Abstract
Enterohemorrhagic Escherichia coli (EHEC) strains comprise a broad group of bacteria, some of which cause attaching and effacing (AE) lesions and enteritis in humans and animals. Non-O157:H7 EHEC strains contain the gene efa-1 (referred to in previous publications as efa1), which influences adherence to cultured epithelial cells. An almost identical gene in enteropathogenic E. coli (lifA) mediates the inhibition of lymphocyte proliferation and proinflammatory cytokine synthesis. We have shown previously that significantly lower numbers of EHEC O5 and O111 efa-1 mutants are shed in feces following experimental infection in calves and that these mutants exhibit reduced adherence to intestinal epithelia compared with isogenic wild-type strains. E. coli O157:H7 strains lack efa-1 but encode a homolog on the pO157 plasmid (toxB/l7095) and contain a truncated version of the efa-1 gene (efa-1′/z4332 in O island 122 of the EDL933 chromosome). Here we report that E. coli O157:H7 toxB and efa-1′ single and double mutants exhibit reduced adherence to cultured epithelial cells and show reduced expression and secretion of proteins encoded by the locus of enterocyte effacement (LEE), which plays a key role in the host-cell interactions of EHEC. The activity of LEE1, LEE4, and LEE5 promoters was not significantly altered in E. coli O157:H7 strains harboring toxB or efa-1′ mutations, indicating that the effect on the expression of LEE-encoded secreted proteins occurs at a posttranscriptional level. Despite affecting type III secretion, mutation of toxB and efa-1′ did not significantly affect the course of fecal shedding of E. coli O157:H7 following experimental inoculation of 10- to 14-day-old calves or 6-week-old sheep. Mutation of tir caused a significant reduction in fecal shedding of E. coli O157:H7 in calves, indicating that the formation of AE lesions is important for colonization of the bovine intestine.
Enterohemorrhagic Escherichia coli (EHEC) strains are zoonotic enteric pathogens of worldwide importance (39). In humans, infection by some EHEC serotypes may cause diarrhea, which may be complicated by hemorrhagic colitis and severe systemic sequelae, including hemolytic-uremic syndrome (45). EHEC strains are closely related to enteropathogenic E. coli (EPEC) strains, which are a leading cause of infantile diarrhea in developing countries, and have many of the EPEC genes implicated in virulence (13, 51).
Ruminants are an important reservoir of EHEC (15, 44), and direct or indirect contact with ruminant feces is the leading antecedent to EHEC infection in humans (17, 30, 43). Natural and experimental infections of calves or sheep with EHEC result in efficient colonization of the intestinal tract, and large numbers of bacteria are shed in the feces for several weeks (3, 5-9, 18, 59, 67, 68). Strategies to reduce the prevalence of EHEC in ruminants offer the potential to lower the incidence of human infections (58). However, little is currently known about the mechanisms underlying intestinal colonization of cattle and sheep by EHEC.
EHEC strains are defined by their ability to produce one or more Shiga toxins and to induce characteristic attaching and effacing (AE) lesions on intestinal epithelia, in which the bacteria adhere intimately to the apical surface of enterocytes on raised actin-rich pedestals and microvilli are locally destroyed. This histopathology is determined by the chromosomal locus of enterocyte effacement (LEE), which encodes a type III protein secretion system (13). One of the LEE-encoded secreted proteins (Tir) is translocated into the host cell plasma membrane, where it acts as a receptor for the bacterial outer membrane protein intimin (10). Intimin is an important colonization factor for E. coli O157:H7 in neonatal calves (7), lambs (67), and adult cattle and sheep (5). Intimin can also bind to β1-integrins (14) and cell surface-localized nucleolin (57); however, the importance of such interactions and of type III secretion in colonization of the ruminant intestine is unclear. Immunization of calves with E. coli O157:H7 type III secreted proteins reduces fecal shedding of the bacteria following experimental infection; however, the contribution of individual secreted proteins in colonization and immunity in ruminants requires detailed investigation (50).
Recently, it was reported that the product of the efa-1 gene (EHEC factor for adherence; referred to in previous publications as efa1) influences intestinal colonization in calves by non-O157:H7 EHEC (59). Efa1 was first identified as a factor influencing adhesion of a clinical O111:H− EHEC strain to cultured epithelial cells (42). EHEC O5 and O111 strains harboring mutations in the efa-1 gene were shed in the feces in significantly lower numbers than the corresponding parent strains following oral inoculation of conventional calves. Furthermore, an EHEC O111:H− efa-1 mutant was impaired in the ability to adhere to the colonic epithelium in calves, as determined by confocal microscopy and direct recovery of bacteria from the mucosa (59).
The mechanism by which Efa1 influences intestinal colonization remains obscure. Mutation of efa-1 in EHEC O5 and O111 reduces the expression and secretion of LEE-encoded effector proteins, suggesting that it may indirectly influence colonization (59). The EHEC O111:H− Efa1 protein is 97.4% identical at the amino acid level to EPEC lymphostatin (LifA), which confers an ability to inhibit lymphocyte proliferation of human peripheral blood lymphocytes and the mitogen-activated synthesis of proinflammatory cytokines (25). LifA also inhibits the proliferation of human and murine intraepithelial lymphocytes, indicating that LifA and Efa1 may influence intestinal colonization by modulating mucosal immunity in the gut (21, 25-27, 32).
The efa-1/lifA gene is present in all non-O157:H7 EHEC serotypes tested so far and in related enteropathogens, such as Citrobacter rodentium and rabbit EPEC (REPEC) (25, 42, 60). Sorbitol-fermenting E. coli O157:H− strains also contain efa-1; however, the intact efa-1 gene is absent from the E. coli O157:H7 strains that have been sequenced (19, 22, 47). The efa-1 gene in REPEC was recently shown to mediate bacterial adherence to cultured epithelial cells, apparently without affecting the secretion of LEE-encoded proteins, implying that it may act as an adhesin in its own right (2). While E. coli O157:H7 lacks the full-length efa-1 gene, a truncated version of efa-1 (efa-1′) is present in the chromosome (z4332 and z4333 in O island 122 of the EDL933 genome). An E. coli O157:H7 mutant carrying a transposon insertion upstream of efa-1′ (G2-B12) showed reduced adherence to human colon carcinoma cells (62; I. Tatsuno, personal communication), indicating that the truncated Efa1 protein may have some of the properties of full-length Efa1. Recent surveys of the prevalence of O island 122 revealed that it is frequently associated with EHEC isolates from serious and/or epidemic disease, and the authors speculated that the truncated efa-1 gene may contribute to virulence (24, 38).
In addition to the product of the truncated efa-1 gene, E. coli O157:H7 also encodes a large predicted protein (ToxB/L7095) that exhibits 28% amino acid identity to Efa1 on the pO157 large plasmid (4, 31). E. coli O157:H7 strains containing derivatives of pO157 that lack toxB exhibit reduced adherence to cultured epithelial cells, possibly as a result of reduced expression and secretion of LEE-encoded type III secreted proteins (63). The toxB gene has also been suggested to be a functional homolog of EPEC lymphostatin, since an E. coli O157:H7 strain with pO157 deleted lacked the ability to inhibit interleukin-2 and interleukin-4 synthesis in mitogen-activated human peripheral blood lymphocytes (25). However, pO157 encodes other secreted cytotoxins, including enterohemolysin (EhxA), a serine protease (EspP), and a metalloprotease (StcE), and it is unclear if ToxB alone mediates the inhibitory effect.
