Abstract
Lactoferrin is an important component of innate immunity through its sequestration of iron, bactericidal activity, and immune modulatory activity. Apolactoferrin (ALF) is the iron-depleted form of lactoferrin and is bactericidal against pneumococci and several other species of bacteria. We observed that lactoferricin (LFN), an 11-amino-acid peptide from the N terminus of lactoferrin, is bactericidal for Streptococcus pneumoniae. Strains of S. pneumoniae varied in their susceptibility to ALF. Lactoferrin is bound to the pneumococcal surface by pneumococcal surface protein A (PspA). Using mutant PspA− pneumococci of four different strains, we observed that PspA offers significant protection against killing by ALF. Knockout mutations in genes for two other choline-binding proteins (PspC and PcpA) did not affect killing by ALF. PspA did not have to be attached to the bacterial surface to inhibit killing, because the soluble recombinant N-terminal half of PspA could prevent killing by both ALF and LFN. An 11-amino-acid fragment of PspA was also able to reduce the killing by LFN. Antibody to PspA enhanced killing by lactoferrin. These findings suggested that the binding of ALF to PspA probably blocks the active site(s) of ALF that is responsible for killing.
Streptococcus pneumoniae is a major cause of pneumonia, meningitis, septicemia, and otitis media (20, 21, 41) and causes about 50,000 fatalities annually in the United States (22). Globally, pneumococci kill at least 1 million children every year (21, 47, 60). Asymptomatic human nasopharyngeal carriage is the major reservoir of pneumococci. Carriage can last from weeks to months and can be found in 40% or more of young children but is found in a lower percentage of adults (3, 49). Acquisition of carriage is generally from carriers of S. pneumoniae, with disease occurring in a subset of newly colonized individuals through invasion of the blood, middle ear, lung, and/or brain (32). Host immunity that minimizes colonization should reduce pneumococcal disease and the spread of pneumococci within the community.
A 7-valent vaccine containing capsular polysaccharides conjugated to a nontoxic variant of diphtheria toxin has successfully reduced invasive infections caused by pneumococci expressing the seven capsular types included in the vaccine (9, 53). The vaccine has been less successful at eliciting protection against carriage, partially because of a postvaccination increase in the rate of carriage of capsular types not present in the vaccine (27, 28). Several pneumococcal proteins have been examined in animals for their ability to serve as pneumococcal vaccines (15). One of these, pneumococcal surface protein A (PspA), is important for full virulence in invasive pneumococcal infections in mice (8, 16, 44). Immunization with PspA elicits protection in mice against invasive infection (13, 14, 61, 62) as well as nasal carriage (10, 13, 14, 61, 62). PspA has successfully undergone a limited human safety trial (12, 45). The human antibodies to PspA elicited during this safety trail were able to protect mice from otherwise fatal pneumococcal sepsis (12, 45). The PspA protein and/or the pspA gene have been shown to be present in all of the over 2,000 clinical isolates of deoxycholate-soluble pneumococci for which capsular types could be identified (25, 26, 38; S. K. Hollingshead, unpublished data). PspAs are divided into two major families based on their immunologic cross-reactivity and sequence diversity (25, 38).
In invasive infections, PspA's role in virulence is thought to be due to its ability to inhibit complement deposition (46, 51, 56). In invasive infections, PspA must be attached to the pneumococcal surface for it to affect virulence. Strains with mutations that result in the secretion of PspA are as avirulent as those making no PspA (51, 66). PspA may have a different role in nasal carriage. A recent study established carriage in human volunteers by using a pneumococcal strain that secreted the N-terminal half of PspA and that did not express surface PspA. Preexisting antibody to PspA appeared to prevent carriage (42). These findings suggested that, in human carriage, PspA is a colonization factor and need not be surface attached. Thus, PspA might act by a very different mechanism in mucosal carriage, where complement concentrations are very low, than in invasive disease, where complement-dependent opsonophagocytosis is critical to protection (19, 50, 56).
A possible explanation for PspA's role in carriage is suggested by its ability to bind lactoferrin (33, 34). Surface binding of lactoferrin to live pneumococci is dependent on PspA (33). Lactoferrin is present in significant quantities (1 to 14 mg/ml) in milk, saliva, and tears and is thought to offer protection against nasal colonization and infection by some bacteria (2, 31, 59). Lactoferrin is a 78-kDa single-chain glycoprotein folded into an N-lobe and a C-lobe. Each lobe contains one iron-binding site. Hololactoferrin (HLF) has one bound metal ion (usually Fe2+ or Fe3+) per lobe. Apolactoferrin (ALF) carries no metal ions (17).
ALF, but not HLF, can be bacteriostatic or bactericidal (5, 40, 63). The bacteriostatic activity of ALF is due in part to its iron chelating ability, which can deplete iron and restrict bacterial growth (48). ALF is also bactericidal against a wide range of bacteria, including Escherichia coli, Vibrio cholerae, Streptococcus mutans, and S. pneumoniae (4, 24, 58). The bactericidal activity of ALF is associated with the N-terminal 47 amino acids of the protein, which is part of the N-lobe (55). Bactericidal peptides from this region are collectively termed lactoferricins (LFNs); peptides as small as 11 amino acids are highly active (23). LFNs contain high concentrations of positively charged residues and one or more tryptophans (23, 24) in a composition reminiscent of antimicrobial peptides which attach to and destabilize negatively charged bacterial membranes (36, 57, 67).
PspA binds to human lactoferrin more strongly than to bovine lactoferrin, suggesting that the binding may be of some benefit to pneumococci, as humans, not cattle, are their natural host (33). Neisseria meningitidis and Neisseria gonorrhoeae acquire iron from HLF and have receptors that bind it more strongly than ALF (30). Pneumococci are unable to acquire iron from HLF, but acquire it instead from heme and hemoglobin (54).
