Abstract
Pathogenic yersiniae (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) harbor a 70-kb virulence plasmid (pYV) that encodes a type III secretion system and a set of at least six effector proteins (YopH, YopO, YopP, YopE, YopM, and YopT) that are injected into the host cell cytoplasm. Yops (Yersinia outer proteins) disturb the dynamics of the cytoskeleton, inhibit phagocytosis by macrophages, and downregulate the production of proinflammatory cytokines, which makes it possible for yersiniae to multiply extracellularly in lymphoid tissue. Y. enterocolitica serotype O:8 belongs to the highly mouse-pathogenic group of yersiniae in contrast to Y. enterocolitica serotype O:9. However, there has been no systematic study of the contribution of Yops to the pathogenicity of Y. enterocolitica O:8 in mice. We generated a set of yop gene deletion mutants of Y. enterocolitica O:8 by using the novel Red cloning procedure. We subsequently analyzed the contribution of yopH, -O, -P, -E, -M, -T, and -Q deletions to pathogenicity after oral and intravenous infection of mice. Here we showed for the first time that a ΔyopT deletion mutant colonizes mouse tissues to a greater extent than the parental strain. The ΔyopO, ΔyopP, and ΔyopE mutants were only slightly attenuated after oral infection since they were still able to colonize the spleen and liver and cause systemic infection. The ΔyopO mutant was lethal for mice, whereas ΔyopP and ΔyopE mutants were successfully eliminated from the spleen and liver 2 weeks after infection. In contrast the ΔyopH, ΔyopM, and ΔyopQ mutants were highly attenuated and not able to colonize the spleen and liver on any of the days tested. The ΔyopH, ΔyopO, ΔyopP, ΔyopE, ΔyopM, and ΔyopQ mutants had only modest defects in the colonization of the small intestine and Peyer's patches. The ΔyopE mutant was eliminated from the small intestine 3 weeks after infection, whereas the ΔyopH, ΔyopP, ΔyopM, and ΔyopQ mutants continued to colonize the small intestine at this time.
Yersiniae that are pathogenic to humans include Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica. Y. pestis is the cause of bubonic plague, which is transmitted by fleas, whereas Y. enterocolitica and Y. pseudotuberculosis cause self-limited food-borne gastrointestinal disease in humans. In the mouse model of infection, however, both Y. enterocolitica and Y. pseudotuberculosis cause systemic disease. Common to all of these species is the presence of a 70-kb virulence plasmid that harbors a type III secretion system (TTSS) and several secreted and translocated proteins called Yersinia outer proteins (Yops) (reviewed by Cornelis [17]). This plasmid-encoded TTSS enables yersiniae to survive and proliferate extracellularly in host lymphatic tissues. Y. enterocolitica and Y. pseudotuberculosis both translocate a set of at least five effector proteins (YopH, YopO/YpkA, YopP/J, YopE, and YopM), whereas Y. enterocolitica is known to translocate a sixth effector called YopT (35). Four of the above-mentioned Yops (YopH, YopE, YopT, and, YopO/YpkA) disturb cytoskeletal dynamics and thereby inhibit phagocytosis by polymorphonuclear leukocytes and macrophages (12, 23, 28, 49). YopH is a phosphotyrosine phosphatase (61) that dephosphorylates focal adhesion kinase (Fak), paxillin, Fyn-binding protein (FYB), p130cas, and SKAP-HOM, thereby disrupting focal adhesions (2, 10, 11, 16, 29, 46, 47), and suppresses the oxidative burst of macrophages (12, 47). YopH has been shown to contribute not only to evasion of the innate immune response but also to the adaptive immune response by impairing T- and B-cell activation (1, 60). YopE is a GTPase-activating protein that acts preferentially on Rac GTPases, which may explain the YopE-associated effect of actin stress fiber destruction (9, 44, 50). YopT is a cysteine protease that preferentially inactivates RhoA GTPases by cleaving at the C-terminal geranylgeranyl-cysteine residue (52, 62). YopO (YpkA in Y. pseudotuberculosis) is an autophosphorylating serine/threonine kinase that interacts with RhoA, Rac and actin (6, 22, 62). YopP/J induces apoptosis of macrophages and inhibits activation of the transcription factor NF-κB, thereby inhibiting tumor necrosis factor alpha and interleukin-8 release by macrophages and epithelial cells (14, 19, 20). YopM is a leucine-rich repeat protein that traffics to the nucleus of infected cells (54), but its target and function are as yet unknown. Recently, it was shown that YopM forms a protein complex with the two cellular kinases PRK2 and RSK1 (39). YopQ (YopK in Y. pseudotuberculosis) is not one of the translocated effector proteins but has been shown to control the translocation of Yop effectors into eukaryotic cells with a yopK mutant hypertranslocating Yops and inducing a larger YopB-dependent pore in eukaryotic cell membranes (33).
Virulence of Yersinia yop mutants has been previously studied mostly in Y. pseudotuberculosis. It is, however, not possible to generally extrapolate these results to Y. enterocolitica since many differences in virulence factor profile and clinical manifestations exist between the two species. Mutations in yopH, ypkA, yopJ, yopE, yopM, and yopK of Y. pseudotuberculosis serotype III have been shown to result in various degrees of attenuation (13, 15, 24, 27, 34, 37, 38, 42). In most cases, the 50% lethal dose was determined in order to assess virulence. Several studies also analyzed colonization of mouse tissue after oral infection (9, 26, 27, 34, 42, 55). However, comparison of data is difficult because different inbred mouse strains, different strains of Yersinia, and different infection doses and routes of application were used.
