Abstract
Helicobacter pylori-induced mucosal inflammation results in high production of interleukin 17 (IL-17), a potent inducer of IL-8 in gastric epithelial cells. The aim of this study was to investigate signaling pathways by which IL-17 regulates IL-8 production in human gastric epithelial cells. Activation of mitogen-activated protein (MAP) kinases in both IL-17-stimulated MKN28 cells and epithelial cells isolated from H. pylori-colonized gastric mucosa was assessed by Western blotting. In IL-17-stimulated MKN28 cells the activation of activatior protein 1 (AP-1), nuclear factor (NF)-IL-6, and NF-κB was also assessed by electrophoretic mobility shift assay. IL-8 production was evaluated by reverse transcription-PCR and enzyme-linked immunosorbent assay (ELISA) both for IL-17-stimulated MKN28 cells treated with specific MAP kinase inhibitors and gastric biopsy cultures treated with a neutralizing IL-17 antibody. Serum from H. pylori-infected patients was tested for immunoglobulin G response to CagA by ELISA. Treatment of MKN28 cells with IL-17 caused activation of extracellular signal-regulated protein kinase 1/2 (ERK 1/2) but not other MAP kinases and had the downstream effects of AP-1 and NF-κB activation and IL-8 synthesis. Blocking ERK 1/2 activity inhibited AP-1-mediated, but not NF-κB-mediated, IL-8 induction. Enhanced activation of ERK 1/2 was seen in gastric epithelial cells isolated from H. pylori-infected patients in comparison to uninfected controls, and this was associated with high IL-8. These effects were even more pronounced in patients seropositive for CagA than in seronegative ones. In gastric biopsy cultures, the addition of a neutralizing IL-17 antibody decreased ERK 1/2 activation, thus resulting in a significant inhibition of IL-8. In H. pylori-colonized gastric epithelial cells, IL-17-induced IL-8 synthesis is associated with and depends at least in part on the activation of ERK 1/2 MAP kinase.
Colonization of gastric epithelial cells by Helicobacter pylori invariably induces an inflammatory mucosal reaction characterized by a massive infiltration of polymorphonuclear leukocytes (PMN). Considerable evidence has been accumulated to indicate that gastric epithelial cell-derived interleukin 8 (IL-8), the major human PMN chemoattractant, plays a key role in the H. pylori-associated acute inflammatory response (3, 7, 9). The exact signaling pathway by which IL-8 synthesis is regulated during H. pylori infection is not fully understood.
IL-8 gene expression is regulated by several pathways. The IL-8 gene promoter region contains several binding sites for various transcription factors, including the activator protein 1 (AP-1) family, nuclear factor (NF)-IL-6, and NF-κB. The involvement of each of these transcription factors in regulating IL-8 gene expression is strictly dependent on the cell type and inducing stimulus.
Mitogen-activated protein (MAP) kinases are a family of ubiquitous, highly conserved, cell signaling molecules represented by three major groups: extracellular signal-regulated protein kinase 1/2 (ERK 1/2), p38, and c-Jun NH2-terminal kinase (JNK) (12). The MAP kinase signal transduction pathway plays a crucial role in many aspects of immune-mediated inflammatory responses. Previous experiments showed that exposure of gastric epithelial cell lines to H. pylori strains induced the production of IL-8 due to the involvement of MAP kinase signaling pathways (26).
During H. pylori infection, the host also mounts an adaptive immune response. There is local production of anti-H. pylori immunoglobulin A (IgA) and IgG, and, importantly, there is a local Th1-cell response with increased synthesis of gamma interferon, tumor necrosis factor alpha, and IL-12, which are thought to be necessary in promoting ongoing mucosal inflammation (4, 11).
IL-17 is a cytokine produced by activated CD4+ T lymphocytes, mainly Th0 and Th1 cells, and exhibits pleiotropic biological activities on various cell types, including macrophages, fibroblasts, and endothelial and epithelial cells (33). In particular, it has been shown that IL-17 is capable of enhancing the transcription of genes encoding a large variety of proinflammatory molecules. Signaling pathways implicated in IL-17-mediated inflammatory responses include AP-1, NF-κB, and MAP kinases (17, 25). We have previously shown that IL-17 is up-regulated in the H. pylori-colonized gastric mucosa and that IL-17 stimulates bioactive IL-8 synthesis in gastric epithelial cells (24).
The aim of this study was to investigate the role of MAP kinase pathway in IL-17-induced IL-8 production in human gastric epithelial cells.
MATERIALS AND METHODS
Culture of MKN28 cells.