We have investigated the possibility that PspA protects pneumococci from killing by ALF and that antibody to PspA may prevent binding of PspA to ALF and thus facilitate killing of pneumococci by ALF.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains of S. pneumoniae were maintained as frozen cultures (−80°C) in Todd-Hewitt Broth containing 0.5% yeast extract (THY) with 10% glycerol. The strains shown in Table 1 include the wild-type parents and their isogenic mutants. Our investigators have described strains EF6796, DBL6A, DBL1, L82016, and EF10197 previously (11); strains BG12730, BG12758, and BG6380 were from Barry M. Gray (University of Illinois at Peoria); EF5668 was from Catharina Svanborg, Lund, Sweden; CH225 was from Minjun Chen, University of Alabama at Birmingham; and MF-V178 was from Kathryn Edwards, Vanderbilt, Nashville, Tenn. Bacteria were grown in a candle jar on blood agar overnight at 37°C. After overnight growth, pneumococci were scraped from the plate and used to inoculate THY broth. Cultures were grown to an optical density at 600 nm (OD600) of 0.5 and then diluted back in THY to an OD of 0.001 to 0.005, regrown, harvested in early exponential phase (OD600 = 0.1 to 0.2), washed, and resuspended in assay solution (AS; 150 mM NaCl, 1 mM MgCl2, 50 μM CaCl2, 1 mM K2PO4; pH 7.2). AS was modified from an earlier solution of Arnold et al. (6). Bacteria were suspended to a concentration of approximately 107 CFU (OD600 = 0.1 to 0.2, or 2 × 107 CFU/ml). Isogenic mutants lacking expression of intact PspA, pneumococcal surface protein C (PspC), or pneumococcal choline-binding protein A (PcpA) were grown in the presence of appropriate antibiotics.
TABLE 1.
Parental and mutant S. pneumoniae strains used in these studies
| Strain | Capsule type | PspA family/clade | Genetic background | Phenotypea | Size (kDa) of expressed PspA | Reference |
|---|---|---|---|---|---|---|
| WU2 | 3 | 1/2 | WU2 | PspA+ | 73 | 65 |
| JY1119 | 3 | WU2 | PspA− | 65 | ||
| JY1123 | 3 | 1/2 | WU2 | PspAtr | 43 | 65 |
| D39 | 2 | 1/2 | D39 | PspA+ | 65 | 65 |
| JY182 | 2 | D39 | PspA− | 65 | ||
| JY53 | 2 | 1/2 | D39 | PspAtr | 43 | 44 |
| KM004 | 2 | 1/2 | D39 | PcpA−** | 65 | This study |
| TRE 108 | 2 | 1/2 | D39 | PspC−** | 65 | 18 |
| TIGR4 | 4 | 2/3 | TIGR4 | PspA+ | 82 | 38 |
| BR61.1 | 2/3 | TIGR4 | PspA−** | 1.2 | 51 | |
| EF3030 | 19 | 1/1 | EF3030 | PspA+ | 68 | 38 |
| TRE141 | 1/1 | EF3030 | PspAtr | 43 | 7 |
tr, truncated by deletion of C-terminal sequence. **, strain where the theoretical expressed product was 1 to 2.5 kDa was scored as a negative phenotype.
Construction of PcpA− strain.
A PcpA− strain was constructed in the D39 genetic background by using insertion-duplication mutagenesis. A 300-bp DNA fragment was amplified using primers JWJ28 (5′-AACTGTTCAAGTGGGTAATGG-3′) and JWJ29 (5′-TGAACTTGAGGAAAAGGTTGAC-3′) corresponding to bp 2276 to 2296 and 2687 to 2666 of pcpA of the TIGR4 sequence (accession number NC003028). The amplified PCR product was cloned into pTOPO cloning vector (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. The insert from the pTOPO plasmid was digested with BamHI and PstI, ligated into pJY4164, and digested with BamHI and PstI. Plasmid pJY4164 contains an erythromycin-resistant marker (1). pcpA mutant strain KM004 was generated by transforming S. pneumoniae strain D39 with pJY4164 containing the 300-bp fragment of the pcpA plasmid as described by Yother et al. with the following modifications: competence factor was synthetically synthesized and was obtained from Zymed Laboratories (San Francisco, Calif.) (37). Cultures were induced with 500 ng of competence factor/ml and incubated at 37°C for 12 min before addition of plasmid DNA. Transformants were plated on erythromycin plates (0.3 μg/ml), and resistant colonies were screened for inserts. The integration of an insert into the chromosome was determined by the PCR amplification of the insert using primer JWJ30 (5′-CCTTTACATCTGTTTTTTGTGG-3′), which binds upstream of the plasmid integration site (bp 1714 and 1735), and primer TTI (5′-GCCACTATCGACTACGCG-3′), which is specific for the plasmid pJY4164. The presence of an expected 973-bp band in the PCR product confirmed the integration of the plasmid in the expected position of the D39 chromosome.
Treatment of S. pneumoniae with ALF and HLF.
Human ALF (L0520) and HLF (L3770) were purchased from Sigma Chemical (St. Louis, Mo.). The individual lots were 87 to 95% pure, with the bulk of the nonlactoferrin molecules being immunoglobulin A. ALF lots were supplied containing from 0.01 to 0.06% iron. HLF was supplied at ≥0.15% iron. To prevent iron contamination of ALF, it was reconstituted in AS, made with Milli Q water that was collected at 18 MΩ. Pneumococci, grown as described above, were washed twice with AS and suspended in AS at 107 CFU/ml prior to the addition of ALF or HLF at the indicated concentrations (similar to those used to kill other bacteria [4]). Control tubes contained bacteria in AS only or were as indicated below. Samples were incubated at 37°C for 1 h followed by plating on blood agar to determine viability. ALF and HLF were filter-sterilized (0.45 μm) prior to use. To convert ALF to HLF, a 1% solution of ALF was made in Milli Q water and was dialyzed against 6% ferrous ammonium sulfate dissolved in Milli Q water for 36 h at 4°C with three changes of buffer, followed by dialysis against Milli Q water at 4°C for 24 h with two changes of buffer.
Treatment of growing S. pneumoniae cells with ALF and HLF.
To determine if ALF can kill actively growing pneumococci, strain D39 was grown in THY as described above and then divided into three 10-ml aliquots (still in THY) to which ALF or HLF was added at concentrations of 1 mg/ml (12.3 μM). All three tubes were incubated at 37°C and monitored at 30-min intervals by measuring the OD600 and by plating 20-μl aliquots of 1:10-serially titrated bacteria on blood plates to determine viability.
