Abstract
Nramp1 is a transporter that pumps divalent cations from the vacuoles of phagocytic cells and is associated with the innate resistance of mice to diverse intracellular pathogens. We demonstrate that sitA and mntH, genes encoding high-affinity metal ion uptake systems in Salmonella enterica serovar Typhimurium, are upregulated when Salmonella is internalized by Nramp1-expressing macrophages and play an essential role in systemic infection of congenic Nramp1-expressing mice.
Salmonella enterica serovar Typhimurium, the causative agent of both a self-limiting gastroenteritis in humans and disseminated infections in the young, elderly, and immunocompromised, causes a severe systemic disease resembling human typhoid fever in mice. Salmonellae are intracellular pathogens that survive within a vacuole inside the host cell that is isolated from normal endosomal trafficking networks, known as the Salmonella-containing vacuole (SCV). Concentrations of divalent cations including Fe2+ and Mg2+ within the SCV are believed to influence Salmonella virulence (11, 22, 23, 25). Nramp1 (natural resistance-associated macrophage protein 1) is a host protein important for natural resistance of mice to S. enterica serovar Typhimurium infections, as mice bearing the wild-type Nramp1G169 allele are inherently resistant to S. enterica serovar Typhimurium, while animals homozygous for the Nramp1D169 allele succumb rapidly (22). Nramp1 functions as a transport system for divalent cations, including Fe2+ and Mn2+, and may influence the pathogenesis of S. enterica serovar Typhimurium by depleting the SCV of divalent cations (10, 14).
The genomes of S. enterica serovar Typhimurium and other pathogens were found to encode homologues of Nramp1 (5, 16, 19), and we began the characterization of mntH, the Salmonella homologue of Nramp1. MntH is a divalent cation transport system with high affinity for Mn2+ and a 10- to 100-fold-lower affinity for Fe2+ (16, 19). A second Salmonella transport system was identified with similar substrate affinities, SitABCD (15). It is appealing to speculate that prokaryotic Nramps and other ion transporters act directly to counter the effects of Nramp1 and compete for the same substrates to influence the course of an active infection. In order to study this question, we chose to investigate the gene expression patterns of these two transport systems and determine their effects on virulence in a modified Nramp1G169 murine typhoid model.
S. enterica serovar Typhimurium strain SL1344 was used as the wild type, and pFZY1 was the parental transcriptional fusion plasmid (17). The transcriptional fusion plasmid pHILA was described previously (25). The mntH::lacZYA transcriptional fusion plasmid, pMLZ104 (16), contains 780 bp of the promoter region of mntH. The transcriptional fusion plasmid pSITA contains 670 bp of the sitA promoter region, constructed by PCR amplification with SL1344 chromosomal DNA and the oligonucleotides sitAFW (5′-CACGCGCGATACGTTTACCAG-3′) and sitARV (5′-CGAAGCTTCGGTAATGCCCATC-3′ [engineered HindIII site underlined]).This PCR product was ligated into pCR2.1 (Invitrogen), digested with EcoRI and HindIII (NEB), and ligated into similarly digested pFZY1. These plasmids were stably maintained in SL1344 upon infection of both HeLa (ATCC CCL-2) and RAW264.7 (ATCC TIB 71) cell lines over a 24-h time course and did not influence bacterial viability (data not shown).
