Abstract
Aging has been associated with a decline in immunocompetence and resistance to infections, partially due to dysregulated NO production by macrophages and deficits in mounting Th2 cell responses. We wondered if these alterations would reverse the immune response in experimental leishmaniasis. Bone-marrow-derived macrophages from 2- and 18-month-old (senescent) C57BL/6 or BALB/c mice showed no marked difference in leishmanicidal functions. In vivo infections of resistant C57BL/6 mice with Leishmania major revealed no difference between senescent and young mice. However, among susceptible BALB/c mice, senescent animals showed less foot-pad swelling than young mice, and 40 to 60% of them even showed healing of ulcers, reduced parasite dissemination, and a Th1 cell response. These changes were associated with a spontaneous release of interleukin-12 (IL-12) by macrophages from aged but not from young mice. Since exogenous microbial stimulation can influence immune responses during aging, we also infected senescent mice who were raised under specific-pathogen-free (SPF) conditions. They showed neither resistance nor a Th1 response, but their macrophages still spontaneously released IL-12. A microbiological analysis showed that conventionally kept mice, but not SPF mice, had experienced infection with murine hepatitis virus (MHV), an infection associated with a Th1-like response. We conclude that for the reversal of the immune response, senescence is the premier requirement but needs to be completed by another mandatory event such as microbial stimulation. One of the age-related, but not environment-related, factors is the spontaneous release of IL-12 by macrophages, while confrontation with MHV presents an environment-related difference, with both having the potential to support a Th1 response.
Morbidity in aging individuals is associated with an increase in susceptibility to many kinds of tumors, autoimmune disease, and infectious pathogens (12, 23). The decline in the immune response toward infectious microbes in senescence is associated with a dysregulation of certain macrophage functions, such as NO release (7, 18) or a reduced generation of oxygen radicals (7). The decline in immune competence is also related to several defined alterations in the T-cell response combined with a subsequent reduction in the generation of antibodies (26). Factors involved in the altered T-cell response in senescent humans or animals include an altered production of T-cell progenitors (stem cell defects and stromal cell defects), decreased levels of newly generated mature T cells (thymic involution), a decreased ratio of naïve to memory cells, and disrupted activation pathways in immune cells, with impairment in early CD4-T-cell activation (25). In addition, elderly humans and aging mice appear to have a deficiency in mounting an antigen-specific Th2 response (30, 35) coupled with a diminished production of interleukin-2 (IL-2) or expression of the IL2 receptor (34). At this point, it would be interesting if this decreased ability for differentiation into Th2 cells (35) would, on the other hand, favor a higher propensity for Th1 responses, and as such, be of advantage in reducing allergic immunoglobulin E (IgE)-mediated reactions or even in fighting infections requiring a Th1 response.
Immunity to infection with Leishmania major in mice requires an L. major-specific Th1 response as well as efficiently functioning dendritic cells and macrophages for resistance (4). Most inbred strains (C3/He and C57BL/6) fulfill these requirements by being armed with functional phagocytes and the ability to mount a Th1 response. However, inbred BALB/c mice are susceptible, primarily because they mount a Th2 instead of Th1 response.
We wondered if age-related alterations of the immune system would result in a different outcome of experimental leishmaniasis. In particular, we analyzed whether (i) effector functions of macrophages decrease with age and thereby influence resistance, (ii) the age-related decline in Th2 responses is dominant enough to reduce the generation of Th2 cells in BALB/c mice or even to bias T-cell differentiation to a Th1 response, and (iii) there is any additional influence by environmental factors during aging.
MATERIALS AND METHODS
Animals.
Female BALB/c and C57BL/6 mice were obtained from Bomholtgard Breeding and Research Centre A/S (Ry, Denmark) and were bred and raised in conventional animal housing. For a second set of experiments, female BALB/c and C57BL/6 mice were bred and raised in specific-pathogen-free (SPF) animal facilities.
Mice were assigned to the group of aged (or senescent) mice once they were 18 months of age. Mice belonging to the group of young mice were 8 to 10 weeks of age.
Parasites and experimental infection.
L. major (MHOM/IL/81/FE/BNI) was cultivated in Schneider's Drosophila medium supplemented with 10% fetal calf serum (FCS), 2% human urine, 2% glutamine, and 1% penicillin-streptomycin.
Preparation of bone-marrow-derived macrophages.
Macrophages were cultured in vitro from bone marrow progenitors. Briefly, mice were killed and their femurs were removed, cleaned of tissue, and flushed with Hanks' balanced salt solution. Erythrocytes were depleted by osmotic shock, and the cells were washed with Hanks' balanced salt solution, collected by centrifugation, and cultured at 107 cells per 10 ml of Dulbecco's modified Eagle's medium containing 2 mM glutamine, 0.1 mM nonessential amino acids, antibiotics, and 10% heat-inactivated FCS (Biochrom, Berlin, Germany). The cells were cultured in Teflon bags (Heraeus, Hanau, Germany) and provided with an additional 10 ml of medium supplemented with 10% L cell-conditioned medium containing macrophage colony-stimulating factor. After 6 days, the Teflon bags were put on ice to loosen adherent cells, which then were gently removed and collected by centrifugation.
Phagocytosis assay and killing rate of macrophages.
L. major was labeled with the fluorescent dye 5 (and 6)-carboxyfluoresceindiacetate succinimidylester (CFDA-SE; Molecular Probes, Leiden, The Netherlands) as previously described (32). Macrophages (after 6 days of culture) were seeded into LabTek culture chamber incubation slides (Nunc, Naperville, Ill.) at 5 × 104 cells per chamber. Fluorescence-labeled L. major was incubated in 10% mouse serum for complement opsonization and then added to the cells at a ratio of 4:1 for 2 h at 37°C.
Thereafter, the cultures were washed to remove extracellular parasites. The initial rate of infection or phagocytosis was evaluated by relating the number of L. major-infected macrophages to the total number of macrophages for at least six chambers. The antileishmanial activity was assessed by monitoring the percentage of macrophages bearing viable L. major again 48 h after infection and activation with gamma interferon (IFN-γ) (100 U/ml). The data presented are the mean values for 100 macrophages for at least six chambers.
NO release.
NO release was measured as the nitrite concentration in the supernatant by a microplate assay using Griess reagent as described previously (8).
