Abstract
Binding of Streptococcus oralis glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to Porphyromonas gingivalis fimbriae was characterized via a biomolecular interaction analysis system. The interaction was specific, and the association constant value was 4.34 × 107 M−1, suggesting that S. oralis GAPDH functions as a dominant receptor for P. gingivalis and contributes to P. gingivalis colonization.
Interaction of Porphyromonas gingivalis, which is a predominant periodontal pathogen, with early plaque-forming bacteria plays an important role with respect to colonization in periodontal pockets (4, 12, 13). P. gingivalis interacts with a variety of other oral gram-positive bacteria, including Actinomyces naeslundii (16), Streptococcus gordonii (5), Streptococcus oralis (7), and Streptococcus sanguinis (14); these intergeneric coaggregations may lead to the initial colonization of P. gingivalis in the oral cavity.
A series of studies demonstrated that P. gingivalis 381 strongly coaggregated with S. oralis ATCC 9811; moreover, its fimbriae were primarily responsible for the interaction, in which several domains of the carboxy terminus of fimbrillin participated (1). A recent paper regarding coadhesin of S. oralis by Maeda et al. (6) revealed that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of S. oralis ATCC 9811 mediated binding to P. gingivalis 381 major fimbriae. GAPDH, which is a tetrameric enzyme of the glycolytic pathway, is responsible for the phosphorylation of glyceraldehyde-3-phosphate, which leads to generation of 1,3-bisphosphoglycerate (15). In addition to its enzymatic activity, GAPDH reportedly possesses multiple binding activities. Pancholi and Fischetti (11) found that the GAPDH of group A streptococci binds to fibronectin, lysozyme, and the cytoskeletal proteins myosin and actin; they noted that it may function in the colonization of those bacteria. In the present study, kinetic interaction of GAPDH of S. oralis ATCC 9811 with P. gingivalis 381 fimbriae was analyzed based on surface plasmon resonance spectroscopy with a biomolecular interaction analysis system (BIAcore).
S. oralis ATCC 9811 was maintained as frozen stocks and was cultured in brain heart infusion broth (BBL Microbiology Systems, Cockeysville, Md.) for 15 h at 35°C in air. Bacterial cells were harvested by centrifugation (High Speed Refrigerated Centrifuge SRX-201; Tomy Seiko Co. Ltd., Tokyo, Japan) at 5,000 × g for 30 min at 4°C; they were subsequently washed three times with 20 mM phosphate buffer supplemented with 0.15 M NaCl (phosphate-buffered saline [PBS] [pH 6.0]) and were suspended in the same buffer.
S. oralis ATCC 9811 GAPDH was purified by mild ultrasonication, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate treatment, ammonium sulfate precipitation, and chromatography with an affinity column coupled with P. gingivalis recombinant fimbrillin (rFimA) as reported previously (6). P. gingivalis 381 rFimA and S. oralis ATCC 9811 recombinant GAPDH (rGAPDH) were prepared in accordance with the methods previously reported (6, 8). Interactions between P. gingivalis rFimA and native and recombinant S. oralis GAPDH were analyzed with a BIAcore 2000 apparatus (Uppsala, Sweden). The carboxymethylated dextran matrix on the CM5 sensorchip (BIAcore) was activated with N-hydroxysuccinimide and N-ethyl-N′-[(3-dimethylamino)-propyl]-carbodiimide hydrochloride (1:1) at a flow rate of 5 μl/min at 37°C. Native GAPDH or rGAPDH (20 μg/ml) in 10 mM sodium acetate buffer (pH 4.8) was immobilized on the matrix, because they were immobilized most on the matrix at pH 4.8 among tested pHs. Although the effect of the pH on the protein conformation was not determined in this study, we confirmed that the coaggregation activity between P. gingivalis and S. oralis was almost the same at pH 4.8 as it was at pH 6.0. To equalize the amount (in moles) of the immobilized proteins, the increase in resonance units (RU) produced by immobilization was manually set at 650× (molecular mass of immobilized protein [40 kDa]/molecular mass of fimbrillin [41 kDa]) RU according to the manufacturer's manual. Excess active sites of the matrix were blocked with 1 M ethanolamine-HCl and washed with 10 mM NaOH. All materials were dissolved in 10 mM PBS (pH 6.0), which also served as a running buffer in the experiments. P. gingivalis rFimA was injected across the active CM5 (GAPDH) and an empty control CM5 surface at a flow rate of 20 μl/min at 37°C. Binding of rFimA was monitored and presented as RU in a sensorgram. One-thousand RU corresponded to a change in the surface concentration of 1 ng/mm2 on the sensory chip. At the end of each run the surface was regenerated by successive injections of 10 mM NaOH. Specific binding profiles of rFimA to the immobilized GAPDH were obtained following subtraction of the response signal from the control surface. Analysis of these kinetic parameters was conducted with BIAevaluation 3.1, a software package (BIAcore), according to the operator's manual. Protein concentration of the samples was determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) utilizing bovine serum albumin as a standard.