In order to clarify the role of the E. coli O157:H7 toxB and truncated efa-1 genes, we investigated the effect of single and combined defined mutations on the transcription and translation of LEE genes and intestinal colonization in calves and sheep.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli O157:H7 strain 85-170 is a spontaneous stx1- and stx2-negative derivative of strain 84-289 (65). 85-170 Nalr is a spontaneous nalidixic acid-resistant derivative of 85-170 that was selected by plating ca. 109 CFU of 85-170 on Luria-Bertani (LB) agar containing 25 μg of nalidixic acid per ml, followed by overnight incubation at 37°C. 85-170 Nalr exhibits normal growth and adhesion characteristics in vitro. Plasmids pCVD442 (11) and pDM4 (37) are positive-selection suicide vectors containing a pir-dependent R6K origin of replication, the mob region from plasmid RP4, and the Bacillus subtilis sacBR genes and confer resistance to ampicillin and chloramphenicol, respectively. pCVD442, pDM4, and derivatives of these plasmids were maintained in E. coli DH5α λpir (49) and in E. coli S17-1λpir for conjugation (56). Plasmids pAJR71 and pAJR74 have been described previously (52) and contain fusions of the LEE1 and LEE4 promoters, respectively, to a promoterless gene encoding an extended-half-life green fluorescent protein (eGFP). Plasmid pAJR70 (52) has single BamHI and KpnI sites 5′ of a promoterless copy of the gene encoding eGFP and was used to create an LEE5 reporter construct. Bacteria were isolated on LB agar and were cultivated in LB broth with appropriate antibiotics at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; and nalidixic acid, 25 μg/ml. For oral inoculation studies bacteria were amplified in brain heart infusion broth for 18 h at 37°C, and the absorbance at 600 nm was adjusted to within 0.05 U.
Cell lines.
HeLa cells (ATCC CCL2) were cultivated in RPMI 1640 buffered with 2 g of sodium bicarbonate per liter and supplemented with 10% (vol/vol) fetal calf serum (PAA Laboratories GmbH, Haidmannweg, Austria) and 0.3 g of l-glutamine per liter. For adhesion assays and fluorescent actin staining, cells were seeded at a concentration of 2 × 105 cells/35-mm dish on glass coverslips and grown for 18 h at 37°C in a humidified 5% CO2 atmosphere.
Routine DNA manipulation.
Standard procedures were used for DNA extraction, cloning, PCR, and verification of mutants by Southern hybridization (54).
Construction of defined E. coli O157:H7 mutants.
A deletion of the entire toxB gene was created by overlapping PCR followed by allelic exchange with the positive-selection suicide vector pCVD442. Sequences flanking the E. coli O157:H7 toxB gene were separately amplified by PCR with Vent proofreading DNA polymerase (New England Biolabs, Beverly, Mass.) by using the primer pairs tox1 (5′ ATATATGTCGACCGCCACAAAATGGCCG 3′)-tox2 (5′ GGAAATCAGCGCTACATATCTATACCCTC 3′) and tox3 (5′ GGGTATAGATATGTAGCGCTGATTTCCTG 3′)-tox4 (5′ ATATATGTCGACGAGAGCATGTGCTGGC 3′) (based on the pO157 sequence; accession number AF074613). The primary PCR products were gel purified and combined in an overlapping PCR (20) by using the flanking primers tox1 and tox4. The secondary PCR product was then cloned into pCVD442 via SalI sites incorporated into the primers. The resulting plasmid, pCVDΔtoxB, was introduced into 85-170 Nalr by conjugation from E. coli S17-1λpir, and merodiploids were isolated on LB agar containing ampicillin and nalidixic acid. Double recombinants were selected by growing merodiploids to the late logarithmic phase in LB medium lacking ampicillin and plating onto LB agar (without NaCl) containing 6% (wt/vol) sucrose at 30°C. Sucrose-resistant colonies were screened for deletions by colony PCR, and recombinants were verified by Southern hybridization. The deletion resulted in juxtaposition of the predicted start and stop codons of toxB.
To create a deletion of the E. coli O157:H7 truncated efa-1 gene, sequences flanking the gene were separately amplified by PCR with Vent DNA polymerase by using the primer pairs efa-10 (5′ ATATATGAGCTCGATGGTCAGGTCGCAG 3′)-efa-11 (5′ GGCAGGATAGTTCAATTACATTTCCGCTTAAA 3′) and efa-12 (5′ GGAAATGTAATTGAACTATCCTGCCGCC 3′)-efa-13 (5′ ATATATGAGCTCCTTAGTTCCCTGTAAGCC 3′) (based on the E. coli O157:H7 EDL933 sequence; accession number AE005174). The primary PCR products were gel purified and combined in an overlapping PCR by using the flanking primers efa-10 and efa-13. The secondary PCR product was then cloned into pDM4 via SacI sites incorporated into the primers. The resulting plasmid, pDM4efa-1′10+13, was introduced into 85-170 Nalr and 85-170 Nalr ΔtoxB by conjugation from E. coli S17-1λpir, and merodiploids were isolated on LB agar containing chloramphenicol and nalidixic acid. Double recombinants were selected essentially as described above, and they were screened for the efa-1′ deletion by colony PCR and verified by Southern hybridization. The deletion removed the open reading frame encoding the N-terminal 433 amino acids of Efa1′ and also removed the start codon and N-terminal 97 amino acids of a contiguous 3′ open reading frame encoding a predicted 275-amino-acid protein that was 100% identical to amino acids 435 to 710 of Efa1.
A tir deletion mutant of strain 85-170 Nalr was used as a control in calf infection studies and was constructed as follows. Sequences flanking the tir gene were separately amplified by using the primer pairs tir1 (5′ ATATATGAGCTCTAGCATCATCGAGAGGG 3′)-tir2 (5′ CCTATTGGTAATCTTGGATCCCATCGTTTCGTC 3′) and tir3 (5′ GAAACGATGGGATCCAAGATTACCAATAGGCAT 3′)-tir4 (5′ ATATATGAGCTCGGGATAACCTTGTCAGG 3′). The primary PCR products were gel purified and combined in an overlapping PCR by using the flanking primers tir1 and tir4. The secondary PCR product was then cloned into pDM4 via SacI sites incorporated into the primers, and the resulting plasmid, pDM4Δtir, was introduced into 85-170 Nalr by conjugation from E. coli S17-1λpir. A double recombinant was selected as described above. The in-frame deletion resulted in juxtaposition of the first and last six codons of the tir gene. The 85-170 Nalr Δtir mutant strain expressed normal levels of intimin, as assessed by Western blotting (data not shown), indicating that the deletion was nonpolar.
Adhesion and fluorescent actin staining assays.