The influence of the complete set of Yops on virulence of Y. enterocolitica in the mouse model has been only partially studied (35, 43) with Y. enterocolitica of serotype O:9. This serotype is only weakly pathogenic for mice (due to lack of the high pathogenicity island encoding the biosynthesis and uptake of the siderophore yersiniabactin [ybt] which contributes to mouse virulence), and therefore mice have to be pretreated with an iron chelator such as desferrioxamine, which not only promotes growth of yersiniae by iron delivery via ferrioxamine uptake but also leads to immunosuppression of the host (5). We generated here a set of yop deletion mutants of Y. enterocolitica of the highly pathogenic O:8 serotype WA-314 (biotype 1B) by the recently reported Red recombination procedure (18) and studied the pathogenicity of these mutants and the course of colonization of the small intestine (SI), Peyer's patches (PP), liver, and spleen over 3 weeks after intravenous and oral infection of C57BL/6 mice.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in the present study are listed in Table 1. Bacteria were cultured aerobically in Luria-Bertani (LB) broth or on LB agar plates (Difco Laboratories, Detroit, Mich.) at 27°C (Yersinia spp.) or 37°C (Escherichia coli). Antibiotics were used at the following concentrations: kanamycin, 25 μg/ml; nalidixic acid, 60 μg/ml; and chloramphenicol, 20 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| Yersinia spp. | ||
| WA-314 | Y. enterocolitica serotype O:8; clinical isolate; pYVO8+ | 32 |
| WA-C | Plasmidless derivative of WA-314 | 32 |
| WA-C(pYV::CM) | WA-C harboring pYV::CM | This study |
| WA-C(pYVΔH) | WA-C harboring pYVΔH | This study |
| WA-C(pYVΔH+H) | WA-C harboring pYVΔH and pYopH | This study |
| WA-C(pYVΔO) | WA-C harboring pYVΔO | This study |
| WA-C(pYVΔP) | WA-C harboring pYVΔP | This study |
| WA-C(pYVΔE) | WA-C harboring pYVΔE | This study |
| WA-C(pYVΔM) | WA-C harboring pYVΔM | This study |
| WA-C(pYVΔT) | WA-C harboring pYVΔT | This study |
| WA-C(pYVΔQ) | WA-C harboring pYVΔQ | This study |
| E. coli | ||
| DH5α | endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U169 (φ80lacZΔM15) | 30 |
| S17-1λpir | pir+tra+ Kanr | 41 |
| Plasmids | ||
| pACYC184 | Low-copy vector; Cmr Tcr | New England Biolabs |
| pACYC177 | Low-copy vector; Kanr Ampr | New England Biolabs |
| pGPCAT | Suicide vector; R6K replicon; Cmr Mob+ | 48 |
| pUC4k | Kanr cassette | Pharmacia-LKB |
| pYV::CM | pYV plasmid harboring Cmr gene from pACYC184 in noncoding region adjacent to yadA | This study |
| pYVΔH | pYV plasmid with yopH replaced by Kmr gene from pACYC177 | This study |
| pYVΔO | pYV plasmid with yopO replaced by Kmr from pACYC177 | This study |
| pYVΔP | pYV plasmid with yopP replaced by Kmr from pACYC177 | This study |
| pYVΔE | pYV plasmid with yopE replaced by Kmr from pACYC177 | This study |
| pYVΔM | pYV plasmid with yopM harboring Kmr from pUC4k | This study |
| pYVΔT | pYV plasmid with yopT replaced by Kmr from pACYC177 | This study |
| pYopHSycH | pACYC184 harboring yopH and sycH | 57 |
Ampr, ampicillin resistance; Tcr, tetracycline resistance.
Nucleic acid manipulations.
Plasmid DNA was isolated with Qiagen kits (Hilden, Germany) according to the manufacturer's recommendations. Restriction enzyme digestions, recovery of DNA fragments from agarose gels, ligations, and transformations were performed as previously described (3). Enzymes and deoxynucleoside triphosphates were purchased from Invitrogen (Karlsruhe, Germany). High-fidelity polymerase was obtained from Roche (Mannheim, Germany). Oligonucleotides were synthesized by Thermo (Ulm, Germany).
Red cloning.
