Abstract
The role of CD4+ T cells in the pathogenesis of ocular toxoplasmosis was investigated in murine models utilizing inbred C57BL/6 mice deficient either in CD4+, CD8+, or B cells (μMT). Severe necrosis and inflammation with replicating parasites were observed in the eyes of control mice after primary ocular infection, and near-normal histology with few tachyzoites was observed in the eyes of mice immunized intraperitoneally with the avirulent ts-4 strain followed by intraocular challenge with the RH strain of Toxoplasma gondii. In contrast, mild inflammation without evidence of necrosis associated with increased parasite burdens were observed in the eyes of CD4 knockout (KO) mice after both primary ocular infection and challenge with RH tachyzoites. CD8 KO mice, as well as μMT mice, demonstrated increased ocular necrosis in response to either primary ocular infection or challenge. The parasite burden was increased in the eyes of both CD8 KO and μMT mice in which the parasite load was even higher. As expected, there were no increases in the levels of immunoglobulin G in serum or aqueous humor in μMT mice, and there was no increase in the levels of gamma interferon and tumor necrosis factor alpha in the sera of CD4 KO mice after both infection and challenge. These results suggest that the ocular inflammatory response to the parasite is mediated primarily by the CD4+-T-cell response. CD8+ T cells and B cells may play an important role in limiting tachyzoite proliferation in the eyes. Mice deficient in CD8+ CD4+ T cells or B cells exhibit diminished vaccine-induced resistance and increased ocular parasite burden after challenge.
Toxoplasma gondii is an obligate intracellular protozoan parasite occurring worldwide in humans and animals. Under normal conditions, this infection is largely asymptomatic, but in immunocompromised individuals such as patients with AIDS the parasite is no longer contained by the host immune response, resulting in the development of toxoplasmic encephalitis (15). T. gondii is also an important cause of ocular disease in both immunosuppressed and immunocompetent individuals and is the most common cause of infectious retinochoroiditis in otherwise-healthy individuals (30). Ocular toxoplasmosis (OT), a potentially blinding disorder, is the most common cause of human retinochoroiditis worldwide (9). About 1 to 3% of AIDS patients will develop OT during the course of their illness (28).
The host immune response against T. gondii can be an innate acute response or an antigen-specific cell-mediated immune response. Cell-mediated immunity is essential for protection against T. gondii (8). Gamma interferon (IFN-γ)-dependent, cell-mediated immunity plays the major role in resistance to toxoplasmic encephalitis (35). The role of CD4+ T cells in the immunopathogenesis of systemic infection with T. gondii is well appreciated (21). In C57BL/6 mice, necrosis of the small intestine after peroral infection with T. gondii is CD4+ T cell dependent and IFN-γ mediated (21).
The pathogenesis of many major infectious diseases of the eye, including onchocerciasis (26), herpes simplex virus keratitis (38), trachoma (1), and pseudomonal keratitis (16), is in part immune mediated. OT may also be considered an immune-mediated process. Previous studies from our laboratory and others have demonstrated the importance of cell-mediated immune response and specific cytokines in this process in T. gondii ocular infection (7, 22). Feron et al. (6) reported that T. gondii-specific T cells in vitreous fluid from patients involve the local inflammatory response of OT. However, the specific function of the T-cell responses in the process of immunopathogenesis of this condition is not fully understood. Because some aspects of the mouse model mimic the aspects of the disease in the eyes of human patients, the present study was designed to better understand the role of CD4+ T cells in the immunopathogenesis of OT by using experimental murine models deficient in CD4+ CD8+ T cells and B cells.
MATERIALS AND METHODS
Parasites.
The RH strain of T. gondii and the temperature-sensitive mutant of RH T. gondii strain (ts-4; kindly provided by Elmer Pfefferkorn, Dartmouth Medical School, Lebanon, N.H.) were used in the present study. In some experiments, RH strain tachyzoites engineered to constitutively express green fluorescent protein (GFP; RH-GFP [kindly provided by John Boothroyd, Stanford University, Stanford, Calif.]) were used. They were maintained by continuous passage in human fibroblasts grown in Dulbecco modified Eagle medium (catalog no. 11965; Gibco, Grand Island, N.Y.) supplemented with 10% newborn calf serum plus antibiotics.
Mice.