Treatment of bacteria with LFN.
Two LFN peptides, LFN1 (GRRRRSVQWCA [amino acids 1 to 11 of human ALF]) and LFN2 (FQWQRNMRKVR [amino acids 21 to 31]) (Research Genetics, Invitrogen, Huntsville, Ala.), were tested for their ability to kill strains D39 and JY182 (PspA− D39) in AS at the concentrations indicated.
Ability of rPspA and a peptide derived from PspA (SM1) to block killing by ALF or LFN.
For inhibition with soluble recombinant PspA (rPspA), conditions were identical to those for ALF treatment of S. pneumoniae in AS, with the exception that rPspA or peptide SM1 was added as inhibitor. rPspA was added to the assay mixture at concentrations of 10 μg/ml (0.2 μM) or 50 μg/ml (1.16 μM). rPspA UAB055 was purified as previously described (43) and diluted into AS. rPspA UAB055 is cloned from strain Rx1, which is a nonencapsulated variant of D39 (44). In the text, we have referred to this as D39 PspA to avoid confusion. The peptide SM1 from the clade-defining region of PspA (residues 269 to 278) and the region known to bind lactoferrin (residues 193 to 288) (33) were synthesized by Research Genetics (Invitrogen), with the sequence NH2-AAKKAELEKT-COOH. SM1 was also suspended in AS at a concentration of 2.2 μg/ml (2 μM). The amino acid positions given are based on the mature protein sequence (64). Note that when the sequences of D39, Rx1, and other pspAs were compared by Hollingshead et al. (38), the sequence numbering included the 31-amino-acid leader peptide.
Killing in the presence of polyclonal anti-PspA.
S. pneumoniae strains D39 and JY182 (PspA− D39) were grown as described above and resuspended in AS at a concentration of 1 × 107 to 2 × 107 CFU/ml. Polyclonal anti-PspA (25), anti-PspC (18), or preimmune rabbit sera were added at dilutions of 1/100 in AS and incubated at 37°C for 30 min. After washing twice with AS, the strains were incubated with 250 μg of ALF/ml (3.1 μM) for 1 h. The two dilutions were then plated on blood agar plates to enumerate surviving CFU.
Statistical analyses.
P values were calculated using the nonparametric Mann-Whitney two-sample rank test with 6 to 18 replicates of each sample. Log CFU killed was determined by subtracting the log CFU of pneumococci incubated in ALF from the log CFU of pneumococci incubated in AS only.
RESULTS
Effect of lactoferrin on S. pneumoniae.
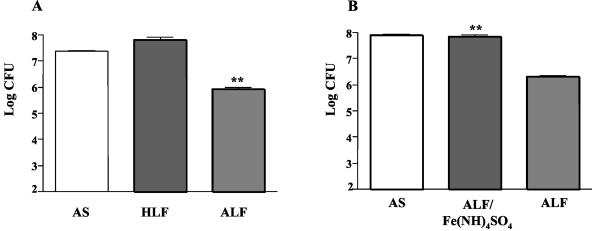
We examined the effects of ALF and HLF on the viability of S. pneumoniae in AS. The two proteins were added separately to tubes of S. pneumoniae strain D39 (capsular type 2, PspA family one) at a concentration of 3.1 μM (250 μg/ml). Treatment with ALF, but not HLF, resulted in a decline in the number of CFU (Fig. 1A). Almost 1 log fewer CFU were observed with ALF treatment compared with the CFU of the bacteria in AS alone (P = 0.007). The number of bacteria after treatment with ALF was 1.8 log below the number of CFU at time zero (data not shown). No bactericidal effect was observed when the culture was incubated with an equimolar concentration of HLF. We also observed that ALF was just as bactericidal when strain D39 was suspended in a 1 mM pH 7.2 potassium phosphate buffer in place of AS. Higher concentrations of phosphate prevented killing by ALF (data not shown). As a further control, we prepared HLF from our ALF by dialyzing it for 36 h against three changes of 6% ferrous ammonium sulfate followed by extensive dialysis against Milli Q water to remove excess unbound iron. This treatment reduced the killing capacity of the ALF by >1.5 log (Fig. 1B). The dialysis of saturated lactoferrin against water was especially important, because free iron is toxic to pneumococci.
FIG. 1.
Effects of ALF and HLF on the growth of strain D39 S. pneumoniae. (A) S. pneumoniae strain D39 was incubated with 3.1 μM (250 μg) ALF, 3.1 μM HLF, or in AS alone. Viability of cells was determined by plating aliquots on blood agar plates after 1 h at 37°C; each bar represents the log10 CFU. (B) Effect of converting ALF to HLF by treatment with 6% ferrous ammonium sulfate (followed by dialysis in water to remove excess iron). Each bar represents the mean log of six replicates from two independent experiments. The presence of ALF resulted in a significant (**) decline in viability (P = 0.007), whereas saturation of ALF with ferrous ammonium sulfate resulted in inhibition of the bactericidal effects of ALF (P < 0.0001). In this figure and all subsequent figures, the error bars represent the standard errors of means.
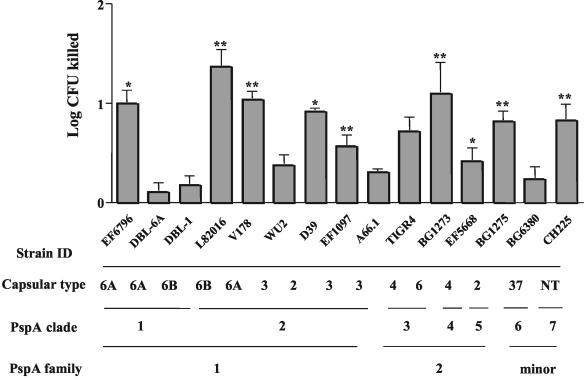
When these studies were expanded to include 14 strains for which the PspA type and capsular type were known, we observed that 3.1 μM ALF could cause at least a 0.5 log killing in 10 of the 14 strains after 1 h at 37°C. Among this relatively small panel of pneumococci, there was no apparent association between susceptibility to lactoferrin-mediated killing and capsular type, PspA clade, or PspA family (Fig. 2).