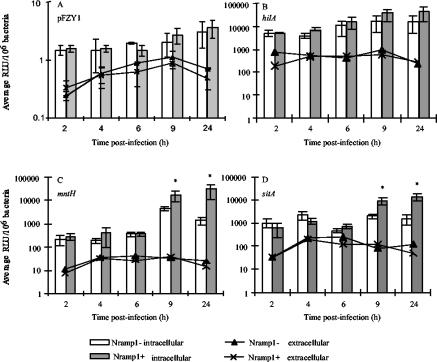
RAW264.7 cells (Nramp1D169) are phenotypically Nramp1−. To determine the basal expression of β-galactosidase from native pFZY1 as a control for copy number and to determine the effect of Nramp1 on the expression of mntH, sitA, and hilA, stably transfected RAW cell lines containing either an empty neomycin-resistant pCB6 vector (Nramp1−) or pCB6 containing the wild-type murine Nramp1G169 allele (phenotypically Nramp1+) were maintained as previously described (9). Chemiluminescent β-galactosidase assays were as previously described (16, 25). Figure 1A shows that expression of β-galactosidase in the absence of a cloned promoter is negligible both inside and outside of the transfected RAW cells and does not change significantly over time or in relation to the Nramp1 status of the cells. This indicates that the copy number of the pFZY1-based plasmids remains constant during the course of these experiments. In contrast, hilA, mntH, and sitA are induced upon reaching the intracellular environment of the Nramp1− cells when compared to expression in Dulbecco's modified Eagle's medium alone, characteristic of Salmonella virulence factors (6, 13, 18, 25). However, upon comparison between expression in the Nramp1+ and Nramp1− cell lines (Fig. 1C and D) mntH and sitA but not hilA are further upregulated in the presence of functional Nramp1. Specifically, expression of mntH and sitA increased approximately 7-fold and 2-fold, respectively, in the Nramp1− cells between 2 and 24 h, and an average of 115-fold and 20-fold over the same time course in the Nramp1+ cell lines. The differences between the gene expression patterns for hilA, sitA, and mntH in the two transfected cell lines indicate that the influence of Nramp1 on Salmonella gene expression in these experiments is specific for mntH and sitA and most likely reflects Nramp1-dependent changes in divalent cation concentration (25).
FIG. 1.
Expression of mntH and sitA is upregulated by intracellular S. enterica serovar Typhimurium in the presence of Nramp1 upon infection of IFN-γ-activated Nramp1− or Nramp1+ transfected RAW 264.7 cells. The Results shown represent the average number of relative light units (RLU) normalized to the number of bacteria present in each well as determined by parallel plate counts and multiplied by 106 to obtain larger numbers for easier interpretation. Results are the average of triplicate experiments performed with duplicate wells. Error bars represent the standard error of the mean. Asterisks indicate statistical significance to P < 0.05 as determined by the Wilcoxon rank sum test for unpaired samples.
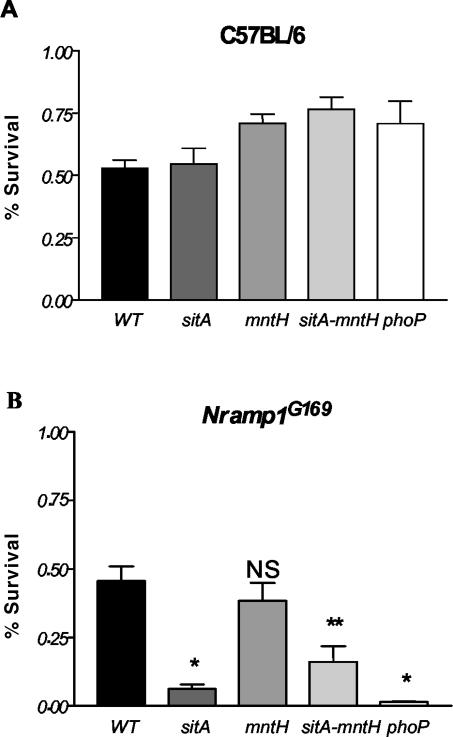
Induction in the intracellular environment is a hallmark of a virulence gene, but we were unable to demonstrate an effect of mntH or sitA, alone or in combination, on Salmonella invasion and replication within HeLa cells or survival within macrophages following phagocytosis by Nramp1− and Nramp1+ RAW264.7 cell lines (data not shown). Wild-type and mutant bacterial survival was further assessed in activated peritoneal macrophages (21). Sodium periodate-elicited peritoneal macrophages were obtained from 6- to 8-week-old C57BL/6 (Nramp1D169) or congenic Nramp1G169 mice, plated at a density of approximately 3 × 105 cells/well in a 96-well plate, and activated with 20-U/ml gamma interferon (IFN-γ) overnight. Bacteria opsonized with normal mouse serum were centrifuged onto the macrophages at a multiplicity of infection of approximately 5:1 and allowed to internalize for 15 min before washing and addition of gentamicin to remove extracellular bacteria. Macrophages were lysed at 0, 4, and 18 h with 0.5% deoxycholate, and bacteria were enumerated by serial dilution and plating onto Luria-Bertani (LB) agar. No difference in survival of mutant and wild-type S. enterica serovar