Abstract
Burkholderia cenocepacia is an opportunistic pathogen that can cause severe lung infections in cystic fibrosis patients. To understand the contribution of B. cenocepacia flagella to infection, a strain mutated in the major flagellin subunit, fliCII, was constructed in B. cenocepacia K56-2 and tested in a murine agar bead model of lung infection. C57/BL6 mice infected with ∼108 wild-type K56-2 bacteria exhibited 40% mortality after 3 days, whereas no mortality was noted in mice infected with the fliCII mutant. Among the mice surviving the infection with either strain, there was no significant difference in the bacterial loads in the lungs and spleen, bacteremia, weight loss, or infiltration of immune effector cells at 3 days postinfection. Similar results were observed at 24 h, prior to expression of the lethality phenotype. KC, a murine interleukin-8 (IL-8) homolog, was elevated in both the bronchoalveolar lavage fluid and serum of mice infected with the wild type compared to the fliCII mutant at 24 h, suggesting that flagella stimulated host cells. To demonstrate that flagella contributed to these responses, the interaction between B. cenocepacia and Toll-like receptor 5 (TLR5) was investigated. Infection of HEK293 cells with heat-killed wild-type K56-2, but not infection with the fliCII mutant, resulted in both NF-κB activation and IL-8 secretion that was dependent upon expression of TLR5. Together, these results demonstrate that B. cenocepacia flagella contribute to virulence in an in vivo infection model, and that induction of host immune responses through interaction with TLR5 may contribute to its overall pathogenic potential.
The cystic fibrosis (CF) lung environment is characterized by increased inflammation, which is exacerbated by bacterial lung infections acquired at an early age (31). Pseudomonas aeruginosa is currently the predominant pathogen infecting individuals with CF and is responsible for most of the morbidity and mortality of these patients. However, in the last 3 decades, an increasing number of CF patients have become infected with bacteria of the Burkholderia cepacia complex (Bcc), which has been reported to infect up to 40% of the patients in some CF centers (24, 54).
The Bcc is a collection of genetically distinct, phenotypically similar bacteria that can be grouped into at least nine species (genomovars). Bcc infections have been associated with three major outcomes in CF patients: asymptomatic carriage, chronic infection, and “cepacia syndrome,” characterized by a rapid decline in pulmonary function and, in some cases, bacteremia and septicemia, resulting in early death (18, 54). All members of the Bcc have been isolated from CF patients, but B. cenocepacia (genomovar III) and B. multivorans (genomovar II) predominate, accounting for 95% of the infections overall in countries where prevalence was monitored. Furthermore, B. cenocepacia epidemic strains, including those of the ET12 lineage, have largely contributed to the incidence of disease in this patient population (32).
B. cenocepacia, like other bacterial pathogens, has many virulence factors that may contribute to disease, including flagella. B. cenocepacia bacteria are motile organisms that possess a single, long, polar flagellum responsible for swimming motility. Members of the Bcc express one of two types of flagellin that are distinguished by size (55 kDa for type I and 45 kDa for type II) and restriction fragment length polymorphism (RFLP) patterns of the fliC gene (19, 38).
Flagella are thought to be important in dissemination of bacteria from local infection sites to other organs (13). In addition to their role in motility, flagella are involved in adherence to and invasion of epithelial cells, formation of biofilms, and the induction of host inflammatory responses (1, 14, 37, 41), all of which have been shown to contribute to virulence in mouse models (5, 16, 46). However, to date, there have been no reports of the involvement of Bcc flagella to pathogenesis in a mouse model of infection.
Flagella have recently been appreciated as a major factor contributing to host inflammatory responses to bacteria (14, 16, 29). It is now recognized that this can result from the interaction of bacterial flagellin with Toll-like receptor 5 (TLR5) (21). TLRs are a family of receptors related to the Drosophila Toll receptors that play a role in innate immunity (35, 45). TLRs recognize pathogen-associated molecular patterns, which are conserved motifs unique to microorganisms. Once a ligand is recognized by the TLR, a signaling cascade is initiated, causing activation of nuclear factor κB (NF-κB), as well as members of the mitogen-activated protein (MAP) kinase family. This pathway activation ultimately results in the transcription of a variety of host defense genes, including those for interleukin-8 (IL-8), IL-6, tumor necrosis factor α, and IL-1 (3, 26, 53).
Cytokines play a major role in CF lung disease. Copious quantities of IL-8, in particular, serve to promote tissue damage and decreased pulmonary function, most profoundly through the recruitment of neutrophils (15, 31, 39). Neutrophil infiltration, in conjunction with other immune mediators, leads to high concentrations of neutrophil oxidants and enzymes in the lung environment, which stimulate mucus secretions, plugging airways and further damaging the lung. This cycle of damage results in a frustrated immune system that, despite a vigorous response, is unable to clear the infection (31, 44).
Levels of IL-8 in bronchoalveolar lavage (BAL) fluid from young CF patients infected with Staphylococcus aureus and P. aeruginosa can be correlated with the bacterial count and severity of infection (7). The Bcc has been shown to induce secretion of IL-8, IL-6, and prostaglandin E (2) in various epithelial cell lines, but there is some debate over which bacterial factors are responsible (17, 42, 43).
The goal of this study was to assess the role of B. cenocepacia flagella in a murine agar bead model of lung infection, by using an isogenic null mutant with a mutation in fliCII, which encodes the major flagellin subunit. We chose the mouse agar bead model (8) since a typical airway application of free organisms leads to rapid clearance of the bacteria, not allowing for study of the infectious process beyond a short time period (4). This model enabled us to examine cytokine production and inflammation in response to infection and led to additional avenues of investigation of the trigger for the inflammatory response.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All bacterial strains were grown at 37°C in Luria broth (LB). Escherichia coli and B. cenocepacia were routinely grown on either Luria agar (LA) containing 1.5% Bacto Agar (Difco, Franklin Lakes, N.J.) or Trypticase soy agar plates (Remel, Lenexa, Kans.). B. cenocepacia was also grown on Pseudomonas Isolation Agar (Difco) made in accordance with the manufacturer's instruction. The medium was supplemented with 10 μg of tetracycline per ml, 1.5 mg of trimethoprim per ml, or 50 μg of kanamycin per ml for E. coli and 300 μg of tetracycline per ml or 100 μg of trimethoprim per ml for B. cenocepacia, as appropriate.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | supE44 hsdR17 recAI endAI gyrA96 thi-1 relA1 | GIBCO |
| SM10 | thi-1 thr leu tonA lacY supE recA RP4-2-Tc::Mu Kmr | 49 |
| B. cenocepacia | ||
| K56-2 | CF respiratory isolate, ET12 lineage | P. Sokol |
| fliCII::Tp | fliCII::Tp derivative of K56-2; Tpr | This study |
| Plasmids | ||
| pCR-XL-TOPO | TA cloning vector for PCR products; Zeor Ampr | Invitrogen |
| pCR2.1-TOPO | TA cloning vector for PCR products; Kmr Ampr | Invitrogen |
| pUCP18Tc | Broad-host-range vector pUCP18 derivative; Tcr | 8 |
| pEX18Tc | Suicide vector; sacB Tcr | 22 |
| p34ETp | Source of Tp cassette | 9 |
| pTOPO-fliCII | pCR-XL-TOPO with 848-bp fliCII fragment; Zeor Ampr | This study |
| pTOPO-fliCII-Tp | pTOPO-fliCII with Tp cassette ligated into a blunted AgeI site; Kmr Zeor Tpr | This study |
| pEX-fliCII-Tp | pEX18Tc with 1.5-kb fragment containing fliCII-Tp; Tcr Tpr | This study |
| pTOPO-fliCII-comp | pCR 2.1-TOPO with 1.2-kb fliCII gene; Kmr Ampr | This study |
| pUCP18Tc-fliCII | pUCP18Tc with 1.2-kb fliCII gene; Tcr | This study |
| pcDNA3.1/Zeo | High-level mammalian expression vector; Zeor Ampr | Invitrogen |
| pEF6::hTLR5 | pGENE/V5-His containing human TLR5 gene; Ampr Zeor | 21 |
| pNF-κB-Luc | NF-κB-luciferase reporter plasmid; Ampr | BD Biosciences Clontech |
| pRL-TK | pRL reporter vector encoding Renilla luciferase; Ampr | Promega |
Ampr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; Tpr, trimethoprim resistance; Zeor, zeocin resistance.