FIG. 2.
Comparison of the bactericidal effect of ALF on strains of S. pneumoniae of different capsular types and PspA clades or families. NT, not typeable by capsular typing. Pneumococci were treated with or without 3.1 μM ALF for 1 h in AS. Log CFU killed was determined by subtracting the log CFU of pneumococci incubated in ALF from the log CFU of pneumococci incubated in AS. Statistically significant killing is indicated by P values (*, P ≤ 0.01; **, P ≤ 0.001). Each bar represents the mean log killing of 18 replicates from three independent experiments.
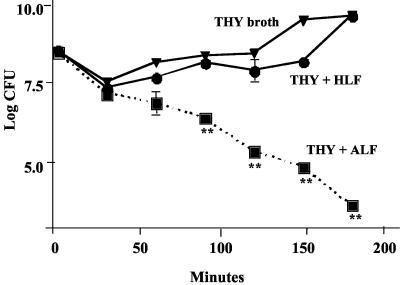
Since AS contained no nutrients, it was important to determine whether ALF would also be able to kill pneumococci under normal growth conditions. In this study we examined the effects of ALF or HLF on the viability of capsular type 2 S. pneumoniae strain D39 suspended in THY broth at 37°C. As a control, D39 was incubated in THY alone. The pneumococci were killed by ALF, but no effect on growth and viability of bacteria was observed in either THY alone or THY containing HLF (Fig. 3). Similar studies with two additional strains of S. pneumoniae (EF3030 and WU2) in THY gave similar results (data not shown).
FIG. 3.
Inhibition of pneumococcal growth in the presence of ALF. D39 S. pneumoniae cells were inoculated into either 10 ml of THY, THY containing 1 mg of ALF/ml (12.4 μM) or 1 mg of HLF/ml (12.4 μM). Samples were taken at the indicated times, serially diluted, and plated at the indicated time points to determine CFU per milliliter. Differences between control and ALF-treated samples that were significant at P = 0.0023 are shown. Data are representative of two independent experiments with six replicates in each experiment.
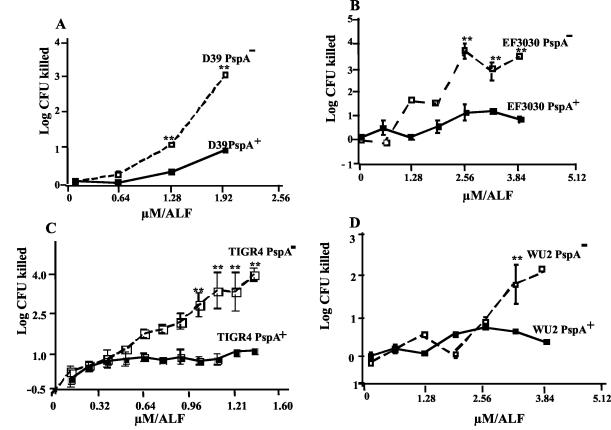
Bactericidal effect of ALF on PspA+ and PspA− strains. To see if binding to PspA might be required for bacterial killing or if it might provide some protection against bacterial killing, we examined four different strains of S. pneumoniae (capsular types 2, 3, 4, and 19F) and their PspA− isogenic mutants for susceptibility to killing by ALF. In each case, more killing was observed for the PspA− than PspA+ strains (Fig. 4). For each pair of strains, the differences in killing between the PspA− and PspA+ strains increased as the ALF concentration increased. One of these three strains, WU2, exhibited such a high innate resistance to killing that even the PspA− strain showed no evidence of being killed in this experiment until the ALF concentration reached 3.1 μM (250 μg/ml). The PspA− mutants of strains D39, WU2, and TIGR4 secreted no PspA. The mutant of EF3030 secreted a truncated 43-kDa N-terminal fragment of PspAs.
FIG. 4.
Killing of PspA-positive and -negative strains over a range of concentrations of ALF. Mutants of D39, TIGR4, and WU2 make no PspA. The mutant of EF3030 makes a 43-kDa secreted fragment whose C-terminal 25 kDa has been deleted. Statistical analysis indicated that there was a significant difference (**, P ≤ 0.004) between ALF-treated parent strains and their isogenic mutants. Each data point represents a mean log CFU of 18 replicates from three independent experiments. The numbers of CFU of each culture at the zero time point were between 1.2 × 107 and 2.5 × 107.
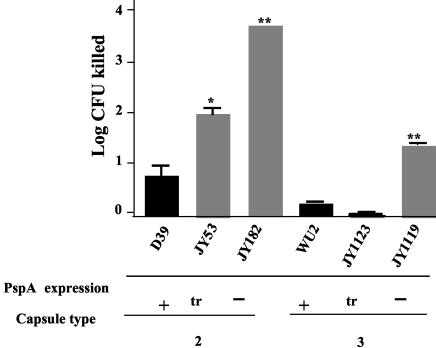
For strains D39 and WU2, we also had variants that either expressed no PspA or expressed an N-terminal fragment of their PspA that was secreted into the medium and therefore was no longer attached to the surface (65). In each case, the strains making surface PspA and those secreting fragments of PspA were more resistant to killing then those making no PspA (Fig. 5). The D39 strain making the truncated PspA showed intermediate resistance to killing by ALF. The WU2 strain making truncated PspA resulted in as much protection against ALF as was afforded by making the whole molecule.
FIG. 5.
Comparison of abilities of ALF to kill PspA-positive strains and their isogenic mutants making either truncated (secreted) PspA or no PspA. Strains JY53 and JY182 are pspA mutants of capsular type 2 strain D39. JY182 makes no PspA, and JY53 makes the N-terminal 43 kDa of D39 PspA. Strains JY1123 and JY1119 are pspA mutants of capsular type 3 strain WU2. JY1119 makes no PspA, and JY1123 makes the N-terminal 43 kDa of WU2 PspA. All strains were incubated with 3.1 μM ALF for 1 h at 37°C. Statistically significant effects of the mutations on killing by ALF are indicated: *, P ≤ 0.01; **, P ≤ 0.002. Each bar represent the mean log and standard error of 18 replicates performed in three independent experiments.