MKN28 cells (gift from R. Zarrilli, University of Naples, Naples, Italy), a gastric epithelial cell line, were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 10 mM l-glutamine, 100 U of penicillin/ml, 100 U of streptomycin/ml, and 1× nonessential amino acid solution (all obtained from Sigma-Aldrich, Milan, Italy) at a concentration of 3 × 106 cells/ml until they reached confluence. Confluent cells were starved in serum-free medium for 12 h to reduce background activation of MAP kinases. Cells were then stimulated with IL-17 at a final concentration of 1 ng/ml (R&D Systems, Minneapolis, Minn.) for 5, 10, 15, 20, 25, 30, and 60 min and 24 h. In parallel, cells were treated with the specific inhibitor of p38 SB203580 at concentrations of 1 and 10 μM for 30 min before the addition of IL-17. Pretreated cells were stimulated with IL-17 for 5, 10, 15, 20, 25, and 60 min. The same protocol as that with inhibitor of p38 SB203580 was used for experiments with MAP kinase inhibitors of ERK 1/2 (PD98059 and U0126) and JNK (SP600125) and a vehicle control (dimethyl sulfoxide [DMSO] at concentration of 0.1%). PD98059 and SP600125 were used at concentrations of 10 and 50 μM, and U0126 was used at 10 μM. All inhibitors were used at generally recommended concentrations (10). All four inhibitors were purchased from Calbiochem (La Jolla, Calif.). All experiments were performed in quadruplicate. At the end, total RNA and proteins were extracted from MKN28 cells to evaluate the expression of IL-8 by reverse transcription-PCR (RT-PCR) and of MAP kinases by Western blotting. Also nuclear proteins were extracted from MKN28 cells and used for electrophoretic gel mobility shift assay (EMSA). Supernatant from 24-h cultures was used to evaluate IL-8 production by enzyme-linked immunosorbent assay (ELISA). Preliminary experiments to test the efficacy of each MAP kinase inhibitor used were performed. For this purpose confluent MKN28 cells were starved in serum-free medium for 12 h and then the MAP kinase inhibitor was added to the culture. After 30 min, medium was supplemented with 10% FBS. MAP kinase inhibitors prevented MAP kinase phosphorylation induced by serum in MKN28 cells (data not shown).
Clinical specimens.
Twenty patients (10 men and 10 women; age range, 19 to 68 years; median, 36 years) who underwent esophagogastroduodenoscopy for dyspeptic symptoms were studied. Only patients with endoscopic findings of normal mucosa or gastric erythema were included in the study. No patient had previously undergone anti-H. pylori treatment or had received antibiotics within the previous 2 months. During endoscopy, eight antral biopsy specimens were taken: one for the urease quick test (Yamanouchi Pharma, Milan, Italy), two for histologic examination, and five for the isolation of epithelial cells. Sections of biopsy specimens were embedded in paraffin and stained with Giemsa to detect H. pylori. A [13C]urea breath test (Cortex, Milan, Italy) was also performed to ascertain H. pylori status. Patients were classified as H. pylori-infected when two or three tests were positive. All three tests were required to be negative to classify patients as noninfected. Thirteen patients had evidence of H. pylori infection, and 7 were classified as H. pylori negative.
Informed consent was obtained from all patients, and the study protocol was approved by the local ethics committee.
Serologic analysis.
Serum samples from each patient were tested for IgG response to CagA by means of a commercial ELISA (CagA IgG EIA Well; Radim, Pomezia, Italy; 93,7% sensitivity and 100% specificity). All patients who were H. pylori negative were also CagA seronegative.
Isolation of gastric epithelial cells.
Epithelial cells were isolated from freshly obtained biopsy specimens. Biopsy specimens were washed in Hanks' balanced salt solution at 4°C and then cut in small pieces (0.2 to 0.4 mm) and washed in 10 ml of Hanks' balanced salt solution with 0.5 mM dithiothreitol (DTT) for 5 min at 4°C under continuous stirring. Tissue was transferred and incubated with 6 ml of chelating buffer (27 mM trisodium citrate, 5 mM Na2HPO4, 96 mM NaCl, 8 mM KH2PO4, 1.5 mM KCl, 55 mM d-sorbitol, 44 mM sucrose) for 7 min at 4°C. The supernatant was collected and run at 800 × g for 5 min at 4°C. Cells were resuspended in 200 μl of phosphate-buffered saline. Isolated cells were counted and checked for viability with 0.1% trypan blue. Viability ranged from 90 to 95%. An aliquot of epithelial cells was used to confirm the nature of isolated cells, by using a cytokeratin 7-specific antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) in Western blotting experiments. Cytokeratin 7 is expressed specifically by epithelial cells and is a useful marker of tissue differentiation (22). To exclude contamination of isolated gastric epithelial cells by lymphocytes and mesenchymal cells, all samples were checked by RT-PCR for the presence of T-cell receptor δ (TCRδ), vimentin (30), and desmin (23) mRNA.
Gastric epithelial cells isolated from four patients without H. pylori infection were resuspended in Hanks' balanced salt solution supplemented with 10% FBS at a concentration of 3 × 105 cells/ml. Cells were then stimulated with IL-17 at a final concentration of 1 ng/ml (R&D Systems) for 15, 30, and 60 min. At the end, total RNA and proteins were extracted from epithelial cells to evaluate the expression of IL-8 by RT-PCR and to evaluate ERK 1/2 phosphorylation by Western blotting.
Gastric biopsy culture.
Additional antral biopsy specimens (n = 3) from 10 H. pylori-infected patients were placed on steel grids in an organ culture chamber at 37°C in 5% CO2-95% O2 with RPMI 1640, 5% FBS, 10 mM l-glutamine, 100 U of penicillin/ml, and 100 U of streptomycin/ml (all obtained from Sigma, St. Louis, Mo.) for 24 h. Biopsy specimens from each patient were cultured with and without a neutralizing IL-17 antibody (10 μg/ml; R&D Systems) or control antibody (anti-cytokeratin 7) to the medium. At the end, organ culture supernatants were collected and used to measure IL-8 production by ELISA. Proteins were extracted from the tissue and utilized to evaluate the expression of MAP kinases by Western blotting.