A breeding pair of CD8 knockout (KO) mice on a C57BL/6 background was kindly provided by T. W. Mak (Amgen Institute, Toronto, Ontario, Canada). Age-matched (7- to 9-week-old) and sex-matched C57BL/6 wild-type (WT), CD4 KO, and B-cell-deficient (μMT) mice of the same genetic background were obtained from The Jackson Laboratory (Bar Harbor, Maine). Animals were bred under specific-pathogen-free conditions at the Animal Research Facility at Dartmouth Medical School.
Immunization and eye inoculation.
Mice were immunized by intraperitoneal (i.p.) injection of 105 ts-4 strain tachyzoites as previously reported (22). CD4 KO, μMT, and WT mice were challenged by eye inoculation of 100 RH tachyzoites at 28 days postimmunization, and CD8 KO mice were challenged at 15 days postimmunization. Primary infection of naive mice was performed by ocular inoculation with 100 RH tachyzoites. Eye inoculation was performed as previously described (12). Briefly, mice were anesthetized with ketamine hydrochloride (40 mg/kg) and xylazine (5 mg/kg) by i.p. injection. After the leaking aqueous fluid was blotted on the right eye, a 5-μl parasite suspension in Dulbecco modified Eagle medium was injected into the anterior chamber under an operating microscope by using a 33-gauge needle attached to a 50-μl syringe (Hamilton, Reno, Nev.).
Histopathology.
At 11 days after inoculation, mice were sacrificed by CO2 asphyxiation, and harvested eyes were immediately fixed in 10% buffered formaldehyde (Polyscience, Warrington, Pa.). Then, 5-μm sections (with a 50- or 100-μm distance between sections) of the eye tissue from each mouse were stained with hematoxylin and eosin and evaluated for inflammatory changes. Pathological changes were scored on a scale of 0 (normal) to 4 according to the method of Hu et al. (12) as follows: 0, normal histology; 1, mild inflammation without necrosis; 2, obvious inflammation without necrosis; 3, strong inflammation with necrosis; and 4, strong necrosis in whole eye section.
Confocal laser scanning microscopy.
Five-μm thick sections (50- or 100-μm distance between sections) of paraffin wax-embedded eye tissue from each mouse infected or challenged with 100 RH-GFP tachyzoites at 11 days were visualized by using a MRC-1024 confocal scanning laser microscope (Bio-Rad, Hercules, Calif.) equipped with a Zeiss Axioskop microscope (Oberkochen, Germany), a 40× plan neofluar 1.3 numerical aperture objective lens, and a 15-mW krypton/argon laser. GFP was detected with a 525 DF32 band-pass filter. The confocal iris setting was 2.0, and the gain was 845.
Levels of IFN-γ and TNF-α in serum.
Mice were bled at 11 days after infection or challenge, and the serum was collected and stored at −70°C until use. The levels of IFN-γ and TNF-α in serum were quantitated by enzyme-linked immunosorbent assay kits (Biosource, Camarillo, Calif.) according to the manufacturer's instructions. Optical density (OD) values were measured at 450 nm, and the cytokine concentrations in serum were determined from the standard curve.
Anti-toxoplasma antibody levels in serum and aqueous fluid.
Approximately 10 to 15 μl of aqueous fluid was withdrawn by using a 27.5-gauge needle via a limbal paracentesis from each naive mouse, and each mouse was immunized i.p. with ts-4 at 28 days. The fluid was stored at −70°C until use. Purified RH parasites (5 × 104/well) were placed in microtiter plates (Nunc-Immuno Plate; Nunc, Roskilde, Denmark), dried overnight, blocked with 5% bovine serum albumin-phosphate-buffered saline (PBS) (Sigma, St. Louis, Mo.), and washed in PBS (pH 7.2)-0.05% Tween 20 (Bio-Rad). Antisera and aqueous fluid were incubated for 2 h at 37°C. Plates were washed and supplemented with a peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG; whole molecule, 1/40,000; Sigma) for 1 h. After a wash step, tetramethyl benzidine substrate in H2O2 (Kirkegaard Perry, Gaithersburg, Md.) was used for development. The reaction was stopped 30 min later by the addition of 2 N H2SO4. OD values were measured at 450 nm with an automatic microplate reader (model 550; Bio-Rad).
Statistical analysis.
Data were analyzed by using the Student t test or the Wilcoxon signed rank test. P values of <0.05 were considered statistically significant.
RESULTS
Decreased ocular inflammation in CD4+-T-cell-deficient mice.