Experimental leishmaniasis.
Experimental leishmaniasis was initiated by the subcutaneous application of 5 × 106 L. major promastigotes (stationary phase) in 20 μl of phosphate-buffered saline into the left hind foot pads of at least five young and five senescent mice per set of experiments. Foot-pad thickness was measured with a metric caliper. Foot pads from five mice from each group were harvested 3 days after infection for immunohistochemical analysis of the early infiltrate. The lymph nodes (LN) and spleen from each mouse were harvested 12 weeks after infection for a determination of the cytokine profile and for a limiting dilution assay (LDA). These experiments were performed three times.
LDA.
Parasite numbers in the spleen were determined 12 weeks after infection as a parameter for systemic spread by use of an LDA (8), modified by the use of leishmania medium as specified above instead of slant blood agar.
Antibodies and immunohistochemical staining.
The following monoclonal antibodies against mouse antigens were purchased from several commercial sources: F4/80 (Biozol, Eching, Germany), BM8 (BMA, Augst, Switzerland), CD4, CD8, GR-1, murine hepatitis virus (MHV) class II (all by BD Pharmingen, Hamburg, Germany), and 8E7 (32). Immunohistochemical staining was performed as described elsewhere (38).
Lymphocyte culture.
The draining inguinal and popliteal LN were collected at 12 weeks postinfection, and T cells were generated as described previously (13). Briefly, the cells were purified by passage over a nylon wool column and a subsequent immunomagnetic depletion of contaminating cells, using monoclonal antibodies against B220, CD11b, CD16/CD32, CD8, GR1, and CD24 (all obtained from BD Pharmingen). After washing and an incubation with goat anti-rat IgG MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), the antibody-negative cell fraction was separated with an automated magnetic cell sorter (autoMACS; Miltenyi Biotec) (13). LN cells were cultured in RPMI 1640 plus 2 mM glutamine, 50 μM mercaptoethanol, and 10% FCS (106 LN cells/ml). After 48 h, the LN cells were restimulated with soluble leishmania antigen (SLA) equivalent to 2 × 106 L. major promastigotes. For control purposes, LN cells were also stimulated with the mitogens phorbol myristate acetate (3 ng/ml) and ionomycine (300 ng/ml). Culture supernatants were collected after 48 h and assayed with an enzyme-linked immunosorbent assay (Pharmingen) for IL-4 (detection limit, <14 pg/ml) and IFN-γ (detection limit, <2.5 pg/ml) (32).
Secretion of IL-12 by macrophages.
Macrophages were seeded into 24-well plates at 2 × 105 cells/well in 2 ml of medium. The macrophages were stimulated for 24 h with lipopolysaccharide (LPS) (20 ng/ml) as a positive control or with L. major opsonized with fresh mouse serum after being primed with IFN-γ (100 U/ml). After 24 h, the concentration of IL-12 in the supernatant was determined by an ELISA, using a paired monoclonal antibody recognizing both the p75 and p40 subunits (detection limit, <5 pg/ml) (R&D Systems, Wiesbaden, Germany).
Microbiological analysis.
Mice from both conventional and SPF facilities were screened by a commercial laboratory for natural murine pathogens according to the guidelines of the Federation of European Laboratory Animal Science Associations by serology, PCR, and culturing (28). In addition, residential bacterial and fungal colonization of the oral cavities, small intestines, and fur of mice from both facilities were analyzed and compared at the Institute of Medical Microbiology (G. Peters) and at the Laboratory for Dermatomicrobiology (C. Sunderkötter, Department of Dermatology, University of Münster).
Statistics.
The means and standard deviations (SD) for all numerical data were calculated. Statistical significance between groups was judged by Student's t test. Comparisons of data sets yielding P values of >0.05 were not considered to be statistically significant.
RESULTS
In order to compare the effector functions of macrophages from aged and young mice, we analyzed phagocytosis, the killing of L. major, and NO production.
Phagocytosis of L. major and killing activity by macrophages from young and aged mice.
We determined the infection rates of macrophages cultured on LabTek culture chambers directly after phagocytosis and 48 h after activation with IFN-γ. Coculturing of L. major with macrophages at a ratio of 4:1 led to an infection rate of 72% in the case of macrophages from young mice and of 67% in the case of macrophages from aged mice (Table 1). Similar results were obtained when the uptake of fluorescently labeled L. major by macrophages was analyzed by fluorescence-activated cell sorting, as 75% (±4%) of the macrophages were infected in young mice versus 66% (±6%) in senescent mice (data not shown). Thus, macrophages from aged mice are capable of phagocytosing L. major at a slightly, but not significantly, reduced rate. Forty-eight hours after incubation in the presence of IFN-γ (100 U/ml), infected macrophages from young mice were able to eliminate 49% of internalized L. major parasites, whereas macrophages from aged mice had killed 51% of ingested L. major parasites (n = 6) (Table 1).
TABLE 1.
Phagocytosis rate and leishmanicidal activity of macrophages from young and senescent micea
| Mouse group | % of L. major-infected macrophages after:
|
Elimination rate (%) | |
|---|---|---|---|
| 4 h | 48 h (plus stimulation with IFN-γ at 100 U/ml) | ||
| Young mice | 72 ± 4 | 37 ± 8 | 49 |
| Senescent mice | 67 ± 3 | 33 ± 9 | 51 |
Labeled L. major (CFDA-SE) parasites were coincubated with macrophages (for 6 days of culture) on LabTek culture chamber incubation slides at an effectortarget ratio of 4:1. Thereafter, the cultures were washed to remove extracellular parasites. The initial rate of infection or phagocytosis was determined by evaluating the percentage of infected cells. The leishmanicidal activity was assessed by again monitoring the percentage of macrophages bearing L. major 48 h after infection and activation with IFN-γ (100 U/ml). The data presented are the mean values ± SD for 100 macrophages for at least six wells.
This indicates that after activation with IFN-γ, macrophages from aged mice present a similar killing rate as macrophages from young mice.
Release of NO by macrophages from young and aged mice.