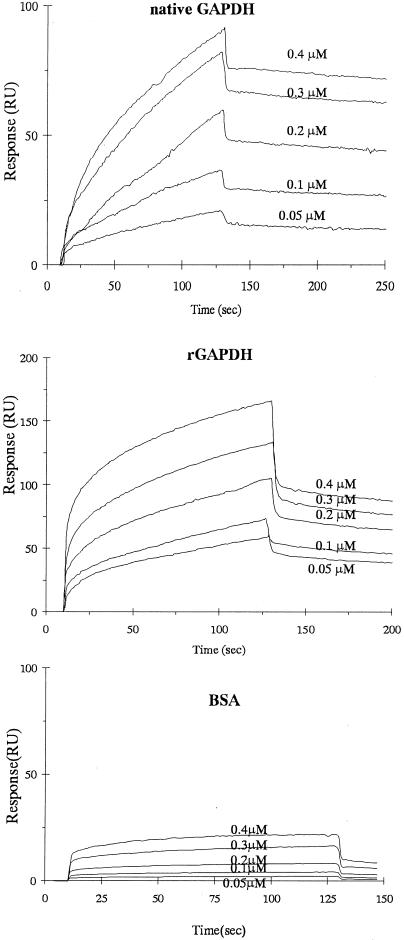
The association rate constants (kass), dissociation rate constants (kdiss), and equilibrium association constants (Ka = kass/kdiss) of native GAPDH and rGAPDH of S. oralis ATCC 9811 for binding of P. gingivalis rFimA are summarized in Table 1. The representative sensorgrams exhibited in Fig. 1 revealed that the resonance response reflecting P. gingivalis rFimA-S. oralis native GAPDH or rGAPDH interaction occurred in an analyte concentration-dependent manner. S. oralis native GAPDH and rGAPDH were characterized by significantly high kass values (2.65 × 104 M−1 s−1 and 7.41 × 104 M−1 s−1, respectively), which indicated rapid association with rFimA. At the dissociation phase, S. oralis native GAPDH and rGAPDH kdiss values were 6.11 × 10−4 M s−1 and 1.1 × 10−3 M s−1, respectively, which were representative of high stabilities. Total affinities were presented as Ka. Ka values of native GAPDH and rGAPDH with rFimA (4.34 × 107 M−1 and 6.75 × 107 M−1, respectively) demonstrated high affinity.
TABLE 1.
Kinetic parameters for P. gingivalis rFimA binding to immobilized S. oralis native GAPDH and rGAPDHa
| Ligand | kass (M−1s−1) | kdiss (s−1) | Ka (M−1) |
|---|---|---|---|
| S. oralis native GAPDH | 2.65 × 104 | 6.11 × 10−4 | 4.34 × 107 |
| S. oralis rGAPDH | 7.41 × 104 | 1.10 × 10−3 | 6.75 × 107 |
P. gingivalis rFimA was the analyte in each case.
FIG. 1.
Sensorgrams of P. gingivalis rFimA binding to immobilized S. oralis native GAPDH, rGAPDH, and bovine serum albumin in kinetic studies. P. gingivalis rFimA solution was injected over S. oralis native GAPDH, rGAPDH, or bovine serum albumin (BSA) on the sensor chip at various concentrations (0.05 to 0.4 μM).
Ka values of many antibody-protein antigen interactions occur within the range 106 to 1010 M−1 (3). In this study, Ka values for P. gingivalis rFimA and S. oralis native GAPDH and rGAPDH were 4.34 × 107 M−1 and 6.75 × 107 M−1, respectively, which indicated that these interactions were specific. P. gingivalis fimbriae reportedly bind to a variety of components, such as epithelial cells, fibroblasts, components of saliva, and several extracellular matrix proteins (2). Nakamura et al. (9) documented the following Ka values between P. gingivalis fimbriae and extracellular matrix proteins: 2.15 × 106 M−1 (laminin), 2.16 × 106 M−1 (fibronectin), 2.26 × 106 M−1 (thrombospondin), 2.76 × 106 M−1 (type I collagen), 3.08 × 106 M−1 (elastin), and 3.79 × 106 M−1 (vitronectin). In addition, Amano (2) reported Ka values between P. gingivalis fimbriae and acidic proline-rich protein, basic proline-rich glycoprotein, statherin, fibrinogen, and hemoglobin of 1.63 × 106 M−1, 1.62 × 106 M−1, 1.48 × 106 M−1, 2.16 × 106 M−1, and 2.43 × 106 M−1, respectively. The Ka value obtained in the present experiment was higher than those of the aforementioned interactions. Consequently, the interaction of P. gingivalis fimbriae with S. oralis GAPDH is considered fairly strong.
