Abstract
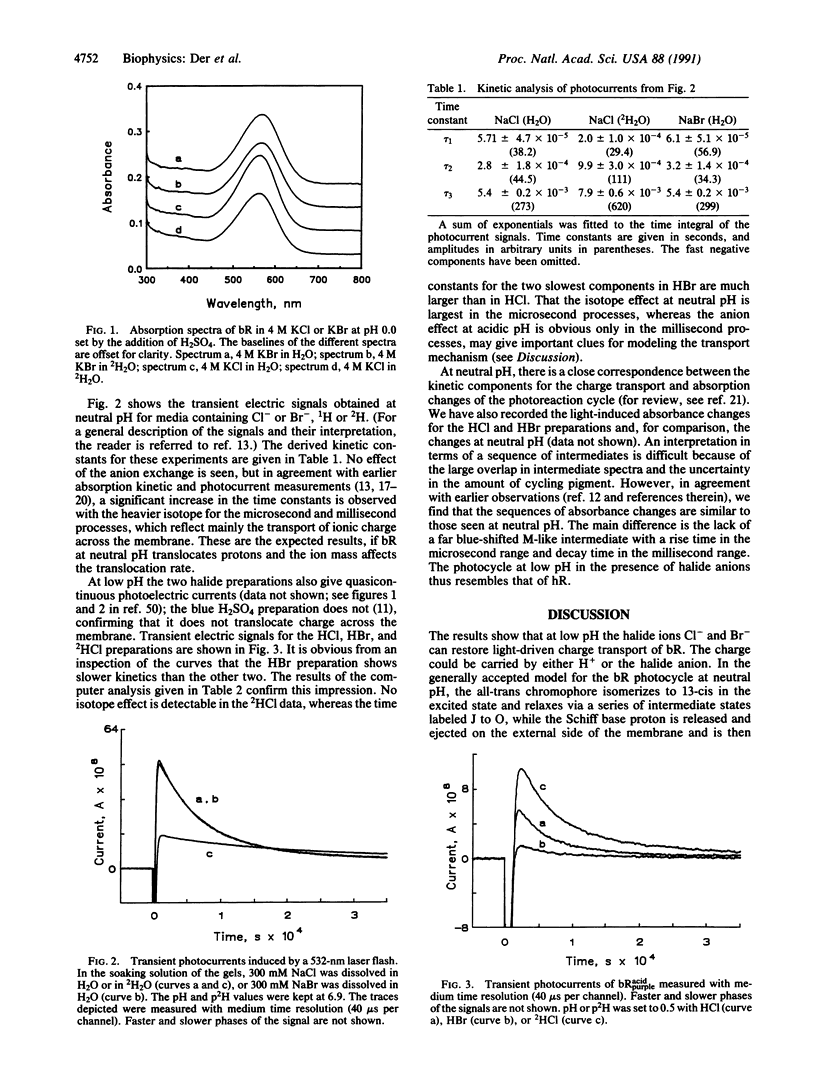
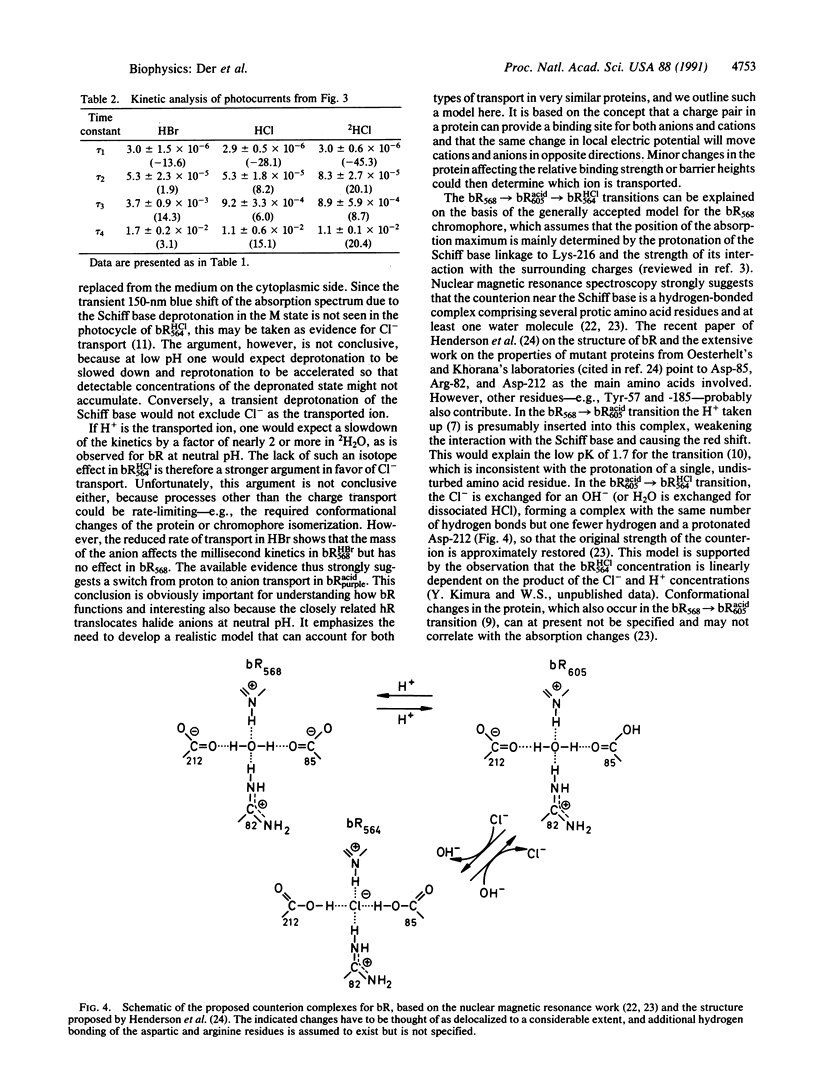
Bacteriorhodopsin (bR568) in purple membrane near pH 2 shifts its absorption maximum from 568 to 605 nm forming the blue protein bRacid605, which no longer transports protons and which shows no transient deprotonation of the Schiff base upon illumination. Continued acid titration with HCl or HBr but not H2SO4 restores the purple chromophore to yield bRHCl564 or bRHBr568. These acid purple forms also regain transmembrane charge transport, but no transient Schiff base deprotonation is observed. In contrast to bR568, no rate decrease of the bRacidpurple transport kinetics is detected in 2H2O; however, the transport rate decreases by a factor of approximately 2 in bRHBr568 compared with bRHCl564. The data indicate that in the acid purple form bR transports the halide anions instead of protons. We present a testable model for the transport mechanism, which should also be applicable to halorhodopsin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Ahl P. L., Rothschild K. J. Millisecond Fourier-transform infrared difference spectra of bacteriorhodopsin's M412 photoproduct. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5221–5225. doi: 10.1073/pnas.84.15.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Skulachev V. P. Time resolution of the intermediate steps in the bacteriorhodopsin-linked electrogenesis. FEBS Lett. 1978 Mar 1;87(1):161–167. doi: 10.1016/0014-5793(78)80157-4. [DOI] [PubMed] [Google Scholar]
- Duschl A., Lanyi J. K., Zimányi L. Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis. J Biol Chem. 1990 Jan 25;265(3):1261–1267. [PubMed] [Google Scholar]
- Dér A., Hargittai P., Simon J. Time-resolved photoelectric and absorption signals from oriented purple membranes immobilized in gel. J Biochem Biophys Methods. 1985 Mar;10(5-6):295–300. doi: 10.1016/0165-022x(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Bogomolni R. A., Mathies R. A. Structure of the retinal chromophore in the hRL intermediate of halorhodopsin from resonance raman spectroscopy. Biochemistry. 