Abstract
We have previously shown that Chlamydia trachomatis inhibits host cell apoptosis and blocks mitochondrial cytochrome c release. We now report that activation of both Bax and Bak, two proapoptotic members of the Bcl-2 family that regulate mitochondrial cytochrome c release, was inhibited in chlamydia-infected cells. This observation has provided new information on the mechanisms of chlamydial antiapoptotic activity.
Urogenital tract infection with Chlamydia trachomatis is a leading cause of sexually transmitted bacterial diseases in the United States. However, the mechanisms of chlamydial pathogenesis are still unclear. It is thought that the inflammatory responses triggered by chlamydia-infected cells may mainly contribute to the chlamydia-induced diseases in humans (15). Chlamydiae have evolved various strategies for long-term survival in the infected host, including hiding inside cytoplasmic vacuoles of host cells, inhibition of phagolysosomal fusion, and evading immune recognition (19). Apoptosis represents a major host defense effector mechanism in both innate and adaptive immunity against microbial infection (2). Others and we have previously demonstrated that chlamydia-infected cells are profoundly resistant to apoptosis induction (4, 6, 7), which may benefit chlamydial long-term survival in the infected host. Although the chlamydial antiapoptotic activity was correlated with inhibition of mitochondrial cytochrome c release in chlamydia-infected cells, the precise mechanisms of chlamydial antiapoptotic activity are still unknown. The present study was designed to map the steps of apoptosis pathways interrupted by chlamydial infection. We measured the effects of chlamydial infection on the activation of two proapoptotic Bcl-2 family members, Bax and Bak, steps occurring upstream of mitochondrial cytochrome c release.
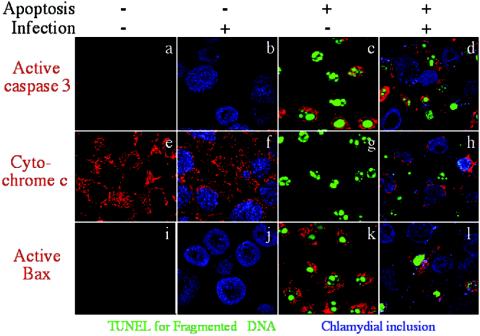
We first evaluated whether chlamydial infection can prevent Bax activation during apoptosis since activation of Bax is known to induce mitochondrial cytochrome c release (10, 11). Upon induction of apoptosis, Bax undergoes a conformational change and translocates to mitochondrial outer membranes, where it inserts itself and mediates the release of cytochrome c from the intermembrane space into the cytosol (18). Also importantly, the Bax conformational change and translocation to mitochondrial membranes can be detected with an NH2 terminus-specific antibody in an immunofluorescence assay (5, 14), which was used in the present study. HeLa cells (American Type Culture Collection, Manassas, Va.) were infected with C. trachomatis serovar L2 at an MOI (multiplicity of infection) of 0.5 (an ∼50% infection rate) for 40 h. Both control and infected cultures were treated with staurosporine at 2 μg/ml (Sigma, St. Louis, Mo.) for 5 h before being processed for triple staining (Fig. 1). The chlamydial inclusions were labeled with either a rabbit or mouse antichlamydial antibody (produced in our own laboratory) in combination with Cy5-conjugated goat anti-rabbit or -mouse immunoglobulin G (IgG; blue; Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). The fragmented DNA was labeled with fluorescein isothiocyanate-tagged dUTP via a terminal transferase reaction with a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling kit (green; Promega, Madison, Wis.). Active caspase 3 (top row), cytochrome c (middle row), or active Bax (bottom row) was labeled with a rabbit anti-active caspase 3 (Promega), a mouse anti-cytochrome c (Pharmingen, San Diego, Calif.), or a rabbit anti-active Bax (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) antibody in combination with Cy3-conjugated goat anti-rabbit or -mouse IgG (red; Jackson ImmunoResearch Laboratories, Inc.). The images for the various colors were acquired individually with an Olympus confocal microscope and overlaid to make tricolor images. Although cells without chlamydial infection were induced to undergo DNA fragmentation (panels c, g, and k), caspase 3 activation (panel c), mitochondrial cytochrome c release (panel g), and Bax activation (panel k), the chlamydia-infected cells were prevented from any of these responses (panels d, h, and l). As we have previously demonstrated (8), the convincing evidence of chlamydial antiapoptotic activity comes from the cultures with both infection and apoptosis induction when the infection rate is kept at ∼50%. As shown in panels d, h, and l of Fig. 1, the apoptosis responses including Bax activation were detected only in uninfected cells and not in infected cells although both cell populations were maintained in the same culture. When these two cell populations from the same culture were counted under an Olympus AX-70 fluorescence microscope for Bax activation, we found that 93% of uninfected cells were induced to express active Bax while only 8% of the chlamydia-infected cells were induced to do so. Five random views with a total of ∼200 cells were counted for each coverslip, and the results were consistent in three independent experiments. These observations have not only confirmed our previous observations that chlamydial infection profoundly inhibits nuclear apoptosis, caspase 3 activation, and mitochondrial cytochrome c release (6, 8) but, more importantly, allowed us to map the chlamydial antiapoptotic activity to Bax, an upstream step of mitochondrial cytochrome c release.
