Abstract
Yersinia pestis and Yersinia pseudotuberculosis are closely related facultative intracellular pathogens. The response regulator PhoP was previously shown to be important for Y. pestis survival in macrophages and for virulence in a murine bubonic plague infection assay. Here the importance of PhoP for Y. pseudotuberculosis pathogenesis was investigated. Y. pseudotuberculosis phoP mutants were unable to replicate in low-Mg2+ medium or in macrophages. phoP+ Y. pseudotuberculosis strains initiated replication in macrophages after a lag period of ∼5 h, as shown by fluorescence microscopy and viable count assays. Y. pseudotuberculosis phoP mutants died at a low rate in macrophages; there was no decrease in viability over the first 5 h of infection, and there was a 10-fold decrease in viability between 5 and 24 h of infection. Trafficking of phagosomes containing phoP+ or phoP mutant Y. pseudotuberculosis was studied by using immunofluorescence microscopy and cathepsin D as a marker for lysosomes. Phagosomes containing phoP mutant Y. pseudotuberculosis acquired cathepsin D at a higher rate than phagosomes containing phoP+ bacteria. However, the increased rate of marker acquisition for phagosomes containing mutant bacteria was only evident ∼5 h after infection, suggesting that phoP mutants are able to retard phagosome maturation during the lag phase of intracellular growth. The results obtained with a Y. pestis phoP mutant were similar to those described above, except that the rates of intracellular killing and trafficking to cathepsin D-positive vacuoles were significantly higher. A Y. pseudotuberculosis phoP mutant was 100-fold less virulent than the wild-type strain in a murine intestinal infection model, suggesting that survival and replication in macrophages are important for Y. pseudotuberculosis pathogenesis.
Yersinia pestis and Yersinia pseudotuberculosis are closely related gram-negative bacterial pathogens. Y. pseudotuberculosis is an enteropathogenic bacterium typically associated with acute infections of mesenteric lymph nodes (5). Twenty-one different O antigen serogroups of Y. pseudotuberculosis have been identified (41). Y. pestis, the agent of bubonic and pneumonic plagues, lacks O antigen and is considered a subspecies of Y. pseudotuberculosis (1, 41). Three biovars of Y. pestis have been identified, biovars Antiqua, Mediaevalis, and Orientalis, each of which has been associated with a major pandemic (32). Y. pseudotuberculosis and Y. pestis encode a number of common virulence determinants, including factors that promote coordinated gene expression and acquisition of iron (32). Another virulence factor common to Y. pestis and Y. pseudotuberculosis is a type III secretion system (TTSS) encoded on a 70-kb plasmid (pCD1 in Y. pestis and pYV in Y. pseudotuberculosis) (34). The plasmid-encoded TTSS is required for sustained bacterial replication in host tissues and exports multiple proteins known as the Yops and LcrV. Several of the Yops are delivered into host cells, where they inhibit phagocytosis and proinflammatory immune responses (22). In addition, LcrV is secreted into the extracellular milieu, where it downregulates inflammation (6).
Y. pseudotuberculosis is typically transmitted via the fecal-oral route (13). The bacteria can cross the intestinal barrier through M cells and reach the underlying Peyer's patches. From the Peyer's patches Y. pseudotuberculosis disseminates to mesenteric lymph nodes, where the bacteria replicate and cause mesenteric lymphadenitis. Y. pseudotuberculosis has been shown to replicate as an extracellular pathogen in lymphoid tissues of mice at late stages of infection (40).
Y. pestis infections are typically transmitted by fleas. Fleas feeding on infected rodents transmit the bacteria to other animals or humans (32). The bacteria disseminate from the site of the fleabite to nearby lymph nodes, where they replicate, resulting in a swollen lymph node (or bubo) that is characteristic of the disease. From the lymph nodes the bacteria spread systemically and colonize organs such as the liver, lungs, and spleen. Infected hosts die of septic shock when large amounts of bacteria from infected organs are released into the bloodstream. Y. pestis can also infect the lungs directly via aerosols and cause pneumonic plague. Pneumonic plague is highly infectious, can be spread from human to human, and is typically fatal.
It is thought that Y. pestis survives and replicates in macrophages at early stages of the infection process in humans and animals (8). In agreement with this idea, Y. pestis can replicate in cultured primary mouse macrophages (8, 36, 43). It is not known if Y. pseudotuberculosis survives or replicates in macrophages in vivo, although a number of different Y. pseudotuberculosis strains can replicate in cultured primary murine macrophages (36, 45). A notable exception to this finding is Y. pseudotuberculosis strain YPIII, which is unable to replicate in macrophages (28, 36). The genetic basis for the difference between YPIII and other Y. pseudotuberculosis strains with respect to the intracellular growth phenotype is not known. Histological analysis of Peyer's patches from rabbits infected with Y. pseudotuberculosis has shown that the bacteria can be internalized by intraepithelial macrophages at the early stages of the infection (15). Therefore, survival and replication in macrophages might play an important role in the Y. pseudotuberculosis infection process.
Macrophages are an important first line of defense of the innate immune system. One of their main functions is to take up bacteria by phagocytosis, digest them, and present their antigens to cells of the adaptive immune system. After a bacterium is internalized in a phagosome, the phagosome undergoes fusion with organelles of the endocytic pathway (e.g., late endosomes and lysosomes) to acquire degradative enzymes (e.g., cathepsin D). As the phagosome matures, the pH drops and ions (e.g., Mg2+) are pumped out of the vacuole. In the fully mature phagosome, or phagolysosome, the bacterium is destroyed and digested (46). A number of pathogenic bacteria that survive and replicate in macrophages have evolved distinct and intricate mechanisms to avoid destruction in macrophage phagolysosomes. Pathogens that can prevent maturation of the phagosome into a phagolysosome include members of Salmonella and Mycobacterium species (12).
The genetic basis for the ability of Y. pestis and Y. pseudotuberculosis to survive and replicate in macrophage phagosomes is largely unknown. Strains cured of the virulence plasmid can replicate in cultured macrophages (36, 43, 45), indicating that the TTSS encoded on the virulence plasmid is not required for intracellular survival. A second distinct TTSS encoded on the chromosomes of Y. pestis and Y. pseudotuberculosis is also dispensable for bacterial replication in cultured macrophages (36). One gene that is known to be required for survival of Y. pestis in macrophages is phoP, which encodes the PhoP response regulator (29).
The importance of PhoP for bacterial survival and replication in macrophages has been extensively studied in Salmonella enterica serovar Typhimurium (14, 18). PhoP is part of a two-component system in which PhoQ is the sensor kinase and PhoP is the response regulator. At low Mg2+ concentrations PhoQ is phosphorylated and the phosphate is transferred to PhoP. PhoP activates a variety of genes, many of which are important for virulence. PhoP-regulated genes allow serovar Typhimurium to adapt to the low-Mg2+ environment of the phagosome (3) and to prevent vacuole maturation to a phagolysosome (17). Genes such as mgtA and mgtB are directly involved in the adaptation to a low-Mg2+ environment since they encode P-type ATPases that function as putative Mg2+ transporters (14, 18).
Although PhoP plays an important role in Y. pestis virulence (29), it is not known if PhoP has a comparable function in Y. pseudotuberculosis. This study was undertaken to determine the importance of PhoP for Y. pseudotuberculosis pathogenesis. We obtained evidence that PhoP in Y. pseudotuberculosis is important for growth in a low-Mg2+ environment and for survival and replication in macrophages. PhoP function appears to be important for Y. pseudotuberculosis to retard the maturation of its vacuole into a phagolysosome. A Y. pseudotuberculosis phoP mutant is 100-fold less virulent than a wild-type strain in a murine intestinal infection model, suggesting that replication in macrophages is important for Y. pseudotuberculosis pathogenesis. Based on similar results obtained with Y. pestis in this study and in another study (29), we concluded that the PhoP function is conserved in Y. pseudotuberculosis and Y. pestis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Y. pseudotuberculosis and Y. pestis strains used in this study are listed in Table 1. YPIII(p−) and YPIII(p+) were obtained from the strain collection of Stanley Falkow. Y. pestis and Y. pseudotuberculosis strains were cultivated at 28°C on heart infusion (HI) (Difco) or Luria-Bertani (LB) agar plates. Cultures were grown in broth with aeration overnight at 28°C. Y. pestis was grown in HI broth. Y. pseudotuberculosis was grown in LB broth or HI broth depending on the experimental conditions. The Escherichia coli strains NovaBlue (Novagen), SM10, and S17-1, the latter two lysogenized with λpir, were grown in LB broth or on LB agar plates at 37°C. Bacterial growth media were supplemented with ampicillin at a concentration of 100 μg/ml, kanamycin at a concentration of 25 μg/ml, or tetracycline at a concentration of 20 μg/ml when appropriate.