Streptococci and actinomycetes are believed to be the major initial colonizers of the pellicle on the tooth surface; furthermore, interactions between these bacteria and their substrata aid the establishment of the early biofilm community (4). Nyvad and Kilian (10) reported that S. oralis, S. sanguinis, and S. mitis represent 60 to 90% of cultivable streptococci within the first 4 h of plaque formation. In the present study, S. oralis ATCC 9811 GAPDH demonstrated high affinity with P. gingivalis fimbriae. Extensive reports have shown that the binding interaction of P. gingivalis and streptococci is multimodal; however, considering the conservation of streptococcal GAPDHs (6) and the high affinity of S. oralis GAPDH binding to P. gingivalis fimbriae, early plaque-forming streptococcal GAPDHs may contribute to P. gingivalis colonization in periodontal pockets.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) (14370694) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Editor: V. J. DiRita
REFERENCES
- 1.Amano, A., T. Fujiwara, H. Nagata, M. Kuboniwa, A. Sharma, H. T. Sojar, R. J. Genco, S. Hamada, and S. Shizukuishi. 1997. Porphyromonas gingivalis fimbriae mediate coaggregation with Streptococcus oralis through specific domains. J. Dent. Res. 76:852-857. [DOI] [PubMed] [Google Scholar]
- 2.Amano, A. 2003. Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease. J. Periodontol. 74:90-96. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson, R., and A. Falt. 1997. Experimental design for kinetic analysis of protein-protein interactions with surface plasmon resonance biosensors. J. Immunol. Methods 200:121-133. [DOI] [PubMed] [Google Scholar]
- 4.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 5.Lamont, R. J., S. G. Hersey, and B. Rosan. 1992. Characterization of the adherence of Porphyromonas gingivalis to oral streptococci. Oral Microbiol. Immunol. 7:193-197. [DOI] [PubMed] [Google Scholar]
- 6.Maeda, K., H. Nagata, Y. Yamamoto, M. Tanaka, J. Tanaka, N. Minamino, and S. Shizukuishi. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect. Immun. 72:1341-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata, H., Y. Murakami, E. Inoshita, S. Shizukuishi, and A. Tsunemitsu. 1990. Inhibitory effect of human plasma and saliva on co-aggregation between Bacteroides gingivalis and Streptococcus mitis. J. Dent. Res. 69:1476-1479. [DOI] [PubMed] [Google Scholar]
- 8.Nagata, H., A. Sharma, H. T. Sojar, A. Amano, M. J. Levine, and R. J. Genco. 1997. Role of the carboxyl-terminal region of Porphyromonas gingivalis fimbrillin in binding to salivary proteins. Infect. Immun. 65:422-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura, T., A. Amano, I. Nakagawa, and S. Hamada. 1999. Specific interactions between Porphyromonas gingivalis fimbriae and human extracellular matrix proteins. FEMS Microbiol. Lett. 175:267-272. [DOI] [PubMed] [Google Scholar]
- 10.Nyvad, B., and M. Kilian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24:267-272. [DOI] [PubMed] [Google Scholar]
- 11.Pancholi, V., and V. A. Fischetti. 1993. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc. Natl. Acad. Sci. USA 90:8154-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickard, A. H., P. Gilbert, N. J. High, P. E. Kolenbrander, and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 13.Slots, J., and R. J. Gibbons. 1978. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect. Immun. 19:254-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinson, M. W., K. Safulko, and M. J. Levine. 1991. Adherence of Porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect. Immun. 59:102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winram, S. B., and R. Lottenberg. 1996. The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology 142:2311-2320. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi, T., K. Kasamo, M. Chuman, M. Machigashira, M. Inoue, and T. Sueda. 1998. Preparation and characterization of an Actinomyces naeslundii aggregation factor that mediates coaggregation with Porphyromonas gingivalis. J. Periodont. Res. 33:460-468. [DOI] [PubMed] [Google Scholar]