FIG. 1.
Correlation of chlamydial antiapoptotic activity with chlamydial blockade of caspase 3 activation, mitochondrial cytochrome c release, and Bax activation. HeLa cells infected with C. trachomatis serovar L2 at an MOI of 0.5 for 40 h were induced to undergo apoptosis with staurosporine. The cultures were then fixed and processed for triple staining with antichlamydial antibodies plus Cy5 conjugates for chlamydial inclusions (blue), terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling for fragmented DNA (green), various primary antibodies plus Cy3 conjugates for active caspase 3 (red; top row), mitochondrial cytochrome c release (red; middle row), or active Bax (red; bottom row). The images for the various colors were acquired individually with an Olympus confocal microscope and overlaid to make tricolor images. The bright granular red staining indicates mitochondrial localization of the labeled molecules. Note that in the cultures with both infection and apoptosis induction (last column), only uninfected cells and not infected cells were induced to undergo DNA fragmentation (panels d, h, and l), caspase 3 activation (panel d), mitochondrial cytochrome c release (panel h), and Bax activation (panel l).
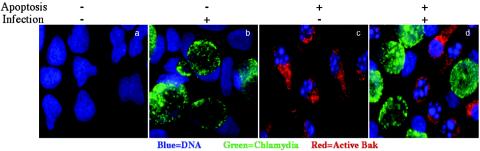
To further understand the mechanism of chlamydial antiapoptotic activity, we evaluated the effect of chlamydial infection on the mitochondrial localization of Bak, another proapoptotic member of the Bcl-2 family. As with Bax, upon apoptosis stimulation, Bak can undergo conformational changes by exposing its cryptic epitope at the NH2 terminus and localize to mitochondria to induce mitochondrial cytochrome c release (1, 17). Bak activation and localization to mitochondria from cell cytosol are also traceable via immunofluorescence staining with antibodies specifically recognizing the NH2-terminal cryptic epitope of Bak molecules (3, 12). We used experimental conditions similar to those described for Fig. 1 to evaluate the effect of chlamydial infection on Bak activation (Fig. 2). The final cultures were stained with the Hoechst DNA dye for visualizing host cell nuclei (blue), a mouse antibody to chlamydia in combination with a Cy2-conjugated goat anti-mouse IgG for visualizing chlamydial inclusions (green), and a rabbit anti-Bak antibody (catalog no. 196150; Calbiochem, La Jolla, Calif.) in combination with Cy3-conjugated goat anti-rabbit IgG for visualizing the activated Bak molecules that are localized to mitochondria (red). The triple staining was observed under an Olympus AX-70 fluorescence microscope, and single-color images were acquired individually and then overlaid to form tricolor images. Again, the convincing evidence comes from the culture with both infection (∼50% infection rate) and apoptosis induction. As shown in the final panel of Fig. 2, although the uninfected HeLa cells were induced to undergo both nuclear condensation and Bak activation, the chlamydia-infected cells in the same culture were prevented from doing so. When both cell populations on the same coverslips were counted for Bak activation in three separate experiments (cell counting was carried out as described above for the Bax experiment), we found that Bak activation was induced in 93% of the uninfected cells but only 7% of the chlamydia-infected cells, demonstrating that chlamydial infection profoundly inhibited staurosporine-induced Bak activation. This observation has allowed us to map the chlamydial antiapoptotic activity to Bak, another upstream step of mitochondrial cytochrome c release.
FIG. 2.