We generated stable Y. enterocolitica Yop mutants by using the λ phage recombinases Redα and Redβ as previously described for E. coli (18). Recombinases were expressed directly in Yersinia. For this purpose, we transformed WA-314 with plasmid pKD46 harboring recombinases redα and redβ, as well as redγ, an inhibitor of bacterial exonucleases. Yersiniae were grown overnight at 27°C, diluted 1:100, and grown to exponential phase in LB medium containing 0.1% arabinose to induce the expression of recombination functions. Yersiniae were made electrocompetent and frozen at −80°C. Recombination fragments consisting of an antibiotic resistance gene with its own promoter, flanked by 50-nucleotide (nt) homology arms, were generated by PCR. The 3′-end 21 nt of each primer were designed to amplify the kanamycin resistance (Kanr) cassette from pACYC177 (forward, 5-TCACTGACACCCTCATCAGTG-3′; reverse, 5′-CGTCAAGTCAGCGTAATGCTC-3′) or the chloramphenicol resistance (Cmr) cassette from pACYC184 (forward, 5′-TGACGGAAGATCACTTCGCAG-3′; reverse, 5′-TTGAGAAGCACACGGTCAC-3′), whereas the 5′ end of each primer contained the 50-nt homology arms. These were designed so that the entire coding region of each yop gene (yopH, -O, -P, -E, and -T) would be replaced by the antibiotic resistance marker. Homology arms were derived from the sequence of pYVa127/90 (GenBank accession number NC_004564) (25). The ΔyopQ mutant used for virulence experiments harbors the first 50 amino acids of YopQ. The ΔyopM mutant was constructed by ligating a PstI-restricted 1.2-kb Kanr cassette cut from pUK4k into the NsiI site of the yopM gene, which was cloned into the suicide vector pGPCAT. Mutagenesis was performed as previously described (58). WA-C(pYV::CM) was constructed by inserting a Cmr cassette amplified from pACYC184 into the noncoding region of the pYV plasmid, upstream of yadA, by the Red cloning procedure. This strain was shown to be as virulent as the unmarked parental strain WA-314 (results not shown). All mutated pYV constructs were transferred to a plasmidless WA-C strain. All strains were passaged through mice before virulence experiments were performed by intraperitoneal infection with 108 CFU and reisolation of yersiniae from the peritoneum after 20 h.
Analysis of Yop secretion.
Secretion of Yop proteins was induced as previously described (57). Yersiniae were grown overnight in LB medium at 27°C, diluted 1:40 in brain heart infusion broth (Difco), and incubated for 2 h at 37°C. Yop expression and/or secretion was induced by the addition of EGTA (5 mM) for Ca2+ chelation, MgCl2 (15 mM), and glucose (0.2%). Bacteria were grown at 37°C for 2 to 3 h and centrifuged (10,000 × g, 15 min), and proteins were precipitated from the culture supernatant with trichloroacetic acid. Released proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (36) on an 11.5% polyacrylamide gel and then stained with Coomassie blue. Immunoblotting was performed as described previously (31) with nitrocellulose sheets (Schleicher & Schuell). Blocking was performed overnight with 5% bovine serum albumin in phosphate-buffered saline (PBS) at 4°C. Polyclonal rabbit anti-YopO, anti-YopQ, and anti-YopT (1:5,000) antisera, as well as a horseradish peroxidase-conjugated secondary antirabbit antibody (Sigma) diluted 1:5,000, were used for immunostaining.
Mouse infections.
Six- to eight-week-old female C57BL/6 mice (Harlan, Winkelmann, Germany) were infected with 108 CFU orally or 4 × 104 CFU intravenously from frozen stock suspensions. Stock suspensions were prepared by growing bacteria to stationary phase in LB medium at 27°C, followed by freezing in 15% glycerol. After appropriate dilutions, bacteria were washed twice with PBS, and mice were fed 50 μl by using a microliter pipette, or 100 μl was injected into the lateral tail vein. Mice were subjected to fasting 16 h prior to oral infection. The actually administered dose was determined by plating serial dilutions on Mueller-Hinton agar for 36 h at 27°C. Mice were sacrificed by CO2 asphyxiation. Liver, spleen, and PP were aseptically removed and homogenized in 5 ml (liver) or 1 ml (spleen and PP) PBS. PP were washed to remove loosely attached bacteria by rinsing them with 2 ml of PBS. The SI was washed with 5 ml of ice-cold PBS. To determine the numbers of CFU/organ, serial dilutions of homogenates were plated on Yersinia selective CIN agar (BD Biosciences, Heidelberg, Germany). P values were determined by using a two-tailed, unpaired Student t test. P values of <0.05 were considered significant. For the competition experiment, a two-tailed paired Student t test was used.
RESULTS
Generation of stable Yop mutants by Red cloning.
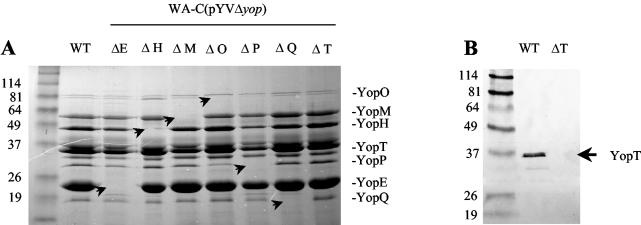
Yersinia yop mutants were generated by replacing the coding region of each yop by a Kanr cassette. This was accomplished by Redα- and Redβ-mediated homologous recombination between the pYV plasmid and a PCR product consisting of the Kanr resistance gene flanked by 50-nt homology arms. Correct replacement of the respective yop genes by the Kanr cassette was verified by PCR and SDS-PAGE of secreted Yop proteins and Western blotting (Fig. 1). To rule out any unwanted recombination in the chromosome due to the action of Redα and Redβ, we transferred all mutated plasmids to the previously pYV-cured strain WA-C. Furthermore, we checked that no unwanted recombination in the mutant pYV plasmids had taken place by restriction endonuclease digestion of the resulting Δyop-pYV plasmids with HindIII and BamHI (results not shown). Two of the mutants (ΔyopQ and ΔyopM) generated by replacement of the entire coding region with a Kanr cassette turned out to be deregulated when grown at 37°C (temperature-sensitive phenotype) and consequently unable to colonize any mouse tissue after oral infection. Therefore, to perform virulence studies, we generated another ΔyopQ mutant by Red cloning, sparing the first 50 amino acids of YopQ. This mutant did not show a growth deficit at 37°C. For yopM we used a mutant that we had already constructed by the suicide vector approach. This mutant harbors a 1.2-kb Kanr cassette at nt 661, expresses a truncated version of YopM of ∼25 kDa (verified by Western blotting), and shows Ca2+- and temperature-dependent growth like that of the wild type.
