Abstract
The role of the neuroendocrine environment in the pathogenesis of enteric bacterial infections is increasingly being recognized. Here we report that norepinephrine augments Escherichia coli O157:H7-induced intestinal inflammatory and secretory responses as well as bacterial adherence to intestinal mucosa in a bovine ligated ileal loop model of infection. Norepinephrine modulation of enteritis and adherence was dependent on the ability of E. coli O157:H7 to form attaching and effacing lesions.
Enterohemorrhagic Escherichia coli (EHEC) causes acute gastroenteritis in humans that may be complicated by life-threatening systemic sequelae depending on serotype- and host-specific factors (35). EHEC strains are defined by their ability to produce one or more Shiga-like toxins and to induce characteristic attaching and effacing (A/E) lesions on intestinal epithelia (33). A/E lesion formation relies on the injection of bacterial effectors via a type III secretion system and is determined by the locus of enterocyte effacement (LEE) (14). The LEE-encoded adhesin intimin is required for the colonization of calves and adult cattle by E. coli O157:H7 (6, 8) and for the colonization of humans by enteropathogenic E. coli (EPEC) (11). Type III secreted proteins also mediate the intestinal colonization of calves by EHEC serotypes O157:H7 and O26:H− (F. Dziva, P. van Diemen, M. Stevens, and T. Wallis, unpublished data), and EspB is required for the carriage and virulence of EPEC in humans (42).
The host factors contributing to the colonization of ruminants and humans by EHEC are poorly understood. Recent studies have suggested that the neuroendocrine environment in the gastrointestinal tract may influence the outcome of infection, since the expression of virulence factors by diarrheagenic E. coli is augmented in vitro by the hormone norepinephrine (NE), which is released by the enteric nervous system under stress (3). NE is taken up by E. coli (23) and stimulates the in vitro expression of the K99 pilus adhesin by enterotoxigenic E. coli (27) as well as growth, iron acquisition, motility, and the expression of Shiga-like toxins and LEE-encoded proteins by EHEC O157:H7 (16, 29, 30, 39). NE also augments the adherence of E. coli O157:H7 to murine cecal explants in vitro (5) and the invasion of the porcine jejunal mucosa (18). NE-containing neurons innervate all levels of the gastrointestinal tract and terminate in neural plexuses and the mucosa (24). The influence of NE released into the gut on the carriage and virulence of gram-negative bacterial pathogens in vivo has received little attention to date.
We have examined the effect of NE on the adherence and enteropathogenicity of E. coli O157:H7 in a bovine ligated ileal loop model of infection. This model permits the simultaneous testing of different treatments in discrete segments of the mid-ileum for their effect on bacterial adherence and the induction of intestinal inflammatory and secretory responses (40). All animal procedures were performed in accordance with the Animals (Scientific Procedures) Act of 1986 and were approved by the local Ethical Review Committee. In four separate experiments, a Friesian bull calf aged 35 to 38 days was subjected to fasting for 12 h prior to surgery, anesthetized with a short-acting barbiturate (Thiovet, 1 g/100 kg of body weight), intubated, and maintained under anesthesia with isoflurane in oxygen for the duration of the experiment. A laparotomy was performed, the mid-ileum was gently flushed with physiological saline, and loops 6 cm in length with 1-cm spacers were ligated with surgical silk. Neutrophils were purified from venous blood, radiolabeled with 111In oxinate, and reinjected via the jugular vein within an hour of inoculation of the loops to permit the quantification of intestinal inflammatory responses as previously described (40).
E. coli O157:H7 strain 85-170 Nalr was used as the inoculum and is a spontaneous nalidixic acid-resistant, stx1- and stx2-lacking derivative of strain 84-289 (43). Bacteria were grown to stationary phase in Luria-Bertani (LB) broth at 37°C for 16 h and adjusted to the same optical density in each experiment (optical density at 600 nm, 1.1). Immediately prior to loop inoculation, a 1 M stock of l-norepinephrine (bitartrate salt; Sigma Chemical Company, St Louis, Mo.) was prepared in phosphate-buffered saline and filter sterilized. Bacterial cultures were supplemented with NE at a final concentration of 50 μM or 5 mM or with an equivalent volume of diluent, and 5 ml (ca. 5 × 109 CFU) was immediately taken up into syringes and injected into the loops. Each treatment was tested in quadruplicate in a semirandomized order in each animal. LB supplemented with 5 mM NE was used as a negative control. Twelve hours after inoculation, enteropathogenesis was assessed in three of the four loops for each treatment with respect to fluid accumulation and infiltration of 111In-labeled neutrophils. A single loop for each treatment was filled ante mortem with 5 ml of 4% (wt/vol) paraformaldehyde in phosphate-buffered saline, excised after death, and then processed for histology as described previously (40).