DNA manipulations.
DNA manipulations and isolations were performed in accordance with standard protocols (34). Restriction enzymes and DNA-modifying enzymes were purchased from New England Biolabs (Beverly, Mass.), Roche (Indianapolis, Ind.), Promega (Madison, Wis.), and Amersham Pharmacia (Piscataway, N.J.). Plasmid DNA was isolated from E. coli with a QIAprep Spin Miniprep kit (QIAGEN, Valencia, Calif.). Plasmid DNA was isolated from B. cenocepacia by phenol-chloroform precipitation as previously described (10). Genomic DNA was isolated from B. cenocepacia by phenol-chloroform precipitation as previously described (57).
Plasmid construction.
Gene replacement plasmid pEX-fliCII-Tp was constructed by first PCR amplifying an 848-bp fragment from the B. cenocepacia K56-2 type II flagellar major subunit (fliCII) gene with primers 5′-CGTGTCGAACGCAAACGATG-3′ (fliCII-1) and 5′-CGTCGGTGATCTGCGACTGA-3′ (fliCII-2), and cloning it into plasmid pCR-XL-TOPO (Invitrogen, Carlsbad, Calif.), creating plasmid pTOPO-fliCII. The fliCII gene fragment was inactivated by inserting an SmaI-digested trimethoprim resistance (Tp) cassette from plasmid p34ETp (9) into a blunted AgeI site, creating plasmid pTOPO-fliCII-Tp. The fliCII-Tp fragment was subcloned into the XbaI and HindIII sites of plasmid pEX18Tc, creating plasmid pEX-fliCII-Tp.
Complementing plasmid pUCP18Tc-fliCII was constructed by first PCR amplifying the fliCII gene with primers 5′-TTCTGAGGCCAGCAATGA-3′ (fliCIIcompF) and 5′-TTACTGCGACCTTGACCTGC-3′ (fliCIIcompR) and cloning it into plasmid pCR2.1-TOPO, creating plasmid pTOPO-fliCII-comp. The fliCII gene was ligated, in the same orientation as the plasmid-encoded promoter, into pUCP18Tc by using the SacI and XbaI sites, creating expression plasmid pUCP18Tc-fliCII.
Allelic exchange in B. cenocepacia.
Biparental matings were performed to transfer pEX-fliCII-Tp from E. coli SM10 to B. cenocepacia K56-2 as previously described (47). Transconjugants were plated onto Pseudomonas Isolation Agar supplemented with 100 μg of trimethoprim per ml. Gene replacements were selected from plates containing 100 μg of trimethoprim per ml and 5% sucrose.
PCR confirmation.
Single colonies were resuspended from LA into 20 μl of LB; 2.5 μl of the resuspension was used in a standard PCR with Taq polymerase and primers fliCII-1 and fliCII-2.
Southern blot hybridization.
Genomic DNA was digested with PstI overnight and separated on a 0.7% agarose gel. The DNA fragments were transferred onto a GeneScreen membrane (Perkin-Elmer Life Sciences, Boston, Mass.) and probed with an ECL (Amersham Biosciences, Piscataway, N.J.)-labeled fliCII gene fragment PCR amplified from B. cenocepacia K56-2 as described above.
Flagellar preparations.
Isolation of flagellar filaments by shearing and centrifugation was performed as previously described (51). Briefly, B. cenocepacia strains were grown overnight in LB, harvested by centrifugation, resuspended in 10% of the original volume of 0.5 M HEPES buffer, and homogenized with a Sorvall Omni Mixer (DuPont, Wilmington, Del.). Deflagellated bacteria were sedimented at 6,000 × g for 15 min, and cell-free supernatants were ultracentrifuged at 100,000 × g for 90 min to pellet the flagellar filaments. Protein pellets were resuspended in phosphate-buffered saline (PBS) and dialyzed with a Slide-A-Lyzer cassette (Pierce, Rockford, Ill.). Eighteen-microgram samples were boiled and separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were visualized with Coomassie blue.
Immunoblot analysis.
Flagellar filament proteins from SDS-PAGE were transferred onto nitrocellulose paper (Bio-Rad, Hercules, Calif.). Blots were probed with Salmonella H antiserum poly a-z antibody (Difco), which recognizes Bcc flagella, and detected with an alkaline phosphatase-conjugated monoclonal anti-rabbit immunoglobulin secondary antibody (Sigma, St. Louis, Mo.). Reactivity was detected by developing blots with 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium (BCIP/NBT) tablets (Sigma).
Microcapillary column liquid chromatography-tandem mass spectrometry.
The 45-kDa band that resulted from flagellar preparations of wild-type K56-2 was extracted from the Coomassie-stained gel and prepared for microcapillary column liquid chromatography-tandem mass spectrometry as described previously (20). The data were analyzed by using the Sequest search algorithm (58). Peptides that were not matched by this algorithm were interpreted manually and searched versus the expressed sequence tag databases with the Sequest algorithm.
Electron microscopy.
Bacteria were taken from LB plates containing 0.4% agar (0.4% LA) and resuspended in PBS. Samples were applied to Formvar and carbon-Formvar-coated copper grids, stained with phosphotungstic acid (pH 7.0), and examined with a Phillips 400T transmission electron microscope operated at 80,000 eV.
Motility assay.
Motility assays were performed by stabbing bacterial colonies into 0.4% LA and incubating the plate overnight at 37°C. Motility was determined by observing growth in a radius around the point of the stab.
Agar bead mouse model.
