Abstract
Recently, we demonstrated that blocking the entry of neutrophils into Borrelia burgdorferi-infected joints in mice deficient in the chemokine receptor CXCR2 prevented the development of experimental Lyme arthritis. Neutrophils were marginalized in blood vessels at the site of infection but could not enter the joint tissue. In the present study, we treated both genetically arthritis-resistant DBA/2J (DBA) and arthritis-susceptible C3H/HeJ (C3H) mice with the neutrophil-depleting monoclonal antibody RB6-8C5 (RB6) to determine the effect on arthritis development. Surprisingly, both DBA and C3H mice treated with RB6 developed arthritis at 1 week postinfection, approximately 1 week earlier than the control-treated C3H mice. The early development of arthritis in the RB6-treated mice was accompanied by an influx into the joints of cells with ring-shaped polymorphonuclear leukocyte (PMN) cell morphology that were negative for the Gr-1 neutrophil maturation marker. RB6 treatment of mice also resulted in increased numbers of B. burgdorferi cells in the joints at 7 days postinfection and earlier expression of the chemokines KC and monocyte chemoattractant protein 1 in the joints compared to control-treated animals. Together, these results suggest that recruitment of neutrophils or PMN-like cells into an infected joint is a key requirement for Lyme arthritis development and that altered recruitment of these cells into the joints of arthritis-resistant mice can exacerbate the development of pathology.
Lyme disease is caused by infection with the spirochete Borrelia burgdorferi and is transmitted through the bite of an infected Ixodes tick (17). Since its recognition in the mid-1970s, Lyme disease has become the most common arthropod-transmitted disease in the United States, with some 18,000 new cases reported each year (19). Arthritis is a common pathology associated with Lyme disease and occurs in about 60% of individuals who are not treated with antibiotics near the time of infection (46). This arthritis can be severe, is typically associated with the presence of spirochetes in the joint tissue, and is characterized by a marked inflammatory cell infiltrate, synovial hypertrophy, and tendonitis (46).
Experimental inoculation of B. burgdorferi into mice recapitulates a portion of the disease spectrum seen in humans (6). Murine genetics control the development of pathology following experimental infection and lead to a spectrum of disease susceptibilities in different mouse strains. While the cellular mechanisms responsible for resistance or susceptibility to Lyme arthritis are unknown, only in BALB/c mice has a direct correlation been found between the development of pathology and the number of spirochetes located within the joints (28). C3H/HeJ (C3H) mice infected with as few as 200 spirochetes go on to develop severe arthritis and harbor large numbers of spirochetes within their ankle tissue (14, 28, 54). Conversely, infected DBA/2J (DBA) and C57BL/6 (B6) mice develop only mild arthritis even when they are infected with as many as 1 × 106 spirochetes despite harboring large numbers of spirochetes in their ankle tissue, like C3H mice (14, 28). The cellular mechanisms responsible for these phenotypic differences are contained within the innate immune response. Studies in which immunodeficient SCID or RAG−/− mice with arthritis-resistant B6 or DBA genetic backgrounds or with arthritis-susceptible C3H genetic backgrounds were used demonstrated that these mice exhibited the disease phenotypes of their wild-type counterparts (15, 44). While a number of studies have demonstrated the ability of pro- or anti-inflammatory cytokines to modulate arthritis severity, the underlying genetic basis for arthritis resistance or susceptibility resides in innate immunity (2-4, 16, 27, 29).
Recently, we reported that differential production of the chemokines KC and monocyte chemoattractant protein 1 (MCP-1) correlated with the development of Lyme arthritis in joints of arthritis-susceptible mouse strains but not in joints of arthritis-resistant mouse strains (13). KC and MCP-1 are powerful chemoattractants for neutrophils and monocytes, respectively, which are cells that make up the majority of the inflammatory infiltrate in the joints of mice experimentally infected with B. burgdorferi. Infection of mice deficient in the receptor for KC (CXCR2) resulted in an inability of neutrophils to enter the joint tissue and blocked the development of arthritis (13). Thus, neutrophil recruitment into the joint tissue appears to be a requirement for development of experimental Lyme arthritis. Neutrophils have been shown to constitute a large percentage of the inflammatory response in humans with Lyme arthritis (52). Similarly, neutrophils have been reported to play key roles in other models of arthritis (1, 31, 33, 40, 42, 51, 53). In experimental Lyme arthritis in mice, some investigators have noted a correlation between neutrophil influx into joint tissue and severe arthritis (43), while other workers have suggested that neutrophils mediate protection against arthritis development through their bactericidal activity in joint tissue (5).
In the present study, we examined the role of neutrophils in the development of experimental Lyme arthritis by treating arthritis-resistant and -susceptible mouse strains with the neutrophil-depleting antibody RB6-8C5 (RB6). To our surprise, both strains of mice began to develop a severe arthritis accompanied by an influx of polymorphonuclear leukocyte (PMN)-like cells and an increase in joint spirochetal loads within the first week after infection. These results suggest that neutrophils are critical for early host defense against spirochetes and support the hypothesis that neutrophils play a role in mediating the development of pathology.
MATERIALS AND METHODS
Experimental infection of mice.
Female C3H and DBA mice that were 4 to 6 weeks old were purchased from The Jackson Laboratories (Bar Harbor, Maine) and housed in microisolator cages under specific-pathogen-free conditions. A virulent, low-passage, clonal isolate of the N40 strain of B. burgdorferi was used in all experiments. Frozen stocks were grown to the log phase in 7 ml of Barbour-Stoenner-Kelly H medium (BSK-H medium) (Sigma-Aldrich, St. Louis, Mo.) by incubating cultures for 5 days at 32°C. Spirochetes were enumerated by dark-field microscopy by using a Petroff-Hausser counting chamber (Hausser Scientific Company, Horsham, Pa.). Mice were inoculated in both hind footpads with 2.5 × 105 B. burgdorferi cells in 50 μl of BSK-H medium. For the dose-response experiments, C3H mice were treated daily with control immunoglobulin G (IgG) or RB6 beginning 1 day prior to infection. Experimental groups of mice were then infected in both hind footpads with increasing log doses (2.5 × 103 to 2.5 × 106 cells) of B. burgdorferi in 50 μl of BSK-H medium and were sacrificed 7 days later.
RB6 treatment of mice.