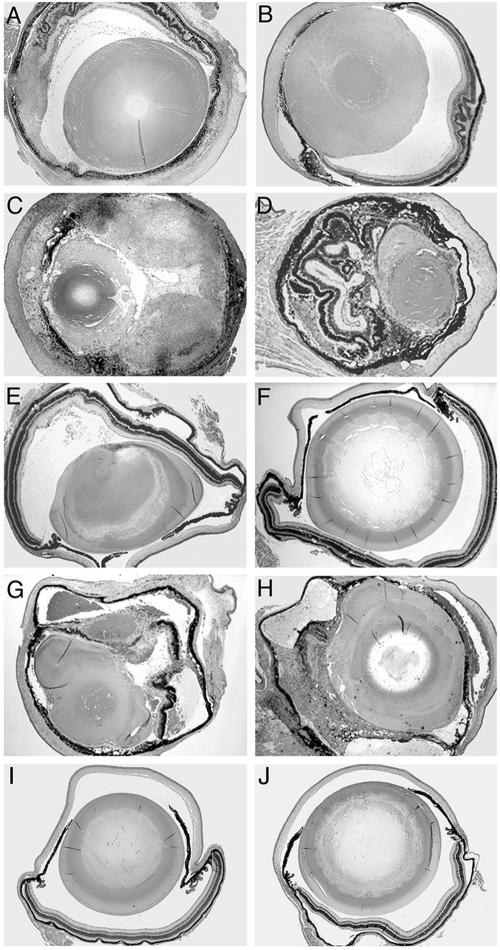
Histological changes (Fig. 1) and inflammatory scores (Table 1) in the eye tissues of genetically deficient and control mice were compared after primary intraocular infection with 100 RH strain tachyzoites or immunization with the ts-4 avirulent parasite strain, followed by challenge with 100 RH tachyzoites at 11 days. In control mice, severe inflammation and necrosis were observed in the eye tissue after primary infection (Fig. 1A). Vaccination with the ts-4 strain protected the eye tissue from necrosis after intraocular challenge (Fig. 1B). Primary ocular parasite infection of μMT mice resulted in increased inflammation and necrosis (Fig. 1C), and the inflammatory score significantly increased (P = 0.043). Vaccination with ts-4, followed by ocular challenge, did not protect μMT from inflammation and necrosis of the eye tissue (Fig. 1D) (P = 0.028). In CD8 KO mice, increased inflammation and necrosis were observed in the eye tissue after either primary ocular infection (Fig. 1G) (P = 0.043) or vaccination, followed by challenge (Fig. 1H) (P = 0.028). In CD4 KO mice, only mild inflammatory cellular infiltration was observed in the eye tissue after primary infection (Fig. 1E) (P = 0.043) and without histological evidence of ocular inflammation or necrosis after vaccination with ts-4, followed by RH strain challenge (Fig. 1F).
FIG. 1.
Pathology of eye tissues from mutant and control mice infected or challenged intracamerally with 100 RH strain T. gondii tachyzoites at 11 days. Shown are the eyes of an infected (A) and challenged (B) control mouse, an infected (C) and challenged (D) μMT mouse, an infected (E) and challenged (F) CD4 KO mouse, an infected (G) and challenged (H) CD8 KO mouse, a normal naive mouse (I), and a normal CD8 KO mouse (J). Six mice were used in each group; this experiment is representative of two performed. Magnification, ×4. Hematoxylin and eosin stain was used.
TABLE 1.
Comparison of parasite numbers and inflammatory scores in the eyes of control and mutant mice after primary infection or ts-4 vaccination and challenge with T. gondiia
| Mouse group | Inflammation score(s)
|
Mean no. of parasites ± SE (106/ml)
|
||
|---|---|---|---|---|
| Infected mice | Challenged mice | Infected mice | Challenged mice | |
| Control | 2,2,3,3,3,3 | 1,0,1,1,1,0 | 3.00 ± 0.42** | 0.30 ± 0.11** |
| B KO | 3,3,3,4,4 | 2,2,2,2,2 | 12.13 ± 2.13** | 5.43 ± 1.55** |
| CD8 KO | 3,3,4,4,3,4 | 2,2,3,4,3,3 | 4.34 ± 0.71** | 3.59 ± 0.43** |
| CD4 KO | 1,0,1,1,1 | 1,1,0,1,1 | 3.65 ± 0.50* | 3.22 ± 0.50** |
Mice were infected or ts-4 vaccinated and challenged intracamerally with 100 RH tachyzoites, and the inflammatory scores and parasite numbers in their eyes were examined at day 11. The details of this experiment are described in the text. Compared to controls (n = 6), μMT mice (n = 5) had significantly increased inflammation and necrosis in the eye tissue after primary infection (P = 0.043) and vaccination with ts-4, followed by ocular challenge (P = 0.028). CD8 KO mice (n = 6) had significantly increased inflammation and necrosis after either primary ocular infection (P = 0.043) or vaccination, followed by challenge (P = 0.028). CD4 KO mice (n = 5) had only mild inflammatory cellular infiltration in the eye tissue after infection (P = 0.043) and without histologic evidence of ocular inflammation after challenge. For parasite numbers, each value represents the mean of four mice±the standard error. Similar results were obtained in two experiments. *, P < 0.05; **, P < 0.01 (compared to control).
