Abstract
Pregnancy-associated malaria (PAM) is an important cause of maternal and neonatal suffering. It is caused by Plasmodium falciparum capable of inhabiting the placenta through expression of particular variant surface antigens (VSA) with affinity for proteoglycans such as chondroitin sulfate A. Protective immunity to PAM develops following exposure to parasites inhabiting the placenta, and primigravidae are therefore particularly susceptible to PAM. The adverse consequences of PAM in primigravidae are preventable by intermittent preventive treatment (IPTp), where women are given antimalarials at specified intervals during pregnancy, but this may interfere with acquisition of protective PAM immunity. We found that Kenyan primigravidae receiving sulfadoxine-pyrimethamine IPTp had significantly lower levels of immunoglobulin G (IgG) with specificity for the type of parasite-encoded VSA—called VSAPAM—that specifically mediate protection against PAM than did women receiving a placebo. VSAPAM-specific IgG levels depended on the number of IPTp doses received and were sufficiently low to be of clinical concern among multidose recipients. Our data suggest that IPTp should be extended to women of all parities, in line with current World Health Organization recommendations.
Pregnancy-associated malaria (PAM) is a major cause of low birth weight (LBW) and maternal anemia among primigravidae in areas of highly endemic parasite transmission (3, 10, 11, 24). PAM is characterized by placental accumulation of Plasmodium falciparum-infected erythrocytes (IE) that can adhere to proteoglycans such as chondroitin sulfate A (CSA) in the placental intervillous space (7, 18, 25). Placental infection frequently occurs in the absence of symptoms and so is unsuspected and undetected.
The ability of IE to inhabit the placenta appears to depend on their expression of a distinct subset (VSAPAM) (2, 7, 8, 14, 17, 23) of the parasite-encoded, clonally variant surface antigens (VSA) that mediate IE sequestration in nonpregnant individuals (reviewed in reference 12). Levels of VSAPAM-specific immunoglobulin G (IgG) increase with parity and are associated with protection against placental parasitemia, LBW, and anemia (6, 23, 24). Together, these findings can explain the sudden reappearance of susceptibility to P. falciparum malaria during the first pregnancy of hitherto clinically immune women and the pronounced reduction in the incidence of PAM with increasing parity (7, 24).
Primigravidae who receive intermittent preventive treatment (IPTp) with sulfadoxine-pyrimethamine are significantly protected from anemia and LBW (19, 21). The effect of IPTp on the development of naturally acquired protective immunity to PAM has not been investigated previously, and thus that was the aim of our study.
MATERIALS AND METHODS
Plasma samples and study population.
We studied third-trimester plasma samples from 281 human immunodeficiency virus-negative primigravidae who had received one (n = 49), two (n = 51), or three (n = 50) doses of sulfadoxine-pyrimethamine or one (n = 39), two (n = 49), or three (n = 43) doses of a placebo as part of a randomized, placebo-controlled IPTp trial (21). The number of doses that women were randomized to receive was related to gestational age at first antenatal attendance (16 to 23 weeks, three doses; 24 to 27 weeks: two doses; 28 to 32 weeks, one dose). The samples studied constituted random subsets of each of the six treatment groups in the original, larger cohort (21), apart from limitations imposed by the need for simultaneous assay and plasma sample availability. The characteristics of the selected donors did not differ significantly from those of the original study cohort (not shown). Samples from eight third-trimester pregnant women without malaria exposure were included as negative controls. Informed consent was obtained from all plasma donors, and ethical clearance was granted by the Kenyan Medical Research Institute-National Ethical Review Committee and by the London School of Hygiene and Tropical Medicine.
Parasites and in vitro selection for adhesion to CSA.
We used three P. falciparum isolates cultured in vitro by standard methodology (23). One (EJ24) was obtained at delivery from the placenta of a woman with PAM. The isolation, adhesion properties, etc., of EJ24 are described in detail elsewhere (20). The second isolate (Busua) was obtained from the peripheral blood of a nonimmune man and was studied with (Busua-CSA) or without (Busua) preceding adhesion selection by repeated panning on CSA in vitro as described in detail elsewhere (17, 23). The third (FCR3, a long-term in vitro culture isolate) (17) was also studied with (FCR3-CSA) or without (FCR3) in vitro selection for CSA adhesion. EJ24, Busua-CSA, and FCR3-CSA all showed the gender-specific and parity-dependent plasma IgG recognition characteristic of VSA expressed by PAM-related parasite isolates (17, 23), whereas unselected Busua and FCR3 did not. The genotypic identities of all of the isolates were regularly confirmed by genotypic profiling at the polymorphic msp1, msp2, and glurp loci (17).
Measurement of variant-specific IgG antibodies.