Total protein extraction and Western blot analysis.
Total proteins were extracted from cells with lysis buffer (50 mM HEPES [pH 7.6], 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 30 mM Na4P2O7, 10% glycerol, 1 mM benzamidine, 1 mM DTT, 10 μg of leupeptin/ml,1 mM phenylmethylsulfonyl fluoride [PMSF] [all obtained from Sigma]). After cell lysis, the supernatant was collected and run at 15,000 × g for 15 min at 4°C.
Nuclear proteins were extracted from MKN28 cells with buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.2 mM EGTA, 1 mM DTT, 10 μg of aprotinin A/ml, 10 μg of leupeptin/ml, 1 mM PMSF, 1 mM Na3VO4, 10 mM Na2F). Cell lysates were centrifuged at 12,000 × g for 1 min at 4°C, and the remaining nuclei were solubilized in buffer C (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1 mM DTT, 10 μg of aprotinin A/ml, 10 μg of leupeptin/ml, 1 mM PMSF).
Protein concentration was determined with a protein assay kit from Bio-Rad (Munich, Germany) by Bradford's assay. Total proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred onto an Immobilon-P membrane (Amersham Life Sciences Inc., Buckinghamshire, England). Ponceau S staining was performed to confirm the equal loading and transfer of proteins. The membranes were blocked overnight at 4°C in Tris-buffered saline with 0.05% Tween 20 (5% nonfat milk in 10 mM Tris-HCl-100 mM NaCl-0.1% Tween 20, pH 7.6). This was followed by incubation at room temperature with MAP kinase antibodies diluted 1/1,000 in blocking buffer for 2 h, and then with a horseradish peroxide-conjugated goat anti-mouse IgG monoclonal antibody diluted 1/3,000 for 1 h (Santa Cruz Biotechnology).
Chemiluminescence luminol reagent (Santa Cruz Biotechnology) was used for detection. A phospho-specific p44/p42 MAP kinase antibody was used to detect ERK 1/2. This antibody detects p44 and p42 MAP kinase (ERK 1 and ERK 2) only when they are activated by phosphorylation at Thr202 and Tyr204. A phospho-specific p38 MAP kinase antibody was used to detect p38 activated by phosphorylation at Thr180 and Tyr182. A phospho-specific p54/p46 MAP kinase antibody was used to detect JNK. This antibody detects p54 and p46 MAP kinase only when they are activated by phosphorylation at Thr183 and Tyr185. After analysis of phosphorylated ERK 1/2, p38, and JNK, blots were stripped and incubated with antibodies recognizing total ERK 1/2, p38, and JNK, followed by incubation with a horseradish peroxidase-conjugated goat anti-mouse IgG monoclonal antibody and detection by chemiluminescence luminol reagent. All phospho-specific and total antibodies against ERK 1/2, p38, and JNK were obtained from Calbiochem.
EMSA.
Nuclear protein-DNA binding studies were carried out for 30 min at 4°C in a 19-μl reaction volume containing 10 mM Tris-HCl, 1 mM DTT, 2.5% glycerol, 1 μg of poly(dI-dC), 50 mM KCl, and 5 mM MgCl2 (all from Sigma); 15 fmol of biotin-labeled oligonucleotide-containing probe; and 5 μg of nuclear proteins. The DNA probe was prepared by annealing two consensus oligonucleotides as follows: AP-1, 5′-GGAAGTGTGATGACTCAGGTTTGCCCT-3′ and 5′-AGGGCAAACCTGAGTCATCACACTTCC-3′; NF-κB, 5′-CTTGCTTTGGAATTTCCTCTGGAATTTCCTCGGA-3′ and 5′-TCCGAGGAAATTCCAGAGGAAATTCCAAAGCAAG-3′; NF-IL-6, 5′-CAGTTGCAAATCGTCAGTTGCAAATCGGAT-3′ and 5′-ATCCGATTTGCAACTGACGATTTGCAACTG-3′; and Sp1, 5′-ATTCGATCGGGGCGGGGCGAGC-3′ and 5′-GCTCGCCCCGCCCCGATCGAAT-3′ (all obtained from Proligo, Paris, France). All oligonucleotides were labeled at the 3′ end with biotin by using a commercially available kit (Pierce, Rockford, Ill.). The binding specificity was confirmed by incubating the nuclear protein samples with unlabeled probe for AP-1, NF-κB, or NF-IL-6 or a nonspecific oligonucleotide (5′-CTCGATACCATATCCACATCCACCACCGAT-3′ or 5′-ATCGGTGGTGGATGTGGATATGGTATCGAG-3′) in 100-fold molar excess to compete binding. For supershift analysis, antibodies (10 μg) against AP-1 (c-Fos and c-Jun) or NF-κB (p65) subunits (Santa Cruz Biotechnology) were included in the binding reaction. Protein-DNA and protein-DNA-antibody complexes were resolved in 6% polyacrylamide gels preelectrophoresed for 30 min at room temperature in 0.25× TBE buffer (22.5 mM Tris-borate, 0.5 mM EDTA, pH 8.3). After being blotted to a membrane, labeled oligonucleotides were detected with a chemiluminescence EMSA kit (Pierce). Comparability of the various nuclear extracts was assessed by EMSA with a biotin-labeled Sp1 probe (data not shown). Gel shifts were performed at least twice with nuclear extract prepared from different batches of MKN28 cells.