Groups of five to eight female 6- to 8-week-old C57/BL6 mice (Jackson Laboratory, Bar Harbor, Maine) were infected with 1.5 × 108 to 9.1 × 108 CFU of B. cenocepacia-infused agar beads per mouse, instilled intratracheally, as previously described (56). Mice were sacrificed at 72 h postinfection, at which time their appearance was monitored and their weight was quantified. BALs were performed, white blood cells (WBCs) in BAL fluid were enumerated, and the percentage of polymorphonuclear leukocytes (PMNs) in that population of cells was determined. Lung and spleen homogenates were plated for determination of bacterial loads (6).
Additionally, groups of 12 mice were infected with 2.75 × 108 to 9.00 × 109 CFU/ml as described above and sacrificed at 24 h postinfection. Appearance and weight were quantified, BALs were performed, and numbers of WBCs and PMNs were determined. All experiments were performed in compliance with federal guidelines and institutional policies.
Murine KC and MIP-2 assays.
Levels of KC and MIP-2 were measured in C57/BL6 BAL fluid or serum collected at the time of sacrifice (24 h) with Quantikine mouse KC and MIP-2 kits (R & D Systems, Minneapolis, Minn.). Fifty microliters of BAL fluid, diluted 1:10, or serum, diluted 1:4 to 1:10, was added to prepared wells and assayed in accordance with KC or MIP-2 kit instructions. Levels of KC and MIP-2 were determined by measuring the A450 of samples and extrapolating the number of picograms of KC and MIP-2 per milliliter from a standard curve performed within the assay.
Transfection of HEK293 cells.
Adherent human embryonic kidney cells (HEK293; American Type Culture Collection, Manassas, Va.) cells were transiently transfected with Superfect transfection reagent (QIAGEN, Valencia, Calif.). Cells were grown in Dulbecco modified Eagle medium (GIBCO, Carlsbad, Calif.) supplemented with 1% penicillin-streptomycin (GIBCO) and 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) at 37°C with 7.5% CO2. HEK293 cells were transfected with 4 μg of pcDNA3.1Zeo (Invitrogen) or pEF6::hTLR5, 4 μg of pNF-κB-Luc (BD Biosciences Clontech, Palo Alto, Calif.), and 2 μg of pRL-TK (Promega) in 100-mm-diameter plates (Falcon, San Jose, Calif.). Transfected cells were split into 24-well plates and 60-mm-diameter dishes. Cells in 24-well plates were stimulated with heat-killed B. cenocepacia as indicated below, whereas cells in 60-mm-diameter dishes were used for protein expression analysis by immunoblotting.
Immunoblot assay for TLR expression.
HEK293 cells were transfected as described above and harvested from 60-mm-diameter plates to detect expression of V5-tagged human TLR5. Cells were lysed in immunoprecipitation buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.4% Nonidet P-40, 10% glycerol, 2 mM EDTA, 1 mM EGTA (36), and a cocktail of protease and phosphatase inhibitors (0.15 U of aprotinin per ml, 1 mM vanadate, 100 μM leupeptin, and 1 mM phenylmethylsulfonyl fluoride) (28). Protein concentrations were determined by bicinchoninic acid protein assay (Pierce). Fifty-microgram samples were boiled and separated on 8% bisacrylamide gels. Separated proteins were transferred to nitrocellulose membranes as indicated above. Blots were probed with anti-V5 antibody conjugated to horseradish peroxidase (Invitrogen) and visualized by Western Lightening ECL labeling (Perkin-Elmer Life Sciences). Blots were also probed with a control antibody, p44/42 MAP kinase (Cell Signaling, Beverly, Mass.), which was detected with anti-rabbit immunoglobulin-horseradish peroxidase secondary antibody (Amersham Biosciences) and developed as indicated above.
HEK293 treatment with B. cenocepacia.
B. cenocepacia strains were grown overnight on LA, resuspended in PBS, and incubated at 65°C for 1 h. Heat-killed samples were plated to confirm that all of the bacteria were nonviable. Transfected HEK293 cells were treated 48 h posttransfection with heat-killed bacteria at an approximate multiplicity of infection of 10:1. Heat-killed bacteria were gently centrifuged onto the cells at 3,220 × g for 5 min to ensure contact. The plates were then incubated at 37°C with 10% CO2 for 18 h. Supernatants were harvested and stored at −20°C for subsequent determination of the IL-8 concentration. Cell monolayers were lysed in dual-luciferase reporter assay system passive lysis buffer (Promega) and stored at −20°C for quantification of luciferase.
Dual-luciferase reporter assay system.
Luciferase production was measured in HEK293 cell lysates with the dual-luciferase reporter assay system (Promega). Relative light units were normalized to the activity of the cotransfected pRL-TK plasmid. For these data, the ratio of NF-κB activation of TLR5-transfected cells to vector-transfected cells was determined and the estimated fold ratios from three separate experiments were compared for statistical analysis.
Human IL-8 cytokine assay.
IL-8 secretion from transfected HEK293 cell supernatants was measured with a Quantikine human IL-8 kit (R & D Systems). Fifty microliters of infected cell culture supernatant was added to prepared wells and assayed in accordance with kit specifications. The level of IL-8 in cell supernatants was determined by measuring the A450 and extrapolating the number of picograms of IL-8 per milliliter from a standard curve performed within the assay. For these data, the ratio of IL-8 secretion from TLR5-transfected cells to vector-transfected cells was determined and the fold ratios from three experiments were compared for statistical analysis.
Determination of albumin in BAL fluid.
Albumin levels were determined with the VetTest Chemistry Analyzer (IDEXX Laboratories, Westbrook, Maine).
Statistical analysis.
Chi-square tests of association were used to determine the relationship between flagellar expression and the rate of mortality observed in C57/BL6 mice 3 days postinfection. Differences between wild-type- and fliCII::Tp mutant-infected mice related to standard markers of lung infection (bacterial loads in the lungs and spleen, numbers of WBCs and percent PMNs in BAL fluid, and percent weight loss) were subjected to analysis of variance (ANOVA) if data were obtained from multiple experiments and to Student t tests otherwise. Similarly, levels of KC in BAL fluid were analyzed by ANOVA by modeling the natural logarithm of the KC level on two factors, infection type (wild type or fliCII::Tp mutant) and experimental variation. The Student t test was used to assess levels of KC in serum. Activation of NF-κB was analyzed by three-factor ANOVA, where the natural logarithm of the average of measurements performed in triplicate served as the response variable. ANOVA factors included type of plasmid, bacterial strain, experimental variation, and an interaction between plasmid and bacterial strain. Model-based fold ratios and 95% confidence intervals were calculated for the comparisons of interest, and statistical tests were performed at the 0.05 level.
RESULTS
Characterization of a K56-2 fliCII null mutant.
