Abstract
Human immunoglobulin G2 (IgG2) responses are gamma interferon (IFN-γ) dependent, and monocyte-derived dendritic cells (mDCs) promote IgG2 production. DCs spontaneously emerge from monocytes in cultures prepared from localized aggressive periodontitis (LagP) patients, and these patients have high levels of IgG2 that is reactive with Actinobacillus actinomycetemcomitans. These results prompted the hypothesis that an interaction between mDCs and A. actinomycetemcomitans promotes IFN-γ production, and IFN-γ is known to promote both immunopathology and protective IgG2. A. actinomycetemcomitans induced mDCs to produce interleukin-12 (IL-12), and the addition of A. actinomycetemcomitans and DCs to cultured peripheral blood lymphocytes elicited high levels of IFN-γ within just 24 h. In contrast, IL-4 was not detectable although DC-derived IL-10 production was apparent. A. actinomycetemcomitans-stimulated macrophages prepared from the same monocytes lacked the ability to induce IL-12 or IFN-γ responses. NK cells of the innate immune system were the primary source of this early IFN-γ, although CD8 T cells also contributed some. The NK cell-derived IFN-γ was IL-12 dependent, and A. actinomycetemcomitans-DC interactions were Toll-like receptor 4 dependent. A. actinomycetemcomitans and A. actinomycetemcomitans lipopolysaccharide (LPS) were more potent than Escherichia coli and E. coli LPS in the ability to induce DC IL-12 and IFN-γ. The ability of A. actinomycetemcomitans-stimulated DCs to induce NK cells to rapidly produce IFN-γ in the absence of detectable IL-4 suggests their potential for skewing responses toward Th1. This may help explain the presence of Th1-associated cytokines in gingival crevicular fluid (GCF) from LagP patients and the high levels of IgG2 in their serum and GCF that is reactive with A. actinomycetemcomitans.
Periodontitis is a chronic inflammatory disease that is largely attributable to infections with gram-negative bacteria and is characterized by both gingival inflammation and alveolar bone resorption. Localized aggressive periodontitis (LagP) is an early-onset form of disease that develops during the circumpubertal period, and the periodontal destruction is most apparent around the first molars and incisors (34). Actinobacillus actinomycetemcomitans is strongly associated with LagP, and this association is supported by the presence of high titers in serum of immunoglobulin G2 (IgG2) that is reactive with surface carbohydrates of A. actinomycetemcomitans (40). LagP patients have features consistent with a Th1 bias, including Th1 cytokines in the gingival crevicular fluid (GCF) (33) and high levels of gamma interferon (IFN-γ)-dependent IgG2 in their sera and GCF (22, 39, 40). Remarkably, the high levels of IgG2 that is reactive with the serotype-specific carbohydrates of A. actinomycetemcomitans and Porphyromonas gingivalis correlate with a significant reduction in the extent and severity of the disease, suggesting that the IgG2 response is protective (5, 14).
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) and are the only APCs that are capable of priming naïve T cells (16, 24, 37). Furthermore, the priming events include the polarization of naïve T cells toward a Th1 or Th2 response (3, 20, 31). DCs, including Langerhans cells and dermal DCs, are found in large numbers in the gingival tissue, and mature CD83+ DCs have been found in gingival tissues from patients with periodontitis (7, 18, 19). Furthermore, dermal DCs, which have similarities with monocyte-derived DCs (mDCs), have been identified in gingival tissue in association with T cells in periodontitis, suggesting a DC-mediated T-cell activation (18). Recent findings also indicated that the pathogen-DC interface plays a major role in skewing responses toward Th1 or Th2 and that this is determined in large measure by the Toll-like receptors (TLRs) on DCs engaged by the pathogen (30). TLR4 agonists are known to promote the production of the interleukin-12 (IL-12)- and IFN-γ-inducible protein 10. In contrast, TLR2 stimulation typically failed to induce these cytokines but may release the IL-12 inhibitory p40 homodimer and conditions that are predicted to favor Th2 development (30). In addition, DCs are potent stimulators of NK cells, and NKT cells are the most abundant IFN-γ-producing cells in the mouse after a lipopolysaccharide (LPS) challenge (11, 13, 26, 28, 43).
Recently, members of our laboratory found that DCs spontaneously emerge in cultures of LagP monocytes and that mDCs selectively promote IgG2 production (2). These results prompted the hypothesis that the initial host-pathogen interface established between A. actinomycetemcomitans and immature DCs might enhance IFN-γ production, which in turn might promote immunopathology as well as protective IgG2 expression (1, 4, 9, 17, 22, 35, 38, 39). To begin testing, we compared the levels of IL-12 production by mDCs and macrophages stimulated with Escherichia coli and A. actinomycetemcomitans. We also compared the abilities of DCs stimulated with these organisms to induce the production of IFN-γ by peripheral blood lymphocytes (PBL) from seronegative subjects. We found that A. actinomycetemcomitans- or A. actinomycetemcomitans LPS-stimulated mDCs induced high levels of IL-12 and that these DCs induced NK cells of the normal PBL to produce IFN-γ in large quantities within just 24 h, in the absence of detectable IL-4. The ability of A. actinomycetemcomitans-stimulated DCs to induce this rapid NK cell response may help to explain the Th1-associated cytokines in LagP GCF and the high levels of IFN-γ-dependent IgG2.
