Abstract
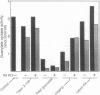
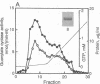
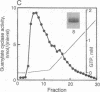
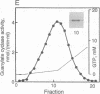
Guanylate cyclase from rod photoreceptors of amphibian (toad, Bufo marinus, and frog, Rana catesbeiana) and bovine retinas was solubilized and purified by a single chromatography step on a GTP-agarose column. Silver staining of purified amphibian enzymes in SDS/polyacrylamide gels disclosed a doublet band (110 and 115 kDa), while the bovine enzyme appeared as a singlet band (110 kDa). The identification of these guanylate cyclases was confirmed using three chromatography systems with the purified enzymes. Specific binding to Con A-Sepharose suggested that rod guanylate cyclase is a glycoprotein. Two-dimensional gel electrophoresis of purified toad, frog, and bovine enzymes resolved two, three, and five variants, respectively, that differed in isoelectric point. Two variants of toad guanylate cyclase showed differences in various characterizations. These data suggest multiple mechanisms for regulation of guanylate cyclase activity in vertebrate rod photoreceptors.
Full text
PDF
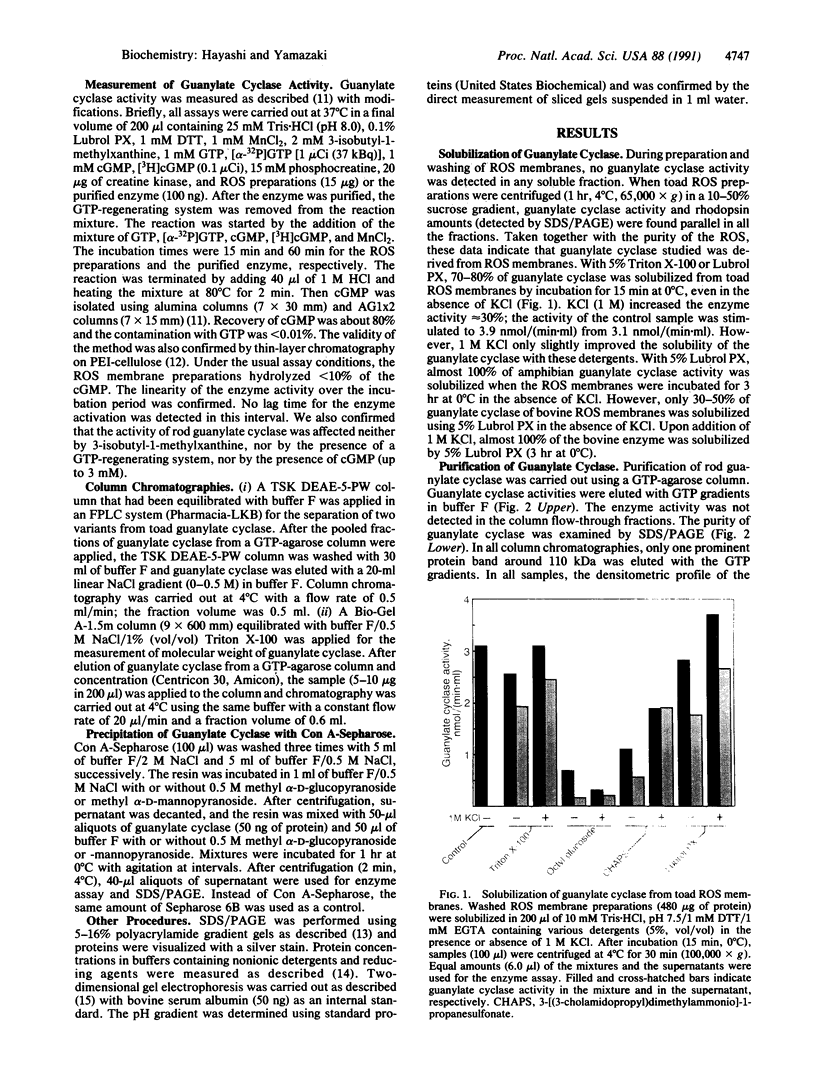
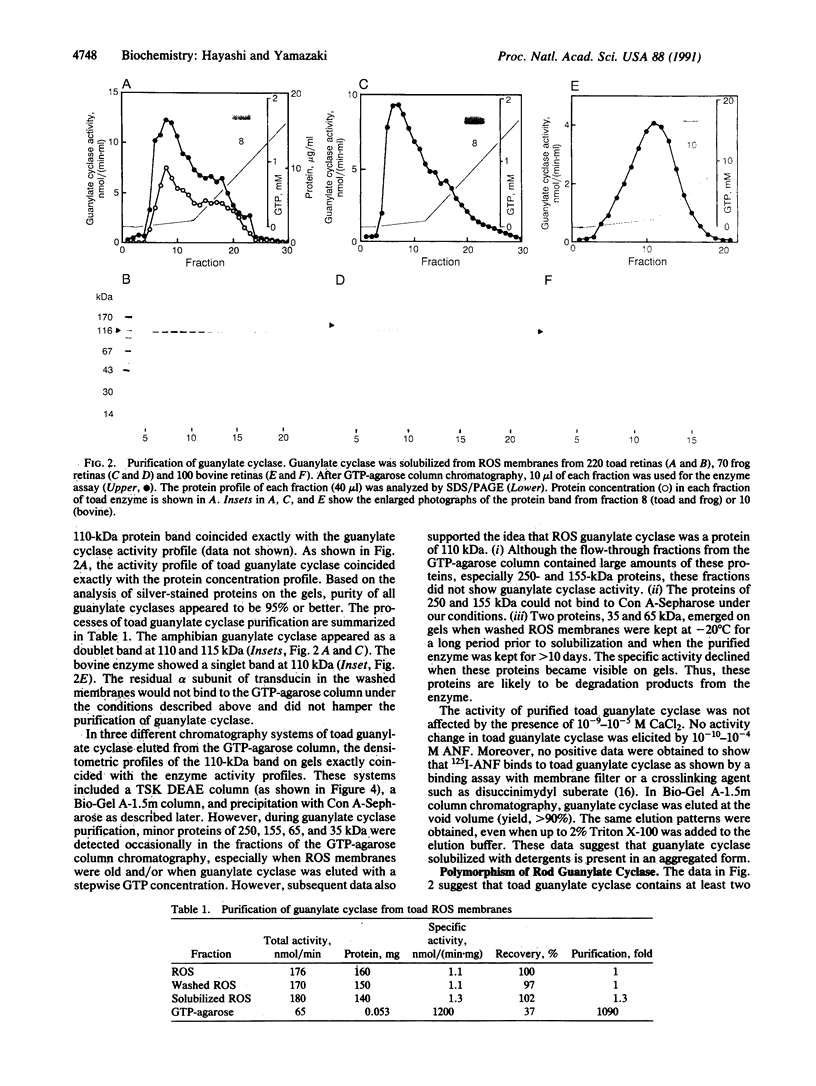
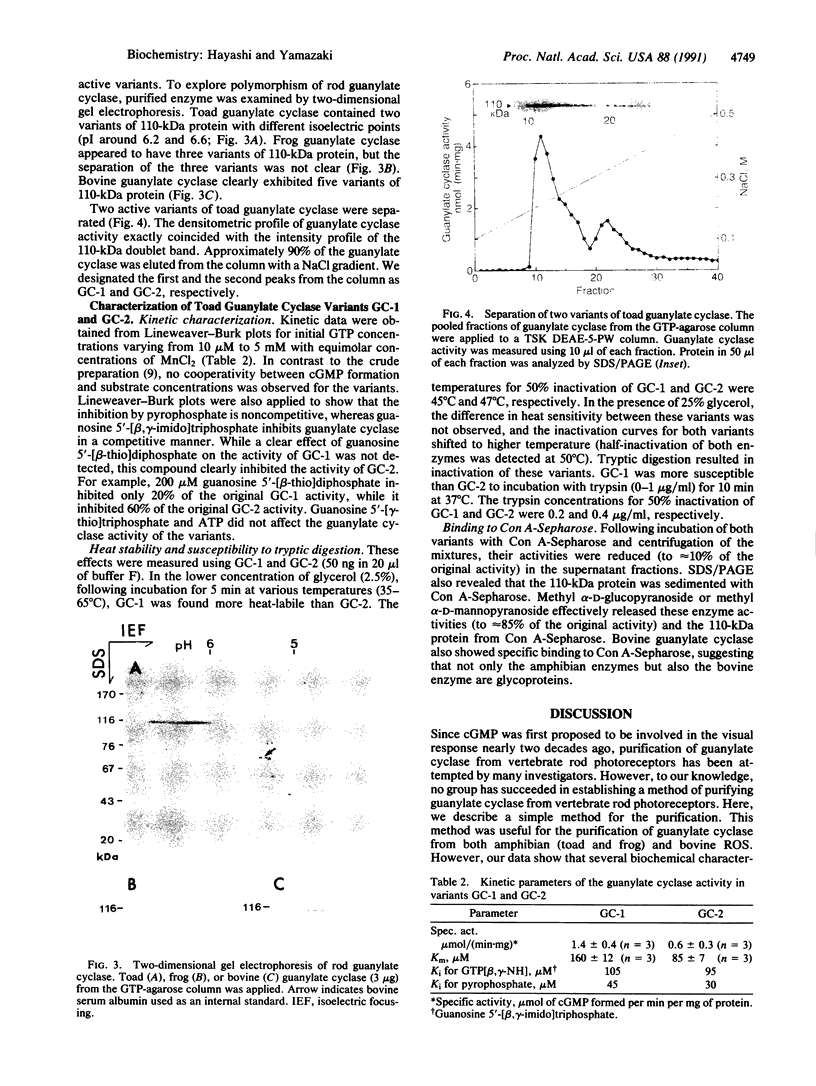

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Walseth T. F., Heyman R. A., Barad M., Graeff R. M., Goldberg N. D. Light-induced increases in cGMP metabolic flux correspond with electrical responses of photoreceptors. J Biol Chem. 1986 Oct 5;261(28):13034–13042. [PubMed] [Google Scholar]
