Abstract
Polyclonal B-cell activation and hypergammaglobulinemia are prominent features of human malaria. We report here that Plasmodium falciparum-infected erythrocytes directly adhere to and activate peripheral blood B cells from nonimmune donors. The infected erythrocytes employ the cysteine-rich interdomain region 1α (CIDR1α) of P. falciparum erythrocyte membrane protein 1 (PfEMP1) to interact with the B cells. Stimulation with recombinant CIDR1α induces proliferation, an increase in B-cell size, expression of activation molecules, and secretion of immunoglobulins (immunoglobulin M) and cytokines (tumor necrosis factor alpha and interleukin-6). Furthermore, CIDR1α binds to Fab and Fc fragments of human immunoglobulins and to immunoglobulins purified from the sera of different animal species. This binding pattern is similar to that of the polyclonal B-cell activator Staphylococcus aureus protein A. Our findings shed light on the understanding of the molecular basis of polyclonal B-cell activation during malaria infections. The results suggest that the var gene family encoding PfEMP1 has evolved not only to mediate the sequestration of infected erythrocytes but also to manipulate the immune system to enhance the survival of the parasite.
Parasites that proliferate in restricted ecological niches such as Plasmodium spp. control the contact with their hosts in order to colonize, divide, and transmit themselves. Chronic infections with Plasmodium falciparum lead to a severely dysregulated immune system, and B cells are overactivated with the subsequent secretion of an array of different autoantibodies (2, 8), the presence of hyperglobulinemia (1), and the frequent occurrence of B-cell tumors (Burkitt's lymphoma) (17). B-cell activation has been reported in studies involving the stimulation of total peripheral lymphocytes with P. falciparum-derived products, and it has been suggested to be the result of direct and indirect mechanisms mediated by T lymphocytes and accessory cells (18, 19). However, the identity of the antigens and mechanisms that lead to polyclonal activation in the course of malaria infection are currently unknown.
It has previously been shown that a large proportion (83%) of fresh isolates of P. falciparum-infected erythrocytes (IE) bind nonimmune immunoglobulins (Igs) though to various degrees (25, 26). One of the domains of the P. falciparum erythrocyte membrane protein 1 (PfEMP1), the cysteine-rich interdomain region 1α (CIDR1α) of FCR3S1.2 (amino acids 395 to 700), binds to CD36, PECAM-1/CD31, and nonimmune Igs (4, 5, 26). Microbial Ig binding proteins (IBPs) are produced by protozoa, viruses, parasites and both gram-positive and gram-negative bacteria (31) and play important physiological roles (20). It has been suggested that during an infectious process these IBPs may act as an evasion mechanism to divert specific antibody (Ab) responses (7, 21). The binding of CIDR1α to nonimmune Igs led us to investigate the interaction between human B cells and P. falciparum-IE and the involvement of CIDR1α. The present study identifies the CIDR1α domain of PfEMP1 as one of the molecules that may be involved in the polyclonal B-cell activation that characterizes human malaria infection. CIDR1α directly binds to and activates purified B lymphocytes in vitro, an interaction that is mediated, at least in part, by binding to surface Igs.
MATERIALS AND METHODS
Medium and reagents.
B-cell cultures were maintained in RPMI 1640 (GIBCO-BRL, Gaithersburg, Md.) with 10% fetal calf serum, 100 U of penicillin per ml, and 2 mM glutamine. P. falciparum cells were cultured according to standard procedures in RPMI medium supplemented with 10% human AB+ Rh+ serum. Phycoerythrin (PE)- or fluorescein (FITC)-conjugated monoclonal Abs (MAbs) and enzyme-linked immunosorbent assay (ELISA) kits were purchased from Becton & Dickinson/Pharmingen (Mountain View, Calif.). Mouse anti-human IgG, IgA, and IgM Abs were purchased from DAKO (Copenhagen, Denmark). Anti-glutathione S-transferase (GST) Abs and human IgM were purchased from Sigma. Ig from different species, human Ig classes, and Ig fragments were purchased from Jackson Immunoresearch Laboratories, Inc. (West Grove, Pa.).
Production of recombinant antigens.