The susceptibility of the PspA− strain was also evaluated over several time points ranging from 30 to 120 min, with 1.25 μM ALF. This dose of ALF was the lowest that gave significant killing against these strains. By 30 min there was 0.75 log more killing of PspA− than PspA+ D39, and this difference did not change with increasing time. For EF3030, significantly (P < 0.05) greater killing of the PspA− pneumococci (0.75 to 0.80 log killing) was not observed until 60 min, and the difference was still increasing at 120 min (data not shown).
Although the kinetics of killing (preceding paragraph) and the amount of ALF required to kill (Fig. 4 and 5) differed for different strains, in all cases the expression of PspA was able to reduce killing by ALF. These data make a robust case for PspA being able to inhibit the ability of ALF to kill pneumococci. The results also demonstrate that PspA need not be surface attached to be able to protect pneumococci against killing by added ALF.
Bactericidal effects of ALF on PspC− and PcpA− S. pneumoniae mutants.
To test the specificity of the effects of the pspA mutations on killing by 3.1 μM ALF, we also examined mutants of D39 unable to make PspC or PcpA. Mature PspC and PcpA of strain D39 are 90- and 79-kDa surface choline-binding proteins (18, 52), but neither contributes to the surface binding of lactoferrin because PspA− pneumococci, which still make PspC and PcpA, do not bind lactoferrin (33). The PspC and PcpA mutants made truncated N-terminal 2.2- and 1.0-kDa proteins. In this study the PspA− strain showed 1.8 log of killing compared with 1 log of killing for the PspA+ strain over 1 h of incubation at 37°C. Neither the pspC nor pcpA mutants resulted in an increase in susceptibility to killing by ALF (data not shown). These data made it clear that the insertion-duplication mutation process in itself does not affect resistance to killing by ALF and that the surface expression of PspC and PcpA is not necessary for protection against killing by ALF.
Recombinant PspA inhibits the bactericidal effects of ALF.
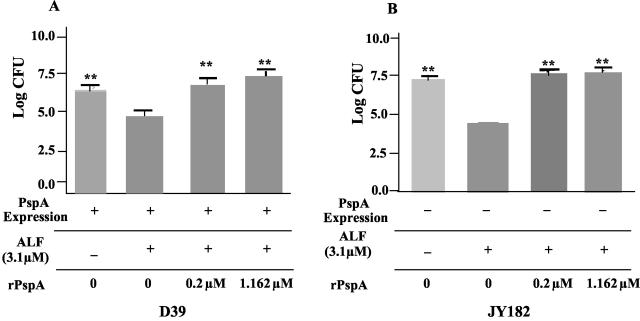
To test the possibility that cell-free PspA could interfere with killing by ALF, we examined the ability of a rPspA, UAB055, to prevent killing by ALF. UAB055 comprises the N-terminal 302 amino acids of the mature PspA from strain D39 (39). This fragment is therefore inclusive of the region of PspA (amino acids 192 to 288) within which the binding site of lactoferrin has been mapped (33). These studies were conducted using viable D39 and its PspA− variant, JY182, as a target strain. In each case, 0.2 μM rPspA was able to cause complete protection against killing by a 15-fold excess (3.1 μM) of ALF (Fig. 6). As controls, we added 3.1 μM human albumin or 1.16 μM rPsaA. Neither of these molecules inhibited killing by ALF (data not shown). The ability of PspA to readily prevent killing by ALF, whether the PspA was bound to the cell surface or in solution, suggests that the PspA may be blocking ALF-mediated killing by neutralizing the site(s) on lactoferrin responsible for the killing. The ability of rPspA to inhibit killing by an excess of ALF suggests that one PspA may bind and inactivate more than one ALF. An alternative, more speculative, explanation might be that the rPspA somehow “catalyzes” a conformational transformation in ALF that prevents it from being able to kill.
FIG. 6.
Inhibition of bactericidal activity of ALF in the presence of soluble 43-kDa rPspA from strain Rx1 for 1 h at 37°C. S. pneumoniae strain D39 (A) and its isogenic PspA− mutant JY182 (B) were incubated with AS, ALF, or ALF in the presence of rPspA for 1 h. **, the viability of the pneumococci was significantly (P ≤ 0.002) different from viability of pneumococci treated with ALF but no rPspA. Data shown are from three independent experiments, and each bar represents the mean log and standard error of a total of 12 replicates.
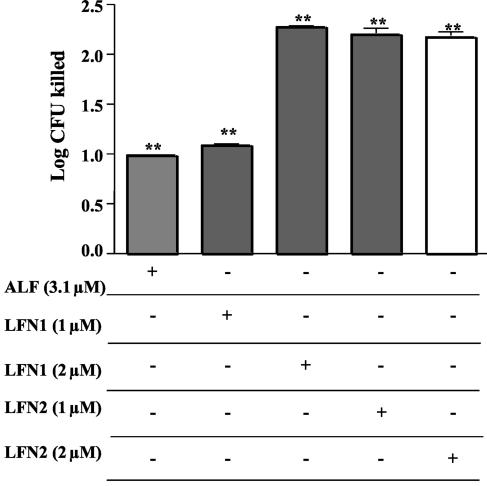
Bactericidal effects of LFN1 and LFN2.
The peptides LFN1, GRRRRSVQWCA (amino acids 1 to 11 of human ALF), and LFN2, FQWQRNMRKVR (amino acids 21 to 31), were able to kill strain D39. Two micromolar concentrations each of LFN1 and LFN2 could cause about 2 logs of killing in AS after a 60-min incubation. This was significantly more killing than was observed with 3.1 μM ALF (Fig. 7).
FIG. 7.
Bactericidal activity of ALF and two LFN molecules compared with that of ALF. LFNs and ALF were incubated with strain D39 at the indicated concentrations in AS. All treatments with ALF, LFN1, or LFN2 caused significant decreases in viability compared with the diluent control (**, P < 0.0023). Treatment with 3.1 μM ALF was less effective than treatment with 2 μM LFN1 or either 1 or 2 μM LFN2 (**, P < 0.0023). Data presented here represent the mean log and standard error of 12 replicates performed in two independent experiments.