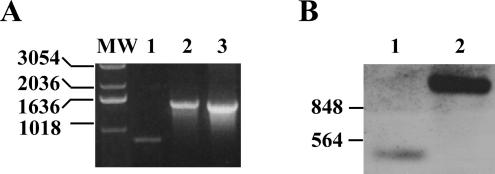
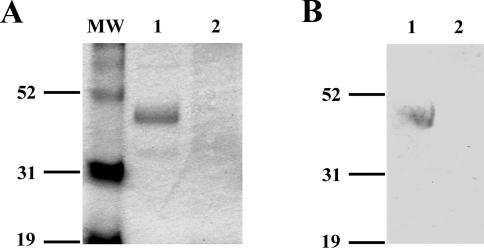
In order to define the contribution of B. cenocepacia flagella to virulence in infection, a null mutant lacking functional flagella was constructed by insertional inactivation of the type II flagellin gene, fliCII, in the chromosome of B. cenocepacia strain K56-2 (Fig. 1). Successful insertion of the Tp cassette was demonstrated by PCR and Southern blot assays, resulting in strain fliCII::Tp (Fig. 2A and B). Whole bacteria resuspended in LB were used for confirmation by PCR (Fig. 2A). Primers fliCII-1 and fliCII-2 (Fig. 1) generated an 848-bp band in wild-type K56-2 and a single band running at approximately 1,500 bp in the fliCII::Tp mutant strain, indicating successful insertion of the 648-bp Tp resistance cassette (Fig. 2A). PstI-digested genomic DNA was probed with a fliCII gene fragment, which resulted in hybridization to a PstI fragment of approximately 400 bp for K56-2 and 1,000 bp for fliCII::Tp, demonstrating successful gene replacement (Fig. 2B). Flagellar preparations of wild-type K56-2 separated by SDS-PAGE and stained with Coomassie blue displayed a band at approximately 45 kDa, consistent with the reported size of Bcc type II flagellin (Fig. 3A). This band reacted with antibody by immunoblotting (Fig. 3B) and was identified by mass mapping to be B. cenocepacia FliCII (data not shown). Similar preparations of fliCII::Tp lacked a 45-kDa band on both the SDS-PAGE gel and the immunoblot (Fig. 3A and B).
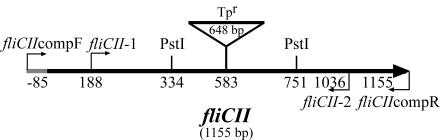
FIG. 1.
Physical map of the B. cenocepacia fliCII gene with insertion of a Tp cassette (Tpr). The fliCII gene was interrupted at the AgeI site at 583 bp with a Tp cassette in the B. cenocepacia K56-2 chromosome. The large arrow indicates the direction of fliCII transcription. Primers generated for gene replacement (fliCII-1 and fliCII-2) and complementation (fliCIIcompF and fliCIIcompR) are represented by directional arrows. Restriction endonuclease sites for PstI used for Southern blot analysis are shown. The numbers refer to nucleotide positions with respect to the fliCII start site.
FIG. 2.
Confirmation of fliCII::Tp by PCR and Southern blot hybridization. (A) PCR was performed on single-colony resuspensions in LB with primers fliCII-1 and fliCII-2, as indicated in Fig. 1. Lanes: 1, K56-2; 2, fliCII::Tp; 3, pTOPO-fliCII-Tp; MW, molecular weight markers (sizes in base pairs are shown at the left). (B) PstI-digested genomic DNA was used for Southern hybridization. DNA was probed with an 848-bp fragment of fliCII generated with primers fliCII-1 and fliCII-2, indicated in Fig. 1. Lanes: 1, K56-2; 2, fliCII::Tp. Sizes of molecular weight markers in base pairs are shown at the left.
FIG. 3.
fliCII::Tp lacks expression of FliCII, as detected by Coomassie staining and immunoblot analysis. Flagella were isolated and separated by SDS-10% PAGE. (A) Coomassie blue staining after SDS-PAGE. Lanes: 1, K56-2; 2, fliCII::Tp; MW, molecular weight markers (sizes in kilodaltons are shown at the left). (B) After SDS-PAGE, proteins were transferred to a nitrocellulose membrane. The membrane was probed with Salmonella H antiserum poly a-z antibody, and reactivity was detected by BCIP/NBT precipitation. Lanes: 1, K56-2; 2, fliCII::Tp. Sizes of molecular weight markers in kilodaltons are shown at the left.
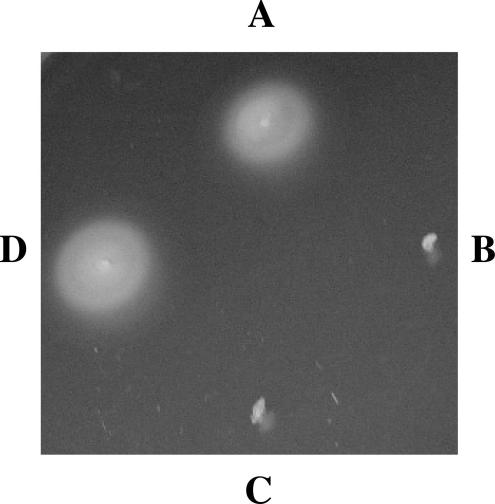
Preparations of K56-2 from 0.4% LA were negatively stained and visualized by electron microscopy. B. cenocepacia K56-2 exhibited singular, long, thin, fragile polar flagella, while flagella were not visualized on fliCII::Tp, as expected (data not shown). When stabbed into 0.4% LA, wild-type K56-2 formed a large, hazy ring around the point of the stab, indicative of swimming motility (Fig. 4). The fliCII::Tp mutant was nonmotile, as demonstrated by lack of growth detected from the point of the stab. This effect was not due to a general growth defect in fliCII::Tp, as the wild type and the fliCII::Tp mutant showed similar growth rates in LB (data not shown). This motility-deficient phenotype was rescued by providing a wild-type copy of the K56-2 fliCII gene in trans on plasmid pUCP18Tc-fliCII, which demonstrated restoration of the fliCII defect to wild-type levels and confirmed the nonpolarity of the mutation. The plasmid vector alone had no effect (Fig. 4).
FIG. 4.
fliCII is necessary for swimming motility. Colonies were stabbed into 0.4% LA, incubated overnight at 37°C, and observed for growth around the point of the stab as a sign of swimming motility. Quadrants: A, K56-2; B, fliCII::Tp; C, fliCII::Tp(pUCP18Tc); D, fliCII::Tp(pUCP18Tc-fliCII).
Virulence of B. cenocepacia in an agar bead model of pulmonary infection.
Wild-type K56-2 and the fliCII::Tp mutant strain were tested for the ability to cause disease in C57/BL6 mice in a mouse agar bead model of infection. Mice were infected intratracheally with B. cenocepacia embedded in agar beads and tested for markers of disease and virulence. It was noted that some mice infected with the wild-type strain exhibited ruffled fur or other signs of morbidity within 24 h postinfection, and consequently, 40% mortality was observed by 3 days postinfection. In contrast, none of the fliCII::Tp-infected mice appeared moribund during the experiment, and all survived to 3 days postinfection, displaying 0% mortality (P = 0.011) (Table 2). The lethality phenotype observed in mice infected with wild-type K56-2 was restored in the fliCII::Tp mutant strain by providing a wild-type copy of fliCII in trans. Infection with fliCII::Tp(pUCP18Tc-fliCII) caused 53% mortality (not significantly different from that of the wild type), while mortality as a result of infection with fliCII::Tp(pUCP18Tc) remained 0% (P = 0.001).