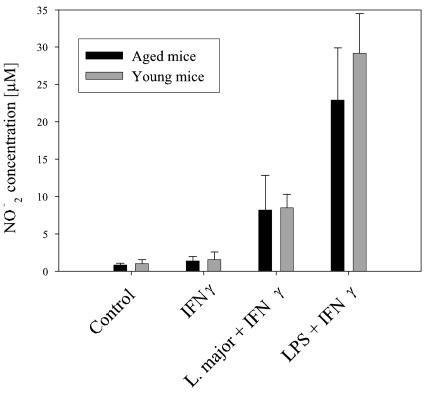
As presented in Fig. 1, NO production by macrophages from senescent mice in response to LPS and L. major was not impaired, consistent with the similar elimination rates of macrophages from senescent and young mice.
FIG. 1.
NO release. NO release was measured in the supernatants of macrophages (106 cells/ml). Macrophages were stimulated with IFN-γ (100 U/ml), LPS plus IFN-γ, or L. major plus IFN-γ or were kept in basal medium for 24 h. Data are means ± SD (n = 6).
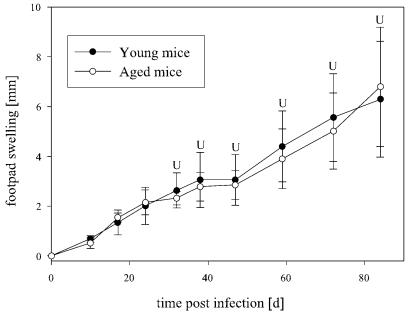
Course of experimental leishmaniasis in young and aged mice.
After infection with 5 × 106 L. major promastigotes in the hind foot pad, senescent C57BL/6 mice showed a similar course for the swelling of foot pads as young C57BL/6 mice (Fig. 2), and there was no marked dissemination of parasites into visceral organs (data not shown).
FIG. 2.
Foot pad swelling (in relation to the noninfected contralateral foot pad) of infected young and senescent C57/BL6 mice and BALB/c mice (data are means ± SD [n = 5]). Foot pads were measured weekly and did not reveal significant differences between young and aged C57BL/6 mice. Foot pads of senescent BALB/c mice showed retarded swelling and ulceration compared to those of young BALB/c mice. Senescent mice even revealed healing of ulcerations in three of five feet in this experiment. U, ulcerated foot pad; (U), healed ulcers. *, P < 0.05 for differences between aged and young mice.
Thus, resistance remained unharmed in senescent C57BL/6 mice in spite of the various deficiencies which have been reported to take place in the general immune response during aging (12, 23, 26).
When young and senescent BALB/c mice were infected, we observed an initially similar increase in foot-pad swelling in both groups during the first 3 weeks postinfection (Fig. 2). In the ensuing weeks, swelling in young BALB/c mice progressed to ulceration of the foot pads beginning after 4 weeks. In senescent BALB/c mice, the swelling progression was slower but also finally resulted in the ulceration (albeit retarded) of all foot pads after 7 weeks. However, when these mice were monitored for longer than 7 weeks, ulceration did not progress or even healed in up to 60% of senescent BALB/c mice after 10 weeks, leaving a scar (Fig. 3). These aged mice also did not show signs of markedly reduced health when their organs were harvested for LDAs at the end of 12 weeks. In contrast, ulceration and a reduction of general health progressed in all young BALB/c mice. With LDAs, we only rarely detected parasites in the spleens of those mice that had healed ulcerations. Among senescent mice without healing, we observed a spectrum from low to occasionally marked dissemination into the spleen. In contrast, all young BALB/c mice were severely parasitized (Fig. 4).
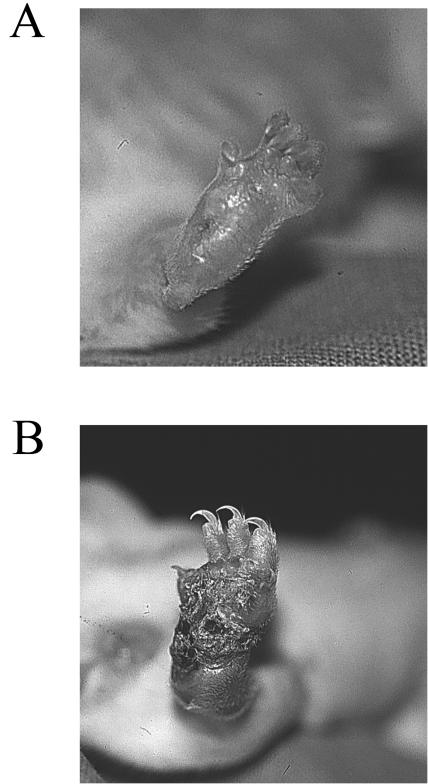
FIG. 3.
Healing of ulcerated foot pad of a senescent BALB/c mouse, but not that of a young BALB/c mouse, 12 weeks after infection with L. major. (A) Foot pad of a senescent mouse which had ulcerated after 7 weeks but started to heal 2 weeks later, leaving a scar and a dilated blood vessel in the central part. (B) Foot pad of a young mouse after 12 weeks of infection showing a large crusted ulcer.
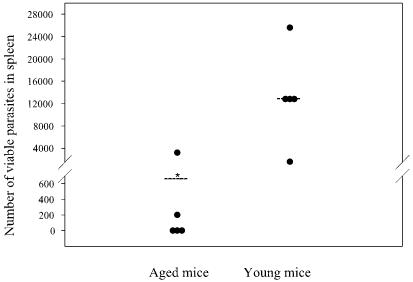
FIG. 4.
LDAs with spleens from young and aged BALB/c mice 12 weeks after infection. We performed an LDA with the spleen from each (n = 5) young and senescent mouse in order to analyze visceralization. We did not detect parasites in the spleens of the three mice with healed ulcerations and detected only a very small number of parasites in another aged mouse, while only one senescent mouse showed marked dissemination into the spleen. In contrast, all of the young mice showed marked to very high dissemination of L. major into the spleen. *, P < 0.05 for differences between young and aged mice.
Thus, senescent mice showed a stronger containment of parasite spread than young mice and presented a spectrum from reduced foot-pad swelling to decreased visceralization and even healing. This is striking as it shows that BALB/c mice are capable of becoming resistant without further treatment when they age.
Lymphokine profile of young and aged mice infected with L. major.