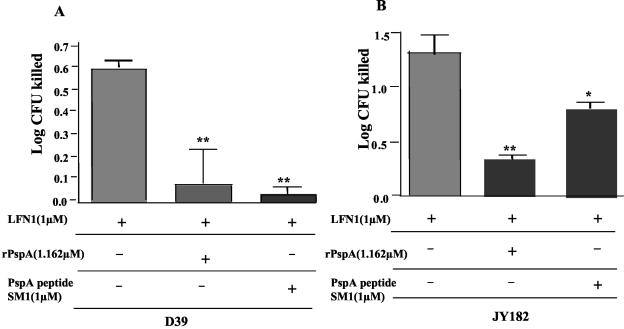
rPspA and its peptide SM1 block the activity of LFN1.
SM1, a 10-amino-acid peptide of Rx1 PspA (amino acids 269 to 278), encodes a sequence highly conserved among family 1 and family 2 PspAs (38) within amino acids 193 to 288 in the N-terminal half of PspA, where lactoferrin is known to bind (33). Using 1 μM SM1, we were able to significantly reduce (1 log10) the killing of PspA− strain D39 pneumococci caused by 1 μM LFN1. Similar results were obtained when PspA+ strain D39 was treated with LFN1 in the presence of SM1 (Fig. 8). SM1 was not, however, able to inhibit killing by ALF (data not shown). The sequence of SM1 was present only once in PspA and does not occur in PspC or PcpA. Another peptide encoding Rx1 amino acids 230 to 244 (SM2) was not protective against LFN1 (data not shown).
FIG. 8.
Effect of rPspA and a 10-amino-acid peptide of PspA (SM1) on the bactericidal activity of LFN1. D39 (A) pneumococci or its PspA− mutant JY182 (B) were incubated with 1 μM LFN1 in the presence or absence of the indicated amounts of rPspA or SM1 for 60 min at 37°C. Surviving CFU were enumerated by plating, and the data are expressed as CFU killed in comparison with a sample lacking LFN1. P values versus D39 parent are indicated as follows: *, P ≤ 0.01; **, P ≤ 0.001. Each bar is the mean of 12 replicates from two independent experiments.
Ability of antibody to PspA to facilitate killing of a strain of PspA+ pneumococci with ALF.
If PspA blocks killing of pneumococci by binding to ALF, then it would be expected that antibody to PspA might prevent binding of ALF to PspA and thereby enhance the killing of pneumococci by ALF. In this study we observed that ALF was causing almost 1 log of killing of D39 pneumococci. If the pneumococci were incubated with a 1/100 dilution of a rabbit antiserum to PspA prior to the addition of ALF, about 2.5 logs of killing were observed (Fig. 9). Thus, the antibody to PspA enhanced the killing by ALF by 1.5 logs. As controls we used (also at 1/100 dilution) a rabbit antiserum to PspC from strain D39 (18), preimmune rabbit sera from the PspA-immune rabbit, and normal mouse serum; none of these enhanced killing by ALF (data not shown).
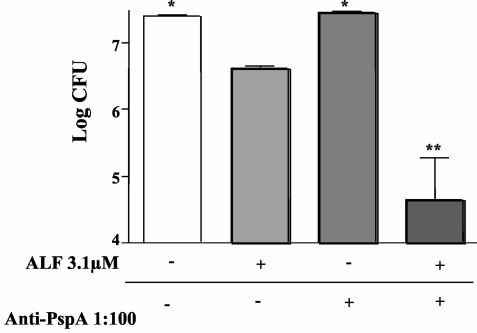
FIG. 9.
Ability of antibody to PspA to facilitate the killing of PspA+ D39 S. pneumoniae. Antibody to PspA was able to enhance killing of pneumococcal strain D39 in the presence of ALF. Bacteria were either incubated in assay buffer alone, 3.1 μM ALF, 1:100 polyclonal anti-PspA, or ALF and polyclonal anti-PspA as indicated in the figure. Statistical significance was calculated for comparisons of each group with the pneumococci treated with ALF (*, P ≤ 0.03; **, P ≤ 0.001). The data are representative of two independent experiments with six replicates in each experiment.
Treatment with ALF in the presence of saliva.
As lactoferrin is an antibacterial protein found in many human secretions, it was important to determine if it is bactericidal in that environment. Early log-phase D39 pneumococci were incubated in two fresh samples of human saliva. In one sample the pneumococci were killed so quickly that it was not possible to study the effects of added ALF. In the other sample we incubated D39 in the saliva for 1 h at 37°C with and without added ALF. At 3.1 μM ALF, we observed 1.3 logs of killing. At 6.2 μM ALF, we observed 2.1 logs of killing (data not shown).
DISCUSSION
The data in this paper confirm and extend the earlier observation (40) that ALF has bactericidal activity against S. pneumoniae. In contrast, HLF was not found to have an effect on pneumococcal viability. PspA has been shown to be the attachment site for lactoferrin binding to the pneumococcal surface (33, 34). In the present paper we have shown that the attachment of ALF to PspA is not necessary for ALF to kill pneumococci. In fact, the killing of pneumococci by ALF is much more efficient if PspA is absent. The effects of mutations in pspA on ALF-mediated killing were specific: the disruption of genes for two other choline-binding surface proteins (pspC and pcpA) failed to show any effect on the bactericidal activity of ALF.
The observations that (i) ALF can kill pneumococci, (ii) virtually all lactoferrin surface binding to pneumococci is via PspA, and (iii) PspA prevents killing by ALF argue that an important role of PspA in the natural history of pneumococcal carriage and/or infections is to prevent this killing of pneumococci by ALF. Our observation that added ALF can kill pneumococci suspended in human saliva strongly supports these conclusions. The demonstration that antibody to PspA can enhance killing of pneumococci by ALF strongly suggests that antibody to PspA may be able to protect against pneumococcal colonization and possibly also against infection.