1987 Oct 20;26(21):6775–6778. doi: 10.1021/bi00395a029. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Souvignier G., Hess B. Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9774–9778. doi: 10.1073/pnas.87.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss T., Grzesiek S., Otto H., Westerhausen J., Heyn M. P. Transmembrane location of retinal in bacteriorhodopsin by neutron diffraction. Biochemistry. 1990 May 22;29(20):4904–4913. doi: 10.1021/bi00472a022. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci. 1990 Mar;15(3):108–112. doi: 10.1016/0968-0004(90)90194-g. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Sherman W. V., Caplan S. R. Kinetic isotope effects in the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1976 Dec 22;2(3):267–276. doi: 10.1007/BF00535372. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kinosita K., Jr, Ikegami A. Structure and function of bacteriorhodopsin. Adv Biophys. 1988;24:123–175. doi: 10.1016/0065-227x(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin, a light-driven electrogenic chloride-transport system. Physiol Rev. 1990 Apr;70(2):319–330. doi: 10.1152/physrev.1990.70.2.319. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Zimányi L., Nakanishi K., Derguini F., Okabe M., Honig B. Chromophore/protein and chromophore/anion interactions in halorhodopsin. Biophys J. 1988 Feb;53(2):185–191. doi: 10.1016/S0006-3495(88)83080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Ormos P. Infrared spectroscopic demonstration of a conformational change in bacteriorhodopsin involved in proton pumping. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):473–477. doi: 10.1073/pnas.88.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Enthalpy changes during the photochemical cycle of bacteriorhodopsin. Biophys J. 1979 Feb;25(2 Pt 1):355–364. doi: 10.1016/s0006-3495(79)85297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C., Lanyi J. K., Callender R. H. Effects of various anions on the Raman spectrum of halorhodopsin. Biophys J. 1989 Mar;55(3):425–431. doi: 10.1016/S0006-3495(89)82836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K., Cragoe E. J., Jr Evidence for a halide-binding site in halorhodopsin. J Biol Chem. 1983 Dec 25;258(24):15158–15164. [PubMed] [Google Scholar]