MATERIALS AND METHODS
Human subjects.
Subjects for this study were obtained by the Clinical Research Center for Periodontal Disease, School of Dentistry, Virginia Commonwealth University, Richmond, Va. PBL were isolated from heparinized whole blood from seronegative nonperiodontitis (NP) patients who had no evidence of attachment loss except for recession on the buccal surfaces of anterior teeth at no more than one site and with no pockets of >3 mm.
Reagents.
Recombinant human IL-4, granulocyte-macrophage colony-stimulating factor (GM-CSF), M-CSF, a monoclonal anti-human IL-12 antibody, and a mouse IgG1 isotype control were obtained from R&D Systems (Minneapolis, Minn.). A monoclonal anti-human TLR4 antibody and a mouse IgG2a isotype control were obtained from eBioscience (San Diego, Calif.). LPS from E. coli (serotype O26:B6) was obtained from Sigma Chemical Co. (St. Louis, Mo.). LPS from A. actinomycetemcomitans Y4 was prepared as previously described (42).
Bacteria.
A. actinomycetemcomitans strain Y4, E. coli strain DH5α, and P. gingivalis strain W83 were employed in this study. In preliminary studies, we found that E. coli (DH5α) was a more potent and reliable inducer of DC IL-12 than a smooth strain of E. coli (O26:B6), which was the source of the commercial LPS used in our study. Because E. coli DH5α was a better stimulator of DC IL-12, it was used for most experiments. A. actinomycetemcomitans and P. gingivalis were grown in brain heart infusion medium (Difco Laboratories, Detroit, Mich.), and E. coli was grown in Luria-Bertani broth (Fisher Scientific, Fairlawn, N.J.). After being cultured, the bacteria were washed three times with phosphate-buffered saline and resuspended to an appropriate concentration in the same buffer. All bacteria, including the controls, were heated at 65°C for 30 min before they were used in cultures. Treatment at this temperature is known to inactivate the A. actinomycetemcomitans leukotoxin.
To study trapping by DCs, we grew the bacteria in 5 ml of medium pulsed with 50 μCi of [3H]oleic acid. After an overnight incubation, E. coli strain DH5α had incorporated 34,400 cpm/107 bacteria, E. coli strain O26:B6 had incorporated 30,400 cpm/107 bacteria, and A. actinomycetemcomitans strain Y4 had incorporated 126,000 cpm/107 bacteria.
Lymphocyte separation.
PBL were obtained from heparinized blood by density centrifugation using lymphocyte separation medium (ICN, Aurora, Ohio). The cells were centrifuged at 400 × g for 20 min, collected from the interface, and washed three times in RPMI 1640 medium (Cellgro, Herndon, Va.). After being washed, the cells were suspended in RPMI 1640 supplemented with 10% fetal calf serum (HyClone, Logan, Utah) and antibiotics for cell culture.
mDC and macrophage cultures.
Adherent monocytes were prepared by culturing PBL (2 × 107 cells/well) in six-well culture plates (35-mm diameter; Costar) for 1 h. The adherent cells were washed and then cultured for 5 days in medium enriched with 10% fetal calf serum, 500 U of recombinant human IL-4/ml, and 800 U of recombinant human GM-CSF/ml for the generation of mDCs or 1,000 U of recombinant human M-CSF/ml for the generation of macrophages (32). In each experiment, monocytes were obtained from a single donor and then some were converted into mDCs and others were converted into macrophages. Different donors were used to reproduce the experiments, but statistics were done within experiments with DCs, macrophages, NK cells, and T cells from the same donor.
Magnetic cell separation.
A magnetic cell separation system (MACS; Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany) was used to separate NK cells and/or CD8 cells from other mononuclear cells. PBL were incubated with antibodies against CD56 and/or CD8 that had been conjugated directly to the Miltenyi microbeads. After being washed, the cell pellet was resuspended and loaded onto LS separation columns that were seated in a strong magnetic field. The labeled cells were trapped in the columns and the cells in the effluent were used as CD56-depleted and/or CD8-depleted cells. The columns were then removed from the separator, and the CD56- or CD8-labeled cells were flushed out and used. The efficiency of depletion was determined by using fluorescein isothiocyanate- or phycoerythrin-conjugated antibodies against CD56 or CD8 (BD Biosciences, San Diego, Calif.) and then was evaluated by flow cytometry (FACScan; Becton Dickinson, Mansfield, Mass.). The depletion of CD56-positive cells was in excess of 95%, and the depletion of CD8-positive cells was over 80%.
Cocultures of mDCs or macrophages with PBL.
A total of 106 PBL were resuspended in 1 ml of RPMI 1640 supplemented with 10% fetal calf serum and cultured in 75-mm2 tubes (Falcon, Lincoln Park, N.J.) at 37°C in a humidified atmosphere containing 5% CO2. Cultures also contained the indicated numbers of mDCs or macrophages from the same subject with an anti-human IL-12 or anti-human TLR4 antibody. The supernatant fluids were collected at the indicated times and the concentration of IFN-γ was measured.