RNA extraction, cDNA preparation, and RT-PCR.
Total RNA was extracted according to the method of Chomczynski and Sacchi (5). RNA samples were quantified by absorbance at 260 nm. RNA integrity was assessed by electrophoresis on a 1.5% agarose gel. cDNA and PCR assays were performed as previously described (23). PCR primers (Genosys, Cambridge, England) were as follows: β-actin, 5′-CGAGGCCCAGAGCAAGAGA-3′ and 3′-CGTGACATTAAGGAGAAGCTGTG-5′; IL-8, 5′-CATGACTTCCAAGCTGGC-3′ and 3′-GTGACTAAGAACCTATGGTG-5′; IL-17 receptor, 5′-GCTTCACCCTGTGGAACGAATC-3′ and 3′-GGAGATGCCCGTGATGAACC-5′; TCRδ, 5′-CTCACCATTTCAGCCTTACAG-3′ and 3′-GGATTAAGGTTTGGTAGG-5′; vimentin, 5′-CCGGAGCTACGTGACTACG-3′ and 3′-GATGTAGTTGGCGAAGCG-5′; desmin, 5′-CAACAAGAACAACGACGC-3′ and 3′-GGCTGGTTTCTCGGAAGT-5′. The level of RNA transcripts was measured by computed densitometry (Image software; National Institutes of Health).
IL-8 ELISA.
A sensitive ELISA (R&D Systems) was used to detect IL-8. The minimum detectable IL-8 concentration was 5 pg/ml. All samples were assayed in duplicate. The amount of IL-8 protein was expressed as picograms per milligram of biopsy specimen proteins in the gastric biopsy culture and as picograms per milliliter of MKN28 cell culture supernatant.
Statistical analysis.
Significance of differences was assessed by one-way analysis of variance (ANOVA) and, when the F value was significant, by Tukey's multiple-comparison test. Differences were considered significant if P was <0.05.
RESULTS
Treatment of MKN28 cells with IL-17 induces ERK 1/2 MAP kinase phosphorylation with the downstream effect of AP-1 activation and IL-8 synthesis.
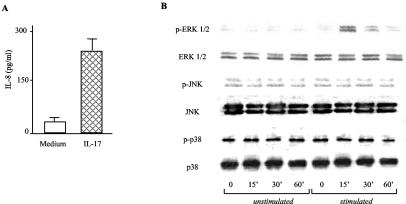
As previously reported (24), stimulation of MKN28 cells with IL-17 significantly increased IL-8 secretion (Fig. 1A). We explored the possibility that IL-17-induced IL-8 production relies on MAP kinase activation. To address this issue, MKN28 cells were treated with IL-17 and then examined for the content of both phosphorylated and total MAP kinases. As shown in Fig. 1B, the addition of IL-17 to MKN28 cell cultures activated ERK 1/2. In contrast, treatment of MKN28 cells with IL-17 resulted in no change in the level of phosphorylated (activated) p38 and JNK.
FIG. 1.
(A) IL-17 induces IL-8 production in MKN28 cells. Cells were cultured in the presence or absence of IL-17 (1 ng/ml) for 24 h. At the end, cell culture supernatants were collected and analyzed for IL-8 content by ELISA. Data indicate mean values ± standard deviations from four representative experiments. (B) IL-17 induces phosphorylation of ERK 1/2 MAP kinases in MKN28 cell cultures. Western blot analysis of phosphorylated and total ERK 1/2, JNK, and p38 was performed on MKN28 cells stimulated with IL-17 (1 ng/ml) for 0, 15, 30, and 60 min. One of four representative experiments is shown.
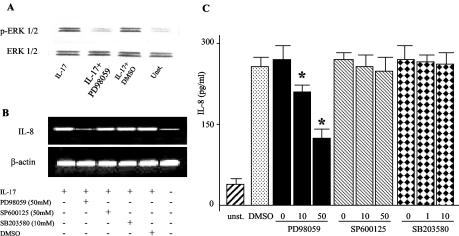
To assess the role of ERK 1/2 activation in IL-17-mediated IL-8 induction, MKN28 cells were stimulated with IL-17 in the presence or absence of PD98059, a specific inhibitor of ERK 1/2. Inhibition of ERK 1/2 activity by PD98059 significantly inhibited IL-17-induced IL-8 expression both at the RNA and protein levels (Fig. 2B and C). Inhibition of IL-8 was seen also in MKN28 cells pretreated with U0126 (data not shown). Results obtained with 50 μM PD98059 were comparable with those obtained with 10 μM U0126. No inhibition in IL-8 synthesis was seen when IL-17-stimulated MKN28 cells were treated with specific inhibitors of p38 and JNK or vehicle (DMSO) (Fig. 2B and C).
FIG. 2.