The cloning and expression of CIDR1α and Duffy binding-like domain 1α (DBL1α) of the cloned strain FCR3S1.2 were conducted as previously described (5). The CIDR1α-GST fusion protein, referred to as CIDR1α, was expressed and purified according to the instructions of the manufacturer. GST produced by the empty vector was used as a control, referred to as GST.
B cells and cell culture.
Buffy coats from the blood of healthy individuals who had not been exposed to malaria were obtained from the blood bank of the Karolinska Hospital. Mononuclear cells were isolated by centrifugation over Lymphoprep (Nycomed Pharma, Oslo, Norway). B cells were isolated by positive selection by using CD19+ magnetic beads and DETACHaBEAD (Dynal, Oslo, Norway) according to the manufacturer's instructions; B-cell purity varied between 94 and 99%. In some experiments B cells were further separated into IgG- and IgM-enriched populations by depleting the IgM- and IgA-positive and the IgG- and IgA-positive cells, respectively, by using M-450 rat anti-mouse coated Dynabeads. The recovered negative fraction usually contained ≤3% of the cells that had formed rosettes. Purified B cells were cultured in round-bottomed 96-well plates (5 × 104 cells/well) in a final volume of 200 μl or in 48-well plates (5 × 105 cells/well) in a final volume of 500 μl and incubated at 37°C in 5% CO2. Cultures were seeded in triplicates (96-well) when the proliferation of cells was studied and in duplicates (48-well) when phenotype, IgM, or cytokine production was analyzed. Unless otherwise specified, the final concentration of GST and CIDR1α was 50 μg/ml each. All experiments were carried out at least three times.
Binding assays.
B cells or IgM-positive B-cell lines were incubated for 1 h at room temperature (RT) in medium containing GST, CIDR1α, or DBL1α at concentrations ranging from 0 to 200 μg/ml. After two washes in phosphate-buffered saline, the cells were incubated with mouse anti-GST Ab for 30 min at 4°C. After two washes, anti-mouse biotinylated Ab was added for 30 min at 4°C, followed by PE-conjugated streptavidin. In binding competition assays, CIDR1α was preincubated with various concentrations of IgG or IgM for 1 h at RT before being added to the B cells (final concentration of CIDR1α, 50 μg/ml). The binding was analyzed by using FACSort. B-cell-erythrocyte binding analysis was carried out as follows: B cells were stained with 1 μg of acridine orange per ml; Percoll-enriched infected (11) and noninfected erythrocytes were stained for 15 min at RT with 0.1 μg of ethidium bromide per ml. Cell mixtures with a final erythrocyte-to-B-cell ratio of 5:1 were seeded into 96-well plates and incubated at 37°C for 1 h; thereafter, the binding was visualized by phase-contrast microscopy and scored in fluorescence light microscopy. In competition studies, B cells were incubated for 1 h with different concentrations of recombinant CIDR1α or GST prior to incubation with IE.
Proliferation assays.
Cultures stimulated with CIDR1α or GST were incubated for 72 h and pulsed with 1 μCi of [3H]thymidine (Amersham, Little Chalfont, United Kingdom) during the last 12 h of the incubation period. As a positive control, phorbol myristate acetate (PMA; Sigma) and ionomycin (Calbiochem) were used at concentrations of 5 ng/ml and 500 ng/ml, respectively. Cells were harvested onto a fiberglass filter, and the [3H]thymidine incorporation was determined by liquid scintillation counting. Results are expressed as mean counts per minute of triplicate samples or as a reactivity index (RI), calculated as follows: RI = counts per minute in stimulated cultures/counts per minute in control cultures. The standard deviations (SD) of the triplicates were generally in the range of 10 to 15% of the mean. In some experiments CIDR1α and GST were incubated for 1 h with soluble human IgM prior being added to the B cells. Results are expressed as percent inhibition, calculated as follows: (1 − counts per minute in cultures of GST or CIDR1α preincubated with IgM/counts per minute in cultures of GST or CIDR1α preincubated in medium alone) × 100.
Phenotypic analysis.
B cells were harvested after culture periods varying from 60 to 72 h. FITC- or PE-conjugated MAbs were added to the cell pellets at saturating concentrations, and the cells were then incubated for 30 min at 4°C. PE- and FITC-conjugated isotype-matched MAbs with irrelevant specificities were used as negative controls. Fluorescence intensity was measured with a FACSort flow cytometer and analyzed by using Cell Quest software (Becton Dickinson).