Adhesion of EHEC strains to HeLa cells was quantified by visual observation essentially as described previously (16, 63). Semiconfluent HeLa cells grown on 22-mm glass coverslips were overlaid with serum-free RPMI 1640 buffered with 2 g of sodium bicarbonate per liter and were inoculated at a multiplicity of infection of approximately 50:1 with fresh stationary-phase LB medium cultures of 85-170 Nalr wild-type and mutant strains adjusted to the same optical density. Cells were incubated for 3 h at 37°C in a humidified 5% CO2 atmosphere, washed three times with 2 ml of phosphate-buffered saline (PBS), and then incubated in fresh medium for an additional 3 h. Cells were washed five times with 2 ml of PBS, fixed, and stained with Hemacolor rapid staining solutions (Merck, Darmstadt, Germany), and multiple images were captured at a magnification of ×400 by using a Leica DMLS microscope with a Polaroid digital microscope camera. For each independent assay 20 randomly selected fields containing 50 or more cells were examined, and the mean number of microcolonies (MC) (comprising eight or more bacteria) per cell was determined. Assays were performed three times, and the microscopic observations were made by a researcher unfamiliar with the identities of the strains tested. The results are given below as the mean number of MC per cell (± standard deviation) from three independent experiments.
Fluorescent actin staining for the detection of filamentous actin under sites of bacterial adhesion to HeLa cells was performed as described previously (28), except that bacteria were incubated on the cells for a total of 8 h.
Detection of EspD by Western blotting.
To measure expression and secretion of the LEE-encoded type III secreted protein EspD, bacteria were grown to the late logarithmic phase (A600, ca. 1.0) in minimal essential medium buffered with 25 mM HEPES (MEM-HEPES). Secreted proteins were precipitated with trichloroacetic acid as described previously (35), and total protein was prepared from 1 ml of each culture by centrifugation and resuspension in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Approximately 25 μg of whole-cell protein was analyzed by Western blotting by using a monoclonal antibody specific for EspD and a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin secondary antibody (Dako, Cambridge, United Kingdom). Bound antibody was visualized after 5 min of incubation with a 1:1 mixture of Pierce (Rockford, Ill.) luminol enhancer solution and Pierce stable peroxide solution. Relative levels of EspD were determined by quantifying luminescence with a Flowgen MultiImage cabinet and MultiImage Spot densitometer software (Flowgen, Ashby de la Zouch, United Kingdom). Three independent experiments were performed, and equivalent loading of proteins was verified by Coomassie blue staining of gels.
Detection of EspA filaments by immunofluorescence microscopy.
To detect EspA filaments, bacteria grown overnight in M9 minimal medium containing 0.2% (wt/vol) glucose were subcultured in MEM-HEPES. Since EspA filament expression is growth phase dependent (52), cultures were sampled at A600 values of 0.4, 0.8, and 1.0. Bacteria were fixed with 0.1% (wt/vol) paraformaldehyde for 5 min at room temperature, and a 20-μl aliquot of each suspension was dried onto a microscope slide at 37°C for 20 min. A 1:100 dilution of EspA antibody (66) in PBS containing 0.1% bovine serum albumin was applied to the slide. After incubation in a humid chamber with gentle rocking for 1 h, the slide was washed three times with PBS containing 0.1% (wt/vol) bovine serum albumin, and then a 1:500 dilution of fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin secondary antibody was applied to the slide. After incubation and washing as described above, the slides were examined by fluorescence microscopy by using appropriate filter sets, and the images were captured by using Leica software. The number of bacteria stained with the EspA antibody was expressed as a proportion of ca. 900 bacteria from three independent experiments (three fields containing ca. 100 bacteria captured per time point per strain in each of three experiments), essentially as described previously (52).
Measurement of LEE promoter activity by using green fluorescent protein plasmid reporter constructs.
Reporter constructs to quantify the activities of the LEE1 and LEE4 promoters (pAJR71 and pAJR74) have been described previously (52). To create an LEE5 reporter construct, the LEE5 (tir) promoter was amplified by PCR by using Lee5 5′ BamHI (5′ CGGGATCCCGCGATAAAGAAACTTAATAAACT 3′) and Lee5 3′ KpnI (5′ CGGGTACCTGAAGGTAATGGAGGTGCAGG 3′) and was cloned into pAJR70 upstream of a promoterless copy of the gene encoding eGFP, yielding pAJR75. To measure the activities of the LEE1, LEE4, and LEE5 promoters, plasmids pAJR71, pAJR74, and pAJR75 were separately transformed into the 85-170 Nalr wild-type strain and ΔtoxB and Δefa-1′ mutant strains by electroporation by using a Bio-Rad Gene Pulser according to the manufacturer's instructions. Transformants were grown overnight in MEM-HEPES containing chloramphenicol and then subcultured 1:50 in fresh, prewarmed medium. The A600 of each of the cultures was monitored by measurement with a spectrophotometer. At intervals, 200-μl portions of the cultures were removed, and the eGFP that had accumulated was quantified by using a FLUOstar Optima fluorescence plate reader (BMG Labtechnologies GmbH, Offenburg, Germany) at appropriate excitation and emission wavelengths. Control bacteria containing no plasmid were used to measure the background fluorescence.
Oral inoculation of calves.
Fifteen 10- to-14-day-old Friesian bull calves were used in this study. The calves were housed in a high-containment accommodation in tanks on tenderfoot mats and were fed milk replacer twice daily, and they had free access to water. Prior to infection the calves were confirmed to be culture negative for EHEC and Salmonella by direct plating of rectal swabs on sorbitol MacConkey agar containing 2.5 μg of potassium tellurite (T-SMAC) per ml and brilliant green agar (Oxoid, Basingstoke, United Kingdom), respectively. While is it acknowledged that T-SMAC may not detect all EHEC, this medium is highly selective for serogroups O157 and O26, which are by far the most prevalent serogroups in cattle in the United Kingdom at present. Presumptive EHEC colonies were screened for stx1 and stx2 genes by PCR by using the primer pairs Stx1F (5′ ATAAATCGCCATTCGTTGACTAC 3′)-Stx1R (5′ AGAACGCCCACTGAGATCATC 3′) and Stx2F (5′ GGCACTGTCTGAAACTGCTCC 3′)-Stx2R (5′ TCGCCAGTTATCTGACATTCTG 3′). Animals excreting stx-positive E. coli were excluded from the study. All calves were obtained from the same farm and received colostrum from their dams for the first 24 to 48 h. After this, no further colostrum was given. Total serum immunoglobulin levels were measured when the calves were 1 or 2 days old as a measure of colostrum intake by the zinc sulfate turbidity assay. Only calves with a zinc sulfate turbidity value of more than 10 were used.
Calves were orally challenged with ca. 1 × 1010 CFU of EHEC in 20 ml of antacid (5% [wt/vol] MgO, 5% [wt/vol] Mg trisilicate, and 5% [wt/vol] NaHCO3 in double-distilled water) just before the morning feeding. Mutants 85-170 Nalr ΔtoxB, 85-170 Nalr Δefa-1′, and 85-170 Nalr ΔtoxB Δefa-1′ were each administered to two calves. The parent strain, 85-170 Nalr, was administered to a total of five calves. An 85-170 Nalr Δtir mutant was administered to four calves. During the next 7 days, the calves were observed for clinical signs, and fecal samples were taken twice daily by rectal palpation. The numbers of viable EHEC per gram of feces were determined by plating triplicate 10-fold serial dilutions onto sorbitol MacConkey agar containing 20 μg of nalidixic acid per ml and 2.5 μg of potassium tellurite per ml (lower limit of detection, 100 CFU/g).