Increased ocular parasite burden in CD4+-T-cell-deficient mice.
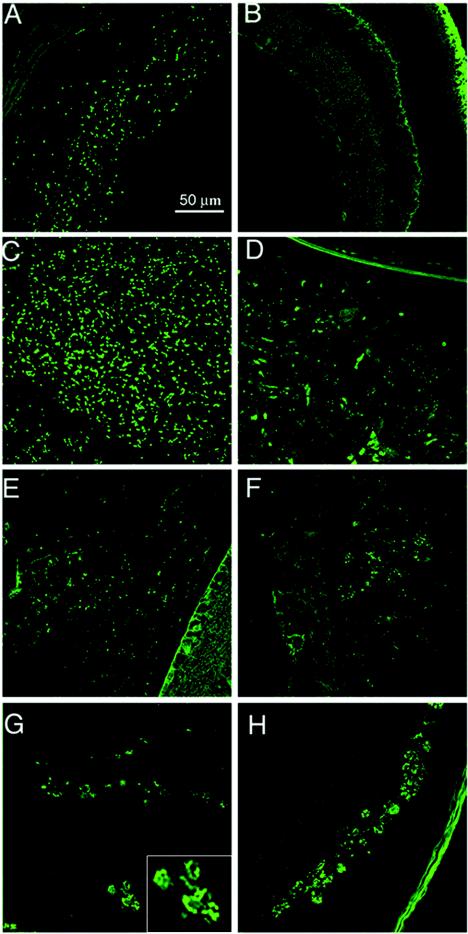
Parasite proliferation in the eye tissues from mutant and control mice was evaluated by confocal microscopy at 11 days after primary infection with 100 RH-GFP tachyzoites or ts-4 immunization, followed by challenge with 100 RH-GFP tachyzoites. As shown in Fig. 2, a quantitative increase in parasite burden was observed after primary infection (Fig. 2A), whereas few parasites were seen after ts-4 vaccination and challenge (Fig. 2B) in the eye tissues of control mice. In contrast, an increased parasite burden was observed after primary infection (Fig. 2E), as well as ts-4 vaccination and challenge (Fig. 2F), in the eye tissues of CD4 KO mice. An increased parasite burden was also observed in the eye tissues of CD8 KO mice after either primary infection (Fig. 2G) or ts-4 vaccination and challenge (Fig. 2H). However, the greatest increased parasite burdens were observed in the eye tissues of μMT mice after either primary ocular infection (Fig. 2C) or ts-4 vaccination and challenge (Fig. 2D), suggesting a potential role for antibody production in control of ocular parasite burden.
FIG. 2.
Parasite replication in the eye tissues of mutant and control mice infected or challenged intracamerally with 100 tachyzoites of RH-GFP at 11 days. Shown are the eyes of an infected (A) and challenged (B) control mouse, an infected (C) and challenged (D) μMT mouse, an infected (E) and challenged (F) CD4 KO mouse, and an infected (G) and challenged (H) CD8 KO mouse. Six mice were used in each group; this experiment is representative of two performed.