- Brown R. E., Jarvis K. L., Hyland K. J. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989 Jul;180(1):136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Fleischman D., Denisevich M. Guanylate cyclase of isolated bovine retinal rod axonemes. Biochemistry. 1979 Nov 13;18(23):5060–5066. doi: 10.1021/bi00590a006. [DOI] [PubMed] [Google Scholar]
- Gonzales L. W., Geel S. E. Thin-layer chromatography of brain adenine nucleoside and nucleotides and determination of ATP specific activity. Anal Biochem. 1975 Feb;63(2):400–413. doi: 10.1016/0003-2697(75)90362-0. [DOI] [PubMed] [Google Scholar]
- Hakki S., Sitaramayya A. Guanylate cyclase from bovine rod outer segments: solubilization, partial purification, and regulation by inorganic pyrophosphate. Biochemistry. 1990 Jan 30;29(4):1088–1094. doi: 10.1021/bi00456a035. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kamisaki Y., Saheki S., Nakane M., Palmieri J. A., Kuno T., Chang B. Y., Waldman S. A., Murad F. Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem. 1986 Jun 5;261(16):7236–7241. [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Regulation of cGMP levels by guanylate cyclase in truncated frog rod outer segments. J Gen Physiol. 1989 Oct;94(4):649–668. doi: 10.1085/jgp.94.