Cytokines and IgM determination.
Supernatants were collected at 24 h (tumor necrosis factor alpha [TNF-α] determination), 48 to 72 h (interleukin-6 [IL-6] and IL-10 determinations), and 10 days (IgM content) after B-cell activation. Cytokines and IgM content were determined by using commercial specific ELISAs, according to the manufacturer's instructions. The sensitivities of the ELISAs were 10 pg/ml, 5 pg/ml, and 8 pg/ml for IL-10, IL-6, and TNF-α, respectively.
Mapping of the Ig binding of CIDR1α.
ELISA plates were coated overnight at 4°C with NaHCO3 buffer containing 5 μg of the Igs or Ig fragments per ml. After blocking for 2 h at RT, GST control or CIDR1α was added in a series of double dilutions at concentrations from 3 to 100 μg/ml. Binding was detected by using an anti-GST Ab and a secondary anti-mouse MAb coupled to alkaline phosphatase. Binding to GST, bovine serum albumin, and phosphate-buffered saline served as internal controls. The absorption was determined from the optical density at 405 nm.
RESULTS
The CIDR1α domain of PfEMP1 is involved in the interaction between B cells and IE. When B lymphocytes from malaria nonimmune donors were cultured together with IE of the PfEMP1-expressing cloned strain FCR3S1.2 (11) at an IE/B-cell ratio of 5:1, large clumps of cells having as many as 30 to 50 IE and B cells were frequently observed (Fig. 1A). In contrast, IE of the sister clone FCR3S1.6 which lacks PfEMP1 (11) did not adhere to B cells (Fig. 1B). No physical interaction was observed between B cells and uninfected erythrocytes (Fig. 1C).
FIG. 1.
IE and CIDR1α bind to B cells. Ethidium bromide-stained P. falciparum-IE (red and yellow) of the FCR3S1.2 subclone (A) or the FCR3S1.6 subclone (B) coincubated with acridine orange-stained B cells (green) at a red blood cell-to-B-cell ratio of 5 to 1 (UV light image). (C) Uninfected erythrocytes coincubated with acridine orange-stained B cells at a red blood cell-to-B-cell ratio of 5 to 1 (UV light image). (D) Fluorescence-activated cell sorter analysis of purified B cells incubated in medium control (black) or medium containing either GST (red), DBL1α (green), or CIDR1α (blue) (50 μg/ml) and stained with biotinylated anti-GST Ab and PE-conjugated streptavidin. (E) Inhibition of CIDR1α binding to B cells with increasing concentrations of soluble IgG (upper panel) and IgM (lower panel). Binding of GST (gray), CIDR1α (black), and CIDR1α preincubated with IgG or IgM at a concentration of 1.5 or 1 mg/ml, respectively (green); with IgM at a concentration of 0.2 mg/ml (red); and with IgG or IgM at a concentration of 0.3 or 0.04 mg/ml, respectively (blue). Results are representative of at least three independent experiments. For further details, see Materials and Methods. (F) Inhibition of IE-B-cell binding by soluble CIDR1α. Values represent percent inhibition of the IE-B-cell binding by preincubation of B cells with CIDR1α in relation to the IE/B-cell ratio of 5:1 in the absence of CIDR1α. Shown are the means ± SD of three independent experiments.
As CIDR1α has been shown to bind to IgM (5), we assessed the binding of recombinant CIDR1α of FCR3S1.2 to freshly isolated B cells or to surface IgM-positive B-cell lines (Daudi and Bjab) by fluorescence-activated cell sorter analysis using FITC-conjugated secondary Abs. B lymphocytes bound CIDR1α, as reflected by a shift in the fluorescence intensity when compared to B cells incubated in medium alone or with the GST control antigen. The binding of the recombinant N-terminal DBL1α, a domain known to lack Ig binding capacity (5), was identical to that of the GST control antigen (Fig. 1D). The binding was also confirmed by indirect fluorescence microscopy (data not shown). The incubation of Daudi cells with increasing concentrations of CIDR1α led to a fluorescence shift proportional to the concentration used (data not shown). Given the binding of CIDR1α to B lymphocytes, we determined its participation in the interaction between IE and B cells. To this end, we performed competition experiments in which B cells were preincubated with increasing concentrations of recombinant CIDR1α before exposure to IE. CIDR1α but not GST competed away in a dose-dependent manner the IE-B-cell binding at concentrations ranging from 0 to 200 μg/ml (Fig. 1E). These results demonstrate the involvement of the CIDR1α domain of PfEMP1 in the IE-B-cell interaction.