TABLE 2.
fliCII is associated with lethality in an agar bead model of infection
| Strain | No. of survivors/total | % Lethalitya |
|---|---|---|
| K56-2 | 12/20 | 40b,d |
| fliCII::Tp | 12/12 | 0b |
| fliCII::Tp (pUCP18Tc) | 15/15 | 0c |
| fliCII::Tp (pUCP18Tc-fliCII) | 7/15 | 53c,d |
P values were determined by chi-square test of association.
P = 0.011.
P = 0.001.
P = 0.43.
The survivors of the infection were sacrificed at day 3 postinfection and assayed for standard markers of lung infection. There were no significant differences between the wild-type survivors and fliCII::Tp-infected mice with respect to CFU counts in lung homogenate, CFU counts in spleen homogenate, percent weight loss, WBC counts in BAL, and percent polymorphonuclear leukocytes in BAL fluid (data not shown). In subsequent experiments, mice were sacrificed at 24 h to assay markers of infection before mortality in the wild-type group occurred; there were no significant differences between wild-type- and fliCII::Tp-infected mice with respect to the aforementioned disease parameters (data not shown).
Markers of inflammation in BAL fluid and serum.
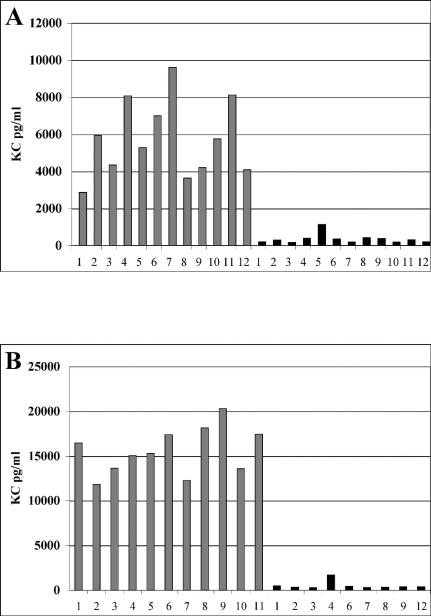
BAL fluid from either wild-type- or fliCII::Tp-infected mice sacrificed at 24 h was assayed for levels of KC, a murine homolog of human IL-8 (Fig. 5A), as a marker of the host chemokine response to the wild type and fliCII::Tp in this model. BAL fluid from wild-type-infected mice contained an average of 5,121 pg of KC per ml, while BAL fluid from fliCII::Tp-infected mice contained an average of 762 pg/ml (P < 0.001). Levels of MIP-2, another murine homolog of IL-8, showed a similar trend in BAL fluid of infected mice (data not shown).
FIG. 5.
KC is elevated in BAL fluid and serum of wild-type-infected mice. The concentration of KC was determined by enzyme-linked immunosorbent assay. (A) BAL fluid was harvested from mice infected with K56-2 (grey bars) or fliCII::Tp (black bars) at 24 h. The results are representative of two independent experiments. (B) Blood was taken from tails of mice infected with K56-2 (grey bars) or fliCII::Tp (black bars) at 24 h.
When counting WBCs in BAL fluid, it was noted that some samples from wild-type-infected mice contained numerous red blood cells. The level of albumin in BAL fluid was determined for both wild-type- and fliCII::Tp-infected mice in an attempt to quantify the amount of blood in the lungs. While both groups showed undetectable levels of albumin (<25 mg/ml), an overall increase in red blood cells in BAL fluid from the wild-type-infected group suggests lung damage as a result of infection.
Blood samples were collected from the tails of infected mice in a single experiment, and the serum was assayed for levels of KC (Fig. 5B). Serum from the wild-type-infected group contained an average of 15,594 pg of KC per ml, while serum from the fliCII::Tp-infected group contained an average of only 536 pg of KC per ml (P < 0.001). The overwhelming difference between KC levels in serum from wild-type- and fliCII-infected mice indicates that a strong immune response was induced by wild-type K56-2 and not by the fliCII::Tp mutant, implicating B. cenocepacia flagella in this process.
B. cenocepacia induces NF-κB activation through human TLR5.
Bacterial flagellin is the only known ligand recognized by TLR5 (21). Flagella from organisms such as P. aeruginosa, E. coli, and Helicobacter pylori, among others, have been shown to be recognized by TLR5 (11, 50, 59). Therefore, we hypothesized that B. cenocepacia flagellin would initiate a signaling cascade through interaction with TLR5, resulting in the downstream events of NF-κB activation and secretion of IL-8. To test this interaction in vitro, a luciferase reporter assay was used to determine the level of NF-κB activation as a consequence of TLR5 expression. HEK293 cells were transiently transfected with pcDNA3.1/Zeo or pEF6::hTLR5 and assayed for NF-κB activation, as determined by luciferase production from an NF-κB-luciferase reporter plasmid (pNF-κB-Luc). Expression of V5-tagged TLR5 in HEK293 cells was confirmed by immunoblot analysis of cell lysates at the time of infection (Fig. 6).
FIG. 6.
Verification of V5-TLR5 expression in HEK293 cells. Transfected HEK293 cells were lysed and separated by SDS-8% PAGE. Separated proteins were transferred to nitrocellulose and probed with anti-V5-horseradish peroxidase antibody or loading control p44/42 MAP kinase antibody. Reactivity of p44/42 MAP kinase was detected by BCIP/NBT precipitation. Lanes: 1, pcDNA3.1/Zeo; 2, pEF6::hTLR5.
To maintain a constant multiplicity of infection and assess the contribution of flagellin in the context of whole bacteria, transfected cells were treated with heat-killed B. cenocepacia, and eukaryotic cell lysates were assayed for luciferase production (Fig. 7). Wild-type K56-2 was able to activate NF-κB in TLR5-transfected cells but not in vector-transfected cells. fliCII::Tp, however, did not cause NF-κB activation in either cell type. The fold activation (TLR5 transfected compared to vector transfected) of NF-κB in wild-type-treated cells was 3.1 times greater than that in cells treated with fliCII::Tp (P < 0.001). fliCII::Tp(pUCP18Tc-fliCII) was able to activate NF-κB in this system to levels similar to wild-type levels of fold activation, demonstrating that activation of NF-κB in TLR5-transfected cells was dependent upon a functional fliCII gene. pUCP18Tc, as anticipated, did not restore the fliCII::Tp defect (data not shown). Taken together, these results demonstrate that flagellum-expressing B. cenocepacia can activate NF-κB through TLR5.
FIG. 7.
Flagellum-expressing B. cenocepacia activates NF-κB through TLR5. HEK293 cells were transiently transfected with pcDNA3.1/Zeo (grey bars) or pEF6::hTLR5 (black bars). Cells were treated with a PBS control or heat-killed bacteria. NF-κB activation was determined by luciferase production (measured in relative light units) 18 h postinfection. These results are representative of three independent experiments. Error bars indicate standard deviations within the experiment.