ELISA.
IFN-γ, IL-4, IL-12 p70, and IL-10 levels in the culture supernatants were evaluated by using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems according to the manufacturer's instructions. The detection limits for the various assays were as follows: 15.6 pg/ml for IFN-γ, 31.5 pg/ml for IL-4 and IL-12 p70, and 62.5 pg/ml for IL-10.
Flow cytometry.
Cell surface CD56, CD8, and CD86 were analyzed by flow cytometry. Cells (106) were incubated with 5 μl of a fluorescein isothiocyanate-labeled anti-CD56, phycoerythrin-conjugated anti-CD8, or Cy-chrome-conjugated anti-CD86 antibody (BD Biosciences) for 30 min on ice in the dark. The cells were washed once and resuspended in phosphate-buffered saline (Gibco, Grand Island, N.Y.) containing 2% fetal bovine serum. The mean fluorescence intensity was then determined by use of a flow cytometer (FACScan; Becton Dickinson). Approximately 10,000 cells were included in each analysis.
Statistical analysis.
All experiments were repeated a minimum of three times and the cultures were done in triplicate. Group means were compared by analysis of variance followed by Tukey's honestly significant difference. Significance was accepted at P values of <0.05.
RESULTS
A. actinomycetemcomitans induced DCs but not macrophages to produce IL-12 p70.
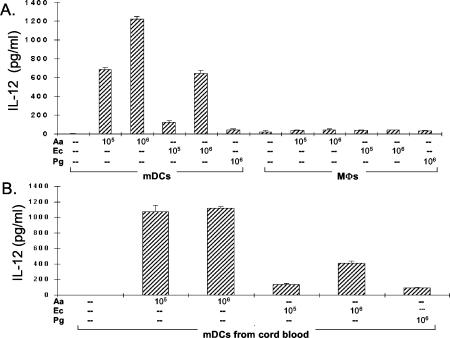
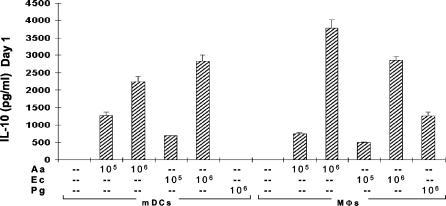
DCs are typically the first APCs to encounter invading microbes, and the engagement of innate pattern recognition receptors (e.g., TLR4 for LPS) leads to DC maturation and antigen presentation. We reasoned that A. actinomycetemcomitans would stimulate immature DCs and that the interaction might promote IFN-γ production. To begin testing this hypothesis, we stimulated immature mDCs from NP subjects with heat-killed A. actinomycetemcomitans, E. coli, or P. gingivalis for comparison. The addition of A. actinomycetemcomitans to immature mDCs dramatically increased the induction of IL-12, and A. actinomycetemcomitans was the most potent stimulator (Fig. 1A). The results in Fig. 1B are for mDCs from human umbilical cord blood, which we reasoned should represent a population with minimal prior exposure to cytokines from activated lymphocytes. The results confirm the remarkable ability of A. actinomycetemcomitans to stimulate IL-12 production by DCs. In marked contrast to the case for mDCs, the addition of A. actinomycetemcomitans to macrophages did not result in increased IL-12 production (Fig. 1A). Induction of the Th2-polarizing cytokine IL-10 by the same bacteria in mDC and macrophage cultures was also studied, and both cell types produced similar levels (Fig. 2). However, a tendency for macrophages to produce slightly more IL-10 was apparent in all three experiments in this series.
FIG. 1.
A. actinomycetemcomitans induces mDCs, but not macrophages, to produce IL-12. MΦ, macrophages; Aa, A. actinomycetemcomitans; Ec, E. coli; Pg, P. gingivalis. (A) mDCs and macrophages (2 × 106) from an NP patient were stimulated with 105 to 106 A. actinomycetemcomitans, E. coli, or P. gingivalis cells. The supernatant fluids were collected 24 h later, and IL-12 levels were measured by ELISA. The data are representative of three experiment of this type and are expressed as means ± standard errors (SE). (B) mDCs from human umbilical cord blood at 2 × 106/DC were stimulated with A. actinomycetemcomitans, E. coli, or P. gingivalis. The supernatant fluids were collected after 24 h, and IL-12 levels were measured by ELISA. The data are representative of three separate experiments of this type and are expressed as means ± SE.
FIG. 2.
A. actinomycetemcomitans and E. coli induce both mDCs and macrophages to produce IL-10. mDCs and macrophages (2 × 106) from an NP patient were stimulated with 105 to 106 A. actinomycetemcomitans, E. coli, or P. gingivalis cells. The supernatant fluids were collected 24 h later, and IL-10 levels were measured by ELISA. The data are representative of three experiments of this type and are expressed as means ± SE. MΦ, macrophages; Aa, A. actinomycetemcomitans; Ec, E. coli; Pg, P. gingivalis.