We next analyzed the functional consequences of the IE-B-cell interaction. Coincubation of highly purified synchronized late-stage IE of FCR3S1.2 with B cells from healthy donors (not previously exposed to malaria) induced proliferation (Fig. 2A) and TNF-α production (Fig. 2B). This B-cell response was specific and dose dependent; uninfected erythrocytes did not activate the cells, and the response correlated with the IE/B-cell ratio. In accordance with the observed lack of B-cell binding, IE of the FCR3S1.6 clone not expressing PfEMP1 did not induce B-cell proliferation or TNF-α production (data not shown).
FIG. 2.
CIDR1α and IE induce B-cell proliferation. Proliferative (A) and TNF-α (B) production response of B cells to increasing numbers of uninfected or P. falciparum-IE. (C) Proliferative response of B cells to increasing concentrations of purified GST and CIDR1α. Values represent mean counts per minute ± SD of a representative experiment. (Inset) Summary of the proliferative response of B cells to 50 μg of GST or CIDR1α per ml. Values represent mean ± standard error of the mean of the RI in 26 independent experiments. (D) Comparison of the proliferative response of B cells to recombinant CIDR1α and DBL1α. Results are representative of three experiments; the SD bars (≤15%) have been omitted. (E) Proliferation of total B cells in response to GST or CIDR1α (50 μg/ml) previously incubated with increasing concentrations of soluble IgM. Values are expressed as percent inhibition of the proliferation of B cells in response to GST or CIDR1α preincubated in medium alone. (F) Comparative proliferative response of total, IgG-positive, and IgM-positive B cells to 50 μg of GST or CIDR1α per ml. Results are representative of five independent experiments. RBC, red blood cells.
Recombinant CIDR1α induces B-cell proliferation and activation.
The stimulatory capacity of recombinant CIDR1α on purified B cells was subsequently studied. Recombinant CIDR1α induced B-cell proliferation in a dose-dependent manner at concentrations varying from 0.5 to 200 μg of CIDR1α per ml and at time points between 48 and 96 h. Figure 2C shows a representative dose titration experiment where optimal B-cell proliferation was seen at concentrations between 33 and 100 μg of CIDR1α per ml. On the basis of these results, a concentration of 50 μg/ml and a time point of 72 h of stimulation were used in further experiments. Results of 26 independent experiments performed with B cells from healthy donors who had not been exposed to malaria (n = 26) are summarized in Fig. 2C (inset). Cells stimulated with CIDR1α proliferated 2.5 times above control levels (RI of 2.5 ± 0.2); in contrast, the control antigen GST only induced minimal proliferation of a magnitude comparable to that occurring in medium alone (RI of 1.2 ± 0.1). CIDR1α without the fusion partner (GST) induced a response of the same magnitude as with the whole fusion protein (data not shown). Conversely, no proliferation was observed in response to the other PfEMP1 domain, the GST recombinant DBL1α domain (Fig. 2D). The response to the positive control PMA-ionomycin was vigorous (RI of 80 ± 25) (data not shown).
To investigate the participation of IgM and IgG in the CIDR1α-B-cell interaction, we performed binding competition experiments in which CIDR1α was incubated with soluble IgM or IgG before being added to the B cells. The results presented in Fig. 1F reveal that soluble IgM and IgG compete out in a dose-dependent manner the binding of CIDR1α to B cells. The proliferation induced by CIDR1α is related to its Ig binding capacity. Accordingly, the proliferation induced was also inhibited by preincubation of CIDR1α with soluble IgM at concentrations ranging from 0.125 to 0.5 μg/ml (Fig. 2E). These concentrations of IgM did not affect the proliferation of B cells activated with PMA-ionomycin (data not shown). We further compared the response of different subpopulations of B cells separated on the basis of IgG and IgM surface expression. Both IgG- and IgM-enriched populations responded to CIDR1α, though the background responsiveness was higher among the IgM-enriched B cells (Fig. 2F). These results show that the CIDR1α domain of PfEMP1 induces the proliferation of B lymphocytes and that the CIDR1α-B-cell interaction is mediated, at least in part, through the binding to surface Ig.