Chlamydial inhibition of Bak activation. HeLa cells with (panels b and d) or without (panels a and c) chlamydial infection and with (panels c and d) or without (panels a and b) apoptosis induction were processed for triple staining. The culture, infection, and apoptosis induction conditions were the same as those described for Fig. 1. However, the host cell nuclei were labeled with a DNA dye (blue), chlamydial inclusions were labeled with a mouse antichlamydial antibody plus a Cy2 conjugate (green), and Bak was labeled with a rabbit primary antibody plus a Cy3 conjugate (red). The images for the various colors were acquired individually with an Olympus AX-70 fluorescence microscope and overlaid to make tricolor images. The granular red staining indicates mitochondrial localization of Bax. Note that although Bak was induced to localize to mitochondria in normal (panel c) and uninfected (panel d) cells, the mitochondrial localization of Bak was completely suppressed in chlamydia-infected cells (panel d). Bak localization to mitochondrial membrane correlates well with nuclear condensation.
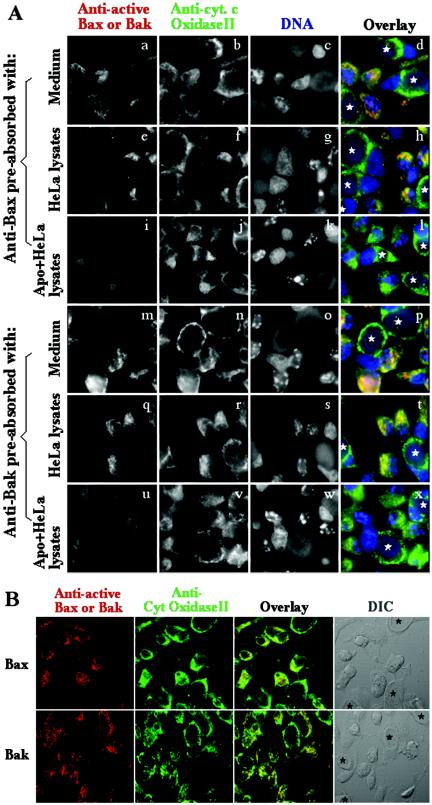
The immunofluorescence staining with the NH2 terminus-specific anti-Bax or -Bak antibodies described here has been extensively used by others for detecting Bax and Bak mitochondrial localization in apoptotic cells (9, 12, 14). In fact, the exact same anti-Bax antibody was previously used to detect Bax activation induced by chlamydial infection (14a). We further carried out a preabsorption experiment to verify the specificity of the anti-Bax and anti-Bak antibodies used in this study (Fig. 3A). HeLa cells infected with chlamydia at an ∼50% infection rate were induced to undergo apoptosis with staurosporine 40 h after infection as described for Fig. 1 and 2. The cell samples were triply stained with the rabbit anti-Bax or -Bak antibodies (visualized with a goat anti-rabbit IgG Cy3 conjugate; red) plus mouse anti-cytochrome c oxidase subunit II (Molecular Probes, Eugene, Oreg.; visualized with a goat anti-mouse IgG Cy2 conjugate; green) plus Hoechst for staining of DNA (blue). To test whether the anti-Bax and anti-Bak antibodies are specific to the activated Bax and Bak molecules in apoptotic cells, we preabsorbed these antibodies with either medium or cell lysates made from either apoptotic or normal HeLa cells. As shown in Fig. 3A, the anti-cytochrome c oxidase II antibody positively stained all of the cells regardless of infection or apoptosis (second column for single-color images, fourth column for tricolor images). The anti-Bax (panels a and d) or anti-Bak (panels m and p) antibodies after preabsorption with medium alone stained the apoptotic cells that were not infected, confirming the results shown in Fig. 1 and 2. More importantly, preabsorption with the apoptotic but not normal HeLa cell lysates completely blocked both the anti-Bax (panel i versus panel e for single-color images, panel l versus panel h for tricolor images) and anti-Bak (panel u versus panel q, panel x versus panel t) antibody staining, which demonstrated that these antibodies specifically recognized the corresponding active Bax and Bak molecules that are present only in apoptotic cells and not in normal cells. To further evaluate whether the activated Bax or Bak molecules are localized to mitochondria in apoptotic cells, we used confocal microscopy to analyze the same sets of samples (Fig. 3B) and found that most of the anti-Bax and anti-Bak staining colocalized with the anti-cytochrome c oxidase subunit II staining. Since cytochrome c oxidase subunit II is known to localize to mitochondrial inner membrane, colocalization of activated Bax and Bak with cytochrome c oxidase subunit II demonstrated that activated Bax and Bak are indeed localized to mitochondria, which is consistent with the observations previously made by others (5, 9, 13, 14, 16, 18).