TABLE 1.
Yersinia strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| KIM10+ | Biovar Mediaevalis, pCD1−, pPCP1− | 33 |
| KIM10+/GFP | KIM10+, p67GFP3.1, Apr | 36 |
| KIM10+phoPΔ | KIM10+phoPΔ127-429 | This study |
| KIM10+phosPΔ/PhoPKIM | KIM10+PhoPΔ, pPhoPKIM, Apr | This study |
| KIM10+phoPΔ/MMB67EH | KIM10+phoPΔ, pMMB67HE, Apr | This study |
| KIM10+phoPΔ/GFP | KIM10+phoPΔ, p67GFP3.1, Apr | This study |
| IP2666c | Serogroup O3, pYV− | 39 |
| IP2666c/GFP | IP2666c, p67GFP3.1, Apr | 36 |
| IP2666cphoPΔ | IP2666c phoPΔ127-429 | This study |
| IP2666cphoPΔ/PhoPKIM | IP2666c phoPΔ, pPhoPKIM, Apr | This study |
| IP2666cphoPΔ/PhoPYPIII | IP2666c phoPΔ, pPhoPYPIII, Apr | This study |
| IP2666cphoPΔ/MMB67EH | IP2666c phoPΔ, pMMB67EH, Apr | This study |
| IP2666cphoPΔ/GFP | IP2666cphoPΔ, p67GFP3.1, Apr | This study |
| YPIII(p+) | Serogroup O3, pYV+, phoPM116H, R117Stop | 4 |
| YPIII(p−) | Serogroup O3, pYV−, phoPT160P | 4 |
| YPIII(p−)/PhoPKIM | YPIII(p−), pPhoPKIM, Apr | This study |
| YPIII(p−)/MMB67EH | YPIII(p−), pMMB67EH, Apr | This study |
| 32777 | Serogroup O1, pYV | 10 |
| 32777/GFP | 32777, p67GFP3.1, Apr | This study |
| 32777phoP::kan | 32777 phoP::kan, Knr | 24 |
| 32777phoP::kan/GFP | 32777phoP::kan, p67GFP3.1, Knr Apr | This study |
| 32777phoP::kan/MMB67EH | 32777phoP::kan, pMMB67EH, Knr Apr | This study |
| 32777phoP::kan/PhoP | 32777phoP::kan, pPhoP, Knr, Apr | This study |
To measure growth of Y. pestis and Y. pseudotuberculosis under Mg2+-limiting conditions, the defined medium TMH (42) was used with the following modifications: MgCl2 was omitted, and the final pH was adjusted to 5.5 (this medium is referred to below as low-Mg2+ TMH). A pH of 5.5 was initially used in an attempt to mimic the low pH of the phagolysosome; however, subsequent experiments indicated that low pH was not an important variable since phoP mutants were able to replicate at pH 5.5 when the medium described above was supplemented with 10 mM MgCl2 (high-Mg2+ TMH). Bacterial cultures grown overnight in HI medium were diluted into low- or high-Mg2+ TMH to obtain an optical density at 600 nm of ∼0.1. Growth at 37°C was recorded by measuring the optical density at 600 nm at different times. The following modifications were made for strains carrying phoP expression vectors. Cultures grown overnight were diluted 1:10 into fresh HI medium containing 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG). The cultures were incubated for 2 h at 28°C to induce phoP expression. The cultures were then diluted into low-Mg2+ TMH supplemented with 100 μM IPTG, and growth at 37°C was recorded as described above.
DNA methods.
Taq polymerase and deoxynucleoside triphosphates (dNTPs) purchased from Roche were used for PCRs. For purification of plasmid DNA, QIAGEN Mini and Midi Prep DNA purification kits were used according to the manufacturer's protocols. Fast Link ligase (Epicentre) was used for ligation of DNA. Vectors were dephosphorylated with shrimp alkaline phosphatase from Roche when necessary. DNA restriction enzymes were purchased from Roche and were used according to the manufacturer's protocol. For cycle sequencing an ABI Prism BigDye Terminator v3.0 Ready Reaction cycle sequencing kit was used.
Sequence analysis of phoP and construction of phoP expression vectors.
The oligonucleotides 5′-GAATTCATGCGGGTTCTGGTTGTGG-3′ (PhoPF) and 5′-CTAGTTGACGTCAAAACGATATCCC-3′ (PhoPR) were used to amplify the phoP open reading frame (ORF) from Y. pestis KIM10+ (phoPKIM) and Y. pseudotuberculosis YPIII(p−) (phoPYPIII) by PCR with an Eppendorf Mastercycler. The PhoPF primer was designed to introduce an EcoRI restriction site (underlined nucleotides in the primer sequence) in front of the start codon of the phoP ORF. The reactions were performed with genomic DNA or whole bacteria as a template in 100 μl of 1× PCR buffer (10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl; pH 8.3) containing 2.5 U of Taq DNA polymerase (Roche), each primer at a concentration of 0.5 μM, and 160 μM dNTPs. The PCR products were cloned into the EcoRV site of the pETBlue-2 cloning vector by using a Perfectly Blunt cloning kit (Novagen). The sequences of the inserts were verified by cycle sequencing by using pETBlueUP and pETBlueDOWN primers as specified in the manufacturer's manual. Additionally, the phoP ORFs from YPIII(p−) (phoPYPIII) and YPIII(p+) [phoPYPIII(p+)] were amplified by using primers PhoP2F (5′ CGTTTACATTGTGACGGCGTC 3′) and PhoP2R (5′ GTTATGCCACTGTGCCAGACTG 3′) and whole bacteria as the template. The PCR product was purified and used for cycle sequencing with the same set of primers. Cycle sequencing was performed with an ABI 3100 automated genetic analyzer by using an ABI PRISM BigDye Terminator cycle sequencing kit (Applied Biosystems). The sequencing data were compared to Yersinia phoP sequences available in GenBank by using BLASTN. The sequences of phoPYPIII and phoPYPIII(p+) were submitted to GenBank.
pETBlue-2 plasmid DNAs containing phoPKIM or phoPYPIII were purified and digested with BamHI and EcoRI to excise the phoP fragments. The resulting phoP fragments were ligated into BamHI- and EcoRI-digested pMMB67EH (16) to place phoP under control of the IPTG-inducible tac promoter. The resulting plasmids were designated pPhoPKIM and pPhoPYPIII. pPhoPKIM and pPhoPYPIII were electroporated into S17 λpir, and transformants were selected with ampicillin. S17 λpir was used to transfer pPhoPKIM and pPhoPYPIII or their parent, pMMB67EH, into different Y. pestis and Y. pseudotuberculosis strains by conjugation. Transconjugants were selected on Yersinia selective medium (Oxoid) supplemented with ampicillin.
Creation of phoP deletion mutants.