FIG. 1.
(A) Coomassie blue-stained SDS-PAGE gel showing secreted proteins of WA-C(pYV::CM) (wild type [WT], lane 1) and Δyop mutants (lanes 2 to 9) precipitated from the culture supernatant. (B) Western blot of secreted proteins from culture supernatant of WA-C(pYV::CM) [WT, lane 1] and WA-C(pYVΔT) (ΔT, lane 2) with a rabbit anti-YopT polyclonal antibody.
Course of colonization and persistence after oral infection.
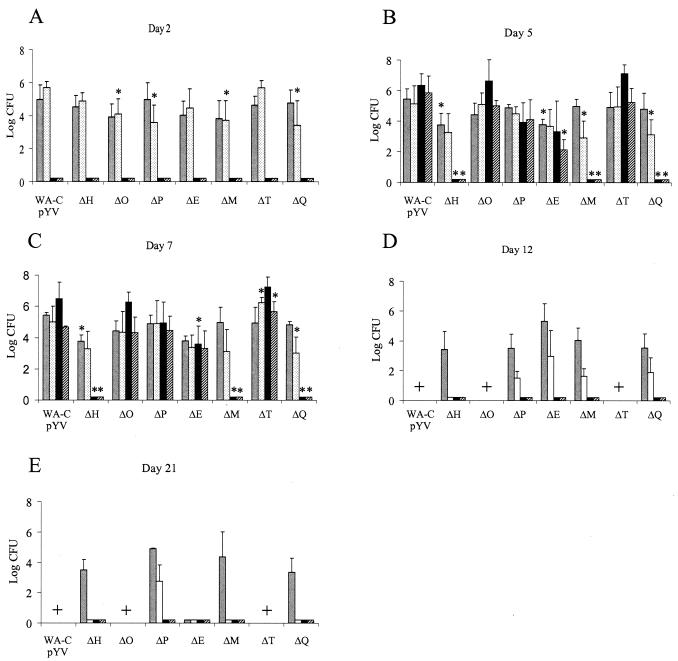
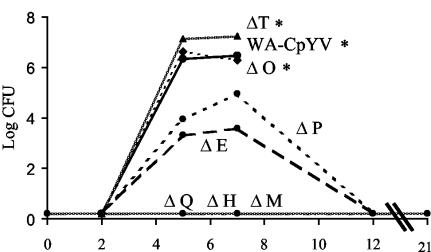
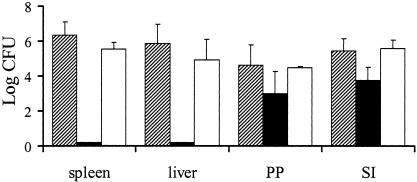
Groups of six C57BL/6 mice were orally infected with 108 CFU of each Δyop mutant (WA-C(pYVΔYop) and WA-C(pYV::CM). The course of colonization was determined by counting the surviving bacteria in the liver, spleen, PP, and SI on days 2, 5, 7, 12, and 21 postinfection by plating tissue homogenates (Fig. 2 and 3). Two days after oral infection, all mutants (ΔyopH, ΔyopO, ΔyopP, ΔyopE, ΔyopM, ΔyopT, and yopQ) and WA-C(pYV::CM) were able to efficiently colonize the SI and PP, with ΔyopH, -O, -P, -E, -M, and -Q mutants showing only moderate defects in colonizing these tissues. The course of infection with WA-C(pYV::CM), as well as the ΔyopO, ΔyopP, ΔyopE, and ΔyopT mutants, was progressive, with bacteria disseminating systemically and forming abscesses in livers and spleens by day 5. By this time all mice infected with these mutants showed severe signs of illness. The ΔyopH, ΔyopM, and ΔyopQ mutants, on the other hand, were highly attenuated and were not able to significantly colonize spleens and livers on any of the days tested (limit of detection of 10 CFU in the spleen and 50 CFU in the liver). By day 7, the ΔyopT, ΔyopO, ΔyopE, and ΔyopP mutants showed the highest colony counts in spleens and livers, with most mice infected with WA-C(pYV::CM), ΔyopT, and ΔyopO mutants dying between days 7 and 12 due to systemic infection and high bacterial load in organs (Fig. 3). The ΔyopE and ΔyopP mutants were, however, successfully eliminated from spleens and livers by day 12. Furthermore, ΔyopP, ΔyopE, ΔyopM, and ΔyopQ mutants were still able to colonize the SI and PP by this time. The ΔyopH mutant was able to colonize only the SI but had been eliminated from the PP by this time. At 3 weeks after infection, ΔyopH, ΔyopM, and ΔyopQ mutants were able to colonize the lumen of the SI only, whereas the ΔyopP mutant continued to colonize both the SI and the PP. To demonstrate that attenuation of our mutants was not due to polar effects caused by the Kanr cassette, we complemented the most highly attenuated mutant (ΔyopH) with a low-copy plasmid (pACYC184) harboring yopH and sycH under their natural promoters (57). At 5 days after oral infection, this strain was able to colonize the spleens and livers of infected mice to an extent comparable to WA-C(pYV::CM) (Fig. 4).
FIG. 2.