The activation of B cells leads to the up-regulation of HLA-DR, adhesion molecules, costimulatory molecules, and other B-cell activation-specific antigens (30). CIDR1α induced a moderate but consistent up-regulation in the expression of HLA-DR, CD23, CD40, CD54, CD58, CD80, and CD86 (Fig. 3A). There were no significant changes in the levels of expression of other markers including surface Ig, CD10, CD11a, CD21, CD69, and CD95 (data not shown). Consistent with this activation, there was also an increase in the size of the CIDR1α-stimulated B cells, reflected by an increase in the forward side scatter (Fig. 3A). Therefore, CIDR1α induces not only proliferation but also the up-regulation of a series of activation antigens on normal B cells.
FIG. 3.
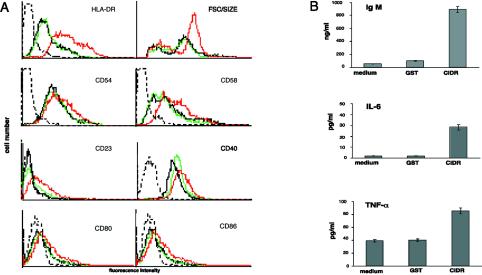
Stimulation with recombinant CIDR1α activates B cells. (A) CIDR1α induces the expression of B-cell activation markers and increased B-cell size. B cells incubated in medium alone (black) and in medium containing 50 μg of either GST (green) or CIDR1α (red) per ml for 72 h were stained with FITC- or PE-conjugated MAbs and analyzed by flow cytometry. Dotted-black-line histograms represent control staining with irrelevant Ab. Results are representative of at least 10 independent experiments. (B) CIDR1α stimulates B cells to produce IgM, IL-6, and TNF-α. Supernatants of B cells cultured in medium alone and in medium containing 50 μg of either GST or CIDR1α per ml were analyzed for the content of TNF-α (24 h), IL-6 (72 h), or IgM (10 days) by specific ELISA. For further details, see Materials and Methods. FSC, forward scatter.
The serum of individuals exposed to P. falciparum malaria is known to contain high levels of Ig and a repertoire of circulating cytokines and autoantibodies that reflect polyclonal B-cell activation (2, 6, 15, 28). Hence, we examined whether CIDR1α could provoke similar B-cell responses. Stimulation with CIDR1α induced a moderate but consistent production of TNF-α and IL-6 compared to levels of the control antigen (Fig. 3B). IL-10 was not detected in any of the supernatants collected either at 24, 48, or 72 h after activation (data not shown). CIDR1α activation also led to an increased production of IgM that was measured 10 days after stimulation (Fig. 3B). Taken together, these results demonstrate that the CIDR1α domain of PfEMP1 induces polyclonal activation in normal peripheral B cells.
The Ig binding pattern of CIDR1α.
Due to the capacity of CIDR1α to bind nonimmune Igs (5) and to activate B cells, we further studied its reactivity with different Igs and Ig fragments. CIDR1α bound equally well to Fab(κ or λ) and to Fc fragments of human Igs as well as to IgG and IgM (Fig. 4A). Furthermore, CIDR1α bound dose-dependently to Igs purified from the sera of different animal species (Fig. 4B). The control antigen (GST) or other similarly produced recombinant domains such as DBL1α and DBL2δ did not bind to any of the Ig or Ig-derived fragments (not shown).
FIG. 4.
Mapping of the Ig binding of CIDR1α by ELISA. (A) Binding of CIDR1α to human Igs and Ig fragments. (B) Binding of CIDR1α to IgG of different species. For further details, see Materials and Methods. OD, optical density.
DISCUSSION
It has previously been shown that IE bind nonimmune Igs and that PfEMP1 can be characterized as a multiadhesive parasite ligand in which the CIDR1α domain mediates binding to several independent host receptors including CD36 and PECAM-1/CD31 and to nonimmune Igs (4, 5). The data presented in this report demonstrate the direct interaction between human B cells and P. falciparum-IE, the involvement of the CIDR1α domain of PfEMP1, and its capacity to activate B cells from nonimmune donors.