Parasite numbers in the eye tissue of the strains of mice were quantified. Eyes were isolated at 11 days after primary intraocular infection or ts-4 vaccination and challenge with 100 RH-GFP tachyzoites. The eye tissue was teased apart, passed through a stainless steel mesh, and incubated with monolayers of human fibroblasts in 25-cm2 flasks at 37°C for 60 h. Parasites were purified from human fibroblast cell culture as previously described (11) and were counted by hemocytometer from four mice in each group (Table 1). Compared to controls, ocular tissue from μMT mice demonstrated the greatest numbers of parasites (106/ml) after either primary infection (P < 0.01) or ts-4 vaccination followed by challenge (P < 0.01). Parasite numbers from the ocular tissue of CD8 KO mice were increased after either primary infection (P < 0.01) or vaccination followed by challenge (P < 0.01). Increased parasite numbers were also observed in the eyes of CD4 KO mice after either primary infection (P < 0.05) or ts-4 vaccination followed by challenge (P < 0.01).
Levels of IFN-γ and TNF-α in serum.
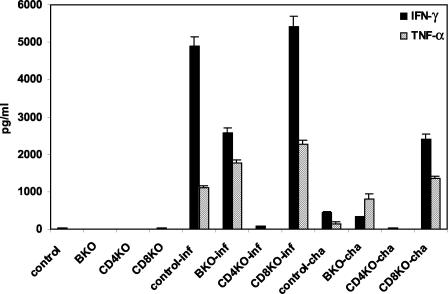
Compared to day 0, there were significant increases of serum levels of IFN-γ and TNF-α in control, μMT, and CD8 KO mice at 11 days after either primary ocular infection (P < 0.01) or ts-4 vaccination and challenge (P < 0.01). However, the levels of IFN-γ and TNF-α in serum were not significantly different in CD4 KO mice after either ocular infection or challenge (Fig. 3).
FIG. 3.
Levels of IFN-γ and TNF-α in serum. Experiments were performed on mutant and control mice infected or challenged intracamerally with 100 RH T. gondii tachyzoites at 11 days. Four mice were used in each group; these data are representative of two separate experiments.
Anti-toxoplasma antibody level.
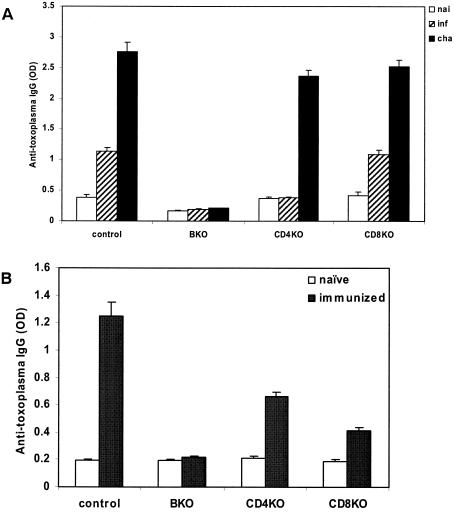
Compared to day 0, the levels of IgG (i.e. the OD value) in serum were significantly increased in control and CD8 KO mice at 11 days after both primary infection (P < 0.05) and vaccination with challenge (P < 0.01). Anti-toxoplasma IgG titers were 1:3,200 to 1:6,400 after infection and 1:12,800 to 1:51,200 after challenge. In CD4 KO mice, the IgG level in serum was significantly increased after challenge (P < 0.01) but not after primary infection. However, anti-toxoplasma antibody level was not detectable in the sera of μMT mice (Fig. 4A). At day 28 after i.p. immunization with ts-4, the levels of IgG in the eye fluid from control, CD4 KO, and CD8 KO mice were all significantly increased (P < 0.01) but not in μMT mice (Fig. 4B).
FIG. 4.
(A) Levels of anti-toxoplasma IgG in serum. Experiments were performed on mutant and control mice infected or challenged intracamerally with 100 RH T. gondii tachyzoites at 11 days. (B) Levels of anti-toxoplasma IgG in eye fluid. Samples were collected from mice vaccinated i.p. with T. gondii ts-4 at 28 days. Four mice were used in each group; these data are representative of two separate experiments.
DISCUSSION
The eye is an immunologically privileged site, and the pathogenesis of OT is complex and as yet poorly understood. We used C57BL/6 background CD4+-T-cell-deficient, CD8+-T-cell-deficient, and μMT murine models to investigate the roles of these cells in the development of pathogenesis of acute OT and in the protective immunity against ocular infection of RH strain tachyzoites of T. gondii. Our data indicate that CD4+ T cells are essential to the pathogenesis of OT.