We used flow cytometry (FCM) to measure levels of VSA-specific IgG in plasma (22). In brief, parasite cultures were enriched for late trophozoite and schizont IE by exposure to a strong magnetic field (22), labeled with ethidium bromide (to identify IE in FCM data analysis), and sequentially exposed to plasma, secondary goat anti-human IgG (Dako, Glostrup, Denmark), and tertiary fluorescein isothiocyanate -conjugated rabbit anti-goat Ig (Dako). FCM data from 10,000 IE were acquired with a Coulter EPICS XL-MCL instrument (Coulter Electronics, Luton, United Kingdom). For each sample, the mean fluorescence index (MFI) was recorded as a measure of VSA-specific IgG reactivity (22). Samples whose forward-scatter and ethidium bromide properties revealed technical problems were excluded from the analyses.
Statistical analysis.
When all of the samples were viewed together, the distribution of VSA-specific IgG levels departed significantly from normality in several cases, particularly with respect to IgG with specificity for PAM-type VSA (24). The statistical significance of differences between IgG levels in exposed and unexposed women was therefore evaluated by Mann-Whitney rank sum test, while dose dependencies were evaluated by Kruskal-Wallis one-way analysis of variance on ranks. Medians, differences between medians, and the associated 95% confidence intervals were calculated as described by Altman et al. (1). SigmaStat version 3.0 (SPSS, Chicago, Ill.) and CIA version 2.0 software (1) were used throughout.
RESULTS AND DISCUSSION
Protective immunity to P. falciparum malaria is acquired following prolonged exposure in areas of endemic parasite transmission. It is accompanied by increases in plasma levels of IgG with specificity for many P. falciparum antigens including parasite-encoded VSA expressed on the surface of IE (Table 1). Naturally acquired protection appears to be mediated mainly by parasite-specific IgG (5, 15), and several studies have pointed to VSA as important targets (4, 13, 16). The evidence in favor of VSA-specific IgG as mediators of acquired protective immunity is strongest and most direct with respect to PAM, which is a major cause of maternal and perinatal suffering in areas of intense parasite transmission (6, 24).
TABLE 1.
Median levels of VSA-specific IgG in P. falciparum-exposed Kenyan third-trimester primigravidae and in pregnant women without malaria exposure
| Parasite line | No. of MFI units (95% confidence interval)
|
Pa | ||
|---|---|---|---|---|
| Kenyan women (n = 281) | Control women (n = 8) | Difference | ||
| Busua | 66.3 (64.7-68.0) | 48.9 (44.6-54.3) | 16.8 (11.1-22.3) | <0.001 |
| FCR3 | 54.9 (51.9-57.0) | 39.9 (36.3-51.2) | 14.1 (6.6-23.8) | <0.001 |
| Busua-CSA | 65.8 (62.3-69.5) | 56.5 (50.9-63.7) | 9.5 (1.1-26.6) | 0.02 |
| FCR3-CSA | 74.1 (65.9-79.8) | 50.1 (48.2-56.1) | 21.6 (6.4-45.1) | <0.001 |
| EJ24 | 56.4 (55.1-58.2) | 44.5 (41.4-55.2) | 11.5 (6.5-17.5) | <0.001 |
Mann-Whitney rank sum test.
IPTp of pregnant women, where women are given antimalarials at specified intervals during pregnancy, effectively protects against the adverse consequences of PAM in malaria-exposed primigravidae (19, 21). Chemoprophylaxis during a first pregnancy can interfere with acquisition of VSAPAM-specific IgG (23) and may therefore compromise acquisition of immunological protection from PAM (6, 24). However, the impact of IPTp on acquisition of PAM immunity is unknown. We therefore compared the plasma levels of VSA-specific IgG among Kenyan primigravidae participating in a randomized, placebo-controlled trial of IPTp (21).