The remarkable ability of A. actinomycetemcomitans to stimulate IL-12 production prompted experiments to determine whether DCs engage A. actinomycetemcomitans more efficiently than E. coli. The bacteria were labeled with [3H]oleic acid, and then labeled bacteria (106/culture) were incubated with DCs at 106/DC in 1 ml of medium. After 1 h, the DCs were washed vigorously and the percentage of bacteria trapped by DCs was determined. Fourteen percent of the most stimulatory E. coli strain DH5α cells, which were used in the experiments for Fig. 1 and 2, had been trapped by DCs in the first hour of incubation and 9% of strain O26:B6 cells had been trapped, but only 2.5% of A. actinomycetemcomitans cells had been trapped. In short, it appears that DCs engage E. coli more efficiently than A. actinomycetemcomitans and that the lower activity of E. coli for stimulating DC IL-12 is not due to a problem in trapping E. coli.
A. actinomycetemcomitans-stimulated mDCs induced early IFN-γ production by PBL.
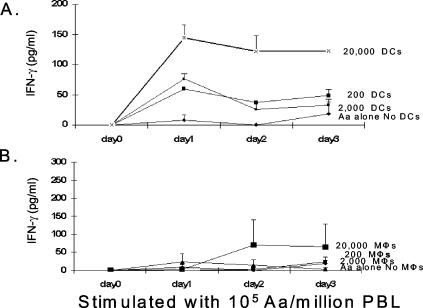
The ability of A. actinomycetemcomitans or A. actinomycetemcomitans LPS to stimulate IL-12 production by mDCs prompted the hypothesis that the addition of mDCs to normal PBL should enhance the IFN-γ response. When mDCs were added to NP PBL and stimulated with A. actinomycetemcomitans, a DC-dependent increase in IFN-γ production was apparent by 24 h (Fig. 3A). In contrast with the case for DCs, the addition of macrophages to PBL stimulated with A. actinomycetemcomitans failed to induce levels of IFN-γ that were significantly above the background, although there was some scatter in the data at days 2 and 3 (Fig. 3B). In most experiments, the simple addition of A. actinomycetemcomitans was sufficient to elicit detectable levels of IFN-γ production; this phenomenon was previously described by Kobayashi et al. (23). This IFN-γ response may be attributable to the presence of some DCs in the PBL, but the addition of more DCs clearly enhanced IFN-γ production. We were unable to detect IL-4 (our detection limit was 31.5 pg/ml) in the first 72 h in DCs or macrophages stimulated with A. actinomycetemcomitans or E. coli.
FIG. 3.
A. actinomycetemcomitans-stimulated mDCs, but not macrophages, induced an early IFN-γ response in PBL. PBL with various numbers of autologous mDCs (A) or macrophages (B) from an NP patient were stimulated with 105 A. actinomycetemcomitans cells. The supernatant fluids were collected after 1, 2, and 3 days, and IFN-γ production was measured by ELISA. Note the remarkable IFN-γ response induced in just 24 h by mDCs (A) but not by macrophages (B). In control cultures in which bacteria were used to stimulate DCs or macrophages alone, we were unable to detect any IFN-γ production. These data are representative of three separate experiments of this type and are expressed as means ± SE. MΦ, macrophages; Aa, A. actinomycetemcomitans.
NK cells as an important source of early IFN-γ.
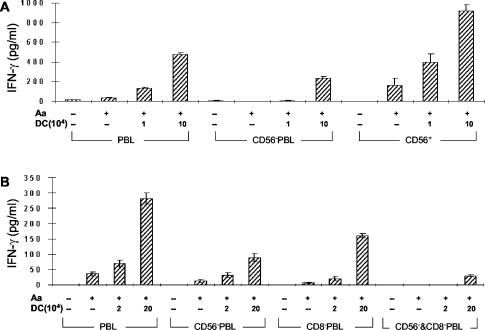
Given the ability of A. actinomycetemcomitans-stimulated DCs to induce IFN-γ production within 24 h and the known ability of NK cells to rapidly respond (43), we sought to determine if this rapid IFN-γ response might be attributable to NK cells (CD56+ cells). We compared IFN-γ production in PBL, PBL depleted of NK cells, and positively selected CD56+ cells treated with A. actinomycetemcomitans, with or without mDCs (Fig. 4A). The depletion of CD56+ cells from PBL significantly reduced the DC-dependent production of IFN-γ. Furthermore, A. actinomycetemcomitans-stimulated mDCs induced a potent IFN-γ response by the CD56+ cells. In contrast, the addition of macrophages failed to increase the production of IFN-γ and even suppressed the background level obtained by adding A. actinomycetemcomitans to CD56+ cells (data not shown). The importance of NK cells was further supported by experiments demonstrating that allogeneic mDCs stimulated with A. actinomycetemcomitans could elicit a potent IFN-γ response after 24 h, indicating a lack of major histocompatibility complex restriction (data not shown). Although the removal of CD56+ cells from PBL did eliminate most IFN-γ production, 25 to 45% remained, indicating that another type of cell also contributed to the early IFN-γ response. Recent reports indicated that DC CD8+-T-cell interaction can result in rapid IFN-γ responses (21), prompting us to examine the effect of removing CD8+ T cells, and as indicated in Fig. 4B, the activity after the NK cells had been removed could be largely attributed to CD8+ cells. Thus, NK cells appear to be the major contributor to the early IFN-γ response in PBL induced by mDCs stimulated by A. actinomycetemcomitans, but CD8+ cells also contribute to the response.