To create an in-frame deletion mutation in phoP, pETBlue-2 plasmid DNA containing phoPKIM was digested with HpaI and SphI to remove nucleotides 127 to 429 of the phoP sequence. After restriction digestion, 1 U of T4 DNA polymerase and 100 μM dNTPs were added to the reaction mixture and incubated for 15 min at 12°C to remove 3′ single-stranded regions. The larger restriction fragment containing pETBlue-2 and the 5′ and 3′ segments of phoPKIM was purified and ligated. The resulting plasmid, pETBlue-2phoPΔ, was digested with BamHI and XbaI. The restriction fragment containing phoPΔ was inserted between the BamHI and XbaI sites of the suicide vector pSB890 (30). The resulting plasmid, pSB890phoPΔ, was electroporated into S17 λpir, and transformants were selected with tetracycline. S17 λpir/pSB890phoPΔ was used to transfer the plasmid to different Yersinia strains by conjugation. Transconjugants were selected with tetracycline on Yersinia selective medium and streaked on LB plates supplemented with tetracycline. Tetr transconjugants were grown for several generations in the absence of tetracycline and then plated on LB agar plates lacking NaCl and supplemented with 5% sucrose to select against the sacB gene carried on pSB890. Sucrose-resistant colonies were tested for the Tets phenotype and for the presence of phoPΔ by using colony PCR and primers for the phoP ORF as specified above. Yersinia strains containing the phoPΔ allele were defective for growth in low-Mg2+ TMH at 37°C.
Analysis of intracellular survival and replication by CFU assay.
The mouse macrophage-like cell line J774A.1 was cultured in Dulbecco's modified Eagle's medium (Gibco) with a high glucose concentration (4,500 μg/ml) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 2 mM l-glutamine, and 1 mM sodium pyruvate at 37°C with 5% CO2. Cells were grown to confluence and detached from the dishes, and 2 × 105 cells were seeded into wells of 24-well tissue culture plates and incubated overnight. Bacterial cultures were grown overnight in HI broth at 28°C with shaking. The cultures were centrifuged for 5 min at 2,000 × g at room temperature, and the pellets were resuspended in phosphate-buffered saline (PBS) (Gibco). Dilutions were made in PBS as needed, and the final dilution was made in complete tissue culture medium (see above). The bacterial numbers were adjusted so that the multiplicity of infection (MOI) was two to five bacteria per cell. After addition of the bacteria to the cells, the 24-well plate was centrifuged for 5 min at 200 × g at room temperature to facilitate bacterial contact with the cells. After 15 min of infection the cells were washed twice with PBS. Some of the wells were used to measure the initial number of cell-associated bacteria by a CFU assay as described below. To the remaining wells, tissue culture medium containing 8 μg of gentamicin per ml was added to kill extracellular bacteria. After 1 h, the medium was removed and replaced with fresh complete tissue culture medium containing 2 μg of gentamicin per ml (for KIM10+-infected cells) or 4.5 μg of gentamicin per ml (for Y. pseudotuberculosis-infected cells), and incubation was continued. At different times thereafter the cells were washed, and the number of intracellular bacteria was determined by a CFU assay. To each well 0.5 ml of 0.1% Triton X-100 in double-distilled H2O was added. After incubation for 10 min at 37°C, the lysates were removed, and the wells were rinsed with 0.5 ml of HI broth. The lysates and HI rinses from each well were pooled in a microcentrifuge tube. The samples were briefly sonicated with a Microson XL200 ultrasonic cell disruptor equipped with a P-1 microprobe (Misonix) to reduce bacterial aggregation. Dilutions were plated on LB agar plates. The plates were incubated at 28°C for 2 days, and the output CFU were enumerated.
Analysis of intracellular survival and replication by immunofluorescence microscopy.
Infection of J774A.1 cells was carried out as described above for the CFU assay, except that macrophages were seeded on glass coverslips. The bacterial strains used for infection contained the p67GFP3.1 plasmid (36), which encodes gfpmut3.1 under the control of an IPTG-inducible tac promoter. One hour prior to fixation green fluorescent protein (GFP) expression was induced with 500 μM IPTG where indicated below. At the end of the incubation period the cells were fixed with 4% paraformaldehyde in PBS for 10 min. This and all subsequent steps were done at room temperature. The fixed cells were incubated in PBS containing 0.2% Triton X-100 for 10 min to permeate membranes and then in PBS containing 3% bovine serum albumin (BSA) to reduce nonspecific binding of antibodies. The fixed cells were incubated in rabbit anti-Yersinia antiserum SB349 (2) diluted 1:1,000 in PBS containing 3% BSA for 40 min to label the bacteria. After two washes with PBS, goat anti-rabbit secondary antibody conjugated to Alexa 594 (Molecular Probes) diluted 1:1,500 in 3% BSA-PBS was added to the fixed cells and incubated for 40 min in the dark. Cells were washed three times with PBS and mounted on glass slides in Airvol (Air Products and Chemicals, Inc.) containing 5% 1,4-diazobicyclo-[2.2.2]-octane (DABCO; Sigma). The slides were examined by phase-contrast and epifluorescence microscopy by using a Zeiss Axioplan2 microscope equipped with a ×100 oil immersion objective (NA 1.3), and images were captured by using a Spot camera (Diagnostic Instruments, Inc.). Alternatively, slides were analyzed by confocal microscopy by using a Leica DM IRE2. Images of the red and green emission signals, as well as the transmission light at a magnification of ×40, were captured separately with Leica's LCS software package. Images were processed by using Adobe Photoshop.
Phagosome trafficking assay.
Infection was carried out as described above for the immunofluorescence microscopy assays. Bacteria were transformed with p67GFP3.1, and GFP expression was maintained in the overnight cultures and during the infection by addition of 100 μM IPTG. The MOIs used were 2 for Y. pestis, 5 for 32777, and 10 for IP2666c, which accounted for the less efficient uptake observed with the latter strain. Cells were fixed at different times with 1 ml of fixing solution (200 μl of 5× PLP buffer, which contained 100 mM morpholineethanesulfonic acid [MES], 350 mM NaCl, 25 mM KCl, 350 mM lysine-HCl, 25 mM MgCl2, and 10 mM EGTA, 45 mg of sucrose, 505 μl of double-distilled H2O, 250 μl of 10% paraformaldehyde, 2.14 mg of NaIO4) for 30 min. Cells were washed with PBS containing 5% sucrose. The fixed cells were incubated in PBS containing 0.2% Triton X-100 for 10 min and in PBS containing 3% goat serum to reduce nonspecific binding of antibodies. Cathepsin D was labeled by incubating the cells in anti-cathepsin D antibody (Scripps Laboratories) diluted 1:200 in PBS containing 3% goat serum for 40 min. After two washes with PBS, goat anti-rabbit secondary antibody conjugated to Alexa 594 (Molecular Probes) diluted 1:1,500 in PBS containing 3% goat serum was added to the fixed cells and incubated for 40 min in the dark. Cells were washed three times with PBS and mounted on glass slides in Airvol containing 5% DABCO. Coverslips were analyzed by confocal microscopy by using a Leica DM IRE2. Images of the red emission and the green emission at a magnification of ×40 were captured separately and combined in one image file by using Leica's LCS software package. The images were analyzed with Adobe Photoshop. Approximately 100 phagosomes were scored for each infection. Phagosomes were scored as cathepsin D positive when the shape of the phagosome was clearly visible in the red channel and colocalized with the GFP emission from the bacteria in the green channel. Data from three independent experiments were collected, and averages and standard deviations were calculated with Microsoft Excel.
Mouse infection experiments.
Eight-week-old female C57BL/6 mice (Iffa Credo, L'Arbresle, France) were challenged by the intragastric route by using a feeding needle and 32777 or 32777phoP::kan (referred to as 32777ΔPhoPA in reference 24) suspended in a 0.2-ml volume. Bacterial inocula were prepared from overnight cultures grown in LB medium at 28°C. The cultures were centrifuged, and the bacterial pellets were washed once and suspended in distilled water. C57BL/6 mice were fasted for 18 h prior to infection. Groups of 10 animals were infected with 107, 108, or 109 CFU of 32777 or 108, 109, or 1010 CFU of 32777phoP::kan. Animals were kept in positive-pressure cabinets, and mortality was monitored daily over a 3-week period. These experiments were carried out in compliance with animal care and use regulations at the Université Lille II. The 50% lethal dose (LD50) was calculated by the method of Reed and Muench (37).
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the phoP gene sequences in YPIII(p−) and YPIII (p+) are AY649416 and AY649417, repectively.