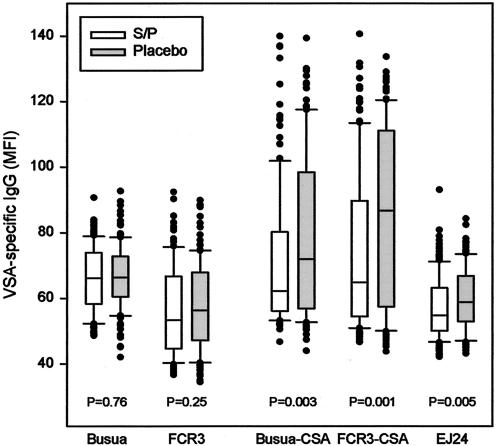
We found that the levels of IgG with specificity for two parasite lines (Busua and FCR3) not expressing the PAM-type VSA implicated in the pathogenesis of PAM were not significantly different between women receiving active drug and those receiving a placebo (Fig. 1). Specifically, the median plasma levels of IgG with specificity for the VSA expressed by the Busua line were essentially the same in women who received sulfadoxine-pyrimethamine as in women who received a placebo (level in placebo group minus level in active drug group, −0.4 [95% confidence interval, −2.8 to 2.0] MFI unit). Similarly, the median plasma level of IgG with specificity for the VSA expressed by the FCR3 line was only marginally lower among women who received sulfadoxine-pyrimethamine than among women who received a placebo (1.8 [95% confidence interval, −5.0 to 1.4] MFI units). We did not detect any statistically significant relationships between the number of active or placebo drug doses received and VSA-specific IgG levels for either of the parasite lines expressing non-PAM-type VSA (Table 2). These results indicate that IPTp has no significant impact on plasma levels of IgG with specificity for VSA not related to PAM. This interpretation is in line with our earlier finding that chemoprophylaxis does not significantly affect the levels of IgG with specificity for non-PAM-type VSA in Cameroonian primigravidae (23).
FIG. 1.
Levels of VSA-specific IgG in the plasma of Kenyan primigravidae receiving (open boxes) or not receiving (shaded boxes) intermittent preventive sulfadoxine-pyrimethamine (S/P) treatment. For each VSA specificity, the center line indicates the median, the box indicates the central 50% of the data points, the bars indicate the central 90% of the data points, and outlying data points are shown individually. The statistical significance of differences in VSA-specific IgG levels between women receiving active drug and placebo is given at the bottom.
TABLE 2.
Relationship between median VSA-specific IgG levels and doses of intermittent preventive sulfadoxine-pyrimethamine treatment or placebo received by Kenyan third-trimester primigravidae
| Parasite line and drug(s) | No. of MFI units (95% confidence interval)
|
Pa | ||
|---|---|---|---|---|
| 1 dose | 2 doses | 3 doses | ||
| Busua | ||||
| S/Pb | 66.9 (63.3-71.9) | 64.3 (60.8-70.1) | 65.8 (62.8-69.3) | 0.80 |
| Placebo | 66.0 (62.3-70.4) | 68.4 (62.9-70.9) | 65.9 (63.4-69.5) | 0.93 |
| FCR3 | ||||
| S/P | 54.1 (47.4-59.6) | 54.8 (48.9-61.5) | 50.8 (48.0-58.7) | 0.97 |
| Placebo | 56.1 (48.4-59.2) | 59.7 (52.5-67.0) | 54.7 (51.2-62.6) | 0.45 |
| Busua-CSA | ||||
| S/P | 73.1 (64.8-80.5) | 61.9 (58.4-69.1) | 58.3 (56.5-61.0) | 0.005 |
| Placebo | 68.8 (60.0-92.9) | 67.6 (59.2-81.3) | 84.1 (72.4-96.6) | 0.10 |
| FCR3-CSA | ||||
| S/P | 75.5 (63.5-92.6) | 72.1 (54.4-90.6) | 56.8 (54.8-61.3) | 0.001 |
| Placebo | 93.8 (62.9-109.4) | 79.8 (61.3-90.1) | 96.5 (76.5-105.5) | 0.57 |
| EJ24 | ||||
| S/P | 57.1 (53.5-61.1) | 55.4 (52.4-60.1) | 53.2 (50.3-55.6) | 0.06 |
| Placebo | 58.0 (54.0-61.4) | 58.3 (55.4-60.5) | 61.0 (55.1-63.7) | 0.68 |
Kruskal-Wallis one-way analysis of variance on ranks.
S/P, sulfadoxine-pyrimethamine.
In marked contrast, plasma levels of IgG with specificity for each of three VSAPAM-expressing isolates (Busua-CSA, FCR3-CSA, EJ24) were markedly and significantly lower in the women who received active drug (median difference for Busua-CSA, 6.4 [95% confidence interval, 1.8 to 11.9] MFI units; median difference for FCR3-CSA, 10.6 [95% confidence interval, 3.7 to 19.0] MFI units; median difference for EJ24, 3.2 [95% confidence interval, 1.0 to 5.3] MFI units) (Fig. 1). In line with this, we have previously shown that chemoprophylaxis can significantly affect levels of VSAPAM-specific IgG in Cameroonian primigravidae without affecting levels of IgG with other VSA specificities (23). Levels of IgG with specificity for the VSA expressed by each of the three VSAPAM-expressing isolates were significantly and inversely related to the number of sulfadoxine-pyrimethamine doses, whereas the number of placebo doses had no effect (Table 2). Titration analysis indicated that the median level of IgG specific for each of the VSAPAM-expressing isolates was largely unaffected in women who received only one sulfadoxine-pyrimethamine dose but was reduced two- to eightfold in women receiving multiple doses (data not shown). For the Busua-CSA isolate, the median level of VSA-specific IgG in women who had received three sulfadoxine-pyrimethamine doses were not significantly different from levels in unexposed control women (difference, 2.3 [95% confidence interval, −1.4 to 7.0] MFI units; P [Mann-Whitney rank sum test] = 0.27). We conclude that multidose IPTp adversely affects acquisition of VSAPAM-specific IgG in primigravidae, leading to significantly lowered plasma levels of VSAPAM-specific IgG at term. This is an issue of potential clinical concern, as there is strong evidence in favor of a causal relationship between VSAPAM-specific IgG levels and protection from untoward consequences of PAM (6, 24). We acknowledge that the timing of IPTp treatments may have contributed to the dose dependency observed here, as women who received three doses received their first dose earlier than two-dose women, who in turn received their first dose earlier than single-dose women. Studies designed specifically to determine the optimal timing and number of IPTp doses are necessary to resolve this issue.