The relationship between lactoferrin binding and pathogenesis is different for pneumococci than for some other bacteria where binding to ALF has been shown to be a prerequisite for its bactericidal effects. The differences in sensitivity of Micrococcus luteus strains to killing by ALF are attributed to the differential binding of ALF to surface proteins of M. luteus (29). In the case of pneumococci, the ALF that binds to PspA appears to be blocked from being able to kill pneumococci.
The ability of cell-free PspA to block ALF-mediated killing indicates that PspA may block killing by binding the active killing site(s) on ALF. Our observation that the molar concentration of rPspA required to block ALF-mediated killing could be less than the concentration of ALF indicates that one PspA molecule can bind and inhibit more than one ALF molecule.
Both of the LFN peptides, LFN1 and LFN2, were also able to kill pneumococci. This suggests that the mechanism that ALF uses to kill pneumococci is probably membrane destabilization similar to that caused by other antibacterial cationic peptides rich in tryptophans (35). The killing activity of LFN1 was strongly inhibited by rPspA and was inhibited almost as well by SM1, a 10-amino-acid peptide of PspA. SM1 is highly conserved in PspA clades 1 to 5, which contain all the PspA sequences in the two major PspA families (38). SM1 is also the only conserved region within the region of Rx1 PspA previously shown to bind lactoferrin (33).
The ability of SM1 to inhibit killing by LFN indicates that the region of PspA that includes SM1 may be particularly important in blocking killing by LFN and possibly by ALF as well. It is also possible that rPspA binds LFN1 at more sites than just SM1. SM1's inability to inhibit killing by ALF may also be an indication that ALF has sites involved in killing that are not recognized by SM1. It is also possible that SM1 is not in exactly the same configuration as the corresponding amino acids in the complete PspA molecule. Our findings also raise the possibility that differences in the PspA expressed, or in the amounts of PspA expressed, may explain some of the variability in susceptibility of different pneumococci to killing by ALF.
Based on the results presented here, it is clear that PspA can prevent killing by ALF regardless of whether it is secreted or surface attached. We have also shown that antibody to PspA can enhance killing by ALF, presumably by preventing ALF from being bound by PspA. Thus, our findings suggest a mechanism by which antibody to PspA in airway secretions might prevent or reduce carriage of pneumococci and offer an explanation to help explain data in the mouse showing that mucosal immunity to PspA can reduce colonization (10, 61).
Acknowledgments
We acknowledge James Watt for his interest in these studies and for critical review of the manuscript, Jason Johnston for making his pcpA PCR primers available to us, Janice King for her technical assistance, and Debasish Chattopadhyay for help in interpreting the inhibition data.
Editor: J. N. Weiser
REFERENCES
- 1.Achen, M. G., B. E. Davidson, and A. J. Hillier. 1986. Construction of plasmid vectors for the detection of streptococcal promoters. Gene 45:45-49. [DOI] [PubMed] [Google Scholar]
- 2.Akin, D. T., M. Q. Lu, S. J. Lu, S. Kendall, J. Rundegren, and R. R. Arnold. 1994. Bactericidal activity of different forms of lactoferrin. Adv. Exp. Med. Biol. 357:61-70. [DOI] [PubMed] [Google Scholar]
- 3.Aniansson, G., B. Alm, B. Andersson, P. Larsson, O. Nylen, H. Peterson, P. Rigner, M. Svanborg, and C. Svanborg. 1992. Nasopharyngeal colonization during the first year of life. J. Infect. Dis. 165(Suppl. 1):S38-S42. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold, R. R., J. E. Russell, W. J. Champion, M. Brewer, and J. J. Gauthier. 1982. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect. Immun. 35:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold, R. R., J. E. Russell, W. J. Champion, and J. J. Gauthier. 1981. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect. Immun. 32:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. The role of pneumococcal surface protein C (PspC) in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. [DOI] [PubMed] [Google Scholar]
- 10.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. A strong association between capsular type and mouse virulence among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 13.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. J. Benjamin. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 14.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. White, K. Prellner, A. Hermansson, P. C. Aerts, H. Van Dijk, and M. J. Crain. 1997. PspA and PspC: their potential for use as pneumococcal vaccines. Microb. Drug Resist. 3:401-408. [DOI] [PubMed] [Google Scholar]
- 15.Briles, D. E., J. C. Paton, and S. K. Hollingshead. 2004. Pneumococcal common proteins and other vaccine strategies, p. 459-469. In M. M. Levine, J. B. Kaper, R. Rappuoli, M. Liu, and M. Good (ed.), New generation vaccines, 3rd ed. Marcel Dekker, Inc., New York, N.Y.