FIG. 3.
Specificity (A) and mitochondrial localization (B) of anti-Bax and anti-Bak antibody staining. HeLa cells infected with C. trachomatis serovar L2 at an ∼50% infection rate for 40 h were induced to undergo apoptosis and processed for triple immunofluorescence staining with rabbit anti-Bax and -Bak antibodies to detect activated Bax and Bak (red; as described in the legends to Fig. 1 and 2) plus a mouse anti-cytochrome c oxidase subunit II antibody to localize mitochondria (green) and Hoechst dye to label DNA (blue) as indicated at the top. Both the anti-Bax and anti-Bak antibodies were subjected to preabsorption with either medium alone, normal HeLa lysates, or apoptotic HeLa lysates as indicated at theleft. Note that although the anti-cytochrome c oxidase subunit II antibody stained all of the cells, the anti-Bax and -Bak antibodies only stained the apoptotic cells and the staining of both Bax and Bak was blocked by preabsorption of the anti-Bax and -Bak antibodies with the lysates made from apoptotic HeLa cells. The chlamydial inclusions are marked with white stars. To more precisely determine the location of the activated Bax and Bak molecules, the samples were also observed under a confocal microscope (B). Hoechst staining requires UV excitation, and our confocal microscope is not equipped with a UV laser. Therefore, only two color images (red for Bax or Bak and green for mitochondrial cytochrome c oxidase subunit II) were acquired. The differential interference contrast (DIC) image can allow us to identify infected cells (marked with black stars). Note that both anti-Bax staining and anti-Bak staining were colocalized with mitochondrial cytochrome c oxidase subunit II to mitochondria in apoptotic cells.
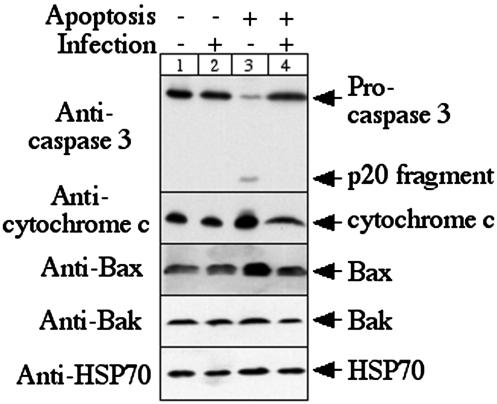
To check whether the chlamydial inhibition of Bax and Bak activation is due to the decreased Bax and Bak protein levels in the infected cells, we compared the total protein levels of caspase 3, cytochrome c, Bax, and Bak between HeLa cells with or without infection and with or without apoptosis induction on a Western blot (Fig. 4). A culture condition similar to that described for Fig. 1 was used for the present experiment except that the infection rate was increased to an MOI of 5 to ensure a greater than 95% infection rate. The large infection dose was designed to limit apoptosis responses in the infected cultures by the uninfected cells. After apoptosis induction, the cultures were lysed with sodium dodecyl sulfate sample buffer for a Western blot assay as previously described (6). Mouse antibodies to caspase 3, cytochrome c (both from Pharmingen), and HSP70 and rabbit antibodies to Bax and Bak (all three from Santa Cruz Biotechnology, Inc.) in combination with horseradish peroxidase-conjugated goat anti-mouse or -rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.) were used for the Western blot assay. Besides obvious cleavage of caspase 3 induced by staurosporine, no significant differences were observed in the cytochrome c, Bax, Bak, and HSP70 protein levels among the various HeLa cultures. Host cell HSP70 served as a loading control since we found that neither chlamydial infection nor apoptosis significantly altered its expression. This observation suggests that chlamydial infection only blocked the mitochondrial localization of Bax and Bak without significantly affecting the total levels of these two proapoptotic Bcl-2 family member proteins.
FIG. 4.
Effects of chlamydial infection on Bax and Bak protein levels. The culture and treatment conditions were similar to those described for Fig. 1, with the exception of an MOI of 5. All four samples were loaded onto the gel with an equivalent number of cells. The anti-caspase 3 antibody recognized both procaspase and processed caspase 3 fragment p20. Host HSP70 detected with a mouse anti-human HSP70 antibody was used to monitor the total proteins loaded onto each lane. Note that there is no significant difference in the total levels of the cytochrome c, Bax, and Bak proteins among the four culture samples although chlamydial infection blocked redistribution of these proteins induced by apoptosis stimulation as shown in Fig. 1 and 2.