RESULTS
Y. pseudotuberculosis YPIII strains carry nonfunctional alleles of phoP.
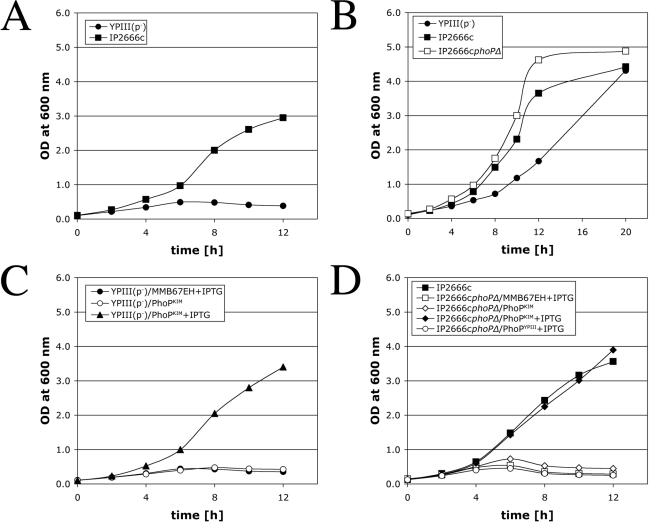
Y. pseudotuberculosis YPIII(p−) (Table 1), unlike other Y. pseudotuberculosis strains, is defective for replication in murine macrophages (36). Serovar Typhimurium mutants that are defective for growth in macrophages (e.g., phoP mutants) also do not replicate in a low-Mg2+ environment (14, 18). To determine if YPIII(p−) is defective for growth in a low-Mg2+ environment, a culture of YPIII(p−) was diluted into a defined medium lacking magnesium (low-Mg2+ TMH) (see Materials and Methods), and bacterial growth was monitored over time by determining the optical density. The same medium was inoculated with IP2666c (Table 1), another Y. pseudotuberculosis strain belonging to the same serogroup (serogroup O3), which is able to replicate in macrophages (36). YPIII(p−) did not grow in low-Mg2+ TMH, while IP2666c did grow in this medium (Fig. 1A and data not shown). YPIII(p−) grew in TMH containing 10 mM MgCl2 (high-Mg2+ TMH), indicating that YPIII(p−) is specifically defective for growth in a low-Mg2+ environment (Fig. 1B).
FIG. 1.
Analysis of Y. pseudotuberculosis strains for growth in low- or high-Mg2+ medium. Bacterial cultures of the strains indicated were diluted into low-Mg2+ TMH (A, C, and D) or high-Mg2+ TMH (B), and growth at 37°C was measured by determining the optical density (OD) at 600 nm at different times. The presence of IPTG in the culture medium is indicated. The results are representative of at least two independent experiments.
To investigate if YPIII(p−) encodes a defective allele of phoP, a DNA fragment containing the phoP ORF from Y. pestis KIM10+ (Table 1) was inserted into a plasmid under the control of an IPTG-inducible promoter. The resulting plasmid (pPhoPKIM) or the empty expression plasmid (pMMB67EH) was introduced into YPIII(p−). Strains containing these plasmids (Table 1) were assayed for growth in low-Mg2+ TMH. YPIII(p−)/PhoPKIM grew in low-Mg2+ TMH containing IPTG but not in low-Mg2+ TMH lacking IPTG (Fig. 1C). YPIII(p−)/MMB67EH did not grow in low-Mg2+ TMH containing IPTG. Therefore, the phenotype of YPIII(p−) (inability to grow in a low-Mg2+ environment) was complemented by expression of phoPKIM in trans.
To directly show that PhoP is required for growth of Y. pseudotuberculosis in a low-Mg2+ environment, a deletion was introduced into phoP in IP2666c, creating IP2666cphoPΔ (Table 1). IP2666cphoPΔ grew in high-Mg2+ TMH (Fig. 2B) but not in low-Mg2+ TMH (Fig. 2D). The inability of IP2666cphoPΔ to grow in low-Mg2+ TMH was rescued by expression of phoPKIM in trans (Fig. 1D), indicating that the mutation introduced into IP2666cphoPΔ was nonpolar. To determine if the phoP ORF in YPIII(p−) encodes a defective product, a DNA fragment containing this ORF (phoPYPIII) was inserted into pMMB67EH under control of the IPTG-inducible promoter. The resulting plasmid (pPhoPYPIII) was transferred into IP2666cphoPΔ, and the resulting strain (Table 1) was assayed for growth in low-Mg2+ TMH containing IPTG. Expression of PhoPYPIII did not rescue growth of IP2666cphoPΔ in low-Mg2+ TMH (Fig. 1D). We therefore concluded that phoPYPIII is a nonfunctional allele of phoP.
FIG. 2.
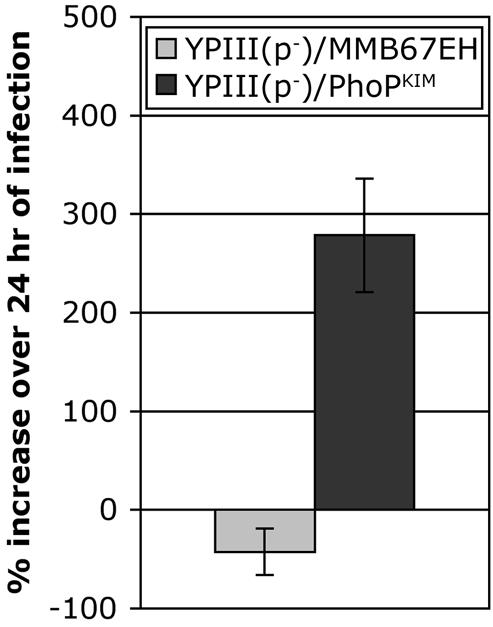
Expression of phoPKIM in trans promotes the survival and growth of YPIII(p−) in macrophages. J774A.1 macrophages were infected with YPIII(p−)/MMB67EH (gray bar) or YPIII(p−)/PhoPKIM (solid bar) at an MOI of 5 in the presence of IPTG. The numbers of CFU recovered from the infected macrophages after 0.25 and 24 h of infection were determined as described in Materials and Methods. The percent increase in CFU was calculated by using the following formula: (CFU at T24 − CFU at T0.25)/(CFU at T24) × 100, where T24 and T24 are 0.25 and 24 h after infection, respectively. The data are the averages ± standard deviations of values obtained from two independent experiments with duplicate wells.
A CFU assay (see Materials and Methods) was carried out to determine if the growth defect of YPIII(p−) in macrophages could be rescued by expression of phoPKIM. YPIII(p−)/PhoPKIMand YPIII(p−)/MMB67EH were used to infect J774A.1 macrophages. The infected macrophages were incubated in the presence of a low concentration of gentamicin to inhibit growth of extracellular bacteria and in the presence of IPTG to induce expression of phoPKIM. As shown in Fig. 2, YPIII(p−)/PhoPKIM grew in macrophages, while the viability of YPIII(p−)/MMB67EH decreased in macrophages. These results suggest that YPIII(p−) is defective for survival and replication in macrophages because it carries a nonfunctional phoP allele.
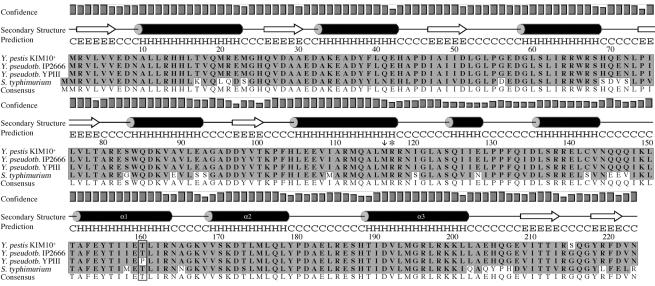
The phoPYPIII ORF was sequenced, and the deduced amino acid sequence of PhoPYPIII was compared with the sequences of PhoPKIM and PhoPIP2666 available in the GenBank database. As shown in Fig. 3, there is one amino acid difference unique to PhoPYPIII, corresponding to a threonine-to-proline substitution at amino acid 160 (T160P). PhoP belongs to the family of winged-helix transcription factors (25). Analysis of the amino acid sequence of PhoPKIM by using PSIPRED structure predictions (26) showed that residue 160 is predicted to lie within an alpha helix (Fig. 3). Additional analysis with SWISS-MODEL (19, 38) to create a three-dimensional model of PhoPKIM revealed that this helix corresponds to the α1 helix found in other winged-helix transcription factors (25). The α1 helix forms the hydrophobic core of the DNA binding domain in winged-helix transcription factors. It forms a structural core, aiding in the positioning of the β-sheets in the winged-helix motif. A T160P substitution in the α1 helix is expected to cause a change in the positioning of residues in the helix and in the contacts that these residues make within the domain. We hypothesize that the T160P substitution results in a conformational change in the positioning of the α2 and α3 helices that make contact with the DNA, thereby disrupting the function of PhoPYPIII. Taken together, our results suggest that YPIII(p−) is defective for survival and replication in macrophages because it encodes a nonfunctional PhoP protein.