Oral inoculation of sheep.
Eighteen 6-week-old cross-bred lambs were randomly divided into two equal groups and supplied with food and water ad libitum, and they were confirmed to be free of EHEC by enrichment culturing as described previously (67). Nine lambs were dosed orally by gavage with 1 × 109 CFU of 85-170 Nalr resuspended in 10 ml of PBS, and a separate group of nine lambs was given a matching dose of 85-170 Nalr ΔtoxB Δefa-1′. Approximately 24 h after administration of the bacteria and as required thereafter for up to 24 days, rectal fecal samples were collected from each group and used for direct plating onto sorbitol MacConkey plates supplemented with 20 μg of nalidixic acid per ml. One lamb from each group was sacrificed on days 3, 8 and 18 postinfection for bacteriological and histological analyses of tissues.
All animal experiments were performed in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the local ethical review committee. The fecal shedding data were statistically analyzed for the effect of mutation by using an F test, with the data taken as repeated measurements (Proc Mixed Statistical Analysis System, 1995; SAS Institute, Cary, N.C.). P values of <0.05 were considered significant.
RESULTS
Mutation of the E. coli O157:H7 toxB and truncated efa-1 genes influences bacterial adherence to cultured HeLa cells but not filamentous actin nucleation.
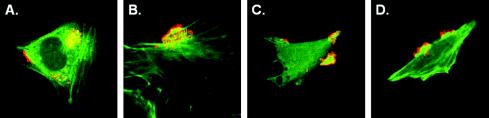
Quantitative adherence assays were performed by using HeLa cells, the 85-170 Nalr ΔtoxB and Δefa-1′ single and double mutants, and the parent strain. Mutation of the E. coli O157:H7 toxB and efa-1′ genes did not alter the in vitro growth rates of the mutants compared to those of the parent strain (data not shown). The numbers of MC comprising eight or more bacteria per HeLa cell in three independent experiments were as follows: for 85-170 Nalr, 0.0784 ± 0.0162 MC/cell; for 85-170 Nalr ΔtoxB, 0.0192 ± 0.0228 MC/cell; for 85-170 Nalr Δefa-1′, 0.0216 ± 0.0250 MC/cell; and for 85-170 Nalr ΔtoxB Δefa-1′, 0.0066 ± 0.0078 MC/cell (means ± standard deviations). Thus, mutation of toxB or efa-1′ alone caused a ca. fourfold reduction in MC formation (for toxB, P = 0.006; for efa-1′, P = 0.007). These phenotypes are comparable to those reported by Tatsuno et al. (62, 63). A double mutant lacking both toxB and efa-1′ exhibited a ca. 12-fold reduction in MC formation compared to the wild-type strain (P = 0.002). Despite forming fewer MC, the mutant strains still nucleated filamentous actin under the sites of attachment to HeLa cells (Fig. 1), indicating that LEE-mediated rearrangement of cytoskeletal actin could still occur.
FIG. 1.
Nucleation of filamentous actin under sites of adherence of the 85-170 Nalr wild-type strain and ΔtoxB and Δefa-1 single and double mutant strains to cultured HeLa cells. Cells were examined by fluorescent actin staining by using Oregon Green 514-phalloidin (green) and a confocal laser scanning microscope. Bacteria were detected with rabbit anti-O157 lipopolysaccharide typing serum and anti-rabbit Ig-Alexa568 (red). (A) 85-170 Nalr; (B) 85-170 Nalr ΔtoxB; (C) 85-170 Nalr Δefa-1′; (D) 85-170 Nalr ΔtoxB Δefa-1′. Representative images are shown. Magnification, ×630.
We are aware that unintended secondary mutations can result from allelic exchange when positive-selection suicide vectors are used (23), and therefore we attempted to trans-complement the defects in MC formation by cloning and expression of the toxB and efa-1′ genes. Several unsuccessful attempts were made to subclone toxB on a ca. 10-kb AatII fragment from pO157 into the low-copy-number vector pACYC184. We obtained the mini-pO157 derivative containing toxB described by Tatsuno et al. (63) (pIC37, kindly supplied by C. Sasakawa, University of Tokyo). However, this plasmid could not be stably maintained in 85-170 Nalr ΔtoxB. When kanamycin-resistant bacteria containing pIC37 were obtained, they exhibited an extremely low growth rate in vitro (data not shown), which prohibited the development of MC in the adherence assay.
Badea et al. recently reported that they were unable to clone the N-terminal 376 amino acids of REPEC efa-1 (2), presumably because of toxicity. We were successful in cloning the truncated efa-1 gene from E. coli O157:H7 (z4332) together with the contiguous open reading frame z4333 in pET30-Ek/Lic (Novagen, Madison, Wis.). However, induction of expression of efa-1′ caused a dramatic decrease in the growth rate, making quantification of MC formation unreliable (data not shown).
Mutation of the E. coli O157:H7 toxB and truncated efa-1 genes reduces expression and secretion of LEE4-encoded proteins.
Expression and secretion of the LEE4-encoded type III secreted protein EspD by the E. coli O157:H7 wild-type strain and ΔtoxB, Δefa-1′, and ΔtoxB Δefa-1′ mutant strains were analyzed by Western blotting by using specific antiserum (Fig. 2). The densities of secreted EspD based on three independent experiments were as follows: for 85-170 Nalr, 1,664 ± 108; for 85-170 Nalr ΔtoxB, 792 ± 123; for 85-170 Nalr Δefa-1′, 1,638 ± 196; and for 85-170 Nalr ΔtoxB Δefa-1′, 201 ± 87 (means ± standard deviations). Thus, mutation of toxB caused a significant reduction in the secretion of EspD (the value was 2.1-fold lower than the wild-type value; P = 0.001). While mutation of efa-1′ alone had little effect (P = 0.82), a cumulative reduction in EspD expression occurred when both toxB and efa-1′ were deleted (the value was ca. 8.2-fold lower than the wild-type and single efa-1′ mutant values; P < 0.001). Similar reductions in the expression of EspD were detected following analysis of whole-cell protein from the wild-type and mutant strains (Fig. 2).
FIG. 2.
Effects of toxB and efa-1′ mutations on expression and secretion of the LEE4-encoded type III secreted protein EspD. Whole-cell and secreted protein fractions of 85-170 Nalr, 85-170 Nalr ΔtoxB, 85-170 Nalr Δefa-1′, and 85-170 Nalr ΔtoxB Δefa-1′ (ca. 25 μg of protein) were resolved by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and probed by using a mouse monoclonal antibody specific for EspD. Equivalent loading of proteins was confirmed by Coomassie blue staining of gels (data not shown). WT, wild type.