Flagellum-expressing B. cenocepacia induces IL-8 secretion through TLR5.
Supernatants from HEK293 cells treated with the wild type and the fliCII::Tp mutant, described above, were harvested and examined for IL-8 secretion (Fig. 8). As with respect to NF-κB activation, wild-type K56-2 was able to induce IL-8 secretion in TLR-5-transfected cells but not vector-transfected cells, while fliCII::Tp was unable to induce secretion of IL-8 in either cell type. The fold induction of IL-8 secretion (TLR5 transfected compared to vector transfected) in wild-type-treated cells was 2.9 times greater than that in fliCII-treated cells (P = 0.039). fliCII::Tp(pUCP18Tc-fliCII) was able to restore the fold induction of IL-8 secretion to levels comparable to those of the wild type, while pUCP18Tc alone did not affect the fliCII::Tp defect in IL-8 secretion (data not shown). These results document that flagellum-expressing B. cenocepacia contributes to IL-8 secretion by host cells through interaction with TLR5.
FIG. 8.
Flagellum-expressing B. cenocepacia induces IL-8 secretion through TLR5. HEK293 cells were transiently transfected with pcDNA3.1/Zeo (grey bars) or pEF6::hTLR5 (black bars). Cells were treated with a PBS control or heat-killed bacteria. Supernatants were harvested, and IL-8 secretion was measured by enzyme-linked immunosorbent assay. The results are representative of three independent experiments. Error bars indicate standard deviations within the experiment.
DISCUSSION
In this study, we created a mutant of B. cenocepacia strain K56-2 with an insertional mutation in the fliCII gene and assessed its role in in vivo infection, as well as its ability to induce host immune responses through interaction with TLR5. We demonstrate that B. cenocepacia flagella serve as virulence determinants in a murine agar bead model of infection, as measured by lethality. Additionally, we have shown that flagellum-expressing B. cenocepacia K56-2 is able to initiate signaling cascades in HEK293 cells expressing TLR5 that can result in NF-κB activation and IL-8 secretion from host cells.
In other bacterial systems, flagella have versatile functions that have been shown to be important for success in environmental niches, as well as in disease processes. Evidence suggests that the role of flagella is not static throughout the progress of infection. In some cases, flagella have been noted to be particularly important in the establishment of initial infection and may not be necessary in later stages (27). This is supported by clinical data that suggest a selective pressure against flagellar expression in the CF lung environment. While many initial P. aeruginosa isolates are motile, those collected from CF patients with chronic P. aeruginosa infections are often found to be aflagellate and nonmotile (30, 33). These results indicate that the loss of flagella may be advantageous to the bacteria in the context of a chronic P. aeruginosa infection.
Despite differences between the bacterial types and infection models used, some of the findings in this study are consistent with a previous report of the pathogenic potential of bacteria expressing flagella in a murine model of infection. Feldman et al. (17) demonstrated that, in contrast to wild-type P. aeruginosa, an isogenic fliC mutant lacking flagella did not cause mortality in an intranasal neonatal model of infection. Additionally, the aflagellate P. aeruginosa fliC mutant strain was found in the blood and spleen, indicating dissemination of infection irrespective of flagellar expression, as seen in the present study. With respect to pneumonia, however, intranasal administration of P. aeruginosa fliC mutants resulted in decreased pneumonia compared to the wild type, whereas we noted no difference between the B. cenocepacia fliCII mutant and wild-type K56-2.
We would not necessarily expect to see a difference in the bacterial lung load in our model, as there is no need for bacterial motility in order to travel to the site of infection, owing to the nature of the agar bead infection model. It is unclear, however, whether the reduction in virulence of the fliCII::Tp mutant was a result of its deficiency in motility or in the flagellar structure itself, as the fliCII null mutant lacks expression of the major flagellin subunit and therefore does not express flagellin. To directly assess the role of motility in virulence in the agar bead model of infection, an isogenic mutant expressing nonfunctional flagella would need to be constructed and tested in future studies. Still, the similarity in the bacterial lung loads of wild-type K56-2-infected and fliCII::Tp mutant-infected mice in our model allows us to assess the contribution of B. cenocepacia flagella to pathogenesis beyond the initial establishment of infection.
B. cenocepacia flagella have been shown to contribute to invasion of lung epithelial cells in vitro in a recent study in which nonmotile mutants of strain J2315 were tested for the ability to adhere to and invade A549 cells. Isogenic mutants with mutations in fliG, a component of the motor-switch complex, and fliI, a conserved ATPase required for protein translocation of flagellar components, were deficient in invasion but not adherence to human lung cells in culture, although the level of wild-type invasion was extremely low. Bacterial motility was shown to contribute to this defect, as more pronounced deficiencies in invasion were observed in the absence of centrifugation of the bacteria onto the cell monolayers (55).
A related study demonstrated a correlation between the invasive potential of the Bcc in in vitro and in vivo infections in this same agar bead model, as measured by dissemination of bacteria to the spleen in the agar bead murine model of infection (6). While it was not investigated here, it is unlikely that the contribution of flagella to invasiveness in vitro correlates to invasive disease in our study, as we observed no statistical difference between dissemination of mice infected with wild-type K56-2 and fliCII::Tp.
In an effort to understand the bacterial and host factors involved in pathogenesis associated with the presence of flagella in this model, the potential interaction between B. cenocepacia flagella and TLR5 was investigated. A conserved amino acid domain of flagellin complementary to human TLR5, discovered by Scatchard plot analysis, was found in 367 bacterial species (25). We identified this consensus sequence in the fliCII gene of J2315 (http:://www.sanger.ac.uk/Projects/B_cenocepacia), the B. cenocepacia sequenced strain (88-LQRIRQLAVQ-97). The same sequence is present in the fliCII gene of strain K56-2. There are two substitutions in B. cenocepacia fliCII in strains J2315 and K56-2, as the consensus sequence contains a V at position 92 and an E at position 94. However, these substitutions did not appear to affect the recognition of flagellum-expressing B. cenocepacia by TLR5 in this model.
Upon treatment with heat-killed wild-type K56-2, NF-κB was activated in HEK293 cells expressing human TLR5 compared to vector-transfected cells, whereas there was almost no induction in TLR5-expressing cells infected with the fliCII::Tp mutant. The paucity of expression of TLR 1-10 in HEK293 cells (23) suggests that NF-κB activation was a direct result of the interaction between flagellum-expressing B. cenocepacia and TLR5. To our knowledge, this is the first report that bacterial products expressed by the Bcc interact with TLRs to cause activation of NF-κB.