Fluid secretion was measured as a ratio of the volume of fluid accumulated to loop length (V/L). Radioactivity associated with the loop contents and mucosa was corrected for differences in loop length. Neutrophil infiltration was expressed as the ratio of total 111In activity to loop length in the test loops to that in the control loop containing 85-170 Nalr plus diluent. The mean value for each treatment in a single animal was determined, and then the means (± standard errors of the means) from the four independent experiments were calculated. The data were statistically analyzed for the effect of treatment, animal, and interactions by two-way analysis of variance (Proc Mixed, Statistical Analysis System [SAS]; SAS Institute, Cary, N.C.). The loop length was included in the analysis as a covariable (Proc GLM, SAS). P values of <0.05 were taken to be significant.
Previously, we and others have reported that inoculation of ileal loops in weaned or gnotobiotic calves with E. coli O157:H7 induces minimal damage to villi and inflammatory and secretory responses that are not significantly greater than those of controls (38, 40). This is consistent with reports that natural and experimental infections of weaned calves with E. coli O157:H7 are asymptomatic (4, 7, 9, 46). Remarkably, NE stimulated a dose-dependent increase in fluid accumulation and the recruitment of 111In-labeled neutrophils in response to E. coli O157:H7 strain 85-170 Nalr in ileal loops (Fig. 1). The elevated secretory response reached significance when NE was used at 5 mM (P < 0.0001) compared to strain 85-170 Nalr in the presence of diluent. The total neutrophil influx was also significantly elevated in the presence of 5 mM NE (P = 0.04) compared to strain 85-170 Nalr in the presence of diluent. At 50 μM NE, the secretory and inflammatory responses induced by 85-170 Nalr were higher than in the presence of diluent but not significantly so (V/L, P = 0.50; neutrophil influx, P = 0.58). LB containing 5 mM NE induced little or no fluid accumulation or neutrophil infiltration, indicating that NE does not induce enteritis per se at this concentration (Fig. 1).
FIG. 1.
Effect of NE on intestinal secretory and inflammatory responses induced by E. coli strains 85-170 Nalr and 85-170 Nalr Δeae Δtir in the mid-ileum of 35- to 38-day-old calves. (A) Fluid accumulation. The ratio of the volume of fluid accumulated to loop length (V/L) for each treatment was determined from triplicate determinations in each calf. The values shown represent the means (± standard errors of the means) of the results for each treatment from four independent experiments. (B) Neutrophil infiltration. Total 111In activity in the contents and mucosa was corrected for loop length and expressed as a ratio of the total activity in loops containing 85-170 Nalr plus diluent. The mean value for each treatment in a single animal was determined, and then the mean neutrophil influx (± standard error of the mean) of the results from the four independent experiments was calculated.
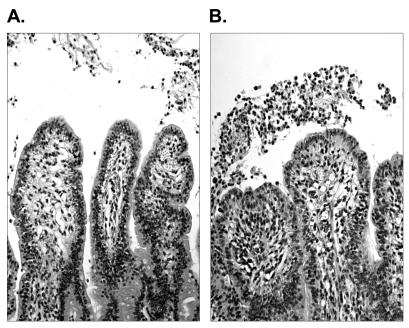
Tissue damage and inflammation were assessed by microscopic analysis of hematoxylin and eosin-stained sections of ileal mucosa from each of the four calves. Consistent with previous reports (38, 40), strain 85-170 Nalr induced little obvious damage to the intestinal epithelium and weak infiltration of neutrophils (Fig. 2A). In contrast, loops inoculated with 85-170 Nalr in the presence of 5 mM NE exhibited a marked infiltration of neutrophils into the lamina propria, submucosa, and intestinal lumen, consistent with the high 111In activity detected (Fig. 2B). Loops inoculated with LB containing 5 mM NE did not show any obvious histological changes.