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Kondo H., Miller W. H. Rod light adaptation may be mediated by acceleration of the phosphodiesterase-guanylate cyclase cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1322–1326. doi: 10.1073/pnas.85.4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., Leitman D. C., Nakane M., Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986 May 5;261(13):5817–5823. [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Sano M., Saito T. Subcellular distribution and properties of guanylate cyclase in rat cerebellum. Biochim Biophys Acta. 1976 Sep 24;444(2):563–570. doi: 10.1016/0304-4165(76)90400-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paul A. K., Marala R. B., Jaiswal R. K., Sharma R. K. Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science. 1987 Mar 6;235(4793):1224–1226. doi: 10.1126/science.2881352. [DOI] [PubMed] [Google Scholar]
- Pepe I. M., Panfoli I., Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+. FEBS Lett. 1986 Jul 14;203(1):73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Cote R. H. Characterization of guanylate cyclase in squid photoreceptors. Vis Neurosci. 1989 Jul;3(1):1–7. doi: 10.1017/s0952523800012451. [DOI] [PubMed] [Google Scholar]
- Yamazaki A., Tatsumi M., Bitensky M. W. Purification of rod outer segment GTP-binding protein subunits and cGMP phosphodiesterase by single-step column chromatography. Methods Enzymol. 1988;159:702–710. doi: 10.1016/0076-6879(88)59065-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki A., Tatsumi M., Torney D. C., Bitensky M. W. The GTP-binding protein of rod outer segments. I. Role of each subunit in the GTP hydrolytic cycle. J Biol Chem. 1987 Jul 5;262(19):9316–9323. [PubMed] [Google Scholar]