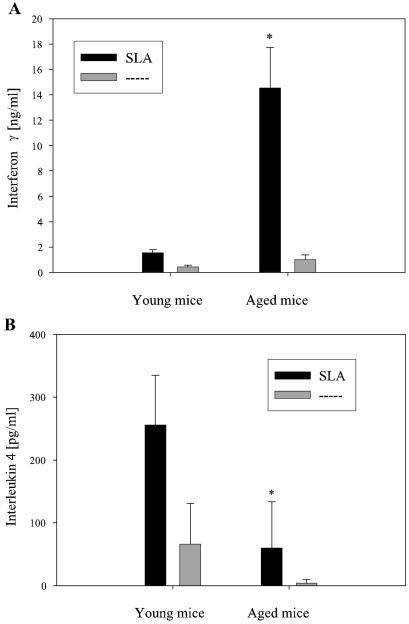
Isolated CD4+ LN cells taken from infected young BALB/c mice and restimulated with SLA regularly showed a cytokine pattern that was typical for a Th2 response (Fig. 5). In contrast, CD4+ LN cells from infected senescent BALB/c mice showed a significant increase in the L. major-specific release of IFN-γ (Fig. 5A). Their release of IL-4 was slightly, but not significantly, less than that from T cells of young infected mice (178 ± 114 pg/ml in senescent mice versus 289 ± 51 pg/ml in young mice). However, when only mice with healed ulcerations were considered for comparison, the release of IL-4 was significantly lower than that from young mice (Fig. 5B). While IL-4 is a characteristic cytokine for a Th2 response, it was the increased release of macrophage-activating IFN-γ which was associated with containment of the parasite load (16).
FIG. 5.
Cytokine assay with LN at 12 weeks postinfection. CD4+ LN cells were restimulated with SLA for 48 h. Subsequently, the IFN-γ and IL-4 contents in the supernatants were determined. CD4+ LN cells from young BALB/c mice that were restimulated with SLA regularly showed a cytokine pattern typical for a Th2 response. In contrast, CD4+ LN cells from infected senescent BALB/c mice showed a significant increase in the L. major-specific release of IFN-γ (A). Their release of IL-4 was not significantly lower than that from T cells of young infected mice (178 ± 114 pg/ml in senescent mice versus 289 ± 51 pg/ml in young mice). However, when only mice with healed ulcerations were considered for comparison (n = 3), their release of IL-4 was significantly lower than that from young mice (B). (A) Data are means and SD (n = 5) for all mice; (B) data are means and SD (n = 3) for mice with healed ulcerations. *, P < 0.05 for differences between young and aged mice. Dashed line, no stimulation.
These results show that during senescence some BALB/c mice are able to mount a Th1 response and to develop resistance. This is remarkable because usually all untreated BALB/c mice infected with high doses of L. major succumb to visceralization.
Composition of the infiltrate.
We did not find any significant differences in the percentages of immunohistochemically stained (F4/80-positive) macrophages between young and senescent mice in the early infiltrate after 3 days, nor did we see significant differences in the local proportions of CD4+ and CD8+ cells (data not shown).
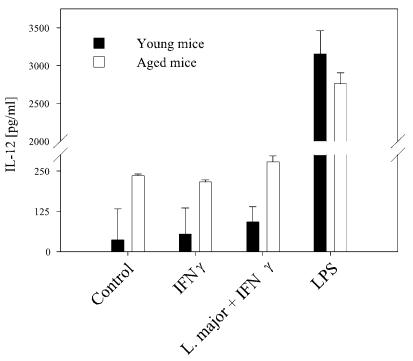
Release of IL-12 from cultured macrophages of young and senescent mice.
Splenic macrophages from aged mice were shown to spontaneously produce IL-12 (36). IL-12 is an important cytokine for innate immunity, especially in experimental leishmaniasis, as it is the decisive inducer of a Th1 response (for a review, see reference 16). We analyzed the ability of bone-marrow-derived macrophages from senescent mice to produce IL-12 in response to infection with L. major after being primed with IFN-γ for 24 h. Compared to macrophages from young mice, cultured macrophages from senescent mice were found to already have a higher spontaneous production of IL-12 (Fig. 6). Since IFN-γ did not lead to a marked stimulation of IL-12 in untreated and L. major-infected macrophages, this difference remained constant in these groups. When macrophages were stimulated with LPS, cells from both young and aged mice showed the capability to respond to this stimulus and to increase the release of IL-12 (Fig. 6).
FIG. 6.
IL-12 secretion by macrophages. Macrophages were stimulated for 24 h with LPS (20 ng/ml) as a positive control, with IFN-γ (100 U/ml), or with L. major opsonized with fresh mouse serum after priming with IFN-γ (100 U/ml). Macrophages from senescent mice showed a slightly raised release of IL-12 compared to those from young mice, and this difference remained apparent after stimulation with L. major or IFN-γ. The secretion of IL-12 was stimulated by LPS in macrophages from aged and young mice. Data are means ± SD (n = 5). *, P < 0.05 for differences between young and aged mice.
Environmental conditions.
Factors influencing the immune response over time encompass exogenous stimulation. It was shown that a continuous exposure to high concentrations of microbes or microbial products in the environment appears to diminish the Th2 cell response (11, 29, 39). On the other hand, an environment with a low level of microbial contents, as found in SPF animal facilities, was shown to improve or to also deteriorate the immune response in various models of inflammation and infection (1, 3, 24, 25). Therefore, we investigated whether the reversal of the immune response would still prevail in senescent mice bred under SPF conditions and thus lacking early and continuous exposure of the immune system to potentially pathogenic microbes. In this experiment, the susceptibility of senescent BALB/c mice did not differ significantly from that of young BALB/c mice. Swelling of the foot pads (Fig. 7) and parasite loads after 12 weeks were similar and were associated with a Th2 response in both groups (data not shown). In resistant C57BL/6 mice, there was also no difference in the immune response between young and aged mice (data not shown), indicating that their genetically determined Th1 responses are independent of exposure to environmental microbes.
FIG. 7.
Foot pad swelling (compared to the noninfected contralateral foot pad) of infected young and senescent BALB/c mice from SPF facilities. Foot pads were measured weekly and revealed no significant change between young and senescent mice, in contrast to the case for mice raised under non-SPF conditions. Data are means ± SD (n = 5). U, ulcerated foot pads.