FIG. 3.
Predicted secondary structure of PhoPKIM and ClustalW alignment of PhoP sequences (single-letter code) from Yersinia species and serovar Typhimurium. The secondary structure was predicted by using PSIPRED. H, α-helix; E, β-sheet; C, coil; Y. pseudotb, Y. pseudotuberculosis; S. typhimurium, S. enterica serovar Typhimurium. The confidence levels for secondary structure assignments are indicated by bar graphs. The amino acid sequences of PhoP proteins for KIM10+ (accession no. NP_669111), IP2666 (accession no. AAK54056), and serovar Typhimurium (accession no. RGEBFT) were obtained from GenBank. The sequence of PhoPYPIII was deduced from the nucleotide sequence of the phoP gene from YPIII(p−) (phoPYPIII) obtained in this study. Sequences were aligned by using the ClustalW alignment function in MacVector. The T160P substitution in PhoPYPIII is enclosed in a box. The arrow at position 116 indicates the first codon in phoPYP1II(p+) that is changed (from M to H) due to a frameshift mutation corresponding to a repeat of nucleotides 341 to 345. The asterisk indicates the position of the stop codon in phoPYP1II(p+) which results from the frameshift mutation.
PhoP is important for survival and replication of Y. pseudotuberculosis in macrophages.
Macrophages infected with IP2666c or IP2666cphoPΔ were analyzed by fluorescence microscopy (see Materials and Methods) to obtain further evidence that PhoP is important for growth of Y. pseudotuberculosis in macrophages. Both strains were transformed with p67GFP3.1, a plasmid that encodes GFP under control of an IPTG-inducible promoter (36). The resulting strains (Table 1) were used to infect J774A.1 macrophages for different periods of time in the presence of a low concentration of gentamicin to inhibit the growth of extracellular bacteria. One hour prior to fixation, IPTG was added to the medium to induce GFP expression in intracellular bacteria. After fixation, the total intracellular bacteria were labeled with anti-Yersinia antibodies and secondary antibodies conjugated to Alexa 594. In a previous study it was found that the numbers of viable Y. pseudotuberculosis cells in macrophages remain constant over the first 4 to 5 h of infection, that bacterial replication begins within 8 h of infection, and that between 8 and 24 h the numbers increase ≥10-fold (36). As expected, IP2666c/GFP underwent significant replication between 5 and 24 h postinfection (Fig. 4, panels A1, A2, C1 to C3, and E1 to E3). By comparison, IP2666cphoPΔ/GFP was clearly defective for replication in macrophages, although GFP-positive intracellular bacteria could be detected at 5 and 24 h (Fig. 4, panels B1, B2, D1 to D3, and F1 to F3). No obvious cytotoxic effect of bacterial replication on the macrophages was observed in these experiments (Pujol, unpublished data). A CFU assay was carried out to measure bacterial replication. J774A.1 macrophages were infected with IP2666c, IP2666cphoPΔ/PhoPKIM, or IP2666cphoPΔ/MMB67EH in the presence of IPTG, and viable intracellular bacteria were quantified after 0.25 or 24 h of incubation. The resulting data were used to calculate a survival index (Table 2). As shown in Table 2, the phoP mutant was defective for survival in macrophages, and the defect could be rescued by expression of phoPKIM in trans.
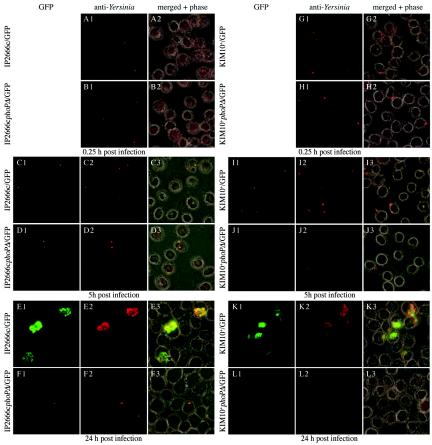
FIG. 4.
Analysis of phoP+ and phoP mutant Y. pseudotuberculosis and Y. pestis strains for survival and replication in macrophages by microscopy. J774A.1 macrophages were infected with IP2666c/GFP (panels A1, A2, C1 to C3, and E1 to E3), IP2666cphoPΔ/GFP (panels B1, B2, D1 to D3, and F1 and F3), KIM10+/GFP (panels G1, G2, I1 to I3, and K1 to K3), or KIM10+phoPΔ/GFP (panels H1, H2, J1 to J3, and L1 to L3). Infections were carried out at MOIs of 2 for Y. pestis and 5 for Y. pseudotuberculosis. The infected cells were fixed after 0.25, 5, or 24 h of infection. One hour before the 5- and 24-h samples were processed, the cells were incubated in the presence of IPTG to induce GFP expression in intracellular bacteria. After fixation, the samples were stained with anti-Yersinia antibody and an Alexa 594-conjugated secondary antibody. The samples were viewed by phase-contrast and epifluorescence microscopy. Representative images were captured by using a digital camera. The GFP and anti-Yersinia signals were merged with phase-contrast images (merged + phase).
TABLE 2.
PhoP is important for survival of Y. pseudotuberculosis and Y. pestis in macrophages
| Strain | Survival indexa |
|---|---|
| IP2666cphoPΔ/MMB67EH | 0.049 |
| IP2666cphoPΔ/PhoPKIM | 0.827 |
| KIM10+phoPΔ/MMB67EH | 0.005 |
| KIM10+phoPΔ/PhoPKIM | 0.738 |
| 32777phoP::kan/MMB67EH | 0.087 |
| 32777phoP::kan/PhoPKIM | 0.811 |
J774A.1 cells were infected with parental strains (IP2666c, 32777, KIM10+) or the phoP mutant strains at an MOI of 5 (IP2666c, 32777) or 2 (KIM10+). Bacterial cultures were grown at 28°C prior to infection. IPTG (500 μM) was present in the medium throughout the infection to induce phoP expression. CFU counts were determined after 0.25 or 25 h of infection as described in Materials and Methods. The values are values from a representative experiment with duplicate wells. The survival index was calculated from the CFU counts by using the following formula: (CFU of mutant at T24/CFU of parent at T24)/(CFU of mutant at T0.25/CFU of parent at T0.25), where T24 and T0.25 are 24 and 0.25 h after infection, respectively.
In order to compare directly the functional importance of PhoP in Y. pseudotuberculosis and Y. pestis, the phoP deletion mutation was introduced into Y. pestis KIM10+. The resulting strain, KIM10+phoPΔ (Table 1), and its parent were assayed for growth in low- or high-Mg2+ TMH. KIM10+ grew in both media, while KIM10+phoPΔ grew only in high-Mg2+ TMH (data not shown). Therefore, PhoP is required for growth of Y. pestis in a low-Mg2+ environment. As determined by fluorescence microscopy, KIM10+/GFP grew in macrophages (Fig. 4, panels G1, G2, I1 to I3, and K1 to K3), while most KIM10+phoPΔ/GFP cells were not viable or destroyed by 5 h (Fig. 4, panels H1, H2, J1 to J3, and L1 to L3). The results of a CFU assay confirmed that the phoP mutant of KIM10+ was defective for intracellular survival and more sensitive to intracellular killing (∼10-fold more sensitive) than the phoP mutant of IP2666c (Table 2). Therefore, phoP mutants of Y. pseudotuberculosis and Y. pestis are both defective for survival and replication in macrophages, although these mutants differ in that the latter is more sensitive to killing in macrophage phagosomes.