FIG. 4.
Contributions of NK cells and CD8+ cells to the early IFN-γ response induced by A. actinomycetemcomitans-stimulated mDCs. CD56−− represents cultures depleted of NK cells, and CD8−− represents cultures depleted of CD8 cells. (A) PBL, CD56+-depleted PBL, and CD56+ cells were held constant at 106 cells per culture with various numbers of autologous mDCs and were stimulated with 106 A. actinomycetemcomitans cells. The supernatant fluids were collected after 24 h, and IFN-γ production was measured by ELISA. The data are representative of three separate experiments of this type, although the response of CD56+ in the absence of DCs was not always seen and is probably attributable to a few DCs contaminating this preparation. The data are expressed as means ± SE. (B) PBL, CD56+-depleted PBL, CD8+-depleted PBL, and both CD56+- and CD8+-depleted cells were held constant at 106 cells per culture with various numbers of autologous mDCs and were stimulated with 106 A. actinomycetemcomitans cells. The supernatant fluids were collected after 24 h, and IFN-γ production was measured by ELISA. These data are representative of three separate experiments of this type. The data are expressed as means ± SE.
DC-derived IL-12 and optimal induction of early IFN-γ response.
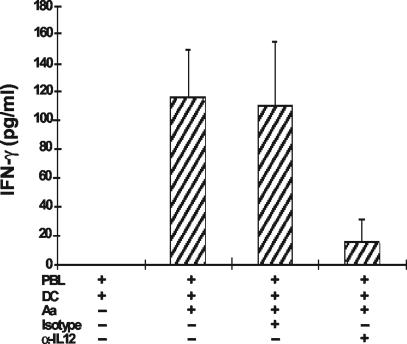
IL-12 is known to stimulate NK cells. To investigate the role of IL-12, we treated PBL (with or without mDCs) with an anti-IL-12 neutralizing antibody followed by stimulation with A. actinomycetemcomitans. As shown in Fig. 5, treatment with an anti-IL-12 antibody markedly inhibited the early production of IFN-γ.
FIG. 5.
IL-12 was critical for optimal IFN-γ production on day 1. PBL with various number of mDCs were treated with or without 10 μg of anti-IL-12 neutralizing antibody/ml, followed by stimulation with 106 A. actinomycetemcomitans cells. The supernatants were collected after 24 h, and IFN-γ was measured by ELISA. Other controls included boiling the anti-IL-12 antibody for 30 min, which eliminated all suppressive activity. These data are representative of three separate experiments of this type and are expressed as means ± SE.
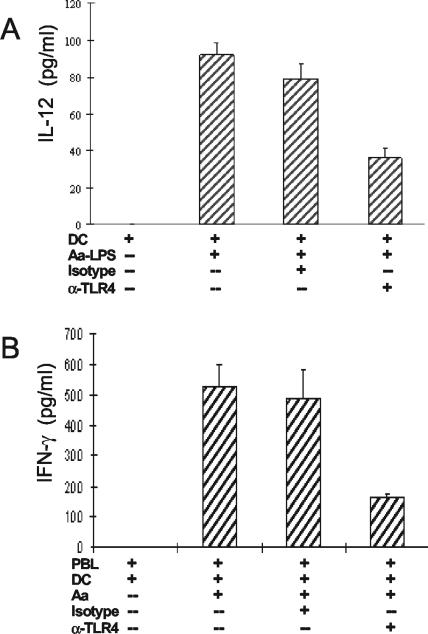
TLR4 involvement in early Th1 cytokine response.
TLR4 agonists such as LPS are known to promote the production of IL-12 by DCs (15), and we reasoned that A. actinomycetemcomitans LPS should promote IL-12 production and that an anti-TLR4 antibody should inhibit the IL-12 response. These predictions were substantiated when mDCs were treated with or without an anti-TLR4 neutralizing antibody followed by stimulation with A. actinomycetemcomitans LPS (Fig. 6A). To confirm that the IFN-γ response induced by intact A. actinomycetemcomitans in PBL was dependent on TLR4, we treated PBL with 105 mDCs, with or without the anti-TLR4 antibody, followed by stimulation with A. actinomycetemcomitans. As predicted, the A. actinomycetemcomitans-induced early IFN-γ response was inhibited by the anti-TLR4 antibody (Fig. 6B).
FIG. 6.