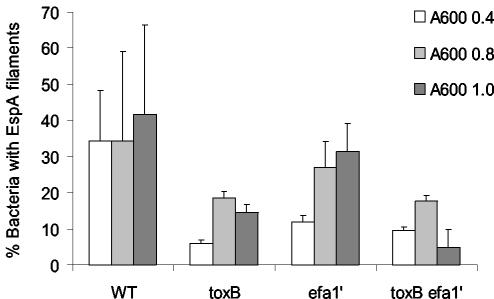
We also analyzed the expression of EspA filaments by the wild type and the ΔtoxB Δefa-1′ mutant strain by indirect immunofluorescence. It was observed previously that EspA filaments are not produced during growth in M9 minimal medium but are induced following subculture in MEM-HEPES (52). Upon induction, not all bacteria in the population express EspA filaments at a given time, and the highest proportion of bacteria with EspA filaments is detected in the late exponential phase (52). We determined the proportion of wild-type and mutant bacteria elaborating EspA filaments at A600 values of 0.4, 0.8, and 1.0. Mutation of toxB and efa-1′ singly or in combination resulted in a reduction in the proportion of bacteria elaborating EspA filaments (Fig. 3); however, the difference was significant only for samples collected at an A600 of 0.4 (for 85-170 Nalr ΔtoxB, P = 0.004; for 85-170 Nalr Δefa-1′, P = 0.013; for 85-170 Nalr ΔtoxB Δefa-1′, P = 0.008). During the late logarithmic phase (A600, 0.8 and 1.0), the extent of EspA filamentation observed with the mutant strains was consistent with the levels of EspD detected by Western blotting (Fig. 2). Since the production of EspA filaments by EHEC is dependent on EspD (29), it is not possible to state whether the observed reduction in EspA filament production in the mutant strains was the result of lower EspD production (Fig. 2), lower EspA production, or a combination of the two.
FIG. 3.
Effect of mutation of toxB and efa-1′ on the formation of EspA filaments. EspA filaments were visualized by indirect immunofluorescence following subculture of bacteria grown in M9 minimal medium into MEM-HEPES. Samples were prepared at A600 values of 0.4, 0.8, and 1.0. The proportions of bacteria expressing EspA filaments were determined as described in Materials and Methods, and the data are the means ± standard deviations for three independent experiments. WT, wild type.
Since production of LEE4-encoded proteins is growth phase dependent (52, 53), it was not possible to trans-complement the defects in EspD or EspA production owing to the inhibitory effect of plasmids containing toxB or efa-1′ on bacterial growth (data not shown).
Mutation of the E. coli O157:H7 toxB and truncated efa-1 genes does not affect the activity of the LEE1, LEE4, and LEE5 promoters.
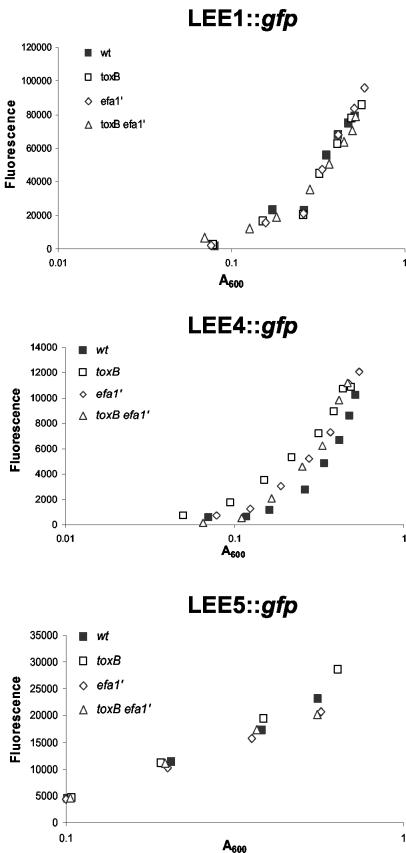
To determine if mutation of toxB and efa-1′ impairs the production of EspD and EspA filaments by reducing the transcription of LEE genes, we separately transformed a series of plasmid-borne eGFP-reporter constructs into the wild-type and mutant strains in order to measure the activity of the LEE1 (ler), LEE4 (sepL), and LEE5 (tir) promoters (pAJR71, pAJR74, and pAJR75, respectively). The emission of fluorescence due to accumulation of eGFP was quantified at several points in the growth cycle. No significant differences in the activities of the LEE1, LEE4, and LEE5 promoters were detected in strains harboring the toxB and efa-1′ mutations compared to the parent strain (Fig. 4). Indeed, the activity of the LEE4 promoter was slightly greater for the 85-170 Nalr ΔtoxB and Δefa-1′ mutant strains than for the parent strain. Thus, mutation of toxB and efa-1′ affects the expression of EspD at a posttranscriptional level.
FIG. 4.
Effect of toxB and efa-1′ mutations on the activity of LEE1, LEE4, and LEE5 promoters. Plasmids pAJR71 (lee1::gfp), pAJR74 (lee4::gfp), and pAJR75 (lee5::gfp) were separately transformed into 85-170 Nalr, 85-170 Nalr ΔtoxB, 85-170 Nalr Δefa-1′, and 85-170 Nalr ΔtoxB Δefa-1′, and the bacteria were grown in MEM-HEPES. At stages in the growth cycle the absorbance of the cultures was recorded, and the fluorescence due to accumulation of eGFP was quantified. The data are the means for two independent measurements. wt, wild type.
Mutation of the E. coli O157:H7 toxB and truncated efa-1 genes does not affect intestinal colonization in calves or sheep.
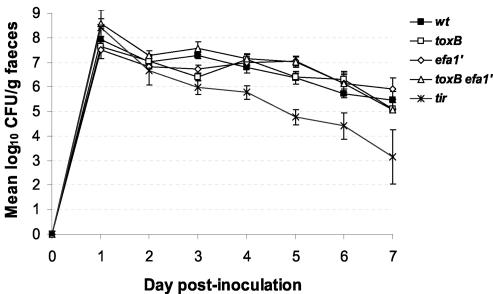
To investigate the role of the E. coli O157:H7 toxB and efa-1′ genes in intestinal colonization, we first inoculated two 10- to 14-day-old conventional Friesian bull calves with E. coli 85-170 Nalr ΔtoxB, 85-170 Nalr Δefa-1′, or 85-170 Nalr ΔtoxB Δefa-1′ by the oral route. Colony PCRs were performed on days 1 and 7 postinoculation by using a minimum of 10 independent nalidixic-acid resistant isolates from each calf, and this analysis confirmed that only the inoculum strain was shed (data not shown). The stx-negative 85-170 Nalr parent strain was administered to five calves and was shed at levels of >105 CFU/g of feces for the duration of the experiment. No significant differences in the shedding patterns were detected between the wild-type and mutant strains (P values at 7 days postinoculation: for ΔtoxB, 0.83; for Δefa-1′, 0.10; and for ΔtoxB Δefa-1′, 0.9) (Fig. 5). As a control, four calves were inoculated with an 85-170 Nalr mutant harboring an in-frame nonpolar deletion in the tir gene. By day 7 postinoculation the 85-170 Nalr Δtir mutant was shed at significantly lower numbers than the wild type (P = 0.004). Indeed, the reduction in shedding of the tir mutant reached significance at just 3 days postinoculation (P = 0.018).
FIG. 5.
Mutation of toxB and efa-1′ does not significantly affect the course of fecal shedding of E. coli O157:H7 in calves. The data are fecal shedding data for duplicate calves inoculated with the 85-170 Nalr ΔtoxB, 85-170 Nalr Δefa-1′, and 85-170 Nalr ΔtoxB Δefa-1′ mutants. Data from five calves infected with 85-170 Nalr and four calves infected with 85-170 Nalr Δtir are shown for comparison. The bacterial recovery from the feces was measured twice each day. The data are daily means ± standard errors of the means. wt, wild type.