Monoclonal antibody RB6 is a rat IgG2B antibody that selectively binds to and depletes mature mouse neutrophils and eosinophils (22, 23). The RB6 hybridoma was a kind gift from Robert L. Coffman (DNAX Research Institute, Palo Alto, Calif.). The antibody was affinity purified from cell culture supernatants by using a protein G column (Pharmacia, Piscataway, N.J.). To deplete mice of neutrophils, 0.2 mg of RB6 was administered intraperitoneally 1 day prior to infection and then daily for 21 days. Control mice were inoculated with an equal volume of normal rat IgG (Sigma). In preliminary experiments, we found that following a single injection of RB6 the number of peripheral blood neutrophils began to rebound by the third day. Thus, mice were given daily injections to ensure continued depletion of neutrophils from the circulation. Neutrophil depletion did not lead to any obvious deterioration in the health of mice throughout the experimental period. Peripheral blood smears were examined on day 0 and weekly thereafter for randomly selected mice for the duration of the neutrophil depletion period. Briefly, blood was collected from the retroorbital plexus, smeared onto a slide, and allowed to dry. The blood smears were stained with Diff-Quick, and at least 200 nucleated cells per slide were counted by an individual experienced in counting blood differentials. Percentages of specific cell types were determined from the total number of cells counted. Neutrophils in RB6-treated mice were depleted so that they accounted for less than 7% of the circulating nucleated cells throughout the experiment.
Assessment of arthritis.
Ankle swelling was monitored by weekly measurement with a metric caliper (Ralmike's Tool-A-Rama, South Plainfield, N.J.) of the thickest anteroposterior portion of the tibiotarsal joint. Baseline ankle diameters were determined immediately prior to infection, and experimental diameters were determined weekly thereafter. Baseline measurements were subtracted from the experimental measurements to determine increases in ankle diameter. To determine arthritis severity scores, a histologic analysis of the right tibiotarsal joint from each mouse was performed following sacrifice at 7 or 21 days postinfection. The joint was excised by cutting just above and below the tibiotarsal joint, fixed in 10% buffered zinc-formalin, and embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin (H&E). The sections were evaluated in a blinded manner by two independent observers and were assessed for arthritis severity on a scale of 0 to 3 (8). A score of 0 indicated normal tissue, 1 and 2 indicated mild and moderate inflammation, respectively, and 3 indicated severe arthritis. The pathology present in histologic sections was characterized by edema and neutrophil and monocyte infiltration into the joints, tendons, and ligament sheaths; hypertrophy and hyperplasia of the synovium; and fibrin exudates. The extent of the observed inflammatory changes formed the basis for the arthritis severity scores.
Culture of B. burgdorferi from tissues.
Experimental samples of blood, heart, spleen, urinary bladder, and skin (ear punch) were aseptically collected from mice infected for 21 days and were cultured at 32°C in BSK-H medium. After 14 days the cultures were scored for the presence of spirochetes by placing 10 μl of supernatant on a microscope slide under a coverslip (22 by 22 mm) and examining 20 high-power fields by dark-field microscopy.
Quantitative assessment of B. burgdorferi in tissues.
Quantitative multiplex real-time PCR was performed as described previously (13) by using the ABI Prism 7700 sequence detection system (PE Applied Biosystems, Foster City, Calif.) to analyze levels of B. burgdorferi DNA present in the tissues of infected mice. Briefly, following mouse sacrifice, ankles were excised, snap frozen in liquid N2, and stored at −80°C. The frozen tissue was pulverized, and the DNA was extracted with a DNEasy tissue kit (QIAGEN, Valencia, Calif.). The DNA was eluted in 100 μl of elution buffer and diluted to a concentration of 50 μg/ml with Tris-EDTA buffer. One microliter of a diluted sample, which has been estimated to contain 1,000 copies of the mouse Nidogen gene (32), was then used in PCRs. The mouse Nidogen gene was used as an endogenous control as described previously (13). Quantification of B. burgdorferi DNA in samples was done by detection of the Flagellin gene by using primers and probes as described previously (34). The Taqman PCR conditions were as follows: 50°C for 2 min, 95°C for 10 min, and then 45 cycles of 95°C for 15 s and 60°C for 1 min. Quantitative multiplex real-time PCR for each sample was performed in duplicate or triplicate for the Flagellin gene, and the results were normalized to copies of Nidogen DNA from the same tube. The B. burgdorferi DNA in each sample was quantified by comparison to a standard curve for known numbers of B. burgdorferi cells. Similarly, normalization of the mouse DNA in each sample was performed by comparison to a standard curve for dilutions of mouse DNA from the same tissue (ear or ankle).
Quantification of cytokine levels in joint extracts.
Joint extracts were prepared by using slight modifications of the method of Kasama et al. (26), as described previously (13). Briefly, the joints were excised by removing the skin and cutting just above and below the ankle joint, and they were snap frozen in liquid nitrogen. The joints were pulverized with a hammer and homogenized on ice in 1 ml of lysis buffer (Hanks balanced salt solution containing 0.2% protease inhibitor cocktail [Sigma] and 0.5% NP-40) by using a tissue homogenizer (IKA Works, Wilmington, N.C.). The homogenized tissues were then centrifuged at 2,000 × g for 10 min at 4°C, and the supernatants were filtered through a 0.45-mm-pore-size filter. The filtrates were diluted in 1.5 ml of lysis buffer, split into aliquots, and stored at −80°C until they were analyzed. A number of murine cytokines and chemokines were then quantified by enzyme-linked immunosorbent assays (ELISA). Interleukin-1β (IL-1β), IL-4, IL-6, IL-10, IL-12p70, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor, and MCP-1 were quantified by using OptEIA ELISA sets (BD Pharmingen, San Diego, Calif.). Macrophage inflammatory protein 1α (MIP-1α), MIP-2, and KC were quantified by using DuoSet ELISA sets (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. Total protein concentrations in the samples were determined by using a protein assay kit (Pierce Chemical Co., Rockford, Ill.). Data are expressed below in picograms of cytokine per milligram of protein.
Immunohistochemistry.