Although C. trachomatis has been reported to possess proapoptotic activity (14a, 14b), it appears that only its antiapoptotic activity is a biologically significant event during chlamydial infection (8). This chlamydial antiapoptotic activity was further correlated with the inhibition of mitochondrial cytochrome c release. The present study has mapped the chlamydial antiapoptotic activity to Bax and Bak, both of which are proapoptotic Bcl-2 family member proteins. It has been shown that activation of either Bax or Bak can result in the release of cytochrome c from mitochondria. Cells deficient in both Bax and Bak but not in one or the other alone are profoundly resistant to apoptosis induction with a wide array of proapoptotic stimuli (17). The observation that chlamydiae blocked the activation of both Bax and Bak upon apoptosis stimulation is consistent with our previous finding that chlamydia-infected cells, like Bak-Bax double-knockout cells, were profoundly resistant to apoptosis induced by a wide spectrum of proapoptotic stimuli (6). The next question is how chlamydiae block the activation of Bax and Bak. We are in the process of both further mapping the chlamydial antiapoptotic activity along the apoptosis pathways and developing cell biological and biochemical assays to identify the potential chlamydial antiapoptotic factors.
Acknowledgments
This work was supported in part by grants (R01 AI47997 and R01HL64883) from the U.S. National Institutes of Health to G. Zhong.
Editor: F. C. Fang
REFERENCES
- 1.Arnoult, D., B. Gaume, M. Karbowski, J. C. Sharpe, F. Cecconi, and R. J. Youle. 2003. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 22:4385-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 3.Bellosillo, B., N. Villamor, A. Lopez-Guillermo, S. Marce, F. Bosch, E. Campo, E. Montserrat, and D. Colomer. 2002. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood 100:1810-1816. [DOI] [PubMed] [Google Scholar]
- 4.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewson, G., R. T. Snowden, J. B. Almond, M. J. Dyer, and G. M. Cohen. 2003. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene 22:2643-2654. [DOI] [PubMed] [Google Scholar]
- 6.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer, S. F., T. Harlander, J. Vier, and G. Hacker. 2004. Protection against CD95-induced apoptosis by chlamydial infection at a mitochondrial step. Infect. Immun. 72:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene, W., Y. Xiao, Y. Huang, G. McClarty, and G. Zhong. 2004. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect. Immun. 72:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths, G. J., L. Dubrez, C. P. Morgan, N. A. Jones, J. Whitehouse, B. M. Corfe, C. Dive, and J. A. Hickman. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimlich, G., A. D. McKinnon, K. Bernardo, D. Brdiczka, J. C. Reed, R. Kain, M. Kronke, and J. M. Jurgensmeier. 2004. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem. J. 378:247-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi, A., S. Shimizu, J. Hirota, T. Takao, Y. Fan, Y. Matsuoka, L. Zhang, Y. Yoneda, Y. Fujii, A. I. Skoultchi, and Y. Tsujimoto. 2003. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell 114:673-688. [DOI] [PubMed] [Google Scholar]
- 13.Makin, G. W., B. M. Corfe, G. J. Griffiths, A. Thistlethwaite, J. A. Hickman, and C. Dive. 2001. Damage-induced Bax N-terminal change, translocation to mitochondria and formation of Bax dimers/complexes occur regardless of cell fate. EMBO J. 20:6306-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nechushtan, A., C. L. Smith, Y. T. Hsu, and R. J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18:2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Perfettini, J. L., J. C. Reed, N. Israël, J. C. Martinou, A. Dautry-Varsat, and D. M. Ojcius. 2002. Role of Bcl-2 family members in caspase-independent apoptosis during Chlamydia infection. Infect Immun. 70:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b.Perfettini, J. L., D. M. Ojcius, C. W. Andrews, Jr., S. J. Korsmeyer, R. G. Rank, and T. Darville. 2003. Role of proapoptotic BAX in propagation of Chlamydia muridarum (the mouse pneumonitis strain of Chlamydia trachomatis) and the host inflammatory response. J. Biol. Chem. 278:9496-9502. [DOI] [PubMed] [Google Scholar]
- 15.Stephens, R. S. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki, M., R. J. Youle, and N. Tjandra. 2000. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103:645-654. [DOI] [PubMed] [Google Scholar]
- 17.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]