In order to evaluate which microbes might have especially influenced the T-cell response in senescent mice, we used a commercial laboratory to screen mice for natural murine pathogens by serology, PCR, and culturing according to the guidelines from the Federation of European Laboratory Animal Science Associations (28). We found that apart from the unapparent confrontation with potentially pathogenic microbes during their lifetimes, sera from young and aged mice from conventional facilities repeatedly tested positive for MHV titers (Table 2), indicating a long-standing exposure to this virus. MHV refers to a large group of single-stranded RNA viruses of the genus Coronavirus with widely varying pathogenicities (6). In addition, we compared residential bacterial colonization of the oral cavities, small intestines, and fur of mice from both facilities. However, we detected no difference in resident microbial colonization between mice from conventional and SPF facilities (microbes in the oral cavity and colon were Staphylococcus aureus, enterococci, Escherichia coli, and spore-forming bacteria; microbes in the fur were mostly S. aureus and E. coli).
TABLE 2.
Microbiological screening of mice from SPF and conventional facilities according to the criteria of FELASA by indirect immunofluorescence (IFA), cultures, and microscopic analysis
| Target organions for test and test type | Method | Result for facility
|
|
|---|---|---|---|
| Conventional | SPF | ||
| Serology (viruses) | |||
| MHV | IFA | +++a | Negative |
| Ectromelia virus | IFA | Negative | Negative |
| Epizootic diarrhea of infant mice | IFA | Negative | Negative |
| Lymphocytic choriomeningitis virus | IFA | Negative | Negative |
| Mouse adenovirus 2 type K87 | IFA | Negative | Negative |
| Murine cytomegalovirus | IFA | Negative | Negative |
| Mouse parvovirus | IFA | Negative | Negative |
| Lactic dehydrogenase virus | Enzymatic | Negative | Negative |
| Minute virus of mice | IFA | Negative | Negative |
| Pneumonia virus of mice | IFA | Negative | Negative |
| Reovirus type 3 | IFA | Negative | Negative |
| Sendai virus | IFA | Negative | Negative |
| Theiler's encephalomyelitis virus | IFA | Negative | Negative |
| Polyomavirus | IFA | Negative | Negative |
| Hantaan virus | IFA | Negative | Negative |
| Mouse thymic virus | IFA | Negative | Negative |
| Bacteriology/mycology | |||
| Citrobacter rodentium | Culture | Negative | Negative |
| Clostridium piliforme | IFA | Negative | Negative |
| Corynebacterium kutscheri | Culture | Negative | Negative |
| Mycoplasma spp. | IFA | Negative | Negative |
| Pasteurella pneumotrophica | IFA | Negative | Negative |
| Streptobacillus moniliformis | Culture | Negative | Negative |
| Streptococcus sp. (B-hemolytic, not group D) | Culture | Negative | Negative |
| Streptococcus pneumoniae | Culture | Negative | Negative |
| Parasitology | |||
| Aspiculuris sp. | Microscopy | Negative | Negative |
| Syphacia sp. | Microscopy | Negative | Negative |
| Coccidia | Microscopy | Negative | Negative |
| Giardia sp. | Microscopy | Negative | Negative |
| Spironucleus muris | Microscopy | Negative | Negative |
| Trichomonada | Microscopy | Negative | Negative |
| Flagellates (other) | Microscopy | Negative | Negative |
| Arthropods | Stereomicroscopy | Negative | Negative |
+++, high titers (>1:80).
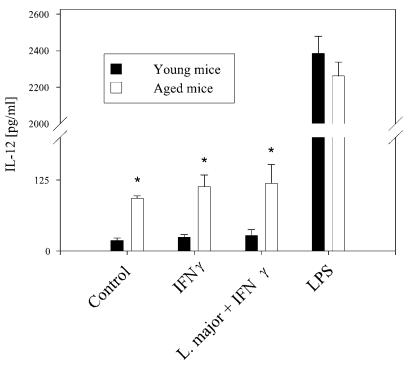
We then analyzed IL-12 release by macrophages from SPF mice that were cultivated and stimulated under the same conditions as those reported for the previous experiments with macrophages from mice from conventional facilities. Again, macrophages from aged mice, in contrast to those from young mice, revealed a spontaneously higher level of IL-12 release (Fig. 8).
FIG. 8.
IL-12 secretion by macrophages from SPF facilities. Macrophages from mice kept under SPF conditions were stimulated for 24 h with LPS (20 ng/ml) as a positive control, with IFN-γ (100 U/ml), or with L. major opsonized with fresh mouse serum after priming with IFN-γ (100 U/ml). As shown for macrophages from senescent mice kept under conventional conditions, macrophages from senescent SPF mice also showed a slightly raised release of IL-12 compared to those from young mice, and this difference remained apparent after stimulation with L. major or IFN-γ. The secretion of IL-12 was stimulated by LPS in macrophages from aged and young mice. Data are means ± SD (n = 5). *, P < 0.05 for differences between young and aged mice.
Thus, the higher spontaneous release of IL-12 from unstimulated macrophages is dependent on senescence but does not appear to be dependent on microbial environmental factors.
Since young BALB/c mice were susceptible, independent of whether they were kept under SPF or conventional conditions, and since the spontaneous release of IL-12 was also independent of these environmental factors, we have evidence that a high microbial exposure alone is not sufficient to prevent a Th2 response. On the other hand, an age-inherent decline of the Th2 cell response in aging BALB/c mice has also been reported for mice under SPF conditions when they were challenged with, e.g., Schistosoma mansoni eggs or the gastrointestinal nematode Nippostrongylus brasiliensis (35). Our results indicate that neither the age-inherent decline of the Th2 cell response nor an early and continuous exposure to pathogenic microbes in conventional animal facilities alone is sufficient to overcome susceptibility toward L. major. Instead, the age-inherent decline of the Th2 cell response in aging BALB/c mice needs to be combined with the exposure of mice to pathogenic microbes to substantially improve the immune response toward L. major.
DISCUSSION
In this study, we showed that senescent BALB/c mice are more efficient than young BALB/c mice in generating a protective immune response toward infection with L. major. The spectrum of this immune response reached from reduced foot-pad swelling and retarded ulceration to a complete reversal of susceptibility, with the emergence of an immunocompetent Th1 cell response, the containment of parasite spread, and the healing of ulcerated foot pads.