FIG. 2.
Light micrographs of hematoxylin and eosin-stained bovine mid-ileal mucosa from ligated loops inoculated with E. coli O157:H7 strain 85-170 Nalr in the presence of diluent (A) or 5 mM NE (B). Magnification, ×250.
We semiquantitatively assessed bacterial adherence to ileal mucosa by confocal microscopy. Tissues were fixed ante mortem and stained for E. coli O157:H7 and F-actin as described previously (40). The percentage of intact villi exhibiting microcolonies (MC) comprised of greater than 5 adherent bacteria was calculated from four nonconsecutive sections from a single loop in each animal, and the mean (± standard error of the mean) was then determined for the four animals. In loops filled with 85-170 Nalr plus diluent, few intact villi exhibited MC (Fig. 3A). In contrast, 5 mM NE stimulated a highly significant increase in the percentage of intact villi exhibiting MC (P < 0.0001), with dense MC of intimately attached bacteria being readily detected (Fig. 3B). No significant difference in the adherence of E. coli O157:H7 to ileal mucosa was detected in loops containing 50 μM NE compared to diluent (P = 0.175). An examination of ileal mucosa exposed to 85-170 Nalr in the presence of 5 mM NE by transmission electron microscopy revealed extensive A/E lesion formation (Fig. 4). No such lesions could be detected on ileal mucosa from loops inoculated with 85-170 Nalr in the presence of diluent (data not shown). Although it is believed that E. coli O157:H7 exhibits a tropism for lymphoid follicle-dense epithelium in the terminal rectum of cattle (34), our data suggest that E. coli O157:H7 can adhere extensively at other intestinal sites and that this may be influenced by the local neuroendocrine environment.
FIG. 3.
Confocal laser scanning micrographs of bovine mid-ileal mucosa from ligated loops inoculated with E. coli O157:H7 strain 85-170 Nalr in the presence of diluent (A) or 5 mM NE (B). F-actin was stained with fluorescein isothiocyanate-conjugated phalloidin (green), and bacteria were detected with rabbit anti-O157 typing serum and anti-rabbit immunoglobulin Alexa 568 (red) as described previously (40). Dense MC of intimately attached bacteria were seen only in the presence of NE. Magnification, ×630. (C) Semiquantitative analysis of bacterial adherence. The percentage of intact villi exhibiting MC of >5 adherent bacteria was calculated from four nonconsecutive sections from a single loop in each animal, and the means (± standard errors of the means) were then determined for the four animals used. At least 50 villi were examined in each nonconsecutive section.
FIG. 4.
Transmission electron micrographs of bovine mid-ileal mucosa showing A/E lesions induced by E. coli O157:H7 in the presence of 5 mM NE. Fixation, staining, and image capture were performed as described previously (41). Scale bars, 10 μm (A) and 5 μm (B).
To assess the importance of A/E lesion formation in NE-induced adherence and enteritis by E. coli O157:H7, we constructed an 85-170 Nalr mutant harboring nonpolar deletions in the genes for intimin (eae) and the type III secreted translocated intimin receptor (tir). Sequences flanking the tir gene were separately amplified by using the primer pairs tir1 (5′-ATATATGAGCTCTAGCATCATCGAGAGGG-3′) plus tir2 (5′-CCTATTGGTAATCTTGGATCCCATCGTTTCGTC-3′) and tir3 (5′-GAAACGATGGGATCCAAGATTACCAATAGGCAT-3′) plus tir4 (5′-ATATATGAGCTCGGGATAACCTTGTCAGG-3′). The primary PCR products were combined in an overlapping PCR (22) by using tir1 and tir4 and the secondary PCR product cloned into the positive-selection suicide vector pDM4 (32) via SacI sites incorporated into the primers. The resulting plasmid was introduced into an existing 85-170 Nalr Δeae mutant (strain ICC170) (13) by conjugation from E. coli S17-1λpir, and a double recombinant was selected as described previously (40). The in-frame deletion results in the juxtaposition of the first and last 6 codons of the tir gene. The 85-170 Nalr Δeae Δtir mutant did not express intimin or Tir as assessed by Western blotting with rabbit polyclonal antisera and did not form MC on HeLa cells or nucleate actin (data not shown).