The expression of PfEMP1 in the IE was required for the binding to B lymphocytes because IE of the sister clone FCR3S1.6 lacking PfEMP1 (11) did not adhere to or activate B cells. Other IE moieties may also participate in this binding, as suggested by the partial competition (≤50%) mediated by recombinant CIDR1α. Although the competition curve did not reach a plateau, the concentrations of recombinant CIDR1α used (up to 200 μg/ml) may exceed the molar concentration present at the surface of IE.
Recombinant CIDR1α bound to and activated B cells; this interaction is mediated, at least in part, through binding to surface IgM and IgG, as indicated by decreased binding and activation following preincubation of CIDR1α with soluble IgM and IgG. The binding of CIDR1α seems to be specific because the binding of another GST recombinant fusion protein, DBL1α, is identical to that of the GST control. It could be argued that since the recombinant antigen was produced in Escherichia coli, the proliferation observed could be due to contaminating endotoxin. We can rule out this possibility because the expression system and purification steps for the control antigen GST and the recombinant DBL1α domain that did not induce proliferation were the same as those used for CIDR1α. Moreover, the response to CIDR1α was not inhibited by polymyxin B, and in contrast to murine B cells, human B cells do not respond to endotoxin or lipopolysaccharide as they do not express the toll-like receptor 4, an essential component of the lipopolysaccharide receptor signaling complex (22). The relatively low magnitude of the proliferative response to CIDR1α observed in our study seems to be inherent to primary human peripheral B cells, yet splenic B cells from Toll 4−/− mice displayed a robust proliferation in response to this antigen (data not shown).
The capacity of PfEMP1 to stimulate B cells seems to be related to the Ig binding property of its domains. Accordingly, the DBL1α domain that lacks the Ig binding capacity (5) did not bind to or induce B-cell proliferation. CIDR1α bound to Ig of different species and to different portions and subclasses of human Igs; this extensive Ig binding pattern is similar to that of other microbial IBPs such as protein A of Staphylococcus aureus (SpA) (10, 24). Crystallographic work has disclosed that the Ig binding domains in SpA are within areas of the polypeptide carrying α-helices and that the Fab- and Fc-binding sites are structurally separated (14). It should be noted that the IgM binding domain of CIDR1α also harbors potential α-helical structures (amino acids 550 to 700) and similar contact residues as those present in protein A, yet the molecular details of the interaction remain to be explored. Taken together, these facts suggest that CIDR1α binds to B cells via the Fab fragments of Ig expressed at the B-cell surface, much as SpA does.
Malaria-induced polyclonal B-cell activation has been reported in vivo (17, 18) and in in vitro studies involving the stimulation of total peripheral blood lymphocytes (16, 18). The identification of P. falciparum-derived products and of the mechanisms responsible for B-cell activation has remained elusive though direct and indirect mechanisms mediated by T cells and accessory cells have been proposed to explain this phenomenon (9, 12, 16, 19). Here we show that CIDR1α directly activates in vitro purified B cells from nonimmune donors and binds to various Ig fragments and to Igs from different species and, therefore, could be defined as a superantigen. Since the proliferative response to CIDR1α was not high (a 2.5-fold increase compared to control antigen and media), the question arises whether this level of response would result in the polyclonal activation seen in vivo during malaria infections. It should be noted that in the present study the proliferation occurred in the absence of accessory cells and cytokines and was consistently observed in 26 of 26 experiments performed with lymphocytes from donors never exposed to malaria previously. We envisage that in vivo the stimulatory capacity of CIDR1α may be enhanced by a multitude of cofactors: splenic B cells would be exposed to CIDR1α presented not only on the IE but phagocytosed and presented as soluble antigen or as immune complexes by follicular dendritic cells and dendritic cells in the presence of cytokines, T helper cells, and costimulatory signals. Additionally, CIDR1α has been shown to induce CD4 T-cell responses in malaria immune and nonimmune donors (3), a factor that in vivo could amplify by many times the magnitude of the response observed in the present study. It must be noted that in the present study purified T cells did not proliferate in response to CIDR1α (data not shown), suggesting that the activation of CD4 Th cells reported by Allsopp et al. (3) may involve cognate recognition on B cells or other antigen-presenting cells.