Immunohistochemistry analyses of ankle sections were performed as described previously (13). Briefly, paraffin sections were fixed in xylene and rehydrated by using ethanol and 1 M phosphate-buffered saline (pH 6.0). Endogenous peroxidases were quenched prior to blocking of nonspecific binding with 1.5% normal rabbit serum (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) diluted in phosphate-buffered saline. Sections were incubated overnight at 4°C with a 1:500 dilution of RB6 (22). Normal rat serum was used as a negative control. A biotinylated rabbit anti-rat IgG antibody was used as the secondary antibody. Following incubation with streptavidin-conjugated horseradish peroxidase, diaminobenzidine tetrahydrochloride (Sigma) was used as the peroxidase substrate. The slides were counterstained with Mayer's hematoxylin solution (Fisher Scientific, Fair Lawn, N.J.), and the stained sections were then dehydrated and mounted.
For myeloperoxidase (MPO) immunohistochemistry analysis the sections were rehydrated as described above, and endogenous peroxidases were blocked with hydrogen peroxide. The sections were pretreated with proteinase K (1:40 dilution) and then blocked with bovine serum albumin. They were then incubated with the primary antibody, a polyclonal rabbit anti-MPO antibody (Dako, Carpinteria, Calif.) diluted 1:200, for 60 min at room temperature. A biotinylated swine anti-rabbit antibody (diluted 1:300) was used as the secondary antibody, and the cells were then treated with streptavidin-conjugated horseradish peroxidase and diaminobenzidine tetrahydrochloride and counterstained as described above. All steps were completed with a Dako autostainer (Dako).
Statistical analysis.
Results are expressed below as means ± standard deviations. Data were analyzed by using Student's t test or analysis of variance followed by the Tukey test for multiple comparisons by using SigmaStat software (SPSS, Inc., Chicago, Ill.). Significance levels were set at α = 0.05.
RESULTS
Neutrophil depletion results in exacerbated arthritis development.
To determine the role of neutrophils in the development of experimental Lyme arthritis, we treated arthritis-resistant DBA mice and arthritis-susceptible C3H mice with the neutrophil-specific monoclonal antibody RB6. Previous reports have shown that treatment of mice with this antibody renders them severely neutropenic for up to 3 days following administration (21, 23, 38). In preliminary studies, we found that neutrophils in the circulation were recovering by 3 days postinjection. Therefore, the mice were treated with antibody on a daily basis. Peripheral blood was collected from mice on days 0, 7, 14, and 21 of infection to determine the extent of the neutropenia. Peripheral blood counts are shown in Table 1. Generally, very few neutrophils (less than 5% of nucleated cells) were found in the peripheral blood of RB6-treated mice through 14 days of infection. By day 21, the levels had risen slightly in both mouse strains to 6 to 7% of the nucleated cells.
TABLE 1.
Peripheral blood counts in control IgG-treated and RB6-treated micea
| Mouse strain | Treatment | Day | % of total counts
|
|||
|---|---|---|---|---|---|---|
| Neutrophils | Lymphocytes | Monocytes | Eosinophils | |||
| C3H | IgG | 0 | 32.2 | 25.7 | 41.9 | 0.7 |
| RB6 | 0 | 1.0 | 42.5 | 56.0 | 0.5 | |
| DBA | IgG | 0 | 23.1 | 23.9 | 53.0 | 0.0 |
| RB6 | 0 | 0.0 | 30.7 | 68.9 | 0.4 | |
| C3H | IgG | 7 | 24.1 ± 7.6 | 46.7 ± 6.0 | 27.8 ± 1.9 | 1.3 ± 0.7 |
| RB6 | 7 | 4.6 ± 3.6 | 44.3 ± 9.9 | 51.2 ± 6.7 | 0.0 ± 0.0 | |
| DBA | IgG | 7 | 25.0 ± 9.4 | 57.1 ± 5.2 | 17.2 ± 5.2 | 0.4 ± 0.6 |
| RB6 | 7 | 0.9 ± 1.3 | 49.2 ± 2.4 | 46.1 ± 1.9 | 0.0 ± 0.0 | |
| C3H | IgG | 14 | 36.0 | 30.7 | 32.9 | 0.4 |
| RB6 | 14 | 0.0 | 44.4 | 55.3 | 0.3 | |
| DBA | IgG | 14 | 27.8 | 38.7 | 33.2 | 0.3 |
| RB6 | 14 | 0.4 | 37.5 | 61.7 | 0.4 | |
| C3H | IgG | 21 | 50.9 ± 0.1 | 22.8 ± 7.0 | 25.4 ± 7.4 | 1.0 ± 0.2 |
| RB6 | 21 | 5.8 ± 0.6 | 48.6 ± 4.0 | 44.9 ± 4.9 | 0.8 ± 0.9 | |
| DBA | IgG | 21 | 33.7 ± 6.0 | 36.6 ± 2.1 | 31.3 ± 8.0 | 0.3 ± 0.2 |
| RB6 | 21 | 6.6 ± 3.9 | 61.4 ± 4.0 | 30.0 ± 1.6 | 2.1 ± 0.5 | |
Mice were given daily intraperitoneal injections of 200 μg of control IgG or RB6 beginning on day −1. Mice were infected with 2.5 × 105 B. burgdorferi cells on day 0 and blood was collected from the retroorbital plexus weekly. Blood smears were stained with Diff-Quick, and at least 200 cells per slide were counted. On days 0 and 14 one mouse per treatment per mouse strain was randomly chosen for bleeding. On days 7 and 21 groups of three mice were bled, and the data are averages ± standard deviations. The data are representative of at least four separate experiments.
Control and neutropenic mice with resistant and susceptible genetic backgrounds were inoculated in both hind footpads with the N40 strain of B. burgdorferi. Neutrophils typically respond rapidly to sites of infection. Therefore, footpad inoculation was used to deliver the spirochetes to a site directly adjacent to the tibiotarsal joints and to avoid the differences in dissemination of spirochetes that can occur between mice of different strains and with different immunological status (7, 45). Ankle swelling is usually indicative of an underlying inflammatory response. Development of pathology was monitored by measuring ankle diameters on a weekly basis (Fig. 1). In this system, pathology in susceptible animals typically develops during the second week of infection (see the curve for IgG-treated C3H mice in Fig. 1A). RB6-treated mice, however, had statistically greater ankle swelling during the first week of infection than control IgG-treated animals (Fig. 1). RB6-treated C3H mice had significantly greater ankle swelling (P < 0.01) than the IgG-treated C3H mice at day 7 postinfection, but the two types of mice had similar levels of ankle swelling at days 14 and 21 (Fig. 1A). RB6-treated DBA mice, however, had exacerbated ankle swelling compared with IgG-treated DBA mice throughout the experimental period (Fig. 1B) (P < 0.001).