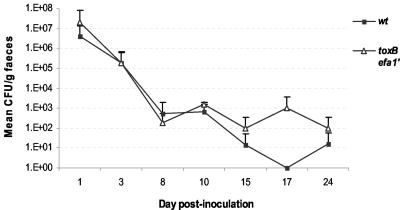
We separately assessed the ability of the 85-170 Nalr ΔtoxB Δefa-1′ mutant to colonize the ovine intestine by orally inoculating groups of nine 6-week-old sheep with the wild-type or double mutant strain, and fecal shedding was quantified for up to 24 days. This model was recently used to establish a role for intimin in persistent colonization of the ovine intestine (67). No significant differences in the shedding patterns were detected between the wild-type and mutant strains at any time tested (Fig. 6).
FIG. 6.
Mutation of both toxB and efa-1′ does not significantly affect the course of fecal shedding of E. coli O157:H7 in 6-week-old sheep. Animals were inoculated with 85-170 Nalr or 85-170 Nalr ΔtoxB Δefa-1′ (nine animals per group), and fecal samples were collected at intervals up to 24 days. One lamb from each group was sacrificed on days 3, 8, and 18 postinfection, and postmortem examinations were performed. The data are means ± standard deviations for different times.
DISCUSSION
It has been reported previously that mutation of the efa-1 gene of EHEC serotypes O5 and O111 results in a significant reduction in fecal shedding of the organisms (P < 0.05 at 7 days postinoculation) (52). E. coli O157:H7 strains lack the full-length efa-1 gene; however, a truncated version of efa-1 is present in the chromosome (19, 47), and a homolog of efa-1 (toxB/l7095) is present in the large plasmid (4, 31). To clarify the role of these genes, we constructed single and double ΔtoxB and Δefa-1′ mutants of E. coli O157:H7 and assessed the phenotypes of the mutants in vitro and in calves and sheep.
Consistent with the observations of Tatsuno et al. (63), we found that deletion of the E. coli O157:H7 toxB gene led to a reduction in the expression and secretion of LEE4-encoded type III secreted proteins and impaired MC formation on cultured epithelial cells. Furthermore, we found for the first time that a defined mutation of the truncated version of efa-1 alone caused a reduction in MC formation on HeLa cells. This is consistent with the finding that a transposon insertion upstream of efa-1′ reduced adherence to Caco-2 cells to 25 to 50% of the wild-type adherence level (62; I. Tatsuno, personal communication). The efa-1′ single mutant expressed and secreted wild-type levels of EspD, and the production of EspA filaments was not reduced significantly during the phase of growth when such filaments are expressed maximally. This is in accordance with the finding that the G2-B12 efa-1′ mutant identified by Tatsuno et al. produced wild-type levels of EspA as assessed by immunoblotting (62). In contrast, deletion of efa-1′ in combination with toxB led to marked reductions in the expression and secretion of EspD and EspA filament production and a further reduction in the ability of the bacteria to form MC on cultured cells, implying that there is a synergistic effect. In all cases the reduction in EspD and EspA expression and secretion was not sufficient to prevent the nucleation of filamentous actin under the sites of E. coli O157:H7 attachment to cultured cells (62, 63; this study). Some bacteria harboring the ΔtoxB Δefa-1′ double mutation still assembled EspA filaments on their surfaces, as detected by indirect immunofluorescence. Since actin condensation under adherent bacteria is dependent on the delivery of LEE-encoded type III secreted effectors, our data indicate that sufficient quantities of LEE4-encoded proteins are still produced and translocated into epithelial cells so that the bacteria can elicit cytoskeletal rearrangements.
We attempted to trans-complement the defects in MC formation and the expression and secretion of EspD and EspA in the mutant strains by cloning and expressing the toxB and efa-1′ genes. Several unsuccessful attempts were made to subclone toxB from pO157, and it is likely that this gene is unstable and/or toxic, as has been reported for the homologous 9.5-kb efa-1 gene (22, 25, 42). A mini-pO157 derivative containing toxB described by Tatsuno et al. (63) could not be stably maintained in 85-170 Nalr ΔtoxB, most likely as a result of incompatibility with the endogenous pO157ΔtoxB plasmid. We were able to clone the truncated efa-1 gene from E. coli O157:H7; however, induction of gene expression restricted the growth rate, probably as a consequence of toxicity, as reported elsewhere (2).
Mutation of toxB caused a defect in the expression and secretion of LEE-encoded type III secreted effectors whose magnitude was similar to that seen in EHEC O5 and O111 efa-1 mutants (59). Type III secretion of EspD was impaired by ca. eightfold in an E. coli O157:H7 mutant harboring deletions of both toxB and efa-1′. Despite this, no significant reduction in fecal shedding of E. coli O157:H7 was seen in calves or sheep infected with the mutant strains. Our data are consistent with the observation that an E. coli O157:H7 strain with the pO157 plasmid which encodes toxB deleted colonizes gnotobiotic piglets and induces enteritis at least as well as the wild type (65). We cannot preclude the possibility that there were differences in the immune status of the animals used in the present study or the possibility that various levels of passively acquired EHEC-specific maternal antibody masked subtle effects of the mutations.
LEE-encoded type III secreted proteins are major virulence factors for several AE pathogens in their target animal species, including EPEC EspB in humans (61), REPEC EspA, EspB, and Tir in rabbits (1, 33), and Citrobacter rodentium EspB in mice (41). Data supporting a role for LEE-encoded type III secreted proteins in the colonization of ruminants by E. coli O157:H7 were recently provided by the finding that immunization of calves with E. coli O157:H7 type III secreted proteins reduces fecal shedding of the bacteria upon subsequent challenge (50). Considerable natural variation in the level of secretion of the LEE-encoded Tir and EspD proteins has been observed among EHEC O157:H7 strains isolated from cattle (35); however, it is not known if this correlates with the ability of the strains to colonize the intestines efficiently. While the E. coli O157:H7 ΔtoxB Δefa-1′ double mutant was impaired in the ability to express and secrete LEE4-encoded proteins, the amount of EspD secreted by this mutant was comparable to the amounts secreted by naturally occurring E. coli O157:H7 strains isolated from cattle (35; Roe and Gally, unpublished observations).
When the ability of E. coli O157:H7 to form AE lesions was abolished through deletion of the tir gene, we observed a significant reduction in fecal shedding of the bacteria following oral inoculation of calves. This indicates that differences in the ability of EHEC strains to colonize the bovine intestine can be detected with our oral challenge model and that the model does not merely measure shedding of the inoculum. Furthermore, it shows for the first time that the translocated intimin receptor is required for intestinal colonization in calves. This implies that the role of intimin in intestinal colonization of calves and sheep may be explained, at least in part, by its ability to bind to its translocated receptor instead of secondary receptors, such as β1-integrins (14) and/or cell surface-localized nucleolin (57). There is strong evidence for the existence of host cell intimin receptors, and the role of such molecules in the carriage and tissue tropism of EHEC merits close investigation.