Time course of colonization and persistence of Y. enterocolitica Δyop mutants and WA-C(pYV::CM) after oral infection of C57BL/6 mice with 108 CFU. Two (A), five (B), seven (C), twelve (D), and twenty-one (E) days after infection, surviving bacteria in the SI (░⃞), PP (□), spleen (▪), and liver (▨) were determined. Values represent the average log CFU per organ for six mice with the standard errors of the means indicated by error bars. The limits of detection were 10 CFU/organ for PP and spleen and 50 CFU/organ for liver and SI. A “+” indicates that the strain was lethal for mice. Asterisks indicate statistical significance (P ≤ 0.05) between colonization of WA-C(pYV::CM) and the mutants.
FIG. 3.
Colonization and persistence in spleens over 3 weeks by Y. enterocolitica Δyop mutants and WA-C(pYV::CM) after oral infection of C57BL/6 mice with 108 CFU. Asterisks indicate death of mice between days 7 and 12 postinfection. ΔyopQ, -H, and -M mutants were below the limit of detection (10 CFU/organ). Values indicate the average log CFU per organ for six mice.
FIG. 4.
Colonization of spleens, livers, PP, and SIs of C57BL/6 mice after oral infection with 108 CFU of WA-C(pYVΔYopH) (▪), WA-C(pYVΔYopH+H) (□), and WA-C(pYV::CM) (▨) on day 5 postinfection.
Virulence of yop mutants after intravenous infection.
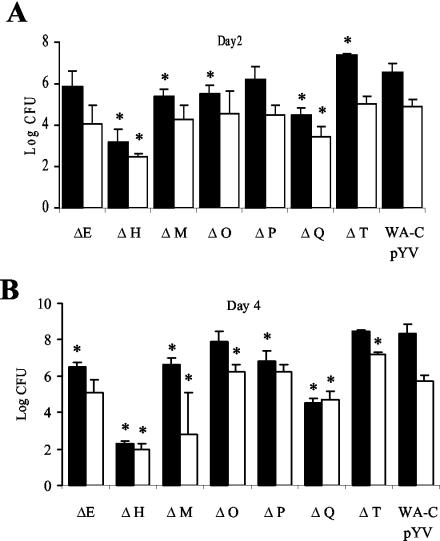
Groups of five C57BL/6 mice were infected with 4.5 × 104 CFU of the Δyop mutants and WA-C(pYV::CM). Surviving bacteria in livers and spleens were determined on days 2 and 4 postinfection (Fig. 5). WA-C(pYV::CM) showed high colony counts in spleens and livers on day 2 (6.53 ± 0.40 and 4.88 ± 0.37 log CFU) with infection progressing by day 4 (8.33 ± 0.54 and 5.73 ± 0.32 log CFU). The ΔyopH mutant was the most highly attenuated mutant, with a 2,100-fold reduction in CFU compared to WA-C(pYV::CM) by day 2. The colony counts were 100-fold lower for ΔyopQ and 13-fold lower for ΔyopM, whereas the ΔyopE and ΔyopP mutants (4.7-fold and 2-fold, respectively) were not dramatically attenuated compared to the parental strain (P > 0.05). The ΔyopT mutant, on the other hand, colonized the spleen to a sevenfold-greater extent on day 2 than did WA-C(pYV::CM). By day 4, infection had progressed for the WA-C(pYV::CM), ΔyopO, ΔyopT, ΔyopM, ΔyopE, and ΔyopP mutants, showing 63-, 229-, 18-, 17-, 4.3-, and 4-fold-higher CFU counts, respectively, in the spleen than on day 2, whereas the CFU counts in the spleens of mice infected with the ΔyopH mutant dropped 40-fold. By day 4 the colony counts in spleens were 219,000-fold lower for the ΔyopH mutant than for WA-C(pYV::CM), 6,300-fold lower for the ΔyopQ mutant, 50-fold lower for the ΔyopM mutant, and 63-fold lower for the ΔyopE and ΔyopP mutants. The ΔyopO mutant showed slightly fewer CFU in the spleen (2.7-fold) but more CFU in the liver (3.2-fold), which was significant only for the liver. The ΔyopT mutant showed higher CFU counts in spleens (1.4-fold) and livers (28-fold) than WA-C(pYV::CM), values that were statistically significant only for livers (P < 0.05). Generally, the CFU counts of the yop mutants and WA-C(pYV::CM) in the livers of infected mice were 10- to 100-fold lower than the CFU counts in the spleens except for the ΔyopH and ΔyopQ mutants on day 4, which showed comparable numbers of bacteria in the liver and spleen.
FIG. 5.
Colonization of spleen (▪) and liver (□) of C57BL/6 mice 2 (A) and 4 (B) days after intravenous infection with 4 × 104 CFU of the indicated Y. enterocolitica Δyop mutants and WA-C(pYV::CM). Each bar represents the mean log CFU for five mice. Asterisks indicate statistical significance between colonization of the Δyop mutants and of the WA-C(pYV::CM) (P ≤ 0.05).
The ΔyopT mutant outcompetes WA-C(pYV::CM).