The activation of B cells in vivo may occur in compartments with relatively high local concentrations of CIDR1α or as the result of direct binding to IE expressing CIDR1α, in which neighboring adhesive moieties of PfEMP1 may further enhance the IE-B-cell interaction. Moreover, blood-borne antigens (and thus malarial antigens related to the erythrocytic phase) are trapped mainly in the spleen, where B cells represent ∼50% of the splenocytes. The Ig binding properties of CIDR1α may serve to amplify its interaction with B cells, making it a key molecule, but potentially not the only one, involved in malaria-induced polyclonal B-cell activation. The fact that Ig-binding parasites (and therefore intravascular superantigens) are frequent in children in areas where malaria is endemic (26) may explain, at least in part, the prominent hypergammaglobulinemia and polyclonal B-cell activation that characterize human malaria (1, 8, 17).
It has been suggested that during an infectious process IBPs of bacteria, viruses, and now CIDR1α of P. falciparum may divert specific Ab responses (21). P. falciparum, one of the most successful human pathogens, has evolved multiple evasion mechanisms including clonal antigenic variation and diversity (23, 29), T-cell antagonism (13), and hindrance of dendritic cell maturation (27). It is tempting to speculate that the polyclonal B-cell activation described here is another virulence mechanism that contributes to the evasion of efficient host responses. The results may suggest that the large var gene family encoding PfEMP1 has evolved not only to mediate the sequestration of IE but also in order to manipulate the immune system and to allow for the survival of the parasite.
Acknowledgments
This work was supported by grants from The Karolinska Institutet, The Swedish Medical Association, The Swedish International Development Cooperation Agency (Sida/SAREC), The Swedish Cancer Society, The Swedish Research Council, and Barncancerfonden.
We thank B. J. Chambers and Victor Fernández for stimulating discussions.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abele, D. C., J. E. Tobie, G. J. Hill, P. G. Contacos, and C. B. Evans. 1965. Alterations in serum proteins and 19S antibody production during the course of induced malaria infections in man. Am. J. Trop. Med. Hyg. 14:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Adu, D., D. G. Williams, I. A. Quakyi, A. Voller, Y. Anim-Addo, A. A. Bruce-Tagoe, G. D. Johnson, and E. J. Holborow. 1982. Anti-ssDNA and antinuclear antibodies in human malaria. Clin. Exp. Immunol. 49:310-316. [PMC free article] [PubMed] [Google Scholar]
- 3.Allsopp, C. E., L. A. Sanni, L. Reubsaet, F. Ndungu, C. Newbold, T. Mwangi, K. Marsh, and J. Langhorne. 2002. CD4 T cell responses to a variant antigen of the malaria parasite Plasmodium falciparum, erythrocyte membrane protein-1, in individuals living in malaria-endemic areas. J. Infect. Dis. 185:812-819. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Q., A. Barragan, V. Fernandez, A. Sundstrom, M. Schlichtherle, A. Sahlen, J. Carlson, S. Datta, and M. Wahlgren. 1998. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 187:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Q., A. Heddini, A. Barragan, V. Fernandez, S. F. Pearce, and M. Wahlgren. 2000. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 192:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtain, C. C., C. Kidson, D. L. Champness, and J. G. Gorman. 1964. Malaria antibody content of gamma 2-7S globulin in tropical populations. Nature 203:1366-1367. [DOI] [PubMed] [Google Scholar]
- 7.Daniel-Ribeiro, C., J. de Oliveira-Ferreira, D. M. Banic, and B. Galvao-Castro. 1989. Can malaria-associated polyclonal B-lymphocyte activation interfere with the development of anti-sporozoite specific immunity? Trans. R. Soc. Trop. Med. Hyg. 83:289-292. [DOI] [PubMed] [Google Scholar]
- 8.Davies, A. H., H. M. Giles, I. A. McGregor, F. A. Pearson, and J. H. Walter. 1956. Effects of heavy and repeated malaria infections of Gambian infants and children: effect of erythrocytic parasitization. Br. Med. J. 22:686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan, A., M. Waterfall, M. Pinder, A. Holder, and E. Riley. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endresen, C. 1979. The binding of protein A of immunoglobulin G and of Fab and Fc fragments. Acta Pathol. Microbiol. Scand. Sect. C 87:185-189. [PubMed] [Google Scholar]