The impact of chemoprophylaxis during a first pregnancy on a second-pregnancy outcome has previously been studied (9). The authors of that study concluded that restriction of chemoprophylaxis to primigravidae does not appear to put recipients at increased risk in subsequent pregnancies. However, many of the women in their study were recruited and given chemoprophylaxis only in the third trimester, making it probable that some already had PAM and had acquired VSAPAM-specific immunity at the time of recruitment. Our data on women receiving only one dose of sulfadoxine-pyrimethamine support this possibility. Secondly, endemicity in Gambia is considerably lower than in our study area, making it likely that a proportion of the Gambian women who received a placebo remained uninfected during their first pregnancy and thus did not acquire VSAPAM-specific immunity. Both factors tend to obscure a protective effect of VSAPAM-specific IgG against maternal anemia and LBW.
In conclusion, our data suggest that multidose IPTp interferes with acquisition of protective immunity to PAM in P. falciparum-exposed primigravidae. Once given, it may therefore be necessary to repeat IPTp in all subsequent pregnancies. This conclusion is in agreement with the most recent World Health Organization recommendations that women of all parities in malaria-endemic areas should be offered IPTp.
Acknowledgments
We are indebted to all of the individuals who donated parasite and plasma samples for this study. We thank all of the KEMRI staff involved in the clinical studies in Kenya. This paper is published with the permission of the Director of KEMRI. Kirsten Pihl, Anne Corfitz, and Maiken Christensen are thanked for excellent technical assistance in the laboratory.
This work received financial support from the Commission of European Union (QLK2-CT-2001-01302, PAMVAC).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Altman, D. G., D. Machin, T. N. Bryant, and M. J. Gardner. 2000. Statistics with confidence. British Medical Journal, London, United Kingdom.
- 2.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gammaglobulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 6.Duffy, P. E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71:6620-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 8.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood, A. M., C. Menendez, P. L. Alonso, S. Jaffar, P. Langerock, S. Lulat, J. Todd, B. M'Boge, N. Francis, and B. M. Greenwood. 1994. Can malaria chemoprophylaxis be restricted to first pregnancies? Trans. R. Soc. Trop. Med Hyg. 88:681-682. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt, H. L., and R. W. Snow. 2001. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 95:569-576. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt, H. L., and R. W. Snow. 2001. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 64:36-44. [DOI] [PubMed] [Google Scholar]
- 12.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 13.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 14.Maubert, B., N. Fievet, G. Tami, C. Boudin, and P. Deloron. 2000. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol. 22:191-199. [DOI] [PubMed] [Google Scholar]
- 15.McGregor, I. A., S. P. Carrington, and S. Cohen. 1963. Treatment of East African P. falciparum malaria with West African human γ-globulin. Trans. R. Soc. Trop. Med. Hyg. 57:170-175. [Google Scholar]
- 16.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. L. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 18.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogerson, S. J., E. Chaluluka, M. Kanjala, P. Mkundika, C. Mhango, and M. E. Molyneux. 2000. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997-99. Trans. R. Soc. Trop. Med. Hyg. 94:549-553. [DOI] [PubMed] [Google Scholar]
- 20.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 21.Shulman, C. E., E. K. Dorman, F. Cutts, K. Kawuondo, J. N. Bulmer, N. Peshu, and K. Marsh. 1999. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet 353:632-636. [DOI] [PubMed] [Google Scholar]
- 22.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 23.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 24.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against the clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363:283-289. [DOI] [PubMed] [Google Scholar]
- 25.Yamada, M., R. Steketee, C. Abramowsky, M. Kida, J. Wirima, D. Heymann, J. Rabbege, J. Breman, and M. Aikawa. 1989. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am. J. Trop. Med. Hyg. 41:161-168. [DOI] [PubMed] [Google Scholar]