The adherence and enteropathogenicity of strain 85-170 Nalr Δeae Δtir was examined in the presence of 5 mM NE. At this concentration, strain 85-170 Nalr Δeae Δtir was significantly less adherent in ileal loops than the parent strain in the presence of 5 mM NE (P < 0.0001), consistent with the roles of intimin and Tir in the colonization of the bovine intestine (Fig. 3C) (6, 8; I. Vlisidou and M. Stevens, unpublished data). The secretory and inflammatory responses induced by the 85-170 Nalr Δeae Δtir mutant in the presence of 5 mM NE were significantly lower than those induced by 85-170 Nalr with 5 mM NE (V/L, P < 0.0001; neutrophil influx, P = 0.0098) (Fig. 1), implying that NE augments EHEC-induced enteritis in a manner dependent on A/E lesion formation. This is consistent with the observation by Sperandio et al. that NE stimulates the expression and secretion of LEE-encoded proteins in vitro (39) and the fact that intimin, Tir, and type III secreted effectors are required for intestinal inflammation in rabbits infected with rabbit EPEC or EHEC O157:H7 (1, 31, 36) and mice infected with Citrobacter rodentium (10, 20).
NE increases the growth of a range of nonpathogenic E. coli isolates of human and environmental origin, and it has been suggested that it may contribute to the pathophysiology of trauma-induced sepsis following surgery (15). Indeed, stress induced by partial hepatectomy, short term starvation, or administration of the noradrenergic neurotoxin 6-hydroxydopamine results in elevated adherence of commensal E. coli to the murine cecal mucosa in vivo (19, 28). Type I fimbriae may play a role in trauma-induced adherence of commensal E. coli to the murine cecum (19). However, modulation of type I fimbriae expression by NE could not explain the increased adherence of E. coli O157:H7 to the bovine ileal mucosa observed in this study, since E. coli O157:H7 strains contain a 16-bp deletion in the promoter region for the major fimbrial subunit and, as such, do not express type I fimbriae (12, 37).
Sperandio et al. have shown that both epinephrine and NE cross talk with a bacterial quorum-sensing system regulating LEE expression and motility (39; reviewed in reference 45). Flagellum synthesis and type III secretion is regulated by an autoinducer (AI-3), the synthesis of which is dependent on LuxS (39). It is not presently clear whether NE activates LEE expression in E. coli O157:H7 by directly substituting for AI-3 or whether it stimulates endogenous AI-3 synthesis or, indeed, the synthesis of autoinducer(s) by the endogenous microflora. It is considered unlikely that epinephrine modulation of LEE expression could have an impact on EHEC carriage and virulence in the intestines, since neurons containing phenylethanolamine N-methyltransferase required for epinephrine synthesis from NE are not found within the gastrointestinal tract (reviewed in reference 26).
It is noteworthy that short-term withdrawal of feed and surgical manipulation of the intestines per se did not stimulate extensive adherence of E. coli O157:H7 to the mid-ileal epithelium (Fig. 3). The amount of NE in the intestinal tract of calves is unknown and will vary between the luminal contents and epithelial interface. It remains to be determined whether the levels of NE that stimulated E. coli O157:H7-induced enteritis and adherence in the present study are physiologically relevant in the context of stress and nutrition. It is naturally hard to imagine millimolar quantities of NE existing in the intestines when plasma levels are typically in the nanomolar to micromolar range; however, it should be remembered that the mesenteric organs contribute over half of all of the NE released and metabolized in the body and strong concentration gradients are likely to exist toward the gastrointestinal epithelium (26). Quantification of free or tissue-associated NE in the gastrointestinal tract is difficult for several reasons (17, 21): (i) microdialysis with probes inserted into the intestinal epithelium or lumen is complicated by clogging of the dialysis membrane with gut contents and the fact that NE levels in the dialysate may not reach equilibrium with the surroundings over time, (ii) quantification of NE in the gut contents requires high-pressure liquid chromatography and recovery during purification steps cannot easily be estimated, (iii) breakdown products of NE are readily detected in the intestines and it is not feasible to calculate how rapidly released NE is degraded through the activity of host and/or bacterial enzymes in the gut.