The oral and intravenous infections suggested that the ΔyopT mutant was slightly more virulent than the wild-type strain. To confirm this, we infected six mice with an equal mixture (108 CFU) of WA-C(pYV::CMΔYopT) and WA-C(pYV::CM) by the intravenous route. Two days later, the mice were killed, and serial dilutions of organs were plated on kanamycin (for selection of the ΔyopT mutant) and chloramphenicol [for selection of WA-C(pYV::CM)] plates. The ΔyopT mutant showed a mean log of 4.23 ± 0.99 CFU in the liver, whereas WA-C(pYV::CM) showed a mean log of 3.05 ± 1.03 CFU. Therefore, the ΔyopT mutant outcompeted WA-C(pYV::CM) by 1.18 log CFU (P = 0.0075). No such difference could be detected for the spleens of mice, with the ΔyopT mutant showing a mean log of 6.61 ± 0.36 CFU and WA-C(pYV::CM) showing a mean log of 6.49 ± 0.34 CFU.
DISCUSSION
The objective of this study was to systematically analyze the contribution of seven Yops of Y. enterocolitica O:8, one of the highly virulent serotypes of biotype IB (New World strains), to virulence by using the same inbred mouse strain, the same route of application, and the same dose of bacteria. Although several studies have dealt with the effects of Yops on virulence, most of these were performed for Y. pseudotuberculosis serotype III, which differs considerably in virulence factor repertoire (e.g., lacking yersiniabactin) and clinical manifestations from Y. enterocolitica. This is exemplified by one study showing that even a plasmidless Y. pseudotuberculosis strain is able to multiply in the spleen after intravenous infection or to survive in human serum, whereas plasmid-cured Y. enterocolitica is rapidly eliminated from this organ or killed by serum (53, 59). Most importantly for the present study, Y. enterocolitica harbors the TTSS effector YopT, which is usually absent in Y. pseudotuberculosis (35), and several differences in a multitude of other virulence factors exist between the different Yersinia strains, e.g., YopP from Y. enterocolitica O:8 is substantially more efficient in suppressing the NF-κB pathway and mediating apoptosis than serotype O:9 (51). Invasin-mediated uptake efficiency in cell culture is much higher for Y. pseudotuberculosis than for Y. enterocolitica (21, 40). YadA and its collagen-binding potential are required for virulence in Y. enterocolitica but not in Y. pseudotuberculosis (45, 56). Furthermore, differences in serum resistance between the two species have been detected (59), and it has been reported that Y. pseudotuberculosis is more resistant to bactericidal cationic peptides (8) and shows increased outer membrane permeability to hydrophobic agents compared to Y. enterocolitica (7). It is therefore not justified to generally extrapolate mouse virulence studies obtained with Y. pseudotuberculosis to Y. enterocolitica.
The study of virulence in the mouse infection model with a naturally high-pathogenicity-island-negative Y. enterocolitica of serotype O:9 and O:3 is further complicated by the fact that mice have to be pretreated with desferrioxamine B (DFO) to achieve mouse virulence, and this pretreatment may influence pathogenicity because of the immunosuppressive effect of DFO (4). The virulence of yopQ and yopM (43) mutants in the mouse model was previously studied by using Y. enterocolitica O:9. Both mutants were shown to be attenuated after intravenous infection of mice pretreated with DFO. Both mutants were able to colonize spleens and livers of Swiss mice for at least 4 days at reduced levels compared to the wild-type strain. We were able to confirm these results here and extend them to the oral infection model, showing for the first time that both of these mutants are highly attenuated and able to colonize only the SI and PP but are not able to disseminate to the spleens and livers of orally infected mice. ΔyopQ and ΔyopM mutants were able to colonize PP for 2 and SI for 3 weeks but the numbers were somewhat lower than for WA-C(pYV::CM). These oral infections are consistent with studies of a yopK mutant in Y. pseudotuberculosis (34). YopQ fine-tuning of translocation or gating of the YopB-induced pore might be essential for effective immunosuppression of the host and systemic spread of yersiniae as proposed by Holmström et al. (33). Oral infections with ΔyopM mutants have not been performed previously. Together with the intravenous infections, our results indicate that YopM and YopQ are mainly involved in the establishment of a systemic infection in mice.
The virulence of ΔyopH, ΔyopO, ΔyopP, and ΔyopE mutants has been previously studied only for Y. pseudotuberculosis. The most striking difference between the virulence of our mutants and that of Y. pseudotuberculosis is that our ΔyopE mutant is only slightly attenuated after oral infection and caused systemic disease with regular seeding of yersiniae to spleens and livers. Furthermore, our ΔyopE mutant was able to colonize the SI and PP at somewhat reduced levels until day 12 postinfection. Y. pseudotuberculosis yopE mutants, on the other hand, were reported to be highly attenuated, being cleared from PP within 4 days and not able to reach the spleens of orally infected mice (34). These differences could be due to the use of a yadA yopE double mutant in that study (34). Another study using Y. pseudotuberculosis showed a markedly reduced ability of yopE mutants to colonize spleens of mice but only minor defects in colonizing the SI and PP (38). Possibly, the additional presence of YopT in Y. enterocolitica could also account for the more virulent phenotype of our yopE mutant since both of these Yops act on Rho GTPases and are involved in the inhibition of phagocytosis. Presumably, a ΔyopT ΔyopE double mutant of Y. enterocolitica is comparable to a yopE mutant of Y. pseudotuberculosis.