This is remarkable, as it shows for the first time that some senescent BALB/c mice are capable of becoming resistant without further treatment. Usually, infected BALB/mice in a wide age range succumb to visceralization without exception, unless they are subjected to exogenous modification of the immune response, such as the neutralization of IL-4 or the application of sufficient IL-12 or IFN-γ (for a review, see reference 16). A recent study of factors influencing L. major infections in IL-4-deficient mice revealed that older but not yet senescent IL-4-deficient BALB/c mice (26 weeks of age) present a markedly slower development of ulcers than young IL-4-deficient BALB/c mice (19). Although the mice in that study were not yet senescent (6 months versus 18 months) and were devoid of IL-4, the results indicate that inherent age-related alterations in the immune response can act complementarily with exogenous modifications (deletion of the gene for IL-4) of the immune system to yield apparent clinical effects.
However, a complete reversal of the immune response did not occur in each of the aged BALB/c mice in our study, and it only took place in senescent mice kept under conventional, not SPF, conditions. This suggests that a continuous exposure to certain microbes or stimulation by their products has an influence on this development (1, 3, 24, 25).
In resistant C57BL/6 mice, senescence was not associated with relevant changes in the immune response toward L. major, regardless of whether mice were reared under conventional or SPF conditions. This is noteworthy in view of the various deficiencies which have been reported to take place in the aging immune response (12, 23, 26).
The mechanisms responsible for longer survival among infected senescent BALB/c mice are not likely to encompass altered functions of their effector cells. Macrophages from senescent mice, on average, showed no significant differences in phagocytosis, killing rate, and NO production. A significant reduction in the generation of NO by macrophages from senescent mice after stimulation with, e.g., IFN-γ, was reported earlier (7, 18). Depressed phagocytic activity has been reported for aging human monocytes (22). However, recent studies have revealed that there is no general age-related reduction in phagocytic functions, but rather a distinct decline depending on the receptors for different pathogens. As such, the reduced phagocytosis of S. aureus by senescent macrophages correlated with a reduction of FcγR III (CD16), while the uptake of gram-negative bacteria did not differ, coinciding with the unchanged expression of CR3 (5). Since CR3 is also a major receptor for L. major, this may explain why we did not find significant age-related differences in the phagocytosis of L. major.
Also, there was no difference in the recruitment of macrophages or lymphocytes to foot pads shortly after infection between young and aged mice. Our results are consistent with observations from previous studies, which demonstrated, in addition, no significant changes in the hematopoiesis of leukocytes during aging (for a review, see reference 37).
In contrast, there were differences in the T-cell response between senescent and young mice that explain the clinical improvement. CD4-positive T cells from those infected senescent mice that revealed resistance had reverted toward a Th1 response. This change in the T-cell response may compensate for some previously reported age-related deficiencies of the T-cell response which we did not analyze in this study but which were shown to include decreased numbers of newly generated mature T cells, a decreased ratio of naïve to memory cells, and impaired activation (26).
There have been conflicting results with regard to the tendency of senescent mice to mount either a Th1- or a Th2-like cytokine response (14). Some studies report a bias for a Th2 response in aging mice (33), while others demonstrate a predominance of murine CD4+ or CD8+ cells releasing IFN-γ (9, 10). Data on the cytokine response by T cells are more consistent when they focus on in vivo murine models. Recent work on two different mouse models involving an in vivo challenge with S. mansoni eggs or the gastrointestinal nematode N. brasiliensis revealed an increased capacity of senescent mice to produce IFN-γ (35) by CD4-positive cells, but also by CD8-positive (Tc1) cells. Similarly, in senescent rats, allergic bronchitis was significantly reduced in correlation with a marked reduction in the expression of mRNAs for Th2 cytokines (15). Our study now shows not only that aging mice may reveal a decline in the Th2 cell response, but also that aging in some cases even leads to a unique reversal to a Th1 response among CD4-positive cells. CD8-positive (Tc1) cells may serve as an additional source of IFN-γ (17, 30), but in the high-dose infection model with L. major, CD4-positive Th1 and Th2 cells are the decisive T cells.
One factor that could facilitate the generation of a Th1 response and contribute to the spectrum of resistance in senescent mice could be the age-related spontaneous release of IL-12 by their macrophages. We found that this release was independent of environmental factors. In previous studies, the spontaneous or LPS-induced release of IL-12 by monocytes or macrophages was also higher or at least unchanged during senescence (20, 36). However, our study also demonstrates that the spontaneous IL-12 release is not sufficient to induce resistance, as it also occurs in senescent, but still susceptible, BALB/c mice from SPF facilities.
Although we cannot present the complete sequence of causal events leading to resistance in senescent BALB/c mice, we were able to identify that reversal to a Th1 cell response was the decisive mechanism and that there are at least two mandatory requirements for this reversal. Our observation that this reversal never took place in mice who were born and raised under SPF conditions led to the hypothesis that an exposure to potentially pathogenic microbes could be a second signal that is required for a complete reversal of the immune response.
In young and aged mice from conventional, but not SPF, facilities, titers for MHV were repeatedly positive, indicating that these mice had been confronted with MHV and prompted to generate an immune response against it.
It has long been hypothesized that human individuals with a higher or more frequent exposure to microbes show a propensity to mount a Th1 response rather than a Th2 response. Some evidence for this theory has recently been provided by a survey which demonstrated that farm children with higher LPS exposures had a significantly reduced prevalence of Th2-associated atopic sensitization than children from municipal areas with less LPS exposure (29). Similarly, changes in the composition of the intestinal microflora were shown to suppress a Th2 response (39). In our study, we did not find differences in the intestinal floras between mice from conventional or SPF facilities, nor was there a difference in bacterial colonization of the oral cavity, small intestine, or fur. Contact with viruses should also facilitate the development of Th1 responses, as increased IFN-γ production by CD8+ T cells was noted for aging human populations which had experienced more viral exposure (Epstein-Barr virus and cytomegalovirus) (2). In our study, mice from conventional facilities were negative for murine cytomegalovirus, but in contrast to mice from SPF facilities, they had positive titers against MHV. MHV refers to a large group of single-stranded RNA viruses of the genus Coronavirus with widely varying pathogenicities. These viruses can infect mice early in life, inducing an immune response (6). The mice in our study were clinically normal and presented no signs of demyelinating diseases in the central nervous tissue and no overt signs of hepatitis or gastrointestinal disease. Since titers against MHV were and had been repeatedly positive in the conventional facilities, we concluded that the mice had been infected earlier in life and had no active infection. Confrontation with MHV could still modulate the immune response (6), as its containment requires a Th1-like response which can be induced even in the absence of IL-12 (27, 31). Remarkably, in humans, an early infection with human hepatitis A virus apparently reduces the risk of developing a Th2 cell-mediated manifestation of atopy later in life (21). These findings do not prove that MHV is the decisive additional factor in the shift to a Th1 response in otherwise susceptible senescent BALB/c mice; however, it clearly indicates that factors triggering a Th1 response are involved in supporting senescence-related resistance (20, 36).