Role of PhoP in retarding phagosome maturation.
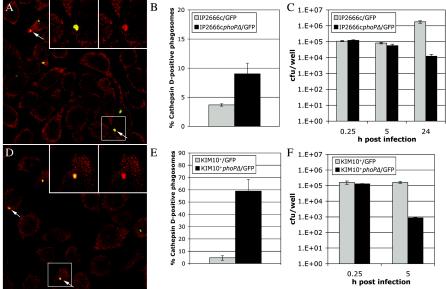
Mills and Finlay have reported that phagosomes containing YPIII(p+) acquire the lysosomal marker cathepsin D and are trafficked to phagolysosomes in J774A.1 macrophages (28). As our findings suggested that YPIII(p−) is a phoP mutant, we used immunofluorescence microscopy and cathepsin D as a marker for lysosomes to determine if phagosomes containing phoP+ or phoP mutant Y. pseudotuberculosis are trafficked differently in macrophages. IP2666c/GFP and IP2666cphoPΔ/GFP were grown in the presence of IPTG and used to infect J774A.1 macrophages in the presence of IPTG to ensure continuous GFP expression during the experiment. At 15 min postinfection the macrophages were incubated in medium containing gentamicin. At different times thereafter, the cells were fixed and stained with an anti-cathepsin D antibody and an Alexa 594 secondary antibody. The samples were examined by confocal microscopy, and cathepsin D-positive phagosomes were identified by an overlap in the green GFP and red Alexa 594 emission signals (Fig. 5A). Very few phagosomes containing either IP2666c/GFP or IP2666cphoPΔ/GFP had acquired cathepsin D at 1.5 h postinfection (data not shown). By 8 h postinfection there was a twofold difference between the percentage of cathepsin D-positive phagosomes in macrophages infected with IP2666c/GFP and the percentage of cathepsin D-positive phagosomes in macrophages infected with IP2666cphoPΔ/GFP (4 versus 8%) (Fig. 5B). A CFU assay was carried out in parallel with the trafficking assay to enumerate viable intracellular bacteria. The results are summarized in Fig. 5C. Between 0.25 and 5 h after infection, the number of viable bacteria remained constant for both IP2666c and IP2666cphoPΔ. Between 5 and 24 h, the number of viable IP2666cphoPΔ cells decreased 10-fold, while the number of viable IP2666c cells increased 10-fold. These results suggest that a Y. pseudotuberculosis phoP mutant can retard phagosome maturation and maintain viability during the several-hour lag phase that precedes intracellular growth. Following the lag phase, mutant bacteria begin to loose the ability to retard phagosome maturation and are killed within phagolysosomes.
FIG. 5.
Analysis of phagosome trafficking in macrophages infected with Y. pseudotuberculosis or Y. pestis by anti-cathepsin D staining and confocal microscopy. IP2666c/GFP, IP2666cphoPΔ/GFP, KIM10+/GFP, and KIM10+phoPΔ/GFP were grown in the presence of IPTG and used to infect J774A.1 macrophages at an MOI of 10 for Y. pseudotuberculosis or 2 for Y. pestis. At different times postinfection the cells were fixed and stained with anti-cathepsin D antibody and a secondary antibody conjugated to Alexa 594. The samples were analyzed by confocal microscopy. (A and D) Representative images of the merged anti-cathepsin D and GFP signals obtained with cells infected for 8 h with IP2666cphoPΔ/GFP (A) or for 1.5 h with KIM10+phoPΔ/GFP (D). The arrows indicate cathepsin D-positive phagosomes. (Insets) Enlargement of the region indicated by the white rectangle. The left inset shows merged GFP and cathepsin D signals, and the right inset shows cathepsin D signals alone. (B) Percentages of cathepsin D-positive phagosomes in macrophages after 8 h of infection with IP2666c/GFP or IP2666cphoPΔ/GFP calculated by using merged images captured at a magnification of ×40. (E) Percentages of cathepsin D-positive phagosomes in macrophages containing KIM10+/GFP or KIM10+phoPΔ/GFP at 1.5 h postinfection determined in the same manner. The data in panels B and E are the averages of three independent experiments. The error bars indicate the standard deviations. (C and F) Numbers of CFU recovered from J774A.1 macrophages infected with IP2666c/GFP or KIM10+/GFP or the corresponding phoP mutants at the times indicated. Each data point represents the average ± standard deviation of the values recovered from triplicate wells. The results are representative of two independent experiments.
To determine if PhoP influences maturation of phagosomes containing Y. pestis, the trafficking experiments described above were repeated by using KIM10+/GFP and KIM10+phoPΔ/GFP. Interestingly, 60% of the phagosomes containing KIM10+phoPΔ/GFP were positive for cathepsin D at 1.5 h postinfection (Fig. 5D and E). In contrast, only 5% of the phagosomes containing KIM10+/GFP were positive for cathepsin D at 1.5 h postinfection (Fig. 5E). The rapid acquisition of cathepsin D by phagosomes containing KIM10+phoPΔ was paralleled by a rapid decrease in viability, and there was a 100-fold decrease in the number of viable bacteria recovered between 0.25 and 5 h after infection (Fig. 5F). These results suggest that phoP mutant Y. pestis cells are rapidly killed in phagolysosomes because they are unable to retard phagosome maturation during the intracellular lag phase.
PhoP is important for Y. pseudotuberculosis virulence.
Oyston et al. (29) have shown that a Y. pestis phoP mutant is less virulent (75-fold less virulent as determined by an LD50 assay) in a murine model of bubonic plague. To determine if PhoP is important for Y. pseudotuberculosis pathogenesis, we utilized 32777, a pYV-containing serogroup O1 strain of Y. pseudotuberculosis. This strain has previously been shown to be highly virulent in a mouse oral infection assay (10). 32777phoP::kan is an isogenic mutant containing a kanamycin resistance gene inserted into phoP (Table 1). Since all previous assays were carried out with pYV-cured Y. pseudotuberculosis phoP mutants, the characteristics of 32777 and 32777phoP::kan were studied by using the different assays described above to confirm that the presence of pYV did not alter the phenotype of a phoP mutant in low-Mg2+ medium or macrophages. 32777 and 32777phoP::kan grew at the same rate in high-Mg2+ TMH, while 32777phoP::kan was specifically defective for growth in low-Mg2+ TMH (J. P. Grabenstein, unpublished data). 32777phoP::kan showed a defect in intramacrophage survival comparable to that observed with other Y. pseudotuberculosis phoP mutants (Table 2; Grabenstein, unpublished). Additionally, expression of PhoPKIM rescued the intracellular survival defect of 32777phoP::kan (Table 2), demonstrating that the phoP::kan mutation was nonpolar. In two independent trafficking assays 27.7% of the phagosomes containing 32777phoP::kan were cathepsin D positive at 8 h postinfection, while 3% of phagosomes containing 32777 were positive at this time (Grabenstein, unpublished). These data demonstrate that a pYV-containing Y. pseudotuberculosis phoP mutant is phenotypically similar to a pYV-cured Y. pseudotuberculosis phoP mutant with respect to growth in low-Mg2+ medium and macrophages.
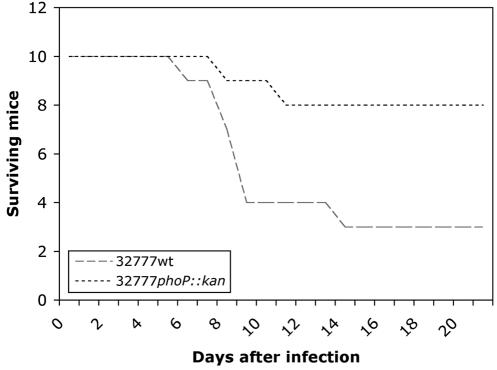
C57BL/6 mice were infected via the intragastric route with different doses of 32777 or 32777phoP::kan, and the LD50 for each strain was determined after 21 days. The LD50 of 32777 in C57BL/6 mice was calculated to be 5 × 108 CFU, while the LD50 of the phoP mutant was at least 100-fold higher. When mice were challenged with 109 CFU of either strain, 70% of the mice infected with 32777 died during the observation period, while 20% of the mice infected with the phoP mutant died (Fig. 6). Therefore, PhoP is important for Y. pseudotuberculosis virulence.