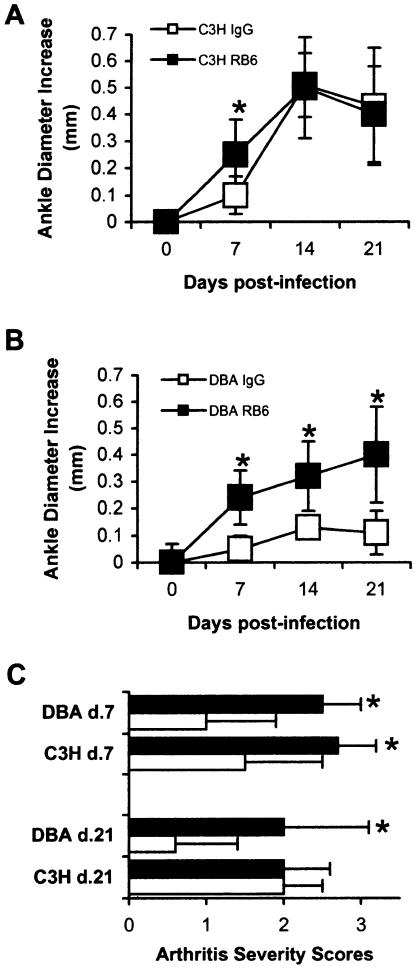
FIG. 1.
Development of experimental Lyme arthritis in control and neutropenic mice. (A and B) Ankle swelling curves for C3H (A) and DBA (B) mice treated with control isotype IgG or RB6. (C) Arthritis severity scores determined from H&E-stained sections of tibiotarsal joints from mice sacrificed on days 7 and 21 of infection. Open symbols and bars, IgG-treated animals; solid symbols and bars, RB6-treated animals. Sections were blindly evaluated and scored by two independent observers on a scale of 0 to 3 as described in Materials and Methods, and the values for each joint were averaged. Mice (five mice per group) were 4 to 6 weeks old when they were infected in both hind footpads with B. burgdorferi. Ankle diameters were measured weekly. The data are means ± standard deviations and are representative of four separate experiments. An asterisk indicates that the P value is <0.05 for a comparison of IgG- and RB6-treated animals.
We also determined histopathological lesion scores as a measure of arthritis severity for mice infected for 7 or 21 days (Fig. 1C). At 7 days postinfection, the RB6-treated C3H mice had significantly higher arthritis severity scores than the IgG-treated C3H mice (P < 0.05). Similarly, the day 7 arthritis severity scores for the RB6-treated DBA mice were significantly higher than the scores for the IgG-treated DBA mice (P < 0.01). By 21 days postinfection the IgG- and RB6-treated C3H mice had similar arthritis severity scores (Fig. 1C). In contrast, the RB6-treated DBA mice still had significantly higher arthritis severity scores than the IgG-treated DBA mice (P < 0.01). These results demonstrate that treatment of mice with RB6 leads to the depletion of neutrophils from the circulation and exacerbation of Lyme arthritis development. RB6-treated C3H mice developed arthritis 1 week earlier than control IgG-treated animals, while the remaining courses of arthritis development were similar. RB6-treated DBA mice, however, developed more severe arthritis than the control IgG-treated DBA mice developed during the first week of infection, and this trend continued throughout the experimental period. Control IgG-treated DBA mice were resistant to arthritis development.
Neutrophil depletion in joint tissue.
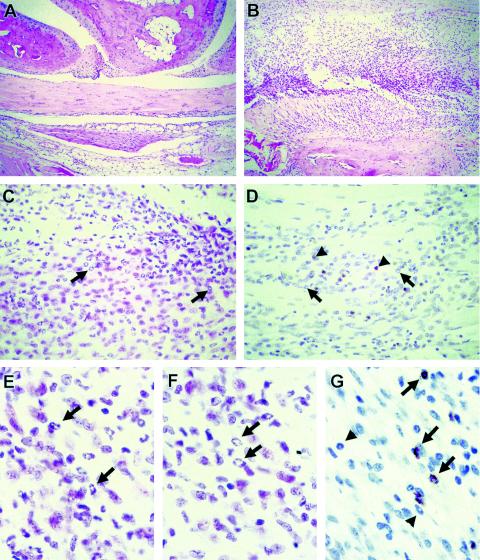
Differential staining of circulating nucleated cells revealed that there was nearly complete depletion of neutrophils following RB6 treatment. To evaluate the level of neutrophil depletion in joint tissues, we examined H&E-stained sections of joints from RB6- and IgG-treated DBA and C3H mice on days 7 and 21 postinfection. In joint sections from mice infected for 7 days, animals with both DBA and C3H genetic backgrounds that were treated with control IgG had mild inflammatory infiltrates consisting of small numbers of both neutrophils and monocytes (Fig. 2A). In contrast, mice treated with RB6 had a much more robust inflammatory response (Fig. 2B), with a large number of cells displaying a ring-shaped nuclear morphology (Fig. 2C). Immunohistochemical staining of joint sections from RB6-treated mice, however, revealed few positively staining cells in the inflammatory infiltrate (Fig. 2D), indicating that the cells were not expressing the neutrophil maturation marker Gr-1 recognized by the RB6 antibody (25). Murine neutrophils display a heterogeneous array of morphogenic cell types, including segmented or segmented ring-shaped nuclei (10), and thus cannot be separated into mature and immature populations based on morphology alone. In mice, cells with ring-shaped nuclei are usually considered to be PMN, but they have recently been shown to include a subset of mononuclear cell (MNC)-like ring cells (10). Separation of these populations on the basis of morphology alone is not always feasible; however, they do have some characteristic features (10). The PMN-like ring cells have a slender ring nucleus that is constricted or lobular and has an irregular contour. In addition, the width of the karyoplasmic ring is smaller than the diameter of the cytoplasmic center. The MNC-like ring cells have a round or ovoid nucleus with a smooth contour, and the karyoplasmic ring is wider than the diameter of the cytoplasmic center. Under high power (Fig. 2E and F) most of the cells with ring-shaped nuclei appeared to be PMN-like cells even though they did not express the Gr-1 maturation marker, which is typical of these cells under normal conditions (10). Thus, under Gr-1-depleting conditions, Gr-1− cells with PMN-like morphology can be dispatched to the site of infection. Immunohistochemical staining revealed that numerous cells with ring-shaped nuclei were positive for MPO (Fig. 2G), indicating that they were of the PMN lineage. Other cells with ring-shaped nuclei, however, were negative or stained weakly for MPO (Fig. 2G), suggesting that these cells were of the monocyte lineage.