Blocking DC-TLR4 inhibited the early Th1 cytokine response. (A) mDCs (2 × 106) were treated with or without 20 μg of anti-TLR4 neutralizing antibody/ml, followed by stimulation with 30 ng of LPS from A. actinomycetemcomitans/ml. The supernatant fluids were collected after 24 h, and IL-12 p70 was measured by ELISA. The data are representative of three separate experiments of this type and are expressed as means ± SE. (B) PBL with 105 mDCs were treated with or without 20 μg of anti-TLR4 neutralizing antibody/ml, followed by stimulation with 106 A. actinomycetemcomitans cells. The supernatant fluids were collected after 24 h, and IFN-γ was measured by ELISA. Other controls included boiling the anti-TLR4 antibody for 30 min, which eliminated all suppressive activity. These data are representative of three separate experiments of this type and are expressed as means ± SE.
Comparisons of the A. actinomycetemcomitans and E. coli LPSs indicated that A. actinomycetemcomitans LPS was a better inducer of DC IL-12 (data not shown), and these results were consistent with the IL-12 data obtained by using whole bacteria (Fig. 1). Similarly, when the A. actinomycetemcomitans and E. coli LPSs were compared for the ability to upregulate the DC costimulatory molecule CD86, A. actinomycetemcomitans LPS was a better stimulator (Table 1). Note that about 1 ng of E. coli LPS was required to significantly upregulate CD86 expression on immature DCs and that a comparable response was obtained with only 0.1 ng of A. actinomycetemcomitans LPS.
TABLE 1.
Comparison of abilities of E. coli and A. actinomycetemcomitans LPS to upregulate CD86 expression by immature DCsa
| Amt (ng) of LPS | Mean fluorescence intensityb
|
P value | |
|---|---|---|---|
| E. coli | A. actinomycetemcomitans | ||
| 0.1 | 94 ± 0.2 | 131 ± 2.7 | <0.01 |
| 1 | 133 ± 3.5 | 147 ± 6.3 | <0.03 |
| 10 | 129 ± 6.3 | 151 ± 7.3 | <0.02 |
Monocytes were cultured with GM-CSF and IL-4 for 5 days, and then various concentrations of LPS from E. coli or A. actinomycetemcomitans were added for 1 day.
The background fluorescence intensity for CD86 on DCs in the absence of any LPS was 102 ± 6.4. Data are means ± SE.
DISCUSSION
The data reported here indicate that A. actinomycetemcomitans-stimulated mDCs secrete IL-12 and promote a rapid IFN-γ response by NK cells. DCs can produce IL-12 in its bioactive form, which is a 70-kDa heterodimer consisting of IL-12 p40 and p35 that must be expressed in the same cell to generate the heterodimer. The assay used here was specific for the 70-kDa heterodimer, which favors the differentiation of precursor Th0 cells into Th1 effectors (12, 27, 36, 41). Furthermore, A. actinomycetemcomitans was a more powerful inducer of IL-12 than E. coli or P. gingivalis (Fig. 1A). Similarly, A. actinomycetemcomitans LPS induced more DC IL-12 than did E. coli LPS, and A. actinomycetemcomitans LPS had more ability to upregulate the costimulatory molecule CD86 on DCs (Table 1). This could be relevant, given that IL-12 and CD86 have been implicated in the ability of bacteria to promote the production of IFN-γ by murine NKT cells and NK cells (43). P. gingivalis LPS is not a good TLR4 stimulator and is capable of interfering with activation by other TLR4 agonists (45), but E. coli stimulates cells well and is frequently used to mature DCs for Th1 responses (29). Thus, the data presented here suggest that A. actinomycetemcomitans might be a better stimulator than E. coli for maturing DCs for Th1 responses designed for use in cancer therapy or for intracellular infections. Our results indicating that bacterium-stimulated mDCs produced IL-12 p70 are also consistent with a critical role for DCs in the rapid production of IFN-γ. Both mDCs and macrophages responded to A. actinomycetemcomitans by producing similar levels of the Th2-polarizing cytokine IL-10 (Fig. 2). According to previous reports, IL-10 inhibits human lymphocyte IFN-γ production by suppressing NK cell stimulatory factor or IL-12 synthesis in accessory cells (10). Thus, mDCs seem to control the type of inflammatory response by secreting two functionally conflicting factors, and the ratio of IL-12 to IL-10 is thought to determine the balance of a Th1 versus Th2 response. The absolute amount of IL-10 was larger than that of IL-12 for both E. coli and A. actinomycetemcomitans, but IL-4 was not found in either case and IL-12 production was preferentially induced by A. actinomycetemcomitans. In short, the cytokine response induced by A. actinomycetemcomitans-stimulated mDCs suggests that there is skewing toward a Th1 response, and these cytokines may help to explain the high levels of IFN-γ-dependent IgG2 that is reactive with A. actinomycetemcomitans in sera and GCF of LagP patients.
A recent report indicated that A. actinomycetemcomitans often stimulates the production of IFN-γ when it is incubated with PBL from seronegative subjects (23). Since A. actinomycetemcomitans stimulates mDCs to secrete IL-12 (Fig. 1), we reasoned that the addition of mDCs to normal PBL should enhance the IFN-γ response. We confirmed that A. actinomycetemcomitans could stimulate IFN-γ production when incubated with normal PBL (Fig. 4B) and found that IFN-γ responses dramatically increased when mDCs were added with the A. actinomycetemcomitans. The observations that high levels of IFN-γ were apparent in just 24 h and that the IFN-γ was largely produced by NK cells (Fig. 3A) extend our understanding of A. actinomycetemcomitans-induced IFN-γ production by normal PBL.