- 11.Fernandez, V., C. J. Treutiger, G. B. Nash, and M. Wahlgren. 1998. Multiple adhesive phenotypes linked to rosetting binding of erythrocytes in Plasmodium falciparum malaria. Infect. Immun. 66:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garraud, O., A. Diouf, I. Holm, C. M. Nguer, A. Spiegel, R. Perraut, and S. Longacre. 1999. Secretion of parasite-specific immunoglobulin G by purified blood B lymphocytes from immune individuals after in vitro stimulation with recombinant Plasmodium falciparum merozoite surface protein-119 antigen. Immunology 97:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert, S. C., M. Plebanski, S. Gupta, J. Morris, M. Cox, M. Aidoo, D. Kwiatkowski, B. M. Greenwood, H. C. Whittle, and A. V. Hill. 1998. Association of malaria parasite population structure, HLA, and immunological antagonism. Science 279:1173-1177. [DOI] [PubMed] [Google Scholar]
- 14.Graille, M., E. A. Stura, A. L. Corper, B. J. Sutton, M. J. Taussig, J. B. Charbonnier, and G. J. Silverman. 2000. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. USA 97:5399-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood, B. M., E. M. Herrick, and E. J. Holborow. 1970. Speckled antinuclear factor in African sera. Clin. Exp. Immunol. 7:75-83. [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood, B. M., A. J. Oduloju, and T. A. Platts-Mills. 1979. Partial characterization of a malaria mitogen. Trans. R. Soc. Trop. Med. Hyg. 73:178-182. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood, B. M., J. H. Playfair, and G. Torrigiani. 1970. Burkitt lymphoma and malaria. Lancet ii:418. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood, B. M., and R. M. Vick. 1975. Evidence for a malaria mitogen in human malaria. Nature 257:592-594. [DOI] [PubMed] [Google Scholar]
- 19.Kataaha, P. K., C. A. Facer, S. M. Mortazavi-Milani, H. Stierle, and E. J. Holborow. 1984. Stimulation of autoantibody production in normal blood lymphocytes by malaria culture supernatants. Parasite Immunol. 6:481-492. [DOI] [PubMed] [Google Scholar]
- 20.Langone, J. J. 1982. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv. Immunol. 32:157-252. [PubMed] [Google Scholar]
- 21.Leonetti, M., J. Galon, R. Thai, C. Sautes-Fridman, G. Moine, and A. Menez. 1999. Presentation of antigen in immune complexes is boosted by soluble bacterial immunoglobulin binding proteins. J. Exp. Med. 189:1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 23.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romagnani, S., M. G. Giudizi, G. del Prete, E. Maggi, R. Biagiotti, F. Almerigogna, and M. Ricci. 1982. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J. Immunol. 129:596-602. [PubMed] [Google Scholar]
- 25.Scholander, C., C. J. Treutiger, K. Hultenby, and M Wahlgren. 1996. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat. Med. 2:204-208. [DOI] [PubMed] [Google Scholar]
- 26.Scholander, C., J. Carlson, P. G. Kremsner, and M. Wahlgren. 1998. Extensive immunoglobulin binding of Plasmodium falciparum-infected erythrocytes in a group of children with moderate anemia. Infect. Immun. 66:361-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 28.Wahlgren, M., K. Berzins, P. Perlmann, and M. Persson. 1983. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin. Exp. Immunol. 54:135-142. [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlgren, M., V. Fernandez, Q. Chen, S. Svärd, and P. Hagblom. 1999. Waves of malarial var-iations. Cell 96:603-606. [DOI] [PubMed] [Google Scholar]
- 30.White, M. W., F. McConnell, G. L. Shu, D. R. Morris, and E. A. Clark. 1991. Activation of dense human tonsilar B cells. Induction of c-myc gene expression via two distinct signal transduction pathways. J. Immunol. 146:846-853. [PubMed] [Google Scholar]
- 31.Widders, P. R. 1990. Fc receptors and the pathogenesis of bacterial infections in animals, p. 375-396. In M. D. P. Boyle (ed.), Bacterial immunoglobulin binding proteins, vol. 1. Academic Press, Inc., San Diego, Calif. [Google Scholar]