It is unclear why mutation of efa-1 in EHEC O5 and O111 impairs intestinal colonization in calves whereas mutation of toxB in EHEC O157:H7 does not, even though type III secretion is similarly affected in the two backgrounds. It is possible that different EHEC serotypes rely to different extents on the LEE-encoded type III secretion system for intestinal colonization. There is increasing evidence that different EHEC serovars colonize the intestines of calves by distinct mechanisms. We and other workers have shown that EHEC serotypes O5, O26, and O111 adhere extensively to the intestinal epithelium in the bovine cecum, colon, and rectum and can readily be seen forming AE lesions (46, 59). In contrast, E. coli O157:H7 is rarely detected in association with the intestinal epithelium at these sites following oral inoculation of calves even though high numbers of organisms may be shed (6, 8, 68). E. coli O157:H7 may exhibit a specific tropism for lymphoid follicle-dense mucosa at the terminal rectum in weaned calves and adult cattle (40); however, preliminary data from our laboratories have indicated that non-O157 EHEC serotypes do not share a tropism for this site (S. W. Naylor, A. J. Roe, and D. L. Gally, unpublished observations). It is not known if E. coli O157:H7 exhibits a specific tropism for the terminal rectum in animals that are the ages we used. Indeed, the preweaned calves and 6-week-old lambs inoculated in this study had not started to ruminate, and it is not clear if lymphoid follicle-dense epithelium has even matured in the terminal rectum at this age. The role of specific E. coli O157:H7 genes in colonization of the terminal rectum and other sites in preweaned, weaned, and adult ruminants awaits detailed investigation.
It is possible that Efa1 influences intestinal colonization of calves by EHEC O5 and O111 by acting independently of the LEE. Given that Efa1 is 97.4% identical to EPEC lymphostatin, this molecule may facilitate colonization by suppressing the activation of gastrointestinal lymphocytes. However, in a recent study it was not possible to detect lymphostatin-like effects on bovine intraepithelial lymphocytes exposed to EHEC serotype O103:H2 in situ in a ligated ileal loop model of infection (36). It is also possible that Efa1 acts as an adhesin per se or influences adhesion indirectly by some other mechanism. Indeed, Badea et al. reported that Efa1 mediates adherence of REPEC to cultured epithelial cells, apparently without affecting the expression and secretion of LEE-encoded proteins (2). Furthermore, Efa1-specific antibody inhibited the adherence of REPEC to cultured cells (2). It may be that ToxB and the truncated version of Efa1 do not influence intestinal colonization by E. coli O157:H7 because they do not share the activities of Efa1.
Our data and those of Tatsuno et al. (63) suggest that Efa1 and ToxB influence the expression of LEE-encoded type III secreted proteins at a posttranscriptional level. Almost all LEE-positive strains tested to date contain either Efa1 or ToxB (25, 42, 60), and indeed in EHEC O26 strain 413/89-1 and REPEC strains RDEC-1 and 83/89, the LEE and efa-1 are adjacent on the chromosome (64, 69) (EHEC O26 LEE accession number AJ277443). This close genetic linkage occurs in many other EPEC and EHEC strains (38) and may imply that Efa1 is required for complete activity of the LEE-encoded type III secretion system. Furthermore, O island 122, which contains truncated efa-1′, is also significantly associated with LEE-positive EHEC strains that cause serious and/or epidemic disease (24, 38). No enzymatic activities have yet been ascribed to the EHEC Efa1, Efa1′, and ToxB proteins. Efa1 and ToxB are highly homologous to large clostridial toxins at the N terminus and share a DXD glycosyltransferase motif (4, 25, 31, 42), and both contain a cysteine protease motif that is present in numerous other secreted bacterial virulence factors (55). The 433-amino-acid truncated Efa1 protein lacks both these motifs, and their relevance to the activities of Efa1 and ToxB remains to be tested.
The mechanism by which Efa1 and ToxB influence the translation of LEE4 transcripts is unclear. For some type III secretion systems in gram-negative bacteria, it has been suggested that the secretion signal for translocated effector proteins is present in the mRNA and that translation and secretion of the proteins may be coupled at the needle complex (48). If such a situation applied to the EHEC LEE-encoded type III secretion apparatus, then it is conceivable that toxB and efa-1′ mutations may influence expression and secretion of LEE4-encoded proteins by interfering with the proper insertion and assembly of the type III secretion apparatus in the membrane and/or the targeting of LEE transcripts.
Interestingly, LifA does not appear to be required for efficient production and secretion of LEE-encoded effector proteins in human or REPEC strains (2, 59). It is noteworthy that the cloned EPEC LEE can confer AE activity upon E. coli K-12 (34), whereas the cloned LEE from E. coli O157:H7 cannot (12), suggesting that there are differences in the control of type III secretion between the strains. The involvement of Efa1, Efa1′, and ToxB in this process is the subject of ongoing studies in our laboratory.
Acknowledgments
We are grateful to Trinad Chakraborty for providing EspD-specific antibody.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council, United Kingdom (grant 201/D17455 to T.W. and M.S.), and the European Union (EU project QLK2-2000-00600 grant to T.W.). D.G. is a Defra Veterinary Research Fellow. A.B., R.M.L., and M.J.W. were supported by Defra contract OZ0706.