Nevertheless, our results demonstrate that NE instilled directly into the intestines can influence the adherence and enteropathogenicity of EHEC. While the concentrations of NE required to provoke this effect may appear high, it is useful to remember that locally high cytokine concentrations at the cell or tissue level may significantly influence the outcome of infection, but this may not be apparent by measuring systemic or free cytokine levels. Recently, Alverdy et al. reported that mice stressed by 30% hepatectomy are more susceptible to Pseudomonas aeruginosa gut-derived sepsis (2), and it is believed that this correlates with increased release of NE into the intestines and the ability of NE to stimulate expression of a key P. aeruginosa virulence factor (PA-I lectin/adhesin) in vitro and in vivo (47). NE also stimulates the invasion of porcine jejunal explants by Salmonella enterica serovar Choleraesuis and E. coli O157:H7 but not nonpathogenic E. coli (18). It therefore seems likely that pathogenic gram-negative bacteria have evolved conserved strategies to sense and respond to host neuroendocrine stress hormones.
It is also important to consider that neuroendocrine hormones, such as the biogenic amine dopamine, are consumed in the diet in quantities that are capable of inducing physiological changes (26). Indeed, dopamine is readily extracted from bananas, and such extracts stimulate the in vitro growth of gram-negative pathogens including E. coli O157:H7 in proportion to the amount of neurochemical present (25, 44). The potential for host- and food-borne neuroendocrine hormones to modulate the outcome of infection has profound implications for our understanding of stress, nutrition, and susceptibility to microbial infection.
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council, (no. 201/D17455 and 201/LKD19295 to T.S.W. and M.P.S.), the Department for the Environment, Food, and Rural Affairs (no. OZ0707 to T.S.W.), and the National Institutes of Health (no. AI-44918 to M.L.).
Editor: A. D. O'Brien
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alverdy, J., C. Holbrook, F. Rocha, L. Seiden, R. L. Wu, M. Musch, E. Chang, D. Ohman, and S. Suh. 2000. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann. Surg. 232:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aneman, A., G. Eisenhofer, L. Olbe, J. Dalenback, P. Nitescu, L. Fandriks, and P. Friberg. 1996. Sympathetic discharge to mesenteric organs and the liver. Evidence for substantial mesenteric organ norepinephrine spillover. J. Clin. Investig. 97:1640-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, C., D. R. Brown, Y. Xie, B. T. Green, and M. Lyte. 2003. Catecholamines modulate Escherichia coli O157:H7 adherence to murine cecal mucosa. Shock 20:183-188. [DOI] [PubMed] [Google Scholar]
- 6.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Med. Biol. 473:173-177. [DOI] [PubMed] [Google Scholar]
- 10.Deng, W., B. A. Vallance, Y. Li, J. L. Puente, and B. B. Finlay. 2003. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48:95-115. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., C. O. Tacket, S. P. James, G. Losonsky, J. P. Nataro, S. S. Wasserman, J. B. Kaper, and M. M. Levine. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Investig. 92:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enami, M., N. Nakasone, Y. Honma, S. Kakinohana, J. Kudaka, and M. Iwanaga. 1999. Expression of type I pili is abolished in verotoxin-producing Escherichia coli O157. FEMS Microbiol. Lett. 179:467-472. [DOI] [PubMed] [Google Scholar]
- 13.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel, G., A. D. Phillips, L. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 15.Freestone, P. P., P. H. Williams, R. D. Haigh, A. F. Maggs, C. P. Neal, and M. Lyte. 2002. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18:465-470. [DOI] [PubMed] [Google Scholar]
- 16.Freestone, P. P., R. D. Haigh, P. H. Williams, and M. Lyte. 2003. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 222:39-43. [DOI] [PubMed] [Google Scholar]
- 17.Grassi, G., and M. Esler. 1999. How to assess sympathetic activity in humans. J. Hypertens. 17:719-734. [DOI] [PubMed] [Google Scholar]