Typhimurium was observed in C57BL/6 macrophages. However, sitABCD and sitABCD mntH mutant S. enterica serovar Typhimurium exhibited significantly reduced survival after 18 h in congenic Nramp1G169 primary macrophages (Fig. 2). In contrast to previously published results (1), we were only able to detect effects of Nramp1 on Salmonella survival in primary macrophages by using mutant bacterial strains, as there was no significant difference in the survival of wild-type S. enterica serovar Typhimurium upon infection of the congenic macrophages; this is probably attributable to differences in experimental protocol and may be influenced by the ability of wild-type Salmonella to resist or antagonize the trafficking of Nramp1 to the SCV (21). The explanation for Nramp1-dependent survival of phoP mutant S. enterica serovar Typhimurium is not known but might be rationalized by known effects of Nramp1 on phagosome acidification (12), which in turn influences PhoPQ activation (2).
FIG. 2.
sitABCD and sitABCD mntH mutant S. enterica serovar Typhimurium exhibit reduced survival in IFN-γ-activated Nramp1+ peritoneal macrophages. The data representing the number of surviving CFU at 18 h divided by the number of CFU at 0 h in C57BL/6 (A) or congenic Nramp1+ (B) macrophages is shown. phoP mutant S. enterica serovar Typhimurium MS7953 (8) was included as a macrophage-sensitive control. Significance comparisons were made between the mutant bacteria compared to the wild-type bacteria within a given cell type. *, P < 0.0001 by two-tailed t test; **, P ∼ 0.0015. NS, not significant.
It remains possible that the lack of effect of mntH and sitA on survival is due to activation of alternative transport systems in these cell types. While this possibility cannot be definitely excluded, the presence of a sitA mntH phenotype in activated primary macrophages but not HeLa cells or RAW cells is more likely attributable to Nramp1 expression. Nramp1 appears to be primarily expressed in the phagocyte (4) and increases with activation (20), and Nramp1 has been shown to actively transport divalent cations out of the SCV under these conditions (14). In contrast, microarray studies of J774 cells indicate that iron, and possibly other divalent cations, is not limiting in cells that do not express Nramp1 (7). Thus, we believe that divalent cation limitation is induced by Nramp1, and, consequently, MntH and SitABCD influence virulence only under these conditions.
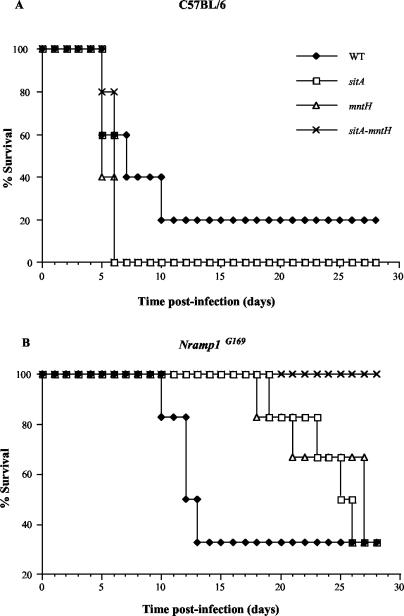
To confirm the role of mntH and sitA in S. enterica serovar Typhimurium pathogenesis, virulence assays were performed in 6- to 8-week-old C57BL/6 or congenic Nramp1G169 mice. Wild-type SL1344 or isogenic sitA::Cm, mntH::Km, or sitA::Cm mntH::Km mutant strains (15, 16) were grown in LB broth supplemented with the desired antibiotic overnight, diluted in phosphate-buffered saline, and administered intraperitoneally to groups of five female mice at a dose of approximately 400 to 800 CFU (C57BL/6; Fig. 3A) or 1,800 to 3,100 CFU (Nramp1G169; Fig. 3B). Different inocula were used to compensate for the ability of Nramp1G169 animals to withstand higher doses of S. enterica serovar Typhimurium than Nramp1D169 animals. Infected animals were monitored daily for survival until the experiment was terminated 28 days postinfection. Figure 3 shows that deletion of mntH or sitA, alone or in combination, had little effect on virulence in susceptible C57BL/6 mice. However, both the mntH and sitA deletion strains were attenuated in Nramp1G169 mice, as only 40% succumbed to infection by days 26 and 28, respectively, while the double mutant was completely avirulent. This demonstrates that during infection of a resistant host, both divalent cation transport systems are required for full virulence of S. enterica serovar Typhimurium, and the effects of these transporters are additive.