The interaction of DCs with A. actinomycetemcomitans, leading to IL-12 production followed by the rapid production of IFN-γ by NK cells, is an important response at the innate level. These cytokines are capable of promoting Th1 development and thus influencing the development of the adaptive immune response. The observation that IFN-γ production was at a high level within just 24 h prompted studies to determine if NK cells were the predominant IFN-γ producers after stimulation with A. actinomycetemcomitans. The depletion of NK cells from PBL decreased the amount of IFN-γ about two-thirds (Fig. 4), and the balance was largely attributable to CD8+ cells. It has previously been reported that CD8+ cells may serve as an early source of IFN-γ (21), and that appears to be the case here.
Certain microbial antigens are known to stimulate IL-12 production, and IL-12 is critical to the optimal induction of IFN-γ production by NK cells and T cells (12, 41). Our studies with a neutralizing anti-IL-12 antibody confirmed that IL-12 is critical for A. actinomycetemcomitans-stimulated DCs to induce early IFN-γ production (Fig. 5). This rapid IFN-γ response also suggests that A. actinomycetemcomitans stimulation favors the differentiation of precursor Th0 cells into Th1 effectors.
LPSs from gram-negative bacteria appear to be important in the pathogenesis of periodontal disease (8). Our data for the neutralizing anti-TLR4 antibody, which has been identified as a signaling receptor of the innate system that is capable of recognizing LPS (45), showed that TLR4 is important in the pathway leading to IL-12 production by mDCs upon A. actinomycetemcomitans LPS stimulation (Fig. 6A). Furthermore, the optimal early IFN-γ response induced in PBL by A. actinomycetemcomitans-stimulated mDCs was also TLR4 dependent (Fig. 6B). These results are consistent with the concepts that LPS is a significant component in the induction of early IFN-γ production by A. actinomycetemcomitans and that LPS-TLR4 interactions on DCs initiate the pathway leading to the production of IFN-γ by NK cells and CD8+ cells. The data reported here indicate that A. actinomycetemcomitans-stimulated mDCs have the ability to promote a rapid IFN-γ response in healthy subjects by stimulating IFN-γ production by cells of the innate immune system. This DC-dependent pathway may help to provide the IFN-γ necessary for the high levels of IgG2 that are associated with less severe periodontal disease (5, 6, 25, 44). However, it is also of some concern that the prolonged production of Th1 cytokines in sites of chronic inflammation has been associated with tissue damage (1, 4, 9, 17, 35, 38). Thus, the DC-driven immune response to A. actinomycetemcomitans could help to induce adaptive immune responses that might have destructive as well as protective effects.
Acknowledgments
We gratefully acknowledge Kimberly Lake and Gail Smith for clinical management of the subjects participating in this study.
This work was supported by grant DE13102 from the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Agostini, C., M. Facco, M. Chilosi, and G. Semenzato. 2001. Alveolar macrophage-T cell interactions during Th1-type sarcoid inflammation. Microsc. Res. Tech. 53:278-287. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, S. E., Y. Ishihara, M. Fakher, S. Al-Darmaki, T. H. Caven, C. P. Shelburne, A. M. Best, H. A. Schenkein, and J. G. Tew. 2002. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect. Immun. 70:2780-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac, A., and R. Medzhitov. 2002. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J. Exp. Med. 195:F19-F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 2001. Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol. Scand. 59:235-243. [DOI] [PubMed] [Google Scholar]
- 5.Califano, J. V., J. C. Gunsolley, K. Nakashima, H. A. Schenkein, M. E. Wilson, and J. G. Tew. 1996. Influence of anti-Actinobacillus actinomycetemcomitans Y4 (serotype b) lipopolysaccharide on severity of generalized early-onset periodontitis. Infect. Immun. 64:3908-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Califano, J. V., R. E. Schifferle, J. C. Gunsolley, A. M. Best, H. A. Schenkein, and J. G. Tew. 1999. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J. Periodontol. 70:730-735. [DOI] [PubMed] [Google Scholar]
- 7.Cirrincione, C., N. Pimpinelli, L. Orlando, and P. Romagnoli. 2002. Lamina propria dendritic cells express activation markers and contact lymphocytes in chronic periodontitis. J. Periodontol. 73:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Daly, C. G., G. J. Seymour, and J. B. Kieser. 1980. Bacterial endotoxin: a role in chronic inflammatory periodontal disease? J. Oral Pathol. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 9.D'Ambrosio, D., A. Iellem, L. Colantonio, B. Clissi, R. Pardi, and F. Sinigaglia. 2000. Localization of Th-cell subsets in inflammation: differential thresholds for extravasation of Th1 and Th2 cells. Immunol. Today 21:183-186. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 5:405-411. [DOI] [PubMed] [Google Scholar]
- 12.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 13.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunsolley, J. C., J. A. Burmeister, J. G. Tew, A. M. Best, and R. R. Ranney. 1987. Relationship of serum antibody to attachment level patterns in young adults with juvenile periodontitis or generalized severe periodontitis. J. Periodontol. 58:314-320. [DOI] [PubMed] [Google Scholar]