Thus, the age-inherent decline of the Th2 cell response, as reported for aging BALB/c mice in an infection model with S. mansoni eggs or N. brasiliensis (35), can also be observed in experimental leishmaniasis, but it is not regularly sufficient to cause reversal to a Th1 response. According to our results, reversal to a Th1 response becomes possible when, e.g., aging mice are not kept under SPF conditions, but rather are exposed at birth and during their lifetimes to an environment with potentially pathogenic microbes, which may lead to innate or specific immune responses.
We were thus able to elaborate two absolutely required conditions for full reversal to a Th1 response, i.e., senescence and an ensuing mandatory condition, such as the reaction to a microbial environment, as follows. (i) Age is absolutely required, as not a single young mouse developed resistance, irrespective of environmental (SPF or conventional) conditions. (ii) A microbial environment is also mandatory, as senescent mice only developed resistance when they were raised under non-SPF conditions. (iii) One clearly age-related immunological difference disclosed in this study was a higher spontaneous release of IL-12 by macrophages from aged mice than from macrophages from young mice. This was independent of the environmental conditions (conventional or SPF facilities). (iv) One difference in the microbial environment was the MHV infection of mice, which was endemic to conventional facilities only and most likely occurred early in life, as the titers were repeatedly positive. In this context, it is remarkable that the resolution of MHV infection is associated with a Th1 response which does not depend on the presence of IL-12 (31, 39). Thus, this could be a supplemental step in light of the observation that the higher spontaneous release of IL-12 alone was not sufficient for reversal of the immune response.
These facts are consistent with the hypothesis derived from human studies that common infections acquired early in life, e.g., because of unhygienic living conditions, appear to reduce the propensity of individuals to develop Th2 cell-mediated immune responses. Although this hypothesis is still speculative, it could be the task of future studies to investigate whether infections with MHV or other microbes in conjunction with certain senescence-related alterations of the immune response generate constellations which explain the different levels of immunologically effective immune response toward L. major.
Acknowledgments
We thank S. Merfeld, E. Nattkemper, and M. Steinert for their skillful technical assistance. We are indebted to Udo Herz and Ulrike Ehrchen for valuable suggestions.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Annacker, O., O. Burlen-Defranoux, R. Pimenta-Araujo, A. Cumano, and A. Bandeira. 2000. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J. Immunol. 164:3573-3580. [DOI] [PubMed] [Google Scholar]
- 2.Bandres, E., J. Merino, B. Vazquez, S. Inoges, C. Moreno, M. L. Subira, and A. Sanchez-Ibarrola. 2000. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(−)CD57(+) subpopulation. Clin. Immunol. 96:230-235. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, M., P. A. Taylor, M. Austin, M. B. Baker, L. B. Schook, M. Rutherford, V. Kumar, E. R. Podack, K. M. Mohler, R. B. Levy, and B. R. Blazar. 1998. Cytokine and cytotoxic pathways of NK cell rejection of class I-deficient bone marrow grafts: influence of mouse colony environment. Int. Immunol. 10:785-790. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 5.Butcher, S. K., H. Chahal, L. Nayak, A. Sinclair, N. V. Henriquez, E. Sapey, D. O'Mahony, and J. M. Lord. 2001. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 70:881-886. [PubMed] [Google Scholar]
- 6.Compton, S. R., S. W. Barthold, and A. L. Smith. 1993. The cellular and molecular pathogenesis of coronaviruses. Lab. Anim. Sci. 43:15-28. [PubMed] [Google Scholar]
- 7.Ding, A., S. Hwang, and R. Schwab. 1994. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J. Immunol. 153:2146-2152. [PubMed] [Google Scholar]
- 8.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 9.Engwerda, C. R., B. S. Fox, and B. S. Handwerger. 1996. Cytokine production by T lymphocytes from young and aged mice. J. Immunol. 156:3621-3630. [PubMed] [Google Scholar]
- 10.Ernst, D. N., W. O. Weigle, D. J. Noonan, D. N. McQuitty, and M. V. Hobbs. 1993. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 151:575-587. [PubMed] [Google Scholar]
- 11.Gerhold, K., K. Blumchen, A. Bock, C. Seib, P. Stock, T. Kallinich, M. Lohning, U. Wahn, and E. Hamelmann. 2002. Endotoxins prevent murine IgE production, T(H)2 immune responses, and development of airway eosinophilia but not airway hyperreactivity. J. Allergy Clin. Immunol. 110:110-116. [DOI] [PubMed] [Google Scholar]
- 12.Ginaldi, L., M. De Martinis, A. D'Ostilio, L. Marini, M. F. Loreto, and D. Quaglino. 1999. Immunological changes in the elderly. Aging (Milan) 11:281-286. [DOI] [PubMed] [Google Scholar]
- 13.Grabbe, S., G. Varga, S. Beissert, M. Steinert, G. Pendl, S. Seeliger, W. Bloch, T. Peters, T. Schwarz, C. Sunderkotter, and K. Scharffetter-Kochanek. 2002. Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J. Clin. Investig. 109:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, S. N., and S. N. Meydani. 2000. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J. Infect. Dis. 182(Suppl. 1):S74-S80. [DOI] [PubMed]