- 16.Briles, D. E., J. Yother, and L. S. McDaniel. 1988. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev. Infect. Dis. 10:S372-S374. [DOI] [PubMed] [Google Scholar]
- 17.Brock, J. H. 2002. The physiology of lactoferrin. Biochem. Cell. Biol. 80:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4):S797-S805. [DOI] [PubMed] [Google Scholar]
- 20.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, and R. F. Breiman. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J. Infect. Dis. 174:986-993. [DOI] [PubMed] [Google Scholar]
- 21.Butler, J. C., E. D. Shapiro, and G. M. Carlone. 1999. Pneumococcal vaccines: history, current status, and future directions. Am. J. Med. 107(1A):69S-76S. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. 1996. Defining the public health impact of drug-resistant Streptococcus pneumoniae: report of a working group. Morb. Mortal. Wkly. Rep. 45:1-20. [PubMed] [Google Scholar]
- 23.Chapple, D. S., C. L. Joannou, D. J. Mason, J. K. Shergill, E. W. Odell, V. Gant, and R. W. Evans. 1998. A helical region on human lactoferrin. Its role in antibacterial pathogenesis. Adv. Exp. Med. Biol. 443:215-220. [PubMed] [Google Scholar]
- 24.Chapple, D. S., D. J. Mason, C. L. Joannou, E. W. Odell, V. Gant, and R. W. Evans. 1998. Structure-function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111. Infect. Immun. 66:2434-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coral, M. C. V., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, and D. E. Briles. 2001. Families of pneumococcal surface protein A (PspA) of Streptococcus pneumoniae invasive isolates recovered from Colombian children. Emerg. Infect. Dis. 7:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crain, M. J., W. D. Waltman, J. S. Turner, J. Yother, D. E. Talkington, L. M. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 28.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greenburg, O. Abramson, P. M. Mendalman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 29.de Lillo, A., L. M. Quiros, and J. F. Fierro. 1997. Relationship between antibacterial activity and cell surface binding of lactoferrin in species of genus Micrococcus. FEMS Microbiol. Lett. 150:89-94. [DOI] [PubMed] [Google Scholar]
- 30.Desai, P. J., E. Garges, and C. A. Genco. 2000. Pathogenic neisseriae can use hemoglobin, transferrin, and lactoferrin independently of the tonB locus. J. Bacteriol. 182:5586-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farnaud, S., and R. W. Evans. 2003. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 40:395-405. [DOI] [PubMed] [Google Scholar]
- 32.Gray, B. M., G. M. Converse III, and H. C. Dillon. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 33.Hakansson, A., H. Roche, S. Mirza, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of the binding of human lactoferrin to pneumococcal surface protein A (PspA). Infect. Immun. 69:3372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerschmidt, S., G. Bethe, P. Remanen, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haug, B. E., M. L. Skar, and J. S. Svendsen. 2001. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Peptide Sci. 7:425-432. [DOI] [PubMed] [Google Scholar]
- 36.Haug, B. E., and J. S. Svendsen. 2001. The role of tryptophan in the antibacterial activity of a 15-residue bovine lactoferricin peptide. J. Peptide Sci. 7:190-196. [DOI] [PubMed] [Google Scholar]
- 37.Haverstein, L. S., G. Coomaraswamy, and D. A. Morrison. 1995. An unmodified heptadecapetide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92:11140-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollingshead, S. K., R. S. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jedrzejas, M. J., S. K. Hollingshead, J. Lebowitz, L. Chantalat, D. E. Briles, and E. Lamani. 2000. Production and characterization of the functional fragment of pneumococcal surface protein A. Arch. Biochem. Biophys. 373:116-125. [DOI] [PubMed] [Google Scholar]
- 40.Leitch, E. C., and M. D. Willcox. 1998. Synergic antistaphylococcal properties of lactoferrin and lysozyme. J. Med. Microbiol. 47:837-842. [DOI] [PubMed] [Google Scholar]
- 41.Marston, B. J., J. F. Plouffe, T. M. File, Jr., B. A. Hackman, S. J. Salstrom, H. B. Lipman, M. S. Kolczak, R. F. Breiman, et al. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch. Intern. Med. 157:1709-1718. [PubMed] [Google Scholar]
- 42.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel, L. S., J. S. Sheffield, P. Delucchi, and D. E. Briles. 1991. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 59:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDaniel, L. S., J. Yother, M. Vijayakumar, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 46.Neeleman, C., P. M. Sibyl, S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Brien, K. L., and H. Nohynek. 2003. Report from a W.H.O. working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 22:E1-E11. [DOI] [PubMed] [Google Scholar]
- 48.Otto, B. R., A. M. Verweij-van Vught, and D. M. MacLaren. 1992. Transferrins and heme compounds as iron sources for pathogenic bacteria. Crit. Rev. Microbiol. 18:217-233. [DOI] [PubMed] [Google Scholar]
- 49.Rello, J., R. Rodriguez, P. Jubert, and B. Alverez. 1996. Severe community-acquired pneumonia in the elderly: epidemiology and prognosis. Clin. Infect. Dis. 23:723-728. [DOI] [PubMed] [Google Scholar]
- 50.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun. 72:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspAs can inhibit complement deposition and confer virulence to a capsular 3 serotype Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Beato, A. R., R. Lopez, and J. L. Garcia. 1998. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol. Lett. 164:207-214. [DOI] [PubMed] [Google Scholar]
- 53.Shinefield, H. R. 2000. Safety and efficacy of the heptavalent pneumococcal conjugate vaccine. Infect. Dis. Children June:12-15. [Google Scholar]
- 54.Tai, S. S., C. J. Lee, and R. E. Winter. 1993. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect. Immun. 61:5401-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomita, M., W. Bellamy, M. Takase, K. Yamauchi, H. Wakabayashi, and K. Kawase. 1991. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy Sci. 74:4137-4142. [DOI] [PubMed] [Google Scholar]
- 56.Tu, A.-H. T., R. L. Fulgham, M. A. McCory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A (PspA) inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel, H. J., D. J. Schibli, W. Jing, E. M. Lohmeier-Vogel, R. F. Epand, and R. M. Epand. 2002. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell. Biol. 80:49-63. [DOI] [PubMed] [Google Scholar]
- 58.Wang, X., S. Hirmo, R. Willen, and T. Wadstrom. 2001. Inhibition of Helicobacter pylori infection by bovine milk glycoconjugates in a BALB/cA mouse model. J. Med. Microbiol. 50:430-435. [DOI] [PubMed] [Google Scholar]
- 59.Ward, P. P., S. Uribe-Luna, and O. M. Conneely. 2002. Lactoferrin and host defense. Biochem. Cell. Biol. 80:95-102. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. 1999. Pneumococcal vaccines. W.H.O. position paper. Wkly. Epidemiol. Rec. 74:177-183. [PubMed] [Google Scholar]
- 61.Wu, H.-Y., M. Nahm, Y. Guo, M. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage and infection with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto, M., L. S. McDaniel, K. Kawabata, D. E. Briles, R. J. Jackson, J. R. McGhee, and H. Kiyono. 1997. Oral immunization with PspA elicits protective humoral immunity against Streptococcus pneumoniae infection. Infect. Immun. 65:640-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison III. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yother, J., and D. E. Briles. 1992. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J. Bacteriol. 174:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yother, J., L. S. McDaniel, M. J. Crain, D. F. Talkington, and D. E. Briles. 1991. Pneumococcal surface protein A: structural analysis and biological significance, p. 88-91. In G. M. Dunny, P. P. Cleary, and L. L. McKay (ed.), Genetics and molecular biology of streptococci, lactococci, and enterococci. American Society for Microbiology, Washington, D.C.
- 67.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]