- Steiner M., Oesterhelt D., Ariki M., Lanyi J. K. Halide binding by the purified halorhodopsin chromoprotein. I. Effects on the chromophore. J Biol Chem. 1984 Feb 25;259(4):2179–2184. [PubMed] [Google Scholar]
- Stern L. J., Khorana H. G. Structure-function studies on bacteriorhodopsin. X. Individual substitutions of arginine residues by glutamine affect chromophore formation, photocycle, and proton translocation. J Biol Chem. 1989 Aug 25;264(24):14202–14208. [PubMed] [Google Scholar]
- Stoeckenius W. The rhodopsin-like pigments of halobacteria: light-energy and signal transducers in an archaebacterium. Trends Biochem Sci. 1985 Dec;10(12):483–486. doi: 10.1016/0968-0004(85)90210-5. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Effect of lipid surface charges on the purple-to-blue transition of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3681–3684. doi: 10.1073/pnas.84.11.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl H. W. Photoelectric measurements of purple membranes. Photochem Photobiol. 1990 Jun;51(6):793–818. [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Photoreactions of bacteriorhodopsin at acid pH. Biophys J. 1989 Dec;56(6):1143–1151. doi: 10.1016/S0006-3495(89)82761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot H. J., Harbison G. S., Herzfeld J., Griffin R. G. Nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin: counterion effects on the 15N shift anisotropy. Biochemistry. 1989 Apr 18;28(8):3346–3353. doi: 10.1021/bi00434a033. [DOI] [PubMed] [Google Scholar]
- de Groot H. J., Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Griffin R. G., Herzfeld J. Solid-state 13C and 15N NMR study of the low pH forms of bacteriorhodopsin. Biochemistry. 1990 Jul 24;29(29):6873–6883. doi: 10.1021/bi00481a017. [DOI] [PubMed] [Google Scholar]