Although NF-κB transcription factors have a variety of roles in the host immune response, their main function in epithelial cells is to induce the expression of proinflammatory genes, including IL-8, a major factor in CF disease (2). Extracellular products from the Bcc have been shown to induce IL-8 secretion, but the particular bacterial components responsible for these effects have been debated in the literature. One report concluded that a protein component(s) from cell-free supernatants of CF, non-CF clinical, and nonclinical strains mediated IL-8 release in lung epithelial cells (42). A more recent report of a study with whole bacteria argued that this induction was likely the result of lipopolysaccharide (43). Yet another study with cell-free supernatants corroborated earlier reports of the Bcc stimulating IL-8 secretion but found that polymyxin B did not inhibit, nor did lipopolysaccharide stimulate, its release and that the effect of the bacterial component was not reduced upon heat treatment, suggesting that it was not a protein product (17). The discrepancies in these reports are not due to differences in the host cells, as A549 cells were used in each experiment, but may be the result of using different Bcc strains and the differences between cell-free supernatants and whole bacteria.
In this report, we have shown that flagellum-expressing B. cenocepacia K56-2 is able to induce secretion of IL-8 in cells expressing TLR5 in a model system. Wild-type K56-2 was able to promote secretion of IL-8 in HEK293 cells transfected with human TLR5 compared to vector-transfected cells, whereas the fliCII::Tp mutant was unable to induce secretion of IL-8 from these cells. Although the experiments in this study were not performed in lung epithelial cells, which have been shown to express TLR5 (G. Soong, A. Muir, S. Sokol, B. Reddy, A. vanHeeckeren, and A. Prince, Abstr. 17th N. Am. Cystic Fibrosis Conf., abstr. 262, 2003), we propose that at least part of IL-8 induction in the cellular immune response caused by B. cenocepacia is due to the ability of TLR5 to induce IL-8 release upon stimulation with the flagellum-expressing Bcc.
TLR5 is expressed predominantly in lung and liver tissues of C57/BL6 mice (48). Although the specific contribution of TLR5 signaling could not be assessed in our mouse model of infection, we noted increased levels of the murine IL-8 homolog KC in BAL fluid and serum from mice infected with wild-type K56-2 compared to those from fliCII::Tp-infected mice. Elevated levels of KC in the lungs of wild-type-infected mice may have been affected by the presence of blood in the lungs, which contained high levels of KC. Thus, given the lethality phenotype in wild-type-infected mice, high levels of KC in BAL fluid may serve as a marker for pathogenesis and poor prognosis in this model.
The collective results from this study suggest that this host response may be due in part to TLR5-induced signaling by K56-2 flagella. It is possible that elevated levels of KC in the lung contribute to lung damage if the overwhelming immune response to the infection crosses the line from protective to destructive. The presence of red blood cells in BALs of wild-type-infected mice but not in those of fliCII::Tp-infected mice suggests that a strong immune response to flagellin can lead to lung damage and, potentially, lethal disease.
KC levels were also elevated in serum from mice infected with wild-type K56-2 compared to those in mice infected with the fliCII::Tp mutant, suggesting that other circulating immune cells such as macrophages, PMNs, and dendritic cells, which also express TLR5, play a role in host IL-8 secretion (40). This also suggests that the immune response to flagellin is not confined to the lung and may contribute to pathogenesis in this model, particularly in disseminated infection.
It was somewhat surprising that we did not observe overt differences in the outcome parameters (including bacterial loads in the lungs and spleen, weight loss, and WBCs and PMNs in BAL fluid) between mice infected with the wild-type and fliCII::Tp strains, given that we observed increased levels of KC in mice infected with the wild-type strain at 24 h postinfection. Since this time point preceded expression of the lethality phenotype, observed in 40% of wild-type-infected mice at 3 days postinfection, we suggest that the levels of KC in the lungs of these mice may not have been high enough to promote PMN infiltration or that PMN recruitment would have been observed at a later time. If we could accurately monitor individual mice for each of these outcome parameters at various times after infection, we anticipate that differences between potential survivors and those mice that were to die might be revealed.
As flagella are important in the initial steps of infection, vaccines directed against flagella hold much promise in the prevention of infection. Its protein nature, position on the surface of the bacteria, and simple isolation and purification may contribute to the success of a flagellin-based vaccine. Antibodies directed against flagella prevent motility and pathogenesis in mouse models (13, 21, 52). In a small vaccine trial, a bivalent P. aeruginosa flagellin vaccine that was administered intramuscularly to CF patients without P. aeruginosa showed promising results. Long-lasting, high titers of antibodies directed against flagella were documented systemically, as well as in the secretory airways of the immune system. Data from ongoing efficacy trials are encouraging (12; G. Doring, Symp. Summary, 17th N. Am. Cystic Fibrosis Conf., S9.1, 2003). In the case of the Bcc, isolates express one of only two types of flagellin (19, 38), and a bivalent vaccine could theoretically recognize any flagellated strain in the complex. In addition to the nature of flagella and their multiple functions in infection, late-stage acquisition of the Bcc by CF patients makes B. cenocepacia flagella a promising vaccine candidate.
It is now appreciated that flagella confer an advantage on pathogenic bacteria beyond motility. In this report, we have shown that B. cenocepacia flagella contribute to pathogenesis in a murine model of infection, as demonstrated by lethality, which is likely due to multiple aspects of flagellar virulence. Specifically, we have demonstrated that B. cenocepacia flagella interact with TLR5 to activate host immune responses, which may contribute to pathogenesis in this model.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1 RO1 HL65898 to C. B. Wilson and J.L.B. and AI050230 to J.B.G.) and the Cystic Fibrosis Foundation (GOLDBE03P0 to J.B.G.). T.A.U. was supported partly by the National Institutes of Health through University of Virginia infectious diseases training grant AI07046.
We thank Zhe Zhang, M. F. Smith, Jr., and V. S. Carl for providing reagents and technical assistance with the NF-κB and IL-8 studies, N. L. Sherman for performing mass mapping of FliCII, W. W. Newcomb for performing the electron microscopy, and C. R. Dean and A. H. Bouton for invaluable insights and helpful suggestions. pEF6::hTLR5 was a kind gift from Theodore Steiner at the University of British Columbia.