- 15.Hilkens, C. M., P. Kalinski, M. de Boer, and M. L. Kapsenberg. 1997. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90:1920-1926. [PubMed] [Google Scholar]
- 16.Inaba, K., J. P. Metlay, M. T. Crowley, and R. M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen, H. K., S. J. Cryz, Jr., H. P. Hougen, C. Moser, and N. Hoiby. 1997. Vaccination promotes Th1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia. A new prophylactic principle. Behring Inst. Mitt. 1997:269-273. [PubMed] [Google Scholar]
- 18.Jotwani, R., and C. W. Cutler. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J. Dent. Res. 82:736-741. [DOI] [PubMed] [Google Scholar]
- 19.Jotwani, R., A. K. Palucka, M. Al-Quotub, M. Nouri-Shirazi, J. Kim, D. Bell, J. Banchereau, and C. W. Cutler. 2001. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J. Immunol. 167:4693-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambayashi, T., E. Assarsson, A. E. Lukacher, H. G. Ljunggren, and P. E. Jensen. 2003. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 170:2399-2408. [DOI] [PubMed] [Google Scholar]
- 22.Kawano, Y., T. Noma, and J. Yata. 1994. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J. Immunol. 153:4948-4958. [PubMed] [Google Scholar]
- 23.Kobayashi, H., T. Nagasawa, M. Aramaki, R. Mahanonda, and I. Ishikawa. 2000. Individual diversities in interferon gamma production by human peripheral blood mononuclear cells stimulated with periodontopathic bacteria. J. Periodont. Res. 35:319-328. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Z., Y. K. Sun, Y. P. Xi, A. Maffei, E. Reed, P. Harris, and N. Suciu-Foca. 1993. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J. Exp. Med. 177:1643-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, H., J. V. Califano, H. A. Schenkein, and J. G. Tew. 1993. Immunoglobulin class and subclass distribution of antibodies reactive with the immunodominant antigen of Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 61:2400-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailliard, R. B., Y. I. Son, R. Redlinger, P. T. Coates, A. Giermasz, P. A. Morel, W. J. Storkus, and P. Kalinski. 2003. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171:2366-2373. [DOI] [PubMed] [Google Scholar]
- 27.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 28.Ogasawara, K., K. Takeda, W. Hashimoto, M. Satoh, R. Okuyama, N. Yanai, M. Obinata, K. Kumagai, H. Takada, H. Hiraide, and S. Seki. 1998. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J. Immunol. 160:3522-3527. [PubMed] [Google Scholar]
- 29.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 31.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 32.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 33.Salvi, G. E., C. E. Brown, K. Fujihashi, H. Kiyono, F. W. Smith, J. D. Beck, and S. Offenbacher. 1998. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J. Periodont. Res. 33:212-225. [DOI] [PubMed] [Google Scholar]
- 34.Schenkein, H. A., and T. E. Van Dyke. 1994. Early-onset periodontitis: systemic aspects of etiology and pathogenesis. Periodontology 6:7-25. [DOI] [PubMed] [Google Scholar]
- 35.Schulze-Koops, H., and J. R. Kalden. 2001. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 15:677-691. [DOI] [PubMed] [Google Scholar]
- 36.Seder, R. A., R. Gazzinelli, A. Sher, and W. E. Paul. 1993. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 90:10188-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sornasse, T., V. Flamand, G. De Becker, H. Bazin, F. Tielemans, K. Thielemans, J. Urbain, O. Leo, and M. Moser. 1992. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J. Exp. Med. 175:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strober, W., B. R. Ludviksson, and I. J. Fuss. 1998. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann. Intern. Med. 128:848-856. [DOI] [PubMed] [Google Scholar]
- 39.Tew, J. G., D. R. Marshall, J. A. Burmeister, and R. R. Ranney. 1985. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect. Immun. 49:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tew, J. G., J.-B. Zhang, S. Quinn, S. Tangada, K. Nakashima, J. C. Gunsolley, H. A. Schenkein, and J. V. Califano. 1996. Antibody of the IgG2 subclass, Actinobacillus actinomycetemcomitans, and early-onset periodontitis. J. Periodontol. 67:317-322. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 42.Ueda, N., M. Koide, M. Ohguchi, Y. Ishihara, T. Noguchi, N. Okahashi, and T. Nishihara. 1998. Involvement of prostaglandin E2 and interleukin-1 alpha in the differentiation and survival of osteoclasts induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4. J. Periodont. Res. 33:509-516. [DOI] [PubMed] [Google Scholar]
- 43.Varma, T. K., C. Y. Lin, T. E. Toliver-Kinsky, and E. R. Sherwood. 2002. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin. Diagn. Lab. Immunol. 9:530-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson, M. E., and R. G. Hamilton. 1992. Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect. Immun. 60:1806-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimura, A., T. Kaneko, Y. Kato, D. T. Golenbock, and Y. Hara. 2002. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human Toll-like receptor 4. Infect. Immun. 70:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]