Inhibition of ERK 1/2 activity down-regulates IL-17-induced IL-8. MKN28 cells were preincubated with various concentrations of ERK 1/2 (PD98059; 10 and 50 μM), p38 (SB203580; 1 and 10 μM) and JNK (SP600125; 10 and 50 μM), inhibitors or vehicle control (DMSO; 0.1%) for 30 min, followed by stimulation with IL-17 (1 ng/ml) for 1 h to evaluate activation of ERK 1/2 and IL-8 transcripts or 24 h to measure IL-8 protein. (A) Phosphorylated ERK 1/2 (top blot) and total ERK 1/2 (bottom blot) were analyzed in proteins extracted from MKN28 cells. PD98059 was used at a concentration of 50 μM. One of four representative experiments is shown. (B) RNA transcripts for IL-8 and β-actin in MKN28 cells. One of four representative experiments is shown. (C) IL-8 protein measured by ELISA in MKN28 cell culture supernatants. Data are mean values ± standard deviations from four independent experiments. *, P < 0.01 for 50 and 10 μM versus 0 μM. Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests.
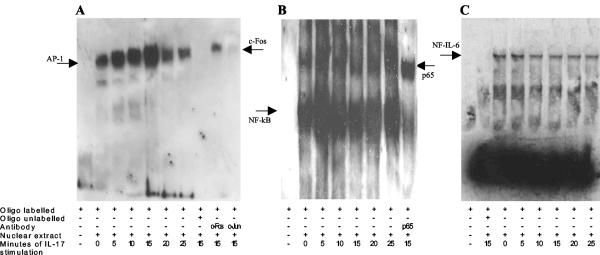
Expression of the IL-8 gene is regulated by the activation of specific transcription factors such as AP-1, NF-IL-6, and NF-κB. To further elucidate the mechanism underlying the response to IL-17, we evaluated the activation of these transcription factors. Treatment of MKN28 cells with IL-17 increased AP-1 and NF-κB DNA-binding activity within 5 min (Fig. 3A and B). The activation of AP-1 sites persisted up to 15 min and then slightly declined, while NF-κB DNA-binding activity declined immediately after 5 min. No changes were found in NF-IL-6 DNA-binding activity at any time point (Fig. 3C). The AP-1 proteins are homodimers and heterodimers composed of basic region-leucine zipper (bZIP) proteins. The best-known components of AP-1, c-Jun and c-Fos proteins, were checked by supershift. It has been demonstrated that AP-1 is composed of c-Fos and, possibly, of another bZIP protein, which is not c-Jun (Fig. 3A).
FIG. 3.
Treatment of MKN28 cells with IL-17 activates AP-1 and NF-κB. EMSA of MKN28 cells stimulated with IL-17 (1 ng/ml) for 5, 10, 15, 20, and 25 min. Biotin-labeled AP-1 (A), NF-κB (B), and NF-IL-6 (C) oligonucleotides probes (oligo) were incubated in the absence (−) or presence (+) of nuclear extract, a 100-fold excess of unlabeled oligonucleotides, NF-κB/p65, or an AP-1 (c-Fos and c-Jun) anti-transcription factor antibody.
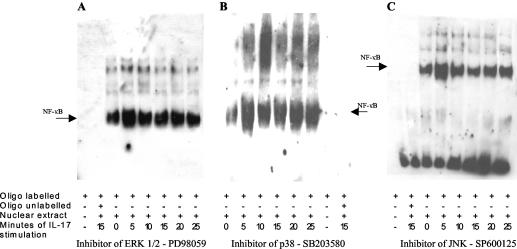
To explore the role of ERK 1/2 MAP kinase in the activation of AP-1 and NF-κB, we performed experiments involving pretreatment of MKN28 cells with specific inhibitors of MAP kinases before stimulation by IL-17. Pretreating MKN28 cells with the specific inhibitor of ERK 1/2 PD98059 completely abolished the activation of AP-1 (Fig. 4A) but did not influence the activation of NF-κB (Fig. 5A). Otherwise, inhibitors of p38 and JNK did not modify either AP-1 (Fig. 4B and C) or NF-κB activation (Fig. 5B and C).
FIG. 4.
Pretreatment of MKN28 cells with specific inhibitor of ERK 1/2 PD98059 completely abolished the activation of AP-1 induced by IL-17. EMSA was performed on MKN28 cells pretreated for 30 min with specific inhibitors of MAP kinases ERK 1/2 (PD98059; 50 μM) (A), p38 (SB203580; 10 μM) (B), and JNK (SP600125; 50 μM) (C) and then stimulated with IL-17 for 5, 10, 15, 20, and 25 min. Biotin-labeled AP-1 oligonucleotides probes (oligo) were incubated in the absence (−) or presence (+) of nuclear extract and a 100-fold excess of unlabeled oligonucleotides.
FIG. 5.
Pretreatment of MKN28 cells with specific inhibitors of MAP kinases does not prevent IL-17-induced NF-κB activation. EMSA was performed on MKN28 cells pretreated for 30 min with specific inhibitors of MAP kinase ERK 1/2 (PD98059; 50 μM) (A), p38 (SB203580; 10 μM) (B), and JNK (SP600125; 50 μM) (C) and then stimulated with IL-17 for 5, 10, 15, 20, and 25 min. Biotin-labeled NF-κB oligonucleotides probe (oligo) were incubated in the absence (−) or presence (+) of nuclear extract and a 100-fold excess of unlabeled oligonucleotides.
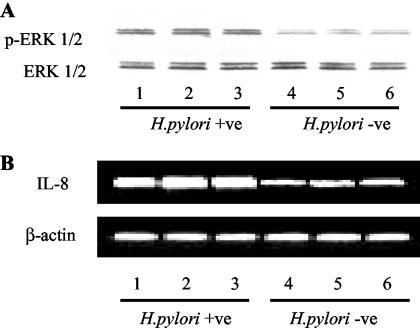
H. pylori colonization of human gastric epithelial cells is associated with increased phosphorylation of ERK1/2 MAP kinase and IL-8 expression.