Editor: V. J. DiRita
REFERENCES
- 1.Arora, S. K., N. Dasgupta, S. Lory, and R. Ramphal. 2000. Identification of two distinct types of flagellar cap proteins, FliD, in Pseudomonas aeruginosa. Infect. Immun. 68:1474-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, A. S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 3.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 4.Chiu, C. H., A. Ostry, and D. P. Speert. 2001. Invasion of murine respiratory epithelial cells in vivo by Burkholderia cepacia. J. Med. Microbiol. 50:594-601. [DOI] [PubMed] [Google Scholar]
- 5.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cieri, M. V., N. Mayer-Hamblett, A. Griffith, and J. L. Burns. 2002. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect. Immun. 70:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dakin, C. J., A. H. Numa, H. Wang, J. R. Morton, C. C. Vertzyas, and R. L. Henry. 2002. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165:904-910. [DOI] [PubMed] [Google Scholar]
- 8.Dean, C. R., and J. B. Goldberg. 2002. Pseudomonas aeruginosa galU is required for a complete lipopolysaccharide core and repairs a secondary mutation in a PA103 (serogroup O11) wbpM mutant. FEMS Microbiol. Lett. 210:277-283. [DOI] [PubMed] [Google Scholar]
- 9.DeShazer, D., and D. E. Woods. 1996. Broad-host-range cloning and cassette vectors based on the R388 trimethoprim resistance gene. BioTechniques 20:762-764. [DOI] [PubMed] [Google Scholar]
- 10.DiGiandomenico, A., M. J. Matewish, A. Bisaillon, J. R. Stehle, J. S. Lam, and P. Castric. 2002. Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol. Microbiol. 46:519-530. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly, M. A., and T. S. Steiner. 2002. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J. Biol. Chem. 277:40456-40461. [DOI] [PubMed] [Google Scholar]
- 12.Doring, G., and F. Dorner. 1997. A multicenter vaccine trial using the Pseudomonas aeruginosa flagella vaccine IMMUNO in patients with cystic fibrosis. Behring Inst. Mitt. 98:338-344. [PubMed] [Google Scholar]
- 13.Drake, D., and T. C. Montie. 1988. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J. Gen. Microbiol. 134:43-52. [DOI] [PubMed] [Google Scholar]
- 14.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virag, G. Ross, F. G. Soriano, C. Szabo, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 15.Escotte, S., O. Tabary, D. Dusser, C. Majer-Teboul, E. Puchelle, and J. Jacquot. 2003. Fluticasone reduces IL-6 and IL-8 production of cystic fibrosis bronchial epithelial cells via IKK-β kinase pathway. Eur. Respir. J. 21:574-581. [DOI] [PubMed] [Google Scholar]
- 16.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink, J., J. H. Steer, D. A. Joyce, A. S. McWilliam, and G. A. Stewart. 2003. Pro-inflammatory effects of Burkholderia cepacia on cystic fibrosis respiratory epithelium. FEMS Immunol. Med. Microbiol. 38:273-282. [DOI] [PubMed] [Google Scholar]
- 18.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 19.Hales, B. A., J. A. Morgan, C. A. Hart, and C. Winstanley. 1998. Variation in flagellin genes and proteins of Burkholderia cepacia. J. Bacteriol. 180:1110-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna, S. L., N. E. Sherman, M. T. Kinter, and J. B. Goldberg. 2000. Comparison of proteins expressed by Pseudomonas aeruginosa strains representing initial and chronic isolates from a cystic fibrosis patient: an analysis by 2-D gel electrophoresis and capillary column liquid chromatography-tandem mass spectrometry. Microbiology 146:2495-2508. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 22.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 23.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 24.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 25.Jacchieri, S. G., R. Torquato, and R. R. Brentani. 2003. Structural study of binding of flagellin by Toll-like receptor 5. J. Bacteriol. 185:4243-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssens, S., and R. Beyaert. 2003. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 28.Kanner, S. B., A. B. Reynolds, and J. T. Parsons. 1989. Immunoaffinity purification of tyrosine-phosphorylated cellular proteins. J. Immunol. Methods 120:115-124. [DOI] [PubMed] [Google Scholar]
- 29.Liaudet, L., C. Szabo, O. V. Evgenov, K. G. Murthy, P. Pacher, L. Virag, J. G. Mabley, A. Marton, F. G. Soriano, M. Y. Kirov, L. J. Bjertnaes, and A. L. Salzman. 2003. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock 19:131-137. [DOI] [PubMed] [Google Scholar]
- 30.Luzar, M. A., M. J. Thomassen, and T. C. Montie. 1985. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect. Immun. 50:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahenthiralingam, E., A. Baldwin, and P. Vandamme. 2002. Burkholderia cepacia complex infection in patients with cystic fibrosis. J. Med. Microbiol. 51:533-538. [DOI] [PubMed] [Google Scholar]
- 33.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 36.Mizel, S. B., and J. A. Snipes. 2002. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J. Biol. Chem. 277:22414-22420. [DOI] [PubMed] [Google Scholar]
- 37.Moens, S., and J. Vanderleyden. 1996. Functions of bacterial flagella. Crit. Rev. Microbiol. 22:67-100. [DOI] [PubMed] [Google Scholar]
- 38.Montie, T. C., and G. B. Stover. 1983. Isolation and characterization of flagellar preparations from Pseudomonas species. J. Clin. Microbiol. 18:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukaida, N. 2003. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L566-L577. [DOI] [PubMed] [Google Scholar]
- 40.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 41.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 42.Palfreyman, R. W., M. L. Watson, C. Eden, and A. W. Smith. 1997. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect. Immun. 65:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddi, K., S. B. Phagoo, K. D. Anderson, and D. Warburton. 2003. Burkholderia cepacia-induced IL-8 gene expression in an alveolar epithelial cell line: signaling through CD14 and mitogen-activated protein kinase. Pediatr. Res. 54:297-305. [DOI] [PubMed] [Google Scholar]
- 44.Richman-Eisenstat, J. B., P. G. Jorens, C. A. Hebert, I. Ueki, and J. A. Nadel. 1993. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am. J. Physiol. 264:L413-L418. [DOI] [PubMed] [Google Scholar]
- 45.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt, C. K., S. C. Darnell, and A. D. O'Brien. 1996. The Salmonella typhimurium flgM gene, which encodes a negative regulator of flagella synthesis and is involved in virulence, is present and functional in other Salmonella species. FEMS Microbiol. Lett. 135:281-285. [DOI] [PubMed] [Google Scholar]
- 47.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 48.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 49.Simon, R., P. U., and A. Puhler. 1983. A broad-host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 50.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 51.Sprott, G. D., S. F. Koval, and C. A. Schnaitman. 1994. Cell fractionation, p. 83. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Kreig (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 52.Stanislavsky, E. S., and J. S. Lam. 1997. Pseudomonas aeruginosa antigens as potential vaccines. FEMS Microbiol. Rev. 21:243-277. [DOI] [PubMed] [Google Scholar]
- 53.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 54.Thomassen, M. J., C. A. Demko, J. D. Klinger, and R. C. Stern. 1985. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am. Rev. Respir. Dis. 131:791-796. [DOI] [PubMed] [Google Scholar]
- 55.Tomich, M., C. A. Herfst, J. W. Golden, and C. D. Mohr. 2002. Role of flagella in host cell invasion by Burkholderia cepacia. Infect. Immun. 70:1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Heeckeren, A. M., J. Tscheikuna, R. W. Walenga, M. W. Konstan, P. B. Davis, B. Erokwu, M. A. Haxhiu, and T. W. Ferkol. 2000. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 161:271-279. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, K. 1998. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y. [Google Scholar]
- 58.Yates, J. R., III, J. K. Eng, and A. L. McCormack. 1995. Mining genomes: correlating tandem mass spectra of modified and unmodified peptides to sequences in nucleotide databases. Anal. Chem. 67:3202-3210. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J., K. Xu, B. Ambati, and F. S. Yu. 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Investig. Ophthalmol. Vis. Sci. 44:4247-4254. [DOI] [PubMed] [Google Scholar]