Sesma, L., V. Montserrat, J. R. Lamas, A. Marina, J. Vazquez, and J. A. Lopez de Castro. 2002. The peptide repertoires of HLA-B27 subtypes differentially associated to spondyloarthropathy (B*2704 and B*2706) differ by specific changes at three anchor positions. J. Biol. Chem. 277:16744-16749. [DOI] [PubMed] [Google Scholar]
- 47.Storkus, W. J., D. N. Howell, R. D. Salter, J. R. Dawson, and P. Cresswell. 1987. NK susceptibility varies inversely with target cell class I HLA antigen expression. J. Immunol. 138:1657-1659. [PubMed] [Google Scholar]
- 48.Sykulev, Y., M. Joo, I. Vturina, T. J. Tsomides, and H. N. Eisen. 1996. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565-571. [DOI] [PubMed] [Google Scholar]
- 49.Toivanen, A., and P. Toivanen. 1997. Reactive arthritis. Curr. Opin. Rheumatol. 9:321-327. [DOI] [PubMed] [Google Scholar]
- 50.Turner, S. J., F. R. Carbone, and R. A. Strugnell. 1993. Salmonella typhimurium delta aroA delta aroD mutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect. Immun. 61:5374-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ugrinovic, S., A. Mertz, P. Wu, J. Braun, and J. Sieper. 1997. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J. Immunol. 159:5715-5723. [PubMed] [Google Scholar]
- 52.Verjans, G. M., J. H. Ringrose, L. van Alphen, T. E. Feltkamp, and J. G. Kusters. 1994. Entrance and survival of Salmonella typhimurium and Yersinia enterocolitica within human B- and T-cell lines. Infect. Immun. 62:2229-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]
- 54.Zemmour, J., A. M. Little, D. J. Schendel, and P. Parham. 1992. The HLA-A,B “negative” mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J. Immunol. 148:1941-1948. [PubMed] [Google Scholar]
- 55.Zhou, M., A. Sayad, W. A. Simmons, R. C. Jones, S. D. Maika, N. Satumtira, M. L. Dorris, S. J. Gaskell, R. S. Bordoli, S. B. Sartor, C. A. Slaughter, J. A. Richardson, R. E. Hammer, and J. D. Taurog. 1998. The specificity of peptides bound to human histocompatibility leukocyte antigen (HLA)-B27 influences the prevalence of arthritis in HLA-B27 transgenic rats. J. Exp. Med. 188:877-886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]