As already demonstrated for MKN28 cells (24), ex vivo human gastric epithelial cells were found to express transcripts of IL-17 receptor (data not shown). Overall data suggest that ERK 1/2 signaling can contribute to the up-regulation of IL-17-induced IL-8 synthesis. To investigate this hypothesis, we first looked at the content of activated ERK 1/2 MAP kinase in gastric epithelial cells isolated from H. pylori-infected patients. As shown in Fig. 6A, enhanced expression of active ERK 1/2 MAP kinase was seen in epithelial cells from H. pylori-infected patients in comparison to that in cells from H. pylori-uninfected patients. Furthermore, the activation of ERK 1/2 MAP kinase was even more pronounced in those that were seropositive for CagA (n = 9) than in those that were seronegative (n = 4) (data not shown).
FIG. 6.
Enhanced activation of ERK 1/2 MAP kinase and IL-8 transcripts in gastric epithelial cells isolated from H. pylori-infected patients. (A) Phosphorylated (top blot) and total (bottom blot) MAP kinase ERK 1/2 was analyzed by Western blotting of proteins extracted from isolated gastric epithelial cells from H. pylori-infected (lanes 1 to 3) and uninfected (lanes 4 to 6) patients. (B) RNA transcripts for IL-8 and β-actin in the same samples were analyzed by RT-PCR. An agarose gel shows RT-PCR products for IL-8 (top) and β-actin (bottom). Examples are representative of four separate experiments analyzing epithelial cells isolated from 13 H. pylori-infected patients and 7 uninfected patients.
Consistently, epithelial cells from H. pylori-infected patients exhibited high levels of IL-8 RNA transcripts (Fig. 6B), and the levels were even more pronounced in those seropositive for CagA (data not shown). No detectable levels of TCRδ, vimentin, and desmin transcripts were found in all tested samples, indicating that there was no contamination by lymphocytes and mesenchymal cells (data not shown).
IL-17 increases ERK 1/2 phosphorylation and IL-8 levels in isolated gastric epithelial cells.
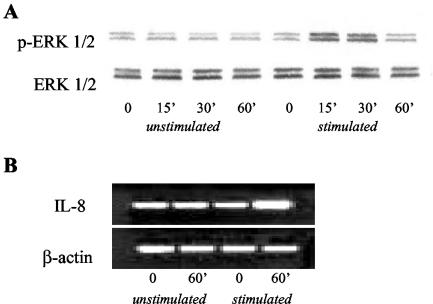
To investigate whether the increase of ERK 1/2 phosphorylation and IL-8 transcripts was due to IL-17, and was independent of H. pylori, isolated gastric epithelial cells from patients without H. pylori infection were stimulated with IL-17. A significant increase of ERK 1/2 phosphorylation and IL-8 transcripts was found (Fig. 7).
FIG. 7.
IL-17 induces phosphorylation of ERK 1/2 MAP kinase and IL-8 transcripts in isolated gastric epithelial cells from patients without H. pylori infection. Gastric epithelial cells were stimulated with IL-17 (1 ng/ml) for 0, 15, 30, and 60 min. (A) Western blot analysis of phosphorylated (top blot) and total (bottom blot) ERK 1/2 MAP kinase is shown. (B) RNA transcripts for IL-8 and β-actin were analyzed by RT-PCR at 0 and 60 min. An agarose gel shows RT-PCR products for IL-8 (top) and β-actin (bottom). Examples are representative of four separate experiments using epithelial cells isolated from four patients not infected with H. pylori.
Neutralization of IL-17 activity reduces ERK 1/2 MAP kinase phosphorylation and down-regulates IL-8 levels in the H. pylori-infected human gastric mucosa.
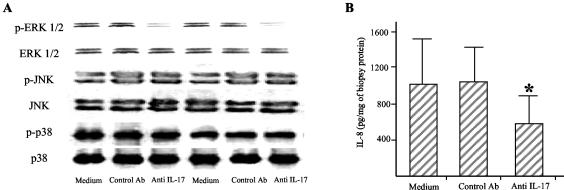
To further examine if IL-17 plays a role in modulating ERK 1/2 activity and IL-8 expression during H. pylori infection, gastric biopsy specimens taken from patients with H. pylori infection were cultured in the presence or absence of a neutralizing IL-17 antibody and then ERK 1/2 MAP kinase and IL-8 production was analyzed. Culturing H. pylori-colonized biopsy specimens with the neutralizing IL-17 antibody decreased ERK 1/2 phosphorylation but neither p38 nor JNK phosphorylation (Fig. 8A). Importantly, treatment of biopsy specimens with an anti-IL-17 antibody also decreased IL-8 secretion (Fig. 8B).
FIG. 8.
(A) An anti-IL-17 antibody significantly decreased the phosphorylation of ERK 1/2 in gastric biopsy cultures. Western blot analysis of phosphorylated and total ERK 1/2, JNK, and p38 MAP kinases was performed on gastric biopsy cultures treated without or with a control (anti-cytokeratin 7; 1 μg/ml) or an anti-IL-17 (10 μg/ml) antibody. One of five representative experiments from two patients is shown. (B) Neutralization of endogenous IL-17 results in down-regulation of IL-8 in gastric biopsy cultures. IL-8 levels were measured in the supernatant by ELISA after 24 h of culture. Data are mean values ± standard deviations from 10 independent experiments. *, P < 0.05 versus medium and control antibodies. Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests.