FIG. 2.
Histopathology of tibiotarsal joints from control and RB6-treated mice. Representative sections were obtained from a control IgG-treated C3H mouse (A) and an RB6-treated mouse (B to G) sacrificed 7 days postinfection. (A and B) H&E-stained sections at a magnification of ×100. (C and D) H&E-stained and immunohistochemically stained sections at a magnification of ×400. The arrows indicate representative cells having ring-shaped nuclear morphology, and the arrowheads indicate representative neutrophils stained with RB6. (E and F) H&E-stained sections at a magnification of ×1,000. The arrows indicate PMN-like cells with ring-shaped nuclei. (G) Immunohistochemically stained sections at a magnification of ×1,000. The arrows indicate representative PMN-like cells that stained positive for MPO. The arrowheads indicate MNC-like ring cells that were negative for MPO staining or exhibited low levels of MPO staining.
Spirochete levels in neutropenic mice.
In vitro, neutrophils are capable of taking up and destroying B. burgdorferi (9, 36). To determine the effect of neutrophil depletion on spirochete numbers in vivo, we examined the presence of B. burgdorferi and the relative numbers in various tissues. Tissue samples were removed from animals infected for 21 days and cultured in BSK-H medium for 14 days. The presence of spirochetes in cultures of tissues was virtually the same for IgG- and RB6-treated mice (Table 2). These results indicate that the overall pattern of dissemination of the spirochetes within infected animals was not altered by the absence of neutrophils in the circulation. To determine the relative numbers of spirochetes in joint tissues in control and neutropenic animals, we used real-time quantitative PCR (13). By day 7 postinfection, tibiotarsal joints from both C3H and DBA mice treated with RB6 had four- to fivefold-greater levels of B. burgdorferi DNA than the joints from control IgG-treated mice (Fig. 3A). By day 21 postinfection, however, the joints (Fig. 3B) and ears (Fig. 3C) from RB6- and control IgG-treated mice contained similar levels of B. burgdorferi DNA. Thus, early depletion of neutrophils resulted in an initial increase in spirochete numbers, which was controlled both locally and systemically at later times.
TABLE 2.
Isolation of B. burgdorferi from selected tissues of control and neutropenic micea
| Strain | Treatment | Culture results
|
||||
|---|---|---|---|---|---|---|
| Blood | Heart | Spleen | Bladder | Ear | ||
| C3H | Control IgG | 3/8 | 5/8 | 0/8 | 8/8 | 8/8 |
| C3H | RB6 | 0/7 | 5/7 | 0/7 | 7/7 | 7/7 |
| DBA | Control IgG | 1/8 | 5/8 | 1/8 | 5/8 | 7/8 |
| DBA | RB6 | 2/8 | 6/8 | 0/8 | 5/8 | 8/8 |
Mice were infected with 2.5 × 105 B. burgdorferi spirochetes and sacrificed at 21 days postinfection. The data are number of cultures positive for B. burgdorferi/number of cultures examined. The data are the results of one of two experiments.
FIG. 3.
B. burgdorferi DNA copy numbers in joint and ear tissues of control IgG- and RB6-treated mice. Mice were infected with 2.5 × 105 B. burgdorferi cells per footpad and treated daily with control IgG or RB6. On day 7 (A) or 21 (B and C), tibiotarsal joints (A and B) or ear samples (C) were collected, and the DNA was isolated and assessed by quantitative real-time PCR as described in Materials and Methods. Samples were normalized by using the single-copy mouse gene Nidogen, and the values are arithmetic means ± standard deviations for 103 single-copy mouse genes. The experiment was conducted twice with similar results by using three to five mice per group. An asterisk indicates that the P value is <0.05 for a comparison of IgG- and RB6-treated animals.
Effect of increased spirochetal loads on arthritis development in neutropenic mice.
The development of Lyme arthritis in C3H and DBA mouse strains is relatively independent of the number of B. burgdorferi cells within the infected joint (6, 14, 28). To determine if the increased number of spirochetes within the joints of the neutropenic mice was responsible for the earlier development of arthritis, we infected control IgG- or RB6-treated C3H mice with increasing log doses of B. burgdorferi. Increasing the infectious dose had little effect on day 7 ankle swelling or arthritis severity scores in control IgG-treated C3H mice (Fig. 4). In contrast, RB6-treated C3H mice exhibited significantly increased ankle swelling and arthritis severity scores (P < 0.01) with increasing infectious doses. Since the neutropenia had a minimal effect on either ankle swelling or arthritis severity scores at the lower doses, it appears that a combination of neutropenia and increased spirochetal loads within the joint tissue is required for the early induction of arthritis.
FIG. 4.
Arthritis development in response to increasing doses of B. burgdorferi in IgG- or RB6-treated C3H mice. C3H mice treated with either IgG or RB6 on day −1 were infected in both hind footpads with increasing doses of B. burgdorferi. On day 7 postinfection ankle swelling (A) and arthritis severity scores (B) were determined. Open bars, IgG-treated mice; solid bars, RB6-treated mice. The values are means ± standard deviations and are representative of two separate experiments performed with three mice per treatment group. (A) An asterisk indicates that the P value is <0.01 for a comparison of IgG- and RB6-treated mice. (B) An asterisk indicates that the P value is <0.01 for a comparison of RB6-treated mice that received 2,500,000 B. burgdorferi (Bb) cells and RB6-treated mice that received 25,000 B. burgdorferi cells.
RB6 treatment alters production of proinflammatory mediators in joints of Borrelia-infected mice.