CD4+ T cells are important in the inflammatory cell response against acute T. gondii infection. In the present study, we observed that eyes from CD4 KO mice did not show necrosis or acute inflammation after either primary infection or ts-4 vaccination and challenge. Experiments using anti-CD4 treatment or class II-deficient C57BL/6 mice by Liesenfeld et al. (21) support a role for CD4+ T cells in determining disease outcome and the development of this inflammatory process in the small intestine. Our data demonstrate that CD4+ T cells are required for the induction of ocular pathogenesis. In addition, we observed that, compared to controls, the number of parasites was much higher in the eyes of CD4 KO mice after either primary infection or vaccination and challenge. CD4+-T-cell depletion not only influences the development of brain cyst in infected mice (4) but also results in a significant increase in the number of brain cysts in vaccinated mice (33). CD4+ T cells have an important role in the maintenance of CD8+-T-cell immunity against T. gondii (4). CD4+ T cells provide a helper function necessary for the generation and/or activity of CD8+ effector cells and for the induction of both protection and CD8+ functional activity (8, 24). Depletion of both CD4+ and CD8+ cells has a greater effect on resistance than the depletion of CD8+ cells alone (8). Feron et al. (6) found that all T. gondii-specific T-cell clones isolated from the vitreous fluid of patients with OT are CD3+ CD4+ T cells. Our results indicate that CD4+ T cells play an important role in complete protective immunity against T. gondii challenge and participate in parasite reduction during ocular infection.
The importance of CD8+ T cells in the host systemic response to this parasite is well appreciated (8). Compared to controls, we observed more severe necrosis with greater multiplication of tachyzoites in the eyes of CD8 KO mice after either primary ocular infection or vaccination with ts-4 and challenge. Depletion of CD8+ T cells significantly decreases both passive immunity and active resistance to lethal infection of T. gondii (8, 37) and, in addition, reduces the cytotoxic-T-lymphocyte activity (18). Vaccinated mice depleted of CD8+ T cells are more susceptible to subsequent challenge (37). Brown and McLeod (3) reported that the formation of cysts of T. gondii in the brain of mice is mediated by CD8+ T cells and restricted by class I. Treatment with the combination of anti-CD4 and anti-CD8 monoclonal antibodies results in an increase of cyst numbers in the ocular lesion (7). Our data indicate that CD8+ T cells are involved in parasite control in both T. gondii-infected and challenged eyes. It has been shown that CD8+ T lymphocytes can lyse extracellular T. gondii tachyzoites and T. gondii-infected target cells in both humans and mice in vitro (17, 40). One observation is that the tachyzoites distributed differently in the eyes between control and mutant mice, especially in CD8 KO mice. Norose et al. (25) noted the differences not only in the number of parasites but also in the distribution of parasites in the various eye parts between WT and IFN-γ KO mice. It has also been reported that OT in immunocompromised host differs from the disease in immunocompetent patients, both clinically and histopathologically (27).
We observed that, compared to controls, μMT mice exhibited more-severe ocular lesions associated with marked increased parasite numbers in the eyes after primary infection, as well as after vaccination and challenge. This heightened increase in parasite burden in the B-cell-deficient mice suggests that B-cell function is important in the control of parasite replication. Antibody is produced systemically and locally in the eye during ocular infection (39). IgG is the major class involved in the humoral immune response against T. gondii parasites (29). B cells are required for vaccination-induced resistance to virulent tachyzoites to produce antibodies that function protectively in vivo by blocking the infection of host cells by tachyzoites (31). T. gondii tachyzoites are rapidly lysed by the activation of complement through the classical pathway in vitro in the presence of specific antibodies (32). Antibodies play a protective role in collaboration with macrophages to kill antibody-coated tachyzoites in vitro (2), and lymphokine-activated killer activity enhances in the presence of antibodies against T. gondii (5). Treatment of tachyzoites with a monoclonal antibody to T. gondii inhibits their intracellular proliferation (23). It has been reported that antibodies play a critical role in prevention of proliferation of tachyzoites in brains and lungs in both primary infection and reactivation of latent infection (14). Our results demonstrate that B cells are involved in protective immunity against ocular infection and challenge, and the presence of T. gondii-specific antibodies correlates with parasite replication in vivo. Consistent with a previous report (13), we also observed that T. gondii-infected CD4 KO mice had low IgG levels in serum in acute OT. CD4+ helper T cells may be needed for generation of high titers of isotype-switched serum antibodies specific for T. gondii antigen (13).