DISCUSSION
This study confirms and expands on our previous work supporting the role of IL-17 in the pathogenesis of H. pylori infection. In particular, we show that IL-17 is a powerful inducer of IL-8 in an epithelial cell line and provide evidence that ERK 1/2 MAP kinase is an essential mediator of IL-17-induced IL-8 production. This is consistent with previous studies showing the ability of IL-17 to up-regulate MAP kinase activity and enhance IL-8 in other cell systems (15, 21, 31). We show that ERK 1/2 activity and IL-8 are expressed at high levels in freshly isolated H. pylori-colonized gastric epithelial cells. Moreover, we demonstrate that IL-17, independently of H. pylori, increases ERK 1/2 phosphorylation and IL-8 transcripts in isolated gastric epithelial cells and that neutralization of IL-17 results in a significant suppression of ERK 1/2 activity and IL-8 synthesis in gastric biopsy cultures. These data suggest the involvement of IL-17 signaling in modulation of IL-8 production. Furthermore, the finding of a more-pronounced activation of ERK 1/2 along with higher IL-8 expression in gastric epithelial cells from patients seropositive for IgG in response to CagA confirms that strain-specific differences may act in the mucosal immune response to H. pylori (8, 29).
Although previous studies have shown that IL-17 can enhance p38 and JNK activity in several cell types (2, 20, 25), we feel that this pathway is not mainly involved in regulating IL-17-induced IL-8 secretion in gastric epithelial cells. In fact, we were able to detect no increase in p38 and JNK phosphorylation in IL-17-treated MKN28 cells. In this context it is relevant to indicate that phosphorylation of either p38α and -β or JNK closely correlates with the activity of these MAP kinases (12). Second, treatment of MKN28 cells with either a p38 or a JNK MAP kinase inhibitor did not modify IL-17-stimulated IL-8 synthesis. In agreement with our observations, Kawaguchi et al. recently showed that induction of IL-8 by IL-17 in primary bronchial epithelial cells relies on activation of ERK 1/2 but not of p38 or JNK (18).
An important finding of our work is that inhibition of ERK 1/2 activity does not completely abolish IL-17-induced IL-8 secretion in MKN28 cells. This is consistent with the demonstration that optimal IL-8 expression relies on the activation of multiple signaling pathways (1, 6, 28). However, it is noteworthy that PD98059 and U0126 only indirectly inhibit ERK 1/2 by blocking MAP kinase kinase (MEK)-dependent phosphorylation. So the observed effects may be MEK dependent as well as ERK dependent.
For a better understanding of the IL-17 signaling pathway, we evaluated the activation of AP-1, NF-IL-6, and NF-κB transcription factors, which regulate the expression of the IL-8 gene. Our experiments show that treatment of MKN28 cells by IL-17 increased AP-1 and NF-κB DNA-binding activity. However, the activation of ERK 1/2 was required to mediate AP-1, but not NF-κB, transcriptional activation. This finding further confirms that the MAP kinase and NF-κB pathways may exert independent regulatory effects on gastric epithelial cell IL-8 production following H. pylori infection (19). The data of the present study do not exclude the possibility that factors other than IL-17 are involved in enhancing both ERK 1/2 activity and IL-8 synthesis in gastric epithelial cells. Indeed, previous studies showed that H. pylori by itself can elicit ERK 1/2 activity and lead to IL-8 secretion in gastric epithelial cells (26, 27, 32).
H. pylori infection leads to a massive infiltration of the gastric mucosa with PMN, which largely depends on the activity of locally released chemoattractants. Several studies have emphasized the possibility that H. pylori can directly promote such a phenomenon by stimulating the release of IL-8 in epithelial cells. Nonetheless, studies of animal models of H. pylori infection clearly show that infection per se does not elicit gastric inflammation, since mice lacking an adaptive immune response show minimal pathology after infection with H. pylori, even though transfer of T cells results in a severe gastritis (13, 14), thus supporting the role of T cells in promoting the recruitment of inflammatory cells within the gastric mucosa. In this context, the demonstration that IL-17, a T-cell-derived cytokine, is up-regulated in H. pylori-associated gastritis and that IL-17 promotes IL-8 secretion in gastric epithelial cells further suggests a role for T cells in promoting the ongoing mucosal inflammation.
The main groups of MAP kinases ERK 1/2, p38, and JNK form a parallel cascade that can be activated simultaneously or independently; the functions of MAP kinases depend on the cell type and immune response. They have to collaborate with each other or with other signal transduction pathways. Findings of this study indicate that IL-17-induced IL-8 production in gastric epithelial cells is mediated by ERK 1/2 through the activation of the AP-1 transcription factor. Although the major role of ERK 1/2-mediated signaling has long been thought to be restricted to cell growth and proliferation, it has become clear that several inflammatory processes involve ERK 1/2 activation. So an important role is attributed to ERK 1/2-dependent regulation of the AP-1 family of transcription factors, which in turn may induce IL-8 gene transcription as well as T-cell activation (16). Both these processes are specific hallmarks of gastric mucosa colonized by H. pylori.