FIG. 6.
Virulence of wild-type and phoP mutant Y. pseudotuberculosis in mice. Wild-type Y. pseudotuberculosis 32777 and the isogenic phoP mutant 32777phoP::kan were used to challenge C57BL/6 mice by the intragastric route. Groups of 10 animals were infected with 109 CFU of a strain, and mortality was monitored for 21 days after challenge.
DISCUSSION
Y. pseudotuberculosis and Y. pestis share a number of characteristics that are important for virulence as a result of their close genetic relatedness (7, 32). A characteristic that is common to Y. pseudotuberculosis and Y. pestis is the ability to replicate in macrophages (36). It was suggested previously that Y. pseudotuberculosis and Y. pestis are likely to have a common genetic basis for the ability to replicate in macrophages (36). As the response regulator PhoP has been shown to be important for survival of Y. pestis in macrophages and for Y. pestis virulence (29), this study was undertaken to evaluate the importance of PhoP for the pathogenesis of Y. pseudotuberculosis. The data presented here show that PhoP is important for survival and replication of Y. pseudotuberculosis in macrophages. Furthermore, we found that PhoP is an important virulence factor of Y. pseudotuberculosis. Our results indicate that survival and replication in macrophages are important for pathogenesis in Y. pseudotuberculosis and Y. pestis infections.
Y. pseudotuberculosis strain YPIII(p−) is unable to grow in macrophages or low-Mg2+ medium. In this regard, YPIII(p−) behaves phenotypically like a phoP mutant of serovar Typhimurium. The results of several complementation tests suggest that YPIII(p−) carries a nonfunctional allele of phoP. Sequence analysis of phoPYPIII revealed that a nucleotide change in this ORF generates a T-to-P substitution at codon 160. A three-dimensional model of PhoPKIM, which belongs to the class of winged-helix transcription factors (25), predicts that the T160P substitution lies within a key α-helix (α1), which forms the hydrophobic core of the DNA binding domain. We speculate that the T160P substitution results in a global conformational change in PhoPYPIII and loss of function. It is currently not clear how and when this codon substitution originated in YPIII. This codon substitution results from an A-to-C transversion, which is a relatively uncommon mutation. YPIII(p+), the presumed parent of YPIII(p−), is also unable to replicate in macrophages (28; Grabenstein, unpublished data). The phoP ORF from YPIII(p+) (Table 1) was sequenced to determine if it carries a nonfunctional phoP allele. Interestingly, we found that phoPYPIII(p+) contains a different mutation, corresponding to a repeat of nucleotides 341 to 345 of phoP, which creates a frameshift mutation and a stop codon at predicted residue 117 (Fig. 3). Therefore, the YPIII(p−) and YPIII(p+) strains used in our study both have nonfunctional alleles of phoP and are unable to replicate in macrophages. Additionally, YPIII(p−) is most likely not derived from YPIII(p+) because of the different nature of the phoP mutations that are present in these strains. A question raised by these observations is whether there is some selective pressure on YPIII strains cultivated in the laboratory to inactivate phoP. As we have shown that PhoP is important for virulence of Y. pseudotuberuculosis, it is possible that YPIII(p+) is partially attenuated due to the mutation in phoP, and we speculate that the virulence of this strain would be increased by restoration of the wild-type phoP sequence.
Y. pseudotuberculosis and Y. pestis mutants carrying site-directed mutations in phoP were also unable to grow in a low-Mg2+ defined medium. In serovar Typhimurium, PhoP activates the expression of high-affinity Mg2+ transporters encoded by the mgtA and mgtB genes. In addition, PhoP-regulated gene expression in serovar Typhimurium is required for downregulation of CorA activity, which prevents the influx of toxic metals, such as Fe(II) (9). The inability of yersinia phoP mutants to grow in low-Mg2+ TMH may therefore result from a combination of Mg2+ starvation and metal toxicity. Bacteria growing in TMH are exposed to high concentrations of Fe(II) because this medium contains 100 μM FeSO4 (42).
Y. pseudotuberculosis phoP mutants were shown to be defective for survival and replication in J774A.1 macrophages. A Y. pestis phoP mutant was also defective for survival and replication in macrophages, as expected from a previous study (29). Therefore, a requirement for PhoP to survive in macrophages is conserved in Y. pseudotuberculosis and Y. pestis. To gain insight into the role of PhoP for survival of yersiniae in macrophages, we studied the trafficking of phagosomes containing phoP+ or phoP mutant bacteria using cathepsin D as a subcellular marker for lysosomes. Evidence that phoP mutant Y. pseudotuberculosis and Y. pestis strains are defective for retarding phagosome maturation was obtained. The concept that phoP+ yersiniae can retard phagosome maturation is supported by the data of Tsukano et al. (45), who reported that Y. pseudotuberculosis inhibits acidification of phagosomes. The results of a previous study indicated that phagosomes containing Y. pestis fuse with lysosomes (44). In this previous study the workers used a different lysosomal marker (thorium dioxide) and a different detection method (transmission electron microscopy) than we used in this study. The previous results (44) are not necessarily in conflict with those reported here, since it is possible that nascent phagosomes containing yersiniae can fuse with lysosomes but as the phagosomes are remodeled by the pathogen, they become secluded from the phagolysosomal pathway. This appears to be the fate of phagosomes containing serovar Typhimurium (21). Interestingly, it has been shown that PhoP is important for serovar Typhimurium to retard phagosome maturation (17). The technique used by Straley and Harmon (44) may be sufficiently sensitive to detect phagosomes that have undergone transient fusion events with lysosomes, while the approach which we used may only detect phagosomes that have undergone sustained fusion events with lysosomes. Cathepsin D may need to accumulate to high levels before it can be easily detected by immunofluorescence microscopy. Studies to better characterize the trafficking of phagosomes containing phoP+ or phoP mutant yersiniae are under way.
Although a general conclusion based on our results is that the function of PhoP is conserved in Y. pseudotuberculosis and Y. pestis, an interesting observation which we made was that a phoP Y. pestis mutant was ∼10-fold more sensitive to killing in macrophages than a phoP Y. pseudotuberculosis mutant (Table 2). The increased rate of intracellular killing of the Y. pestis phoP mutant was paralleled by an increased rate of phagosomal cathepsin D staining. The data suggest that PhoP is required for Y. pestis to retard phagosome maturation during the intracellular lag phase, while PhoP is dispensable for Y. pseudotuberculosis to delay phagosome maturation during the intracellular lag phase. The mechanistic basis behind this difference is not known. It is possible that Y. pseudotuberculosis encodes a mechanism that can function in the absence of PhoP to retard phagosome maturation during the intracellular lag phase. After Y. pseudotuberculosis exits the lag phase, it appears that PhoP function becomes important for retarding phagosome maturation. This hypothetical backup mechanism could be unique to Y. pseudotuberculosis or may have been lost in Y. pestis due to genetic decay. One known difference between Y. pseudotuberculosis and Y. pestis is that the lipopolysaccharide (LPS) of Y. pestis lacks O antigen due to the presence of several mutations in genes required for O antigen biosynthesis (41). O antigen could play a role in retarding maturation of phagosomes containing Y. pseudotuberculosis during the intracellular lag phase. It has been shown that the O antigen of Brucella suis is involved in the ability of this intracellular pathogen to avoid phagosome maturation in macrophages (35). The presence of O antigen appears to promote entry of B. suis into macrophages via lipid rafts, resulting in the formation of phagosomes that avoid fusion with lysosomes (35). If O antigen allows Y. pseudotuberculosis to delay phagosome maturation, then mutants defective for O antigen and PhoP should be rapidly killed in macrophages. Mutants of YPIII(p+) defective for O antigen biosynthesis have been isolated and shown to be attenuated for virulence in mouse infection assays (23, 27). It is conceivable that these mutants, which are missing O antigen and a functional PhoP protein, are attenuated due to increased sensitivity to intracellular killing.