We observed significant increase in the serum production of IFN-γ and TNF-α in μMT, CD8 KO, and control mice at 11 days after both ocular infection and challenge. However, the production of IFN-γ and TNF-α in CD4 KO mice was not significantly different after either infection or vaccination and challenge. T-cell immunity is dependent on the induction of an effective IFN-γ and CD8+-T-cell response (34); treatment with either anti-IFN-γ or anti-TNF-α monoclonal antibodies results in a dramatic increase in the intensity of ocular lesions associated with the presence of the parasite and the severity of inflammatory response (7), whereas IFN-γ mediates development of the immunopathology and contributes to early death after T. gondii infection (21). Both TNF-α and IFN-γ are involved in the development of the small intestine pathology (20). IFN-γ acts synergistically with TNF-α to activate the inflammatory response and establish the type of immunity that limits parasite proliferation (19). Our data reveal that whereas the increased levels of IFN-γ and TNF-α in serum correspond to the development of ocular necrosis in the infected and challenged μMT, CD8 KO, and control mice, these cytokines also correspond to the protective role in the challenged control mice. IFN-γ is likely produced predominantly by CD4+ T cells (36). Neutralization of either IFN-γ or CD4+ T cells during peroral infection prevents severe necrosis of the ilea and acute mortality (21). Our data further suggest that CD4+ T cells are the major source of the production of IFN-γ and TNF-α and the outcome of ocular pathogenesis.
Host resistance to T. gondii infection is multifaceted and may involve a number of different cellular and humoral immune responses (10). Our findings suggest that the CD4+-T-cell compartment is fundamental to the induction of inflammation associated with ocular disease. The murine model utilized in the present study could be applied to better understand the mechanism of trafficking and persistence of these inflammatory cells in this immune privileged site.
Acknowledgments
We thank Mark S. Hu and Dominique Buzoni-Gatel for helpful suggestions and Franck J. D. Mennechet, Wen Li, and Nicolas Rachinel for support in providing valuable technical information. We thank Kenneth A. Orndorff for excellent technical assistance in confocal observation.
This study was supported by grants A119613, A130000, and TW01003 from the National Institutes of Health.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abu el-Asrar, A. M., K. Geboes, K. F. Tabbara, S. A. al-Kharashi, L. Missotten, and V. Desmet. 1998. Immunopathogenesis of conjunctival scarring in trachoma. Eye 12:453-460. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S. E., S. C. Bautista, and J. S. Remington. 1976. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin. Exp. Immunol. 26:375-380. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, C. R., and R. McLeod. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438-3441. [PubMed] [Google Scholar]
- 4.Casciotti, L., K. H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannemann, B. R., V. A. Morris, F. G. Araujo, and J. S. Remington. 1989. Assessment of human natural killer and lymphokine-activated killer cell cytotoxicity against Toxoplasma gondii trophozoites and brain cysts. J. Immunol. 143:2684-2691. [PubMed] [Google Scholar]
- 6.Feron, E. J., V. N. Klaren, E. A. Wierenga, G. M. Verjans, and A. Kijlstra. 2001. Characterization of Toxoplasma gondii-specific T cells recovered from vitreous fluid of patients with OT. Investig. Ophthalmol. Vis. Sci. 42:3228-3232. [PubMed] [Google Scholar]
- 7.Gazzinelli, R. T., A. Brezin, Q. Li, R. B. Nussenblatt, and C. C. Chan. 1994. Toxoplasma gondii: acquired OT in the murine model, protective role of TNF-α and IFN-γ. Exp. Parasitol. 78:217-229. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 9.Gilbert, R. E., D. T. Dunn, S. Lightman, P. I. Murray, C. E. Pavesio, P. D. Gormley, J. Masters, S. P. Parjer, and M. R. Stanford. 1999. Incidence of symptomatic toxoplasma eye disease: etiology and public health implications. Epidemiol. Infect. 123:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakim, F. T., R. T. Gazzinelli, E. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310-2316. [PubMed] [Google Scholar]