Neutrophils are capable of producing a number of both pro- and anti-inflammatory mediators (18), and these mediators have been shown to influence the development of pathology in other models of infectious disease (11, 20, 35, 37, 39, 47, 49). Recently, we demonstrated the importance of the production of KC and MCP-1 in inflammatory lesions in joints of susceptible mice infected with B. burgdorferi (13). Differential production of these two chemokines in resistant and susceptible animals became detectable between 7 and 10 days postinfection and correlated with the onset of arthritis. To determine if RB6 treatment altered the production of inflammatory mediators in B. burgdorferi-infected animals, we measured the production of a panel of cytokines and chemokines in ankle homogenates at days 7 and 21 postinfection. Figure 5 shows the production of representative cytokines and chemokines at day 7 postinoculation in ankles of IgG- or RB6-treated animals that were infected with B. burgdorferi or were sham infected (BSK-H medium only). Ankles from sham-infected animals were included to control for the trauma of footpad inoculation. There was no difference in the production of IL-4, IL-6, IFN-γ, or IL-12 in the joints between IgG- and RB6-treated animals (Fig. 5A to D). Although there was a trend toward higher IL-6 levels in the RB6-treated B. burgdorferi-infected mice, it was not statistically significant. However, we did find significantly higher levels of KC and MCP-1 in the ankles of RB6-treated mice than in the ankles of the IgG-treated mice (Fig. 5E and F) (P < 0.05). Joints from both DBA and C3H RB6-treated mice produced high levels of KC and MCP-1 that correlated with arthritis development. No differences were found in the levels of IL-1β, IL-10, granulocyte-macrophage colony-stimulating factor, TNF-α, MIP-1α, and MIP-2 in joints of IgG- and RB6-treated mice (data not shown). At day 21 postinfection we found no differences in the joint production of any of the cytokines or chemokines, although there was a trend toward higher levels of IL-6, KC, and MCP-1 in the joints of RB6-treated animals (data not shown). The cytokines or chemokines were not produced systemically as none of the serum samples taken from control or RB6-treated C3H or DBA mice on day 7 or 21 postinfection consistently contained threshold levels (data not shown).
FIG. 5.
Cytokine production in ankles from B. burgdorferi- or sham-infected mice 7 days postinfection. Open bars, sham-infected mice; solid bars, B. burgdorferi-infected mice. The mice were treated with control IgG or RB6, and the levels of IL-4 (A), IL-6 (B), IFN-γ (C), IL-12 (D), KC (E), and MCP-1 (F) in ankle homogenates were determined by ELISA as described previously (13). The data are means ± standard deviations. Five mice were sacrificed at each time, and each ankle was processed individually. The data are representative of two separate experiments. An asterisk indicates that the P value is <0.001 for a comparison of IgG- and RB6-treated mice for each strain.
DISCUSSION
In a recent report, we demonstrated that entry of neutrophils into the joint tissue was required for development of experimental Lyme arthritis (13). Joint tissue from arthritis-susceptible C3H mice produced high levels of the chemokines KC and MCP-1 in a manner that correlated with the development of severe arthritis. Infection of mice deficient in the receptor for KC (CXCR2) resulted in an inability of neutrophils to enter the joint tissue and a decrease in arthritis severity. In the present study, we investigated the effect of neutrophil depletion on the development of Lyme pathology in arthritis-resistant and -susceptible mouse strains. Based on our previous observations we expected that the neutropenic mice would be prevented from developing severe arthritis. Surprisingly, neutropenic mice belonging to both mouse strains began developing arthritis earlier than the control-treated mice, and genetically resistant DBA mice also developed severe arthritis like C3H mice. Histologic examination of the tibiotarsal joints from the neutropenic mice at 7 days postinfection revealed the presence of numerous cells with ring-shaped morphology, which are typically considered polymorphonuclear neutrophils (10), within the inflammatory infiltrates. Immunohistochemistry revealed that most of these cells did not express the Gr-1 neutrophil maturation antigen recognized by RB6 and thus were not affected by the antibody treatment. These cells with ring-shaped nuclei were likely to consist of a mixture of granulocytes and monocytes and their precursors, but under high-power magnification morphologically they appeared to be mostly PMN-like cells (10). Many of these cells also stained positive for MPO, providing further evidence of their PMN lineage. Recently, it was demonstrated that murine bone marrow contains a large number of functionally competent neutrophils with ring-shaped morphology that serve as a reservoir for rapidly replacing circulating neutrophils during infection (12). In the present study the functional capability of these cells was not directly tested; however, since there was an increased spirochetal burden in the joints of RB6-treated mice, it is likely that these PMN-like cells at least had a decreased capacity for bactericidal activities. The proximity of bone marrow to the site of infection may also be important for the recruitment of Gr-1− PMN-like cells as this was not reported to occur in other infectious disease models in which neutrophils were depleted from infected soft tissues, such as lungs or livers (22, 23, 30, 35, 48). The contribution that these cells might make to the development of pathology is currently unknown.
Neutrophils have been implicated in arthritis pathology in a number of other animal models, including the K/BxN mouse rheumatoid arthritis model (53), streptococcal cell wall-induced arthritis (41), adjuvant-induced arthritis (40), and collagen-induced arthritis (24). In each of these models, depletion of circulating neutrophils resulted in a decrease in arthritis severity and joint swelling. Neutrophils are known to produce a number of proinflammatory cytokines that can drive the development of pathology, as well as chemokines capable of recruiting antigen-specific T and B cells into the inflamed joint (18). In contrast, in a model of Staphylococcus aureus-induced septic arthritis, depletion of neutrophils resulted in a higher frequency of arthritis, as well as increased levels of TNF-α, IL-6, and IFN-γ, compared to the control animals (51). The increased frequency of arthritis, however, was likely due to the 100- to 1,000-fold increase in the bacterial burden in tissues from the neutrophil-depleted mice (51). In the present study, we found a four- to fivefold increase in spirochete loads in joints of RB6-treated mice compared to the loads in IgG-treated mice at 7 days postinfection. Infection of IgG-treated C3H mice with increasing doses of B. burgdorferi did not result in development of arthritis at 7 days postinfection, demonstrating that the increased spirochetal burden alone was not sufficient to cause the earlier development of pathology seen in the RB6-treated mice. Infection of RB6-treated mice with increasing doses of B. burgdorferi had little effect at low doses but had a dramatic effect on ankle swelling and arthritis severity scores at higher doses. This suggests that a combination of altered neutrophil responses and increased spirochetal burden is required for the earlier development of pathology seen in the RB6-treated mice. Another possibility is that in the normal mice, the neutrophils efficiently maintained the spirochete levels below a threshold that prevented the development of pathology. In the RB6-treated mice, the PMN-like cells were not functionally competent to contain the growth of spirochetes, allowing them to cross the bacterial burden threshold and cause pathology. This simplistic view, however, does not explain why C3H and DBA mice develop different levels of arthritis yet have similar levels of spirochetes within their joint tissues (14), although different bacterial burden thresholds in different mouse strains might be an explanation. It also does not explain how blocking the entry of neutrophils into the joint tissue in CXCR2-deficient mice prevented the development of arthritis, even though the joints contained the same number of spirochetes as the joints of the wild-type mice that developed severe arthritis (13). It appears that there is a requirement for neutrophils in the development of Lyme arthritis and that the PMN-like cells can fulfill this requirement, even though they are deficient in bactericidal capacity. Thus, during infectious arthritis the continued persistence of bacteria in the joint may result in sustained recruitment and activation of neutrophils and ultimately in development of pathology and chronic inflammation. Conversely, during autoimmune arthritis, timely removal of neutrophils and their proinflammatory mediators may tip the balance toward anti-inflammatory mediators and allow inflammation resolution and healing.