Editor: A. D. O'Brien
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badea, L., S. Doughty, L. Nicholls, J. Sloan, R. M. Robins-Browne, and E. L. Hartland. 2003. Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34:205-215. [DOI] [PubMed] [Google Scholar]
- 3.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Med. Biol. 473:173-177. [DOI] [PubMed] [Google Scholar]
- 9.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eaeA deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, S. J., J. Yu, and J. B. Kaper. 1999. The cloned locus of enterocyte effacement from enterohemorrhagic Escherichia coli O157:H7 is unable to confer the attaching and effacing phenotype upon E. coli K-12. Infect. Immun. 67:4260-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, G., A. D. Phillips, L. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 14.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. A. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 15.Gansheroff, L. J., and A. D. O'Brien. 2000. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA 97:2959-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gansheroff, L. J., M. R. Wachtel, and A. D. O'Brien. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 67:6409-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohaemorrhagic Escherichia coli, p. 739-761. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 18.Hall, G. A., D. J. Reynolds, N. Chanter, J. H. Morgan, K. R. Parsons, T. G. Debney, A. P. Bland, and J. C. Bridger. 1985. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet. Pathol. 22:156-163. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 20.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 21.James, S. P., and J. M. Klapproth. 1996. Major pathways of mucosal immunity and inflammation: cell activation, cytokine production and the role of bacterial factors. Aliment. Pharmacol. Ther. 10(Suppl. 2):1-9. [DOI] [PubMed] [Google Scholar]
- 22.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H-. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, J. R., H. A. Lockman, K. Owens, S. Jelacic, and P. I. Tarr. 2003. High-frequency secondary mutations after suicide-driven allelic exchange mutagenesis in extraintestinal pathogenic Escherichia coli. J. Bacteriol. 185:5301-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmali, M. A., M. Mascarenhas, S. Shen, K. Ziebell, S. Johnson, R. Reid-Smith, J. Isaac-Renton, C. Clark, K. Rahn, and J. B. Kaper. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klapproth, J. M., I. C. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klapproth, J. M., M. S. Donnenberg, J. M. Abraham, and S. P. James. 1996. Products of enteropathogenic E. coli inhibit lymphokine production by gastrointestinal lymphocytes. Am. J. Physiol. 271:G841-G848. [DOI] [PubMed] [Google Scholar]
- 27.Klapproth, J. M., M. S. Donnenberg, J. M. Abraham, H. L. Mobley, and S. P. James. 1995. Products of enteropathogenic Escherichia coli inhibit lymphocyte activation and lymphokine production. Infect. Immun. 63:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohaemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kresse, A. U., M. Rohde, and C. A. Guzman. 1999. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun. 67:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locking, M. E., S. J. O'Brien, W. J. Reilly, E. M. Wright, D. M. Campbell, J. E. Coia, L. M. Browning, and C. N. Ramsay. 2001. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol. Infect. 127:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino, K., K. Ishii, T. Yasunaga, M. Hattori, K. Yokoyama, C. H. Yutsudo, Y. Kubota, Y. Yamaichi, T. Iida, K. Yamamoto, T. Honda, C. G. Han, E. Ohtsubo, M. Kasamatsu, T. Hayashi, S. Kuhara, and H. Shinagawa. 1998. Complete nucleotide sequences of 93-kb and 3.3-kb plasmids of an enterohemorrhagic Escherichia coli O157:H7 derived from Sakai outbreak. DNA Res. 5:1-9. [DOI] [PubMed] [Google Scholar]
- 32.Malstrom, C., and S. James. 1998. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect. Immun. 66:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchés, O., J.-P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 35.McNally, A., A. J. Roe, S. Simpson, F. M. Thomson-Carter, D. E. E. Hoey, C. Currie, T. Chakraborty, D. G. E. Smith, and D. L. Gally. 2001. Differences in levels of secreted locus of enterocyte effacement proteins between human disease-associated and bovine Escherichia coli O157. Infect. Immun. 69:5107-5114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Menge, C., I. Stamm, P. M. van Diemen, P. Sopp, G. Baljer, T. S. Wallis, and M. P. Stevens. 2004. Phenotypic and functional characterisation of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic Escherichia coli infection. J. Med. Microbiol. 53:573-579. [DOI] [PubMed] [Google Scholar]
- 37.Milton, D. L., R. O'Toole, P. Hörstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morabito, S., R. Tozzoli, E. Oswald, and A. Caprioli. 2003. A mosaic pathogenicity island made up of the locus of enterocyte effacement and a pathogenicity island of Escherichia coli O157:H7 is frequently present in attaching and effacing E. coli. Infect. Immun. 71:3343-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien, S. J., G. K. Adak, and C. Gilham. 2001. Contact with farming environment as a major risk factor for Shiga toxin (verocytotoxin)-producing Escherichia coli O157 infection in humans. Emerg. Infect. Dis. 7:1049-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paiba, G. A., J. C. Gibbens, S. J. Pascoe, J. W. Wilesmith, S. A. Kidd, C. Byrne, J. B. Ryan, R. P. Smith, M. McLaren, R. J. Futter, A. C. Kay, Y. E. Jones, S. A. Chappell, G. A. Willshaw, and T. Cheasty. 2002. Faecal carriage of verocytotoxin-producing Escherichia coli O157 in cattle and sheep at slaughter in Great Britain. Vet. Rec. 150:593-598. [DOI] [PubMed] [Google Scholar]
- 45.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson, G. R., K. J. Bazeley, J. R. Jones, R. F. Gunning, M. J. Green, A. Cookson, and M. J. Woodward. 1999. Attaching and effacing lesions in the large intestine of an eight-month-old heifer associated with Escherichia coli O26 infection in a group of animals with dysentery. Vet. Rec. 145:370-373. [DOI] [PubMed] [Google Scholar]
- 47.Perna, N. T., G. Plunkett, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 48.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 49.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 51.Roe, A. J., and D. L. Gally. 2000. Enteropathogenic and enterohaemorrhagic Escherichia coli and diarrhea. Curr. Opin. Infect. Dis. 13:511-517. [DOI] [PubMed] [Google Scholar]
- 52.Roe, A. J., H. Yull, S. W. Naylor, M. J. Woodward, D. G. Smith, and D. L. Gally. 2003. Heterogeneous surface expression of EspA translocon filaments by Escherichia coli O157:H7 is controlled at the posttranscriptional level. Infect. Immun. 71:5900-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenshine, I., S. Ruschkowski, and B. B. Finlay. 1996. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect. Immun. 64:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Shao, F., P. M. Merritt, Z. Bao, R. W. Innes, and J. E. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 56.Simon, R., U. Preifer, and A. Puhler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 57.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 58.Stevens, M. P., P. M. van Diemen, F. Dziva, P. W. Jones, and T. S. Wallis. 2002. Options for the control of enterohaemorrhagic Escherichia coli in ruminants. Microbiology 148:3767-3778. [DOI] [PubMed] [Google Scholar]
- 59.Stevens, M. P., P. M. van Diemen, G. Frankel, A. D. Phillips, and T. S. Wallis. 2002. Efa1 influences colonization of the bovine intestine by Shiga toxin-producing Escherichia coli serotypes O5 and O111. Infect. Immun. 70:5158-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szalo, I. M., F. Goffaux, V. Pirson, D. Pierard, H. Ball, and J. Mainil. 2002. Presence in bovine enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) Escherichia coli of genes encoding for putative adhesins of human EHEC strains. Res. Microbiol. 153:653-658. [DOI] [PubMed] [Google Scholar]
- 61.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamaru, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of mini-Tn5Km2 insertion mutants of enterohemorrhagic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 68:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatsuno, I., M. Horie, H. Abe, T. Miki, K. Makino, H. Shinagawa, H. Taguchi, S. Kamiya, T. Hayashi, and C. Sasakawa. 2001. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infect. Immun. 69:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tauschek, M., R. A. Strugnell, and R. M. Robins-Browne. 2002. Characterization and evidence of mobilization of the LEE pathogenicity island of rabbit-specific strains of enteropathogenic Escherichia coli. Mol. Microbiol. 44:1533-1550. [DOI] [PubMed] [Google Scholar]
- 65.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wales, A. D., F. A. Clifton-Hadley, A. L. Cookson, M. P. Dibb-Fuller, C. M. Hayes, R. M. La Ragione, J. M. Roe, K. A. Sprigings, G. R. Pearson, and M. J. Woodward. 2001. Preliminary observations on E. coli O157:H7 in sheep. Epidemiology of verocytotoxigenic E. coli, p. 105-113. In G. Duffy, P. Garvey, J. Coia, Y. Wasteson, and D. A. MacDowell (ed.), Verocytotoxigenic E. coli in Europe, vol. 5. Concerted action CT98-3935. Teagasc, Dublin, Ireland.
- 67.Woodward, M. J., A. Best, K. A. Sprigings, G. R. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Row, J. C. Low, and R. M. La Ragione. 2003. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293:299-308. [DOI] [PubMed] [Google Scholar]
- 68.Wray, C., I. M. McLaren, L. P. Randall, and G. R. Pearson. 2000. Natural and experimental infection of normal cattle with Escherichia coli O157. Vet. Rec. 147:65-68. [DOI] [PubMed] [Google Scholar]
- 69.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]