- 15.Ide, K., H. Hayakawa, T. Yagi, A. Sato, Y. Koide, A. Yoshida, M. Uchijima, T. Suda, K. Chida, and H. Nakamura. 1999. Decreased expression of Th2 type cytokine mRNA contributes to the lack of allergic bronchial inflammation in aged rats. J. Immunol. 163:396-402. [PubMed] [Google Scholar]
- 16.Jankovic, D., Z. Liu, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 17.Khan, N., N. Shariff, M. Cobbold, R. Bruton, J. A. Ainsworth, A. J. Sinclair, L. Nayak, and P. A. Moss. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169:1984-1992. [DOI] [PubMed] [Google Scholar]
- 18.Kissin, E., M. Tomasi, N. McCartney-Francis, C. L. Gibbs, and P. D. Smith. 1997. Age-related decline in murine macrophage production of nitric oxide. J. Infect. Dis. 175:1004-1007. [DOI] [PubMed] [Google Scholar]
- 19.Kropf, P., S. Herath, V. Weber, M. Modolell, and I. Muller. 2003. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 25:439-447. [DOI] [PubMed] [Google Scholar]
- 20.Lio, D., C. D'Anna, F. Gervasi, L. Scola, M. Potestio, G. Di Lorenzo, F. Listi, A. Colombo, G. Candore, and C. Caruso. 1998. Interleukin-12 release by mitogen-stimulated mononuclear cells in the elderly. Mech. Ageing Dev. 102:211-219. [DOI] [PubMed] [Google Scholar]
- 21.Matricardi, P. M., F. Rosmini, L. Ferrigno, R. Nisini, M. Rapicetta, P. Chionne, T. Stroffolini, P. Pasquini, and R. D'Amelio. 1997. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ 314:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mege, J. L., C. Capo, B. Michel, J. L. Gastaut, and P. Bongrand. 1988. Phagocytic cell function in aged subjects. Neurobiol. Aging 9:217-220. [DOI] [PubMed] [Google Scholar]
- 23.Miller, R. A. 1996. The aging immune system: primer and prospectus. Science 273:70-74. [DOI] [PubMed] [Google Scholar]
- 24.Ohmoto, Y., M. J. Wood, H. M. Charlton, K. Kajiwara, V. H. Perry, and K. J. Wood. 1999. Variation in the immune response to adenoviral vectors in the brain: influence of mouse strain, environmental conditions and priming. Gene Ther. 6:471-481. [DOI] [PubMed] [Google Scholar]
- 25.Ohteki, T., R. Okuyama, S. Seki, T. Abo, K. Sugiura, A. Kusumi, T. Ohmori, H. Watanabe, and K. Kumagai. 1992. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J. Immunol. 149:1562-1570. [PubMed] [Google Scholar]
- 26.Pawelec, G., Y. Barnett, R. Forsey, D. Frasca, A. Globerson, J. McLeod, C. Caruso, C. Franceschi, T. Fulop, S. Gupta, E. Mariani, E. Mocchegiani, and R. Solana. 2002. T cells and aging, January 2002 update. Front. Biosci. 7:d1056-d1183. [DOI] [PubMed] [Google Scholar]
- 27.Pope, M., S. W. Chung, T. Mosmann, J. L. Leibowitz, R. M. Gorczynski, and G. A. Levy. 1996. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2, response, whereas pre-existing antibody partially protects against primary infection. J. Immunol. 156:3342-3349. [PubMed] [Google Scholar]
- 28.Rehbinder, C., P. Baneux, D. Forbes, H. van Herck, W. Nicklas, Z. Rugaya, and G. Winkler. 1996. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab. Anim. 30:193-208. [DOI] [PubMed] [Google Scholar]
- 29.Riedler, J., C. Braun-Fahrlander, W. Eder, M. Schreuer, M. Waser, S. Maisch, D. Carr, R. Schierl, D. Nowak, and E. von Mutius. 2001. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358:1129-1133. [DOI] [PubMed] [Google Scholar]
- 30.Saurwein-Teissl, M., T. L. Lung, F. Marx, C. Gschosser, E. Asch, I. Blasko, W. Parson, G. Bock, D. Schonitzer, E. Trannoy, and B. Grubeck-Loebenstein. 2002. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893-5899. [DOI] [PubMed] [Google Scholar]
- 31.Schijns, V. E., B. L. Haagmans, C. M. Wierda, B. Kruithof, I. A. Heijnen, G. Alber, and M. C. Horzinek. 1998. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J. Immunol. 160:3958-3964. [PubMed] [Google Scholar]
- 32.Schonlau, F., K. Scharffetter-Kochanek, S. Grabbe, B. Pietz, C. Sorg, and C. Sunderkotter. 2000. In experimental leishmaniasis deficiency of CD18 results in parasite dissemination associated with altered macrophage functions and incomplete Th1 cell response. Eur. J. Immunol. 30:2729-2740. [DOI] [PubMed] [Google Scholar]
- 33.Shearer, G. M. 1997. Th1/Th2 changes in aging. Mech. Ageing Dev. 94:1-5. [DOI] [PubMed] [Google Scholar]
- 34.Shi, J., and R. A. Miller. 1993. Differential tyrosine-specific protein phosphorylation in mouse T lymphocyte subsets. Effect of age. J. Immunol. 151:730-739. [PubMed] [Google Scholar]
- 35.Smith, P., D. W. Dunne, and P. G. Fallon. 2001. Defective in vivo induction of functional type 2 cytokine responses in aged mice. Eur. J. Immunol. 31:1495-1502. [DOI] [PubMed] [Google Scholar]
- 36.Spencer, N. F., and R. A. Daynes. 1997. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5− B cells: possible involvement in age-associated cytokine dysregulation. Int. Immunol. 9:745-754. [DOI] [PubMed] [Google Scholar]
- 37.Sunderkotter, C., H. Kalden, and T. A. Luger. 1997. Aging and the skin immune system. Arch. Dermatol. 133:1256-1262. [DOI] [PubMed] [Google Scholar]
- 38.Sunderkotter, C., M. Kunz, K. Steinbrink, G. Meinardus-Hager, M. Goebeler, H. Bildau, and C. Sorg. 1993. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J. Immunol. 151:4891-4901. [PubMed] [Google Scholar]
- 39.von Mutius, E., C. Braun-Fahrlander, R. Schierl, J. Riedler, S. Ehlermann, S. Maisch, M. Waser, and D. Nowak. 2000. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin. Exp. Allergy 30:1230-1234. [DOI] [PubMed] [Google Scholar]