The effect of neutrophil depletion on arthritis development in Lyme disease has not been studied directly previously. Barthold and de Souza (5) targeted granulocytes for depletion using cyclophosphamide treatment during B. burgdorferi infection of mice. They reported increased arthritis severity in cyclophosphamide-treated C3H mice at 14 days postinfection compared to the arthritis in untreated mice. Genetically resistant B6 mice had an increased incidence of arthritis following cyclophosphamide treatment, but the arthritis severity scores were similar to those of the untreated control B6 mice. These results suggest that granulocytes might play an important role in Lyme arthritis development; however, this interpretation is limited by the nonspecificity of the action of cyclophosphamide. Cyclophosphamide is particularly damaging to neutrophils, although it is not specific for them, and it also causes pancytopenia. Barthold and de Souza speculated that granulocytes might regulate Lyme arthritis development through their bactericidal activity (5). In the present study, we extended these findings by demonstrating specifically that neutrophils do play a role in limiting borrelial growth during the early part of the infection. However, this bactericidal activity alone is not enough to control the development of pathology. In conjunction with our previous study (13), the present results demonstrate that neutrophil presence within the infected joint tissue is required for the development of Lyme arthritis and that altered recruitment or activation of neutrophils can exacerbate arthritis severity in resistant strains of mice.
Neutrophils and monocytes make up the majority of the inflammatory infiltrate in the infected joint during experimental Lyme arthritis (6). We have recently demonstrated that the recruitment of these cells into the joint during B. burgdorferi infection is mediated via production of the chemokines KC and MCP-1 (13). KC and MCP-1 were produced in the joints of arthritis-susceptible C3H mice but not in the joints of arthritis-resistant B6 or DBA mice during the second week of infection, at a time that correlated with the development of severe arthritis (13). In the present study, treatment of mice with RB6 resulted in earlier production of KC and MCP-1 by day 7 postinfection in joints of both genetically arthritis-resistant DBA mice and genetically arthritis-susceptible C3H mice. This early expression of KC and MCP-1 correlated with the early development of arthritis in both mouse strains. These results again suggest that KC and MCP-1 expression may be required for the development of experimental Lyme arthritis, although in our previous study infection of CXCR2−/− mice but not infection of CCR2−/− mice (CCR2 is the receptor for MCP-1) altered the development of arthritis (13). Levels of MCP-1 at inflammatory sites are regulated by receptor binding and internalization (50), and similar mechanisms are likely for other chemokines as well. Thus, in the joints of RB6-treated mice the increased expression of KC and MCP-1 by day 7 postinfection likely resulted from the decreased uptake of these chemokines by neutrophils and/or other cell types. Furthermore, our results suggest that neutrophils may provide an early mechanism for protection against inflammation as their depletion results in early expression of KC and MCP-1 in joints and exacerbated recruitment of inflammatory cells. In genetically resistant mouse strains this early neutrophil protection appears to be sustained, preventing the production of KC and MCP-1 and resulting in only a mild inflammatory response to B. burgdorferi infection. In genetically susceptible mouse strains the early neutrophil protection is eventually overcome, resulting in the production of KC and MCP-1, the recruitment of more inflammatory cells, and the development of severe arthritis. Further experiments are required to elucidate the mechanisms responsible for the differential regulation of neutrophils in this model system.
Resistance or susceptibility to the development of experimental Lyme arthritis has been correlated with the production of certain anti- or proinflammatory cytokines, especially IL-4 and IFN-γ (2-4, 16, 27, 29). Restimulation of splenocytes or lymph node cells from arthritis-susceptible C3H mice resulted in the production of high levels of IFN-γ and low levels of IL-4, while cells from arthritis-resistant DBA or B6 mice produced lower levels of IFN-γ and higher levels of IL-4 (27, 29). In a recent study, we measured the levels of 12 cytokines and chemokines directly from the tibiotarsal joints of B. burgdorferi-infected C3H and DBA or B6 mice (13). Surprisingly, virtually no differences in cytokine production were found (with the exception of MCP-1 and KC) between resistant and susceptible mouse strains, whether production was measured at the protein level or at the mRNA level by quantitative real-time PCR. In the present study, we again found no differences in the levels of IL-4, IL-6, IL-12, and IFN-γ between C3H and DBA mice with or without RB6 treatment, when the levels were measured directly from the infected joint, despite clear differences in the inflammatory responses. The regulatory mechanisms controlling disease resistance or susceptibility in this model system remain obscure, but they appear to depend upon the presence and involvement of neutrophils.
Acknowledgments
We thank Robert Coffman and Sara Smelt for the RB6 hybridoma, Howard Wilson and Don Connor for help with the digital images, and Jeff Mitchell and David Lee for a critical review of the manuscript and helpful discussions.