- 11.Haque, S., I. Khan, A. Haque, and L. Kasper. 1994. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect. Immun. 62:2908-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, M. S., J. D. Schwartzman, A. C. Lepage, I. A. Khan, and H. L. Kasper. 1999. Experimental OT induced in naive and preinfected mice by intracameral inoculation. Ocul. Immunol. Inflamm. 7:17-26. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, L. L., and P. C. Sayles. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect. Immun. 70:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 15.Kasper, L. H., T. Matsuura, S. Fonseka, J. Arruda, J. Y. Channon, and I. A. Khan. 1996. Induction of gamma-delta T cells during acute murine infection with Toxoplasma gondii. J. Immunol. 157:5521-5527. [PubMed] [Google Scholar]
- 16.Kernacki, K. A., R. P. Barrett, J. A. Hobden, and L. D. Hazlett. 2000. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J. Immunol. 164:1037-1045. [DOI] [PubMed] [Google Scholar]
- 17.Khan, I. A., K. A. Smith, and L. H. Kasper. 1988. Induction of antigen-specific parasiticidal cytotoxic T-cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J. Immunol. 141:3600-3605. [PubMed] [Google Scholar]
- 18.Khan, I. A., K. H. Ely, and L. H. Kasper. 1994. Antigen-specific CD8+ T-cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 152:1856-1860. [PubMed] [Google Scholar]
- 19.Khan, I. A., M. E. Eckel, E. R. Pfefferkorn, and L. H. Kasper. 1988. Production of gamma interferon by cultured human lymphocytes stimulated with a purified membrane protein (P30) from Toxoplasma gondii. J. Infect. Dis. 157:979-984. [DOI] [PubMed] [Google Scholar]
- 20.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 21.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T-cell dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, F., S. Huang, and L. H. Kasper. 2003. Interleukin-10 and pathogenesis of murine OT. Infect. Immun. 71:7159-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 79:11-20. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 25.Norose, K., H. S. Mun, F. Aosai, M. Chen, L. X. Piao, M. Kobayashi, Y. Iwakura, and A. Yano. 2003. IFN-gamma-regulated Toxoplasma gondii distribution and load in the murine eye. Investig. Ophthalmol. Vis. Sci. 44:4375-4381. [DOI] [PubMed] [Google Scholar]
- 26.Pearlman, E., J. H. Lass, D. S. Bardenstein, M. Kopf, F. E., Jr. Hazlett, E. Diaconu, and J. W. Kazura. 1995. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness). J. Exp. Med. 182:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, F., and R. McLeod. 1999. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol. Today 15:51-57. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers, C. A., and J. R. Harris. 1996. Ocular toxoplasmosis in HIV infection. Int. J. STD AIDS 7:307-309. [DOI] [PubMed] [Google Scholar]
- 29.Ronday, M. J. H., J. V. Ongkosuwito, A. Rothova, and A. Kijlstra. 1999. Intraocular anti-Toxoplasma gondii IgA antibody production in patients with OT. Am. J. Ophthalmol. 127:294-300. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki, M. G., J. Arana, and A. G. Leite. 2000. Ocular involvement in systemic toxoplasmosis: a case report. Braz. J. Infect. Dis. 4:301-306. [PubMed] [Google Scholar]
- 31.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber, R. D., and H. A. Feldman. 1980. Identification of the activator system for antibody to Toxoplasma as the classical complement pathway. J. Infect. Dis. 141:366-369. [DOI] [PubMed] [Google Scholar]
- 33.Scorza, T., S. D'Souza, M. Laloup, J. Dewit, J. De Braekeleer, H. Verschueren, M. Vercammen, K. Huygen, and E. Jongert. 2003. A GRA1 DNA vaccine primes cytolytic CD8+ T cells to control acute Toxoplasma gondii infection. Infect. Immun. 71:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subauste, C. S., A. H. Koniaris, and J. S. Remington. 1991. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J. Immunol. 147:3955-3959. [PubMed] [Google Scholar]
- 35.Suzuki, Y. 2002. Host resistance in the brain against Toxoplasma gondii. J. Infect. Dis. 185:S58-S65. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, Y., A. Sher, G. Yap, D. Park, L. E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946. [PubMed] [Google Scholar]
- 38.Thomas, J., and B. T. Rouse. 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 16:375-386. [DOI] [PubMed] [Google Scholar]
- 39.Turunen, H. J., P. O. Leinikki, and K. M. Saari. 1983. Demonstration of intraocular synthesis of immunoglobulin G Toxoplasma antibodies for specific diagnosis of toxoplasmic chorioretinitis by enzyme immunoassay. J. Clin. Microbiol. 17:988-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yano, A., F. Aosai, M. Ohta, H. Hasekura, K. Sugane, and S. Hayashi. 1989. Antigen presentation by Toxoplasma gondii-infected cells to CD4+ proliferative T cells and CD8+ cytotoxic cells. J. Parasitol. 75:411-416. [PubMed] [Google Scholar]