Our ΔyopO and ΔyopT mutants behaved most like WA-C(pYV::CM) after oral and intravenous infections, and in fact most mice succumbed to oral infection with these mutants at around day 7 just like the parental strain. For a yopO mutant, this is consistent with a recent study with Y. pseudotuberculosis (38) but contrasts with another (27) in which a ΔyopO mutant failed to cause a systemic infection. The mutant used in the latter study, however, also harbored a mutation in yadA, which could have had an effect on colonization of the spleen. There is only scarce evidence for the role of YopT in animal models, with one preliminary study showing that a yopT mutant was not affected in its capacity to colonize PP on day 2 after oral infection (35). Surprisingly, our yopT mutant was not attenuated and even showed higher colonization of mouse tissues after oral and intravenous infection than WA-C(pYV::CM). This is a surprising finding in light of the fact that YopT and YopO have a strong influence on phagocytosis resistance in cell culture models (28). YopO binds RhoA and Rac and YopT modifies RhoA and presumably Rac, which may also change binding properties to YopO. Possibly, YopT is redundant in this animal model, and its functions are taken over by other Yops, such as YopO and YopE. Another possibility is that the functions of these effector proteins become apparent only after several weeks of infection, when the adaptive immune system becomes effective. Since C57BL/6 mice succumb to infection after 1 week, it is not possible to study these effects in this model. We are therefore generating double mutants for yopT and other yop genes to test this hypothesis.
Our yopP mutant colonized gut tissues, as well as spleens and livers, at reduced levels but was not impaired in its ability to cause systemic disease, with bacteria regularly colonizing the livers and spleens of all mice after oral infection. By day 12 most mice had eliminated bacteria from spleens and livers, but colonization of gut tissues continued for at least 3 weeks. The role of yopJ in virulence has been previously studied only for Y. pseudotuberculosis. One study claims yopJ to be dispensable for virulence (27), and another shows that a yopJ mutant is highly attenuated, with only two of three PP and one of five spleens being colonized after oral infection (42). These differences could be due to the different Yersinia species used, the different mouse strains used, or different doses of applied bacteria.
Our ΔyopH mutant was by far the most attenuated mutant in both oral and intravenous infections. ΔyopH mutants were only slightly deficient in colonizing the SI and PP initially and were not able to disseminate and cause systemic disease. ΔyopH mutants were eliminated from the PP by day 12, earlier than the other surviving yop mutants, but colonization of the gut lumen continued for at least 3 weeks. Intravenous infections showed that the ΔyopH mutant initially implants into spleens and livers, but colony counts dramatically decrease between days 2 and 4. This indicates that YopH plays important roles in PP, as well as during the systemic stage of disease.
In summary, the ΔyopH, ΔyopO, ΔyopP, ΔyopE, ΔyopM, and ΔyopQ mutants had only modest defects in colonization of the SI and PP. This is consistent with results obtained for Y. pseudotuberculosis (38) and indicates that no single effector Yop is absolutely necessary for colonizing the SI or translocating to the underlying PP. YopH, YopQ, and YopM of Y. enterocolitica are important virulence factors for inducing systemic disease in mice, whereas YopO, YopP, and YopE are dispensable for yersiniae to reach the spleen and liver. The presence of YopT on the other hand even seems to slightly decrease virulence of Y. enterocolitica in this model.
Acknowledgments
We thank Laryssa Teplytska for excellent technical assistance.
This study was supported by Deutsche Forschungsgemeinschaft DFG (SFB 1887 and Graduiertenkolleg “Infektion und Immunität”). J.H. and H.R. were supported by the Deutsche Forschungsgemeinschaft (priority program “novel vaccination strategies”; HE1297/9-2 and RU838/1-2).
Editor: J. B. Bliska
REFERENCES
- 1.Alonso, A., N. Bottini, S. Bruckner, S. Rahmouni, S. Williams, S. P. Schoenberger, and T. Mustelin. 2003. Lck dephosphorylation at Y394 and inhibition of T-cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 6:4922-4928. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, K., K. E. Magnusson, M. Majeed, O. Stendahl, and M. Fallman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M. 1987. Current protocols in molecular biology. Greene Publishing Associates, Brooklyn, N.Y.