This work was supported in part by National Institutes of Health grant R01 AR44042.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Adkison, A. M., S. Z. Raptis, D. G. Kelley, and C. T. N. Pham. 2002. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Investig. 109:363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., S. W. Barthold, S. Samanta, J. Ryan, and E. Fikrig. 1999. Selective anti-inflammatory action of interleukin-11 in murine Lyme disease: arthritis decreases while carditis persists. J. Infect. Dis. 179:734-737. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., D. H. Persing, M. Rincon, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguita, J., M. Rincon, S. Samanta, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1998. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J. Infect. Dis. 178:1512-1515. [DOI] [PubMed] [Google Scholar]
- 5.Barthold, S. W., and M. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 9.Benach, J. L., H. B. Fleit, G. S. Habicht, J. L. Coleman, E. M. Bosler, and B. P. Lane. 1984. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J. Infect. Dis. 150:497-507. [DOI] [PubMed] [Google Scholar]
- 10.Biermann, H., B. Pietz, R. Dreier, K. W. Schmid, C. Sorg, and C. Sunderkotter. 1999. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J. Leukoc. Biol. 65:217-231. [DOI] [PubMed] [Google Scholar]
- 11.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. [DOI] [PubMed] [Google Scholar]
- 12.Boxio, R., C. Bossenmeyer-Pourie, N. Steinckwich, C. Dournon, and O. Nusse. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75:604-611. [DOI] [PubMed] [Google Scholar]
- 13.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 14.Brown, C. R., and S. L. Reiner. 1998. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect. Immun. 66:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 18.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. 2002. Lyme Disease—United States, 2000. Morbid. Mortal. Wkly. Rep. 51:29-31. [PubMed] [Google Scholar]
- 20.Chen, L., T. Watanabe, H. Watanabe, and F. Sendo. 2001. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31:265-275. [DOI] [PubMed] [Google Scholar]
- 21.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 24.Fava, R. A., C. Gates, and A. S. Townes. 1993. Critical role of peripheral blood phagocytes and the involvement of complement in tumour necrosis factor enhancement of passive collagen-arthritis. Clin. Exp. Immunol. 94:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 26.Kasama, T., R. M. Strieter, N. W. Lukacs, P. M. Lincoln, M. D. Burdick, and S. L. Kunkel. 1995. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J. Clin. Investig. 95:2868-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 28.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mednick, A. J., M. Feldmesser, J. Rivera, and A. Casadevall. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 33:1744-1753. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki, S., A. Matsukawa, S. Ohkawara, K. Takagi, and M. Yoshinaga. 2000. Neutrophil infiltration as a crucial step for monocyte chemoattractant protein (MCP)-1 to attract monocytes in lipopolysaccharide-induced arthritis in rabbits. Inflamm. Res. 49:673-678. [DOI] [PubMed] [Google Scholar]
- 32.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niki, Y., H. Yamada, S. Seki, T. Kikuchi, H. Takaishi, Y. Toyama, K. Fujikawa, and N. Tada. 2001. Macrophage- and neutrophil-dominant arthritis in human IL-1α transgenic mice. J. Clin. Investig. 107:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 37:1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson, P. K., C. C. Clawson, D. A. Lee, D. J. Garlich, P. G. Quie, and R. C. Johnson. 1984. Human phagocyte interactions with the Lyme disease spirochete. Infect. Immun. 46:608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedel, D. D., and S. H. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers, H. W., and E. R. Unanue. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 61:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romani, L., A. Mencacci, E. Cenci, G. Del Sero, F. Bistoni, and P. Puccetti. 1997. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J. Immunol. 158:2356-2362. [PubMed] [Google Scholar]
- 40.Santos, L. L., E. F. Morand, P. Hutchinson, N. W. Boyce, and S. R. Holdsworth. 1997. Anti-neutrophil monoclonal antibody therapy inhibits the development of adjuvant arthritis. Clin. Exp. Immunol. 107:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schimmer, R. C., D. J. Schrier, C. M. Flory, J. Dykens, D. K. Tung, P. B. Jacobson, H. P. Friedl, M. C. Conroy, B. B. Schimmer, and P. A. Ward. 1997. Streptococcal cell wall-induced arthritis. Requirements for neutrophils, P-selectin, intercellular adhesion molecule-1, and macrophage-inflammatory protein-2. J. Immunol. 159:4103-4108. [PubMed] [Google Scholar]
- 42.Schrier, D. J., R. C. Schimmer, C. M. Flory, D. K. Tung, and P. A. Ward. 1998. Role of chemokines and cytokines in a reactivation model of arthritis in rats induced by injection with streptococcal cell walls. J. Leukoc. Biol. 63:359-363. [DOI] [PubMed] [Google Scholar]
- 43.Seiler, K. P., Y. Ma, J. H. Weis, P. S. Frenette, R. O. Hynes, D. D. Wagner, and J. J. Weis. 1998. E and P selectins are not required for resistance to severe murine Lyme arthritis. Infect. Immun. 66:4557-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon, M. M., R. Wallich, and M. D. Kramer. 1996. Borrelia burgdorferi infection of inbred strains of mice provides insights into cellular and molecular parameters of pathogenesis and protection of Lyme disease: a viewpoint. J. Spirochet. Tick-Borne Dis. 3:45-52. [Google Scholar]
- 45.Smith, A. L., D. S. Beck, L. J. Kim, G. M. Hansen, Jr., and S. W. Barthold. 1993. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect. Immun. 61:4493-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 47.Tacchini-Cottier, F., C. Zweifel, Y. Belkaid, C. Mukankundiye, M. Vasei, P. Launois, G. Milon, and J. A. Louis. 2000. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 165:2628-2636. [DOI] [PubMed] [Google Scholar]
- 48.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 49.Tateda, K., T. A. Moore, M. W. Newstead, W. C. Tsai, X. Zeng, J. C. Deng, G. Chen, R. Reddy, K. Yamaguchi, and T. J. Standiford. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 69:2017-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tylaska, L. A., L. Boring, W. Weng, R. Aiello, I. F. Charo, B. J. Rollins, and R. P. Gladue. 2002. CCR2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine 18:184-190. [DOI] [PubMed] [Google Scholar]
- 51.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willis, A. A., R. F. Widmann, J. M. Flynn, D. W. Green, and K. B. Onel. 2003. Lyme arthritis presenting as acute septic arthritis in children. J. Pediatr. Orthop. 23:114-118. [PubMed] [Google Scholar]
- 53.Wipke, B. T., and P. M. Allen. 2001. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167:1601-1608. [DOI] [PubMed] [Google Scholar]
- 54.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]