FIG. 3.
mntH and sitA are required for full virulence of S. enterica serovar Typhimurium upon infection of Nramp1G169 mice. (A) C57BL/6. (B) Nramp1G169. Solid diamonds, SL1344 wild type; open squares, sit::Cm; open triangles, mntH::Km; crosses, sitA::Cm mntH::Km. This experiment was performed three times with essentially identical results. Representative results are shown.
A previous study failed to demonstrate a role of MntH in Salmonella virulence following intravenous infection of congenic 129/Sv Nramp1D169 mice (3). However, our observations indicate that both the MntH and Sit transport systems play an important role in Salmonella virulence following intraperitoneal infection of C57BL/6-derived mice expressing a functional Nramp1 locus. As our previous work demonstrated that the sitA::Cm mntH::Km mutant is severely deficient in Mn2+ uptake but displays no signs of Fe2+ deficiency in vitro (15), this suggests that Nramp1-mediated Mn2+ depletion may be responsible for the observed attenuation of S. enterica serovar Typhimurium in the congenic Nramp1G169 mice. However since the affinities of Nramp1, MntH, and SitABCD for Mn2+ and Fe2+ as well as their precise time course of function in vivo remain to be determined, we cannot rule out the possibility that levels of both divalent cations within the SCV are important for S. enterica serovar Typhimurium pathogenesis and are influenced by the activity of Nramp1.
It should be noted that we have demonstrated a role for MntH and SitABCD following intraperitoneal infection of mice but have not investigated the role of mntH and sitA in the intestinal phase of infection. There is evidence indicating that iron levels are quite high in the gastrointestinal tract (24), which suggests that MntH and SitABCD would not be important at this stage of infection. The possibility remains that the mntH and sitA mutants may cause pathology by unhindered replication in intestinal epithelial cells. However, as mutant strains attenuated following intraperitoneal infection are virtually always attenuated following oral infection as well, we believe that these genes are unlikely to be essential for the intestinal phase of infection.
In summary, S. enterica serovar Typhimurium requires both of the divalent cation transport systems, MntH and SitABCD, for full virulence in wild-type Nramp1G169 mice following intraperitoneal infection. Genes encoding these transport systems are expressed by intracellular S. enterica serovar Typhimurium within the macrophage and are upregulated in the presence of functional Nramp1. Although there was no apparent effect on intracellular bacterial survival during infection of cell lines, the studies in activated primary macrophages and in congenic mice confirm that transport of divalent cations such as Mn2+ or Fe2+ is essential for Salmonella virulence and demonstrate that host and pathogen compete for essential metals within the SCV by both homologous and paralogous mechanisms.
Acknowledgments
We thank B. Zwilling (Ohio State University) for the gift of the C57BL/6 and congenic Nramp1G169 mice.