- 18.Green, B. T., M. Lyte, A. Kulkarni-Narla, and D. R. Brown. 2003. Neuromodulation of enteropathogen internalization in Peyer's patches from porcine jejunum. J. Neuroimmunol. 141:74-82. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickson, B. A., J. Guo, R. Laughlin, Y. Chen, and J. C. Alverdy. 1999. Increased type 1 fimbrial expression among commensal Escherichia coli isolates in the murine cecum following catabolic stress. Infect. Immun. 67:745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 21.Hjemdahl, P. 1993. Plasma catecholamines-analytical challenges and physiological limitations. Baillieres Clin. Endocrinol. Metab. 7:307-353. [DOI] [PubMed] [Google Scholar]
- 22.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 23.Kinney, K. S., C. E. Austin, D. S. Morton, and G. Sonnenfeld. 2000. Norepinephrine as a growth stimulating factor in bacteria-mechanistic studies. Life Sci. 67:3075-3085. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren, O. 2000. Sympathetic input into the enteric nervous system. Gut 47(Suppl. 4):iv33-iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyte, M. 1997. Induction of gram-negative bacterial growth by neurochemical containing banana (Musa × paradisiaca) extracts. FEMS Microbiol. Lett. 154:245-250. [DOI] [PubMed] [Google Scholar]
- 26.Lyte, M. 2004. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol. 12:14-20. [DOI] [PubMed] [Google Scholar]
- 27.Lyte, M., A. K. Erickson, B. P. Arulanandam, C. D. Frank, M. A. Crawford, and D. H. Francis. 1997. Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli. Biochem. Biophys. Res. Commun. 232:682-686. [DOI] [PubMed] [Google Scholar]
- 28.Lyte, M., and M. T. Bailey. 1997. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J. Surg. Res. 70:195-201. [DOI] [PubMed] [Google Scholar]
- 29.Lyte, M., B. Arulanandam, K. Nguyen, C. Frank, A. Erickson, and D. Francis. 1997. Norepinephrine induced growth and expression of virulence associated factors in enterotoxigenic and enterohemorrhagic strains of Escherichia coli. Adv. Exp. Med. Biol. 412:331-339. [DOI] [PubMed] [Google Scholar]
- 30.Lyte, M., B. P. Arulanandam, and C. D. Frank. 1996. Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J. Lab. Clin. Med. 128:392-398. [DOI] [PubMed] [Google Scholar]
- 31.Marchés, O., J.-P. Nougayrède, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milton, D. L., R. O'Toole, P. Hörstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga-toxin producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritchie, J. M., C. M. Thorpe, A. B. Rogers, and M. K. Waldor. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 71:7129-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roe, A. J., C. Currie, D. G. Smith, and D. L. Gally. 2001. Analysis of type 1 fimbriae expression in verotoxigenic Escherichia coli: a comparison between serotypes O157 and O26. Microbiology 147:145-152. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu, K. S., and C. L. Gyles. 2002. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 66:65-72. [PMC free article] [PubMed] [Google Scholar]
- 39.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens, M. P., O. Marches, J. Campbell, V. Huter, G. Frankel, A. D. Phillips, E. Oswald, and T. S. Wallis. 2001. Intimin, Tir, and Shiga toxin 1 do not influence enteropathogenic responses to Shiga toxin-producing Escherichia coli in bovine ligated intestinal loops. Infect. Immun. 70:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens, M. P., P. M. van Diemen, G. Frankel, A. D. Phillips, and T. S. Wallis. 2002. Efa1 influences colonization of the bovine intestine by Shiga toxin-producing Escherichia coli serotypes O5 and O111. Infect. Immun. 70:5158-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waalkes, T. P., A. Sjoerdsma, C. R. Creveling, H. Weissbach, and S. Udenfriend. 1958. Serotonin, norepinephrine, and related compounds in bananas. Science 127:684-750. [PubMed] [Google Scholar]
- 45.Winzer, K., and P. Williams. 2003. Escherichia coli gets the message. Nat. Med. 9:1118-1119. [DOI] [PubMed] [Google Scholar]
- 46.Wray, C., I. M. McLaren, L. P. Randall, and G. R. Pearson. 2000. Natural and experimental infection of normal cattle with Escherichia coli O157. Vet. Rec. 147:65-68. [DOI] [PubMed] [Google Scholar]
- 47.Wu, L., C. Holbrook, O. Zaborina, E. Ploplys, F. Rocha, D. Pelham, E. Chang, M. Musch, and J. Alverdy. 2003. Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the quorum sensing signaling system. Ann. Surg. 238:754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]