The elucidation of the multiple signaling pathways used by H. pylori would provide better understanding and rational approaches for the control of the H. pylori-related inflammatory processes in the gastric mucosa.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aihara, M., D. Tsuchimoto, H. Takizawa, A. Azuma, H. Wakebe, Y. Ohmoto, K. Imagawa, M. Kikuchi, N. Mukaida, and K. Matsushima. 1997. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect. Immun. 65:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awane, M., P. G. Andres, D. J. Li, and H. C. Reinecker. 1999. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 162:5337-5344. [PubMed] [Google Scholar]
- 3.Baggiolini, M., A. Watz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 6.Chu, S. H., H. Kim, J. Y. Seo, J. W. Lim, N. Mukaida, and K. H. Kim. 2003. Role of NF-κB and AP-1 on Helicobacter pylori-induced IL-8 expression in AGS cells. Dig. Dis. Sci. 48:257-265. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree, J. E., J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, P. N. Primrose, and I. J. D. Lindley. 1994. Interleukin 8 expression in Helicobacter pylori, normal and neoplastic mucosa. J. Clin. Pathol. 47:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabtree, J. E., A. Covacci, S. M. Farmery, Z. Xiang, D. S. Tompkins, S. Perry, I. J. Lindley, and R. Rappuoli. 1995. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J. Clin. Pathol. 48:41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe, S. E., L. Alvarez, M. Dytoc, R. H. Hunt, M. Muller, P. Sherman, J. Patel, Y. Jin, and P. B. Ernst. 1995. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology 108:65-74. [DOI] [PubMed] [Google Scholar]
- 10.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 12.Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. [DOI] [PubMed] [Google Scholar]
- 13.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh, H. G., C. C. Loong, and C. Y. Lin. 2002. Interleukin-17 induces src/MAPK cascades activation in human renal epithelial cells. Cytokine 19:159-174. [DOI] [PubMed] [Google Scholar]
- 16.Jorritsma, P. J., J. L. Brogdon, and K. Bottomly. 2003. Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J. Immunol. 170:2427-2434. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic, D. V., J. A. Di Battista, J. Martel-Pelletier, F. C. Jolicoeur, Y. He, M. Zhang, F. Mineau, and J. P. Pelletier. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J. Immunol. 60:3513-3521. [PubMed] [Google Scholar]
- 18.Kawaguchi, M., F. Kokubu, H. Kuga, S. Matsukura, H. Hoshino, K. Ieki, T. Imai, M. Adachi, and S. K. Huang. 2001. Modulation of bronchial epithelial cells by IL-17. J. Allergy Clin. Immunol. 108:804-809. [DOI] [PubMed] [Google Scholar]
- 19.Keates, S., A. C. Keates, M. Warny, R. M. Peek, P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 20.Kehlen, A., K. Thiele, D. Riemann, and J. Langner. 2002. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin. Exp. Immunol. 127:539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laan, M., J. Lotvall, K. F. Chung, and A. Linden. 2001. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br. J. Pharmacol. 133:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauwers, G. Y., J. Furman, L. E. Michael, U. J. Balis, and P. S. Kubilis. 2001. Cytoskeletal and kinetic epithelial differences between NSAID gastropathy and Helicobacter pylori gastritis: an immunohistochemical determination. Histopathology 39:133-140. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Z. L., A. Lilienbaum, G. Butler-Brown, and D. Paulin. 1989. Human desmin-coding gene: complete nucleotide sequence, characterization and regulation of expression during myogenesis and development. Gene 78:243-254. [DOI] [PubMed] [Google Scholar]
- 24.Luzza, F., T. Parrello, G. Monteleone, L. Sebkova, M. Romano, R. Zarrilli, M. Imeneo, and F. Pallone. 2000. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 165:5332-5337. [DOI] [PubMed] [Google Scholar]
- 25.Martel-Pelletier, J., F. Mineau, D. V. Jovanovic, J. A. Di Battista, and J. P. Pelletier. 1999. Mitogen-activated protein kinase and nuclear factor κB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated protein kinase (MAPKAPK). Arthritis Rheum. 42:2399-2409. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 27.Mitsuno, Y., H. Yoshida, S. Maeda, K. Ogura, Y. Hirata, T. Kawabe, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 49:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 29.Peek, R. M., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 30.Perreau, J., A. Lilienbaum, M. Vasseur, and D. Paulin. 1988. Nucleotide sequence of the human vimentin gene and regulation of its transcription in tissues and cultured cells. Gene 62:7-16. [DOI] [PubMed] [Google Scholar]
- 31.Shalom-Barak, T., J. Quach, and M. Lotz. 1998. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kB. J. Biol. Chem. 273:27467-27473. [DOI] [PubMed] [Google Scholar]
- 32.Yamada, H., T. Aihara, and S. Okabe. 2001. Mechanism for Helicobacter pylori stimulation of interleukin-8 production in a gastric epithelial cell line (MKN 28): roles of mitogen-activated protein kinase and interleukin-1β. Biochem. Pharmacol. 61:1595-1604. [DOI] [PubMed] [Google Scholar]
- 33.Yao, Z., S. L. Painter, F. C. Fanslow, D. Ulrich, B. M. Macduff, M. K. Spriggs, and R. J. Armitage. 1995. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155:5483-5486. [PubMed] [Google Scholar]