This work was supported by operating grants to B.B.F. from the Canadian Institutes of Health Research (CIHR) and the Howard Hughes Medical Institute (HHMI). B.B.F. is a CIHR Distinguished Investigator, an HHMI International Research Scholar, and a University of British Columbia Peter Wall Distinguished Professor. Grant support to F.C.F. was from NIH grant AI39557. Grant support to M.E.M. was from NIH grant GM61748. M.L.Z. is supported by a CIHR Doctoral Research Award.
Editor: A. D. O'Brien
REFERENCES
- 1.Ables, G. P., D. Takamatsu, H. Noma, S. El-Shazly, H. K. Jin, T. Taniguchi, K. Sekikawa, and T. Watanabe. 2001. The roles of Nramp1 and Tnfa genes in nitric oxide production and their effect on the growth of Salmonella typhimurium in macrophages from Nramp1 congenic and tumor necrosis factor-alpha−/− mice. J. Interferon Cytokine Res. 21:53-62. [DOI] [PubMed] [Google Scholar]
- 2.Alpuche Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 89:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, E., I. Bergevin, D. Malo, P. Gros, and M. F. M. Cellier. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6032-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cellier, M., C. Shustik, W. Dalton, E. Rich, J. Hu, D. Malo, E. Schurr, and P. Gros. 1997. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukoc. Biol. 61:96-105. [DOI] [PubMed] [Google Scholar]
- 5.Cellier, M. F., I. Bergevin, E. Boyer, and E. Richer. 2001. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 17:365-370. [DOI] [PubMed] [Google Scholar]
- 6.Daigle, F., J. E. Graham, and R. Curtiss III. 2001. Identification of Salmonella typhi genes expressed within macrophages by selective capture of transcribed sequences (SCOTS). Mol. Microbiol. 41:1211-1222. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 8.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 9.Govoni, G., F. Canonne-Hergaux, C. G. Pfeifer, S. L. Marcus, S. D. Mills, D. J. Hackam, S. Grinstein, D. Malo, B. B. Finlay, and P. Gros. 1999. Functional expression of Nramp1 in vitro in the murine macrophage line RAW264.7. Infect. Immun. 67:2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govoni, G., and P. Gros. 1998. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 47:277-284. [DOI] [PubMed] [Google Scholar]
- 11.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackam, D. J., O. D. Rotstein, W. Zhang, S. Gruenheid, P. Gros, and S. Grinstein. 1998. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 188:351-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 14.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 17.Koop, A. H., M. E. Hartley, and S. Bourgeois. 1987. A low-copy-number vector utilizing beta-galactosidase for the analysis of gene control elements. Gene 52:245-256. [DOI] [PubMed] [Google Scholar]
- 18.Lee, A. K., C. S. Detweiler, and S. Falkow. 2000. OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J. Bacteriol. 182:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 20.Searle, S., N. A. Bright, T. I. Roach, P. G. Atkinson, C. H. Barton, R. H. Meloen, and J. M. Blackwell. 1998. Localisation of Nramp1 in macrophages: modulation with activation and infection. J. Cell Sci. 111:2855-2866. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 22.Vidal, S. M., E. Pinner, P. Lepage, S. Gauthier, and P. Gros. 1996. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 157:3559-3568. [PubMed] [Google Scholar]
- 23.Wilmes-Riesenberg, M. R., B. Bearson, J. W. Foster, and R. Curtis III. 1996. Role of the acid tolerance response in virulence of Salmonella typhimurium. Infect. Immun. 64:1085-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wosten, M. M., L. F. Kox, S. Chamnongpol, F. C. Soncini, and E. A. Groisman. 2000. A signal transduction system that responds to extracellular iron. Cell 103:113-125. [DOI] [PubMed] [Google Scholar]
- 25.Zaharik, M. L., B. A. Vallance, J. L. Puente, P. Gros, and B. B. Finlay. 2002. Host-pathogen interactions: host resistance factor Nramp1 up-regulates the expression of Salmonella pathogenicity island-2 virulence genes. Proc. Natl. Acad. Sci. USA 99:15705-15710. [DOI] [PMC free article] [PubMed] [Google Scholar]