It is currently not known how PhoP retards phagosome maturation. In serovar Typhimurium, over 40 genes that have diverse functions and are directly or indirectly under the control of PhoP have been identified. Products of the pmr and pag genes modify the lipid A portion of LPS, making the outer membrane less negatively charged and less permeable for cationic antimicrobial peptides (14). Other gene products, such as the Mg2+ transporters encoded by the mgt genes, are required for the adaptation of serovar Typhimurium to the low-Mg2+ environment of the phagosome (3). Homologues of the mgt, pmr, and pag genes are present in the Y. pestis genome (11, 31), so it is possible that the products of these genes have similar functions in serovar Typhimurium and yersiniae. The LPS core of Y. pestis appears to be modified in a phoP-dependent fashion (20). Therefore, it is conceivable that yersinia phoP mutants are defective for retarding phagosome maturation due to an inability to acquire a nutrient or to modify the membranes or surfaces within the phagosome. This in turn could prevent the bacterium from synthesizing or properly secreting a factor(s) that is required to retard phagosome maturation. In this context, PhoP-regulated gene expression could be indirectly involved in the process of retarding phagosome maturation. Alternatively, it is possible that PhoP activates an unidentified gene(s) whose product functions directly to retard phagosome maturation.
A murine intestinal infection model was used to demonstrate that PhoP is an important virulence factor of Y. pseudotuberculosis. Inactivation of phoP resulted in a ∼100-fold increase in the LD50 of Y. pseudotuberculosis. This increase is the same magnitude as that reported for a Y. pestis phoP mutant in a mouse bubonic plague infection model (29). The phoP mutant was not defective for expression or secretion of Yop proteins under standard growth conditions (Grabenstein, unpublished), suggesting that the phoP mutant is selectively impaired for intracellular pathogenesis. It is possible that survival and replication in macrophages allow Y. pseudotuberculosis and Y. pestis numbers to increase while the organisms are protected from the actions of neutrophils (8). Thus, survival and replication in macrophages may allow yersiniae to thwart innate immune responses. Yersiniae may also circumvent the development of adaptive immunity by surviving in macrophage phagosomes. It has been shown that a serovar Typhimurium phoP mutant is more efficiently processed for class II antigen presentation by macrophages than the parental phoP+ strain (47). The more efficient antigen processing of a serovar Typhimurium phoP mutant could be directly related to the inability of the mutant to avoid phagosome maturation (17). As we have shown that PhoP is important for yersiniae to avoid maturation of phagosomes to cathepsin D-positive vacuoles, it is possible that Y. pseudotuberculosis or Y. pestis phoP mutant strains induce an earlier adaptive immune response than parental phoP+ bacteria. The induction of an earlier adaptive immune response could contribute to the significant decrease in virulence of phoP mutant yersiniae. Further studies are needed to determine if there is a connection between the ability of yersiniae to replicate in macrophages and their ability to avoid innate and adaptive immune responses.
Acknowledgments
We thank members of the Bliska laboratory for comments on the manuscript and Kwadwo Bonsu for helping establish the cathepsin D staining assay.
This work was supported by Public Health Service grant AI48507 awarded to J.B.B.
Editor: D. L. Burns
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 3.Blanc-Potard, A. B., and E. A. Groisman. 1997. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16:5376-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carniel, E. 2002. Plasmids and pathogenicity islands of Yersinia. Curr. Top. Microbiol. Immunol. 264:89-108. [PubMed] [Google Scholar]
- 8.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of fleaborne plague. J. Immunol. 85:348-363. [PubMed] [Google Scholar]
- 9.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44:561-571. [DOI] [PubMed] [Google Scholar]
- 10.Collyn, F., M. A. Lety, S. Nair, V. Escuyer, A. Ben Younes, M. Simonet, and M. Marceau. 2002. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect. Immun. 70:6196-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2:365-377. [DOI] [PubMed] [Google Scholar]
- 13.El-Maraghi, N. R. H., and N. S. Mair. 1979. The histopathology of enteric infection with Yersinia pseudotuberculosis. Am. J. Clin. Pathol. 71:631-639. [DOI] [PubMed] [Google Scholar]
- 14.Ernst, R. K., T. Guina, and S. I. Miller. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J. Infect. Dis. 179:S326-S330. [DOI] [PubMed] [Google Scholar]
- 15.Fujimura, Y., T. Kihara, and H. Mine. 1992. Membranous cells as a portal of Yersinia pseudotuberculosis entry into rabbit ileum. J. Clin. Electron Microsc. 25:35-45. [Google Scholar]
- 16.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 17.Garvis, S. G., C. R. Beuzon, and D. W. Holden. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell. Microbiol. 3:731-744. [DOI] [PubMed] [Google Scholar]
- 18.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 20.Hitchen, P. G., J. L. Prior, P. C. Oyston, M. Panico, B. W. Wren, R. W. Titball, H. R. Morris, and A. Dell. 2002. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol. Microbiol. 44:1637-1650. [DOI] [PubMed] [Google Scholar]
- 21.Holden, D. W. 2002. Trafficking of the Salmonella vacuole in macrophages. Traffic 3:161-169. [DOI] [PubMed] [Google Scholar]
- 22.Juris, S. J., F. Shao, and J. E. Dixon. 2002. Yersinia effectors target mammalian signalling pathways. Cell. Microbiol. 4:201-211. [DOI] [PubMed] [Google Scholar]
- 23.Karlyshev, A. V., P. C. Oyston, K. Williams, G. C. Clark, R. W. Titball, E. A. Winzeler, and B. W. Wren. 2001. Application of high-density array-based signature-tagged mutagenesis to discover novel Yersinia virulence-associated genes. Infect. Immun. 69:0-7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marceau, M., L. Dubuquoy, C. Caucheteux-Rousseaux, B. Foligne, P. Desreumaux, and M. Simonet. 2004. Yersinia pseudotuberculosis anti-inflammatory components reduce trinitrobenzene sulfonic acid-induced colitis in the mouse. Infect. Immun. 72:2438-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Hackert, E., and A. M. Stock. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269:301-312. [DOI] [PubMed] [Google Scholar]
- 26.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 27.Mecsas, J., I. Bilis, and S. Falkow. 2001. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect. Immun. 69:2779-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills, S. D., and B. B. Finlay. 1998. Isolation and characterization of Salmonella typhimurium- and Yersinia pseudotuberculosis-containing phagosomes from infected mouse macrophages: Y. pseudotuberculosis traffics to terminal lysosomes where they are degraded. Eur. J. Cell Biol. 77:35-47. [DOI] [PubMed] [Google Scholar]
- 29.Oyston, P. C. F., N. Dorrell, K. Williams, S.-R. Li, M. Green, R. W. Titball, and B. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and the downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 31.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 32.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry, R. D., M. L. Pendrak, and P. Schuetze. 1990. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 172:5929-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 35.Porte, F., A. Naroeni, S. Ouahrani-Bettache, and J. P. Liautard. 2003. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 71:1481-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 38.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonet, M., and S. Falkow. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonet, M., S. Richard, and P. Berche. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skurnik, M., A. Peippo, and E. Ervela. 2000. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol. Microbiol. 37:316-330. [DOI] [PubMed] [Google Scholar]
- 42.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Straley, S. C., and P. A. Harmon. 1984. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect. Immun. 45:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straley, S. C., and P. A. Harmon. 1984. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 45:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukano, H., F. Kura, S. Inoue, S. Sato, H. Izumiya, T. Yasuda, and H. Watanabe. 1999. Yersinia pseudotuberculosis blocks the phagosomal acidification of B10.A mouse macrophages through the inhibition of vacuolar H+-ATPase activity. Microb. Pathog. 27:253-263. [DOI] [PubMed] [Google Scholar]
- 46.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wick, M. J., C. V. Harding, N. J. Twesten, S. J. Normark, and J. D. Pfeifer. 1995. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol. Microbiol. 16:465-476. [DOI] [PubMed] [Google Scholar]