- 4.Autenrieth, I. B., E. Bohn, J. H. Ewald, and J. Heesemann. 1995. Deferoxamine B but not deferoxamine G1 inhibits cytokine production in murine bone marrow macrophages. J. Infect. Dis. 172:490-496. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., R. Reissbrodt, E. Saken, R. Berner, U. Vogel, W. Rabsch, and J. Heesemann. 1994. Desferrioxamine-promoted virulence of Yersinia enterocolitica in mice depends on both desferrioxamine type and mouse strain. J. Infect. Dis. 169:562-567. [DOI] [PubMed] [Google Scholar]
- 6.Barz, C., T. N. Abahji, K. Trülzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 7.Bengoechea, J. A., K. Brandenburg, U. Seydel, R. Diaz, and I. Moriyon. 1998. Yersinia pseudotuberculosis and Yersinia pestis show increased outer membrane permeability to hydrophobic agents which correlates with lipopolysaccharide acyl-chain fluidity. Microbiology 144(Pt. 6):1517-1526. [DOI] [PubMed] [Google Scholar]
- 8.Bengoechea, J. A., B. Lindner, U. Seydel, R. Diaz, and I. Moriyon. 1998. Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bactericidal cationic peptides than Yersinia enterocolitica. Microbiology 144(Pt. 6):1509-1515. [DOI] [PubMed] [Google Scholar]
- 9.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 10.Black, D. S., A. Marie-Cardine, B. Schraven, and J. B. Bliska. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2:401-414. [DOI] [PubMed] [Google Scholar]
- 11.Black, D. S., L. G. Montagna, S. Zitsmann, and J. B. Bliska. 1998. Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol. Microbiol. 29:1263-1274. [DOI] [PubMed] [Google Scholar]
- 12.Bliska, J. B., and D. S. Black. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bliska, J. B., K. L. Guan, J. E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 88:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolin, I., and H. Wolf-Watz. 1988. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol. Microbiol. 2:237-245. [DOI] [PubMed] [Google Scholar]
- 16.Burridge, K., C. E. Turner, and L. H. Romer. 1992. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119:893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis, G. R. 2002. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell. Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 18.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 20.Denecker, G., S. Totemeyer, L. J. Mota, P. Troisfontaines, I. Lambermont, C. Youta, I. Stainier, M. Ackermann, and G. R. Cornelis. 2002. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 70:3510-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dersch, P., and R. R. Isberg. 2000. An immunoglobulin superfamily-like domain unique to the Yersinia pseudotuberculosis invasin protein is required for stimulation of bacterial uptake via integrin receptors. Infect. Immun. 68:2930-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dukuzumuremyi, J. M., R. Rosqvist, B. Hallberg, B. Akerstrom, H. Wolf-Watz, and K. Schesser. 2000. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275:35281-35290. [DOI] [PubMed] [Google Scholar]
- 23.Fallman, M., K. Andersson, S. Hakansson, K. E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsberg, A., and H. Wolf-Watz. 1988. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol. Microbiol. 2:121-133. [DOI] [PubMed] [Google Scholar]
- 25.Foultier, B., and G. R. Cornelis. 2003. DNA sequence and analysis of the pYVa127/90 virulence plasmid of Yersinia enterocolitica strain A127/90. Res. Microbiol. 154:553-557. [DOI] [PubMed] [Google Scholar]
- 26.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730-732. [DOI] [PubMed] [Google Scholar]
- 27.Galyov, E. E., S. Hakansson, and H. Wolf-Watz. 1994. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J. Bacteriol. 176:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamid, N., A. Gustavsson, K. Andersson, K. McGee, C. Persson, C. E. Rudd, and M. Fallman. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27:231-242. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 31.Heesemann, J., U. Gross, N. Schmidt, and R. Laufs. 1986. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect. Immun. 54:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heesemann, J., and R. Laufs. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmstrom, A., J. Petterson, R. Rosqvist, S. Hakansson, F. Tafazoli, M. Fallman, K. E. Magnusson, H. Wolf-Watz, and A. Forsberg. 1997. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol. Microbiol. 24:73-91. [DOI] [PubMed] [Google Scholar]
- 34.Holmström, A., R. Rosqvist, H. Wolf-Watz, and A. Forsberg. 1995. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect. Immun. 63:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915-929. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Leung, K. Y., B. S. Reisner, and S. C. Straley. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logsdon, L. K., and J. Mecsas. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 71:4595-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald, C., P. O. Vacratsis, J. B. Bliska, and J. E. Dixon. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278:18514-18523. [DOI] [PubMed] [Google Scholar]
- 40.Miller, V. L. Yersinia invasion genes and their products. ASM News 58:26-32.
- 41.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulder, B., T. Michiels, M. Simonet, M. P. Sory, and G. Cornelis. 1989. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect. Immun. 57:2534-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 45.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 63:4837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson, C., R. Nordfelth, K. Andersson, A. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 48.Roggenkamp, A., H. R. Neuberger, A. Flugel, T. Schmoll, and J. Heesemann. 1995. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol. Microbiol. 16:1207-1219. [DOI] [PubMed] [Google Scholar]
- 49.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defense. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 50.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruckdeschel, K., K. Richter, O. Mannel, and J. Heesemann. 2001. Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-κB suppression and apoptosis induction in macrophages. Infect. Immun. 69:7652-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao, F., P. M. Merritt, Z. Bao, R. W. Innes, and J. E. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 53.Simonet, M., D. Mazigh, and P. Berche. 1984. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt 47 × 106. J. Med. Microbiol. 18:371-375. [DOI] [PubMed] [Google Scholar]
- 54.Skrzypek, E., C. Cowan, and S. C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051-1065. [DOI] [PubMed] [Google Scholar]
- 55.Straley, S. C., and M. L. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 57:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamm, A., A. M. Tarkkanen, T. K. Korhonen, P. Kuusela, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10:995-1011. [DOI] [PubMed] [Google Scholar]
- 57.Trülzsch, K., A. Roggenkamp, M. Aepfelbacher, G. Wilharm, K. Ruckdeschel, and J. Heesemann. 2003. Analysis of chaperone-dependent Yop secretion/translocation and effector function using a mini-virulence plasmid of Yersinia enterocolitica. Int. J. Med. Microbiol. 293:167-177. [DOI] [PubMed] [Google Scholar]
- 58.Trülzsch, K., A. Roggenkamp, C. Pelludat, A. Rakin, C. Jacobi, and J. Heesemann. 2001. Cloning and characterization of the gene encoding periplasmic 2′,3′-cyclic phosphodiesterase of Yersinia enterocolitica O:8. Microbiology 147:203-213. [DOI] [PubMed] [Google Scholar]
- 59.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Z. Y., J. C. Clemens, H. L. Schubert, J. A. Stuckey, M. W. Fischer, D. M. Hume, M. A. Saper, and J. E. Dixon. 1992. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267:23759-23766. [PubMed] [Google Scholar]
- 62.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]