Abstract
Polymorphonuclear neutrophils (PMN) exposed to Mycobacterium tuberculosis display bactericidal responses and produce inflammatory proteins. This PMN-mediated inflammatory response is regulated by an activation of the apoptotic program, which collaborates to avoid tissue injury. In vitro, circulating PMN from patients with tuberculosis (TB) show an increased spontaneous apoptosis, and M. tuberculosis-induced activation accelerates the PMN apoptosis. In this study, we evaluated the mechanisms involved in spontaneous and M. tuberculosis-induced apoptosis. We demonstrate that apoptosis of PMN is not induced by lipoarabinomannan or by a whole-cell lysate of M. tuberculosis and that neither tumor necrosis factor alpha nor CD11b, CD14, and Fcγ receptors are involved. Apoptosis of PMN from patients with active TB (TB-PMN) is induced by the interaction with the whole M. tuberculosis via Toll-like receptor 2 (TLR2), and, in contrast to spontaneous apoptosis, it involves the p38 mitogen-activated protein kinase (MAPK) pathway. These results correlate with a high expression of phosphorylated p38 (p-p38) in circulating TB-PMN and with the ability of M. tuberculosis to induce in vitro the expression of p-p38 in PMN. Therefore, when the bacterial burden is low, TB-PMN could be detecting nonopsonized M. tuberculosis via TLR2, leading to the activation of the p38 MAPK pathway, which in turn would induce PMN activation and apoptosis. This mechanism needs further confirmation at the site of infection.
The influx of polymorphonuclear neutrophils (PMN) to the lung is one of the first events in the pathogenesis of pulmonary tuberculosis (TB). Bacterial products elicit the up-regulation of the β2 integrin CD11b, as well as the release of proinflammatory cytokines by PMN, thus contributing to the recruitment of leukocytes at the site of infection, amplifying the immune response. The inflammatory reaction that follows infection is mediated principally by monocytes, PMN, and endothelial cells. The central role of PMN in inflammation and host defense has long been thought to be restricted to phagocytosis and bacterial killing. However, in the past few years, several studies have revealed that PMN produce a variety of inflammatory proteins upon appropriate stimulation (11), and they exhibit a granuloma-regulatory function (38). PMN exposed to mycobacteria display bactericidal responses (9, 19, 25), but the PMN-mediated inflammatory response is regulated by activation of the apoptotic program, which helps to avoid tissue injury (15).
It is well known that phagocytosis of Mycobacterium tuberculosis involves different receptors on the phagocytic cell which bind to either opsonized or nonopsonized bacilli. M. tuberculosis may be internalized through either the type A scavenger (45) or Fcγ receptors (6). Nevertheless, nonopsonized M. tuberculosis can bind directly to the complement receptor 3 (CR3, CD11b/CD18) and CR4. In addition, recent studies have shown that Toll-like receptors (TLRs) are also involved in cellular recognition of mycobacteria. TLRs are members of the interleukin-1 (IL-1) receptor superfamily and signal via similar mechanisms, with initiation of proinflammatory gene transcription through pathways including NF-κB and mitogen-activated protein kinase (MAPK) cascades (34). Both the release of cytokines and the response of PMN to cytokines and other proinflammatory agents (27, 46), as well as activation of PMN (10), involve the MAPK pathway, that is, the p38 MAPK and extracellular signal-regulated kinases (ERK). Moreover, recent studies indicate the involvement of tyrosine phosphorylation events in signaling pathways that results in PMN apoptosis (43, 44).
It has been demonstrated that PMN from healthy purified protein derivative-positive individuals undergo rapid apoptosis when stimulated with M. tuberculosis or tumor necrosis factor alpha (TNF-α) (20) and that M. tuberculosis-induced apoptosis is dependent on the generation of reactive oxygen intermediates generated by PMN during phagocytosis of opsonized M. tuberculosis (30). On the other hand, we have demonstrated an increased spontaneous apoptosis in vitro of PMN from patients with active TB (TB-PMN), which is related to the activation state of these cells in the circulation (2, 3). Furthermore, M. tuberculosis-induced activation accelerates in vitro the apoptosis of TB-PMN and PMN from healthy individuals (N-PMN) (3).
In the present study, we have investigated the mechanisms involved in spontaneous and M. tuberculosis-induced apoptosis of TB-PMN and N-PMN. We have demonstrated that when a low ratio of nonopsonized M. tuberculosis to PMN is employed, apoptosis of TB-PMN is induced by the whole bacteria via TLR2 and involving the p38 MAPK pathway, in contrast to the case for spontaneous apoptosis. These results are in accordance with the high expression of the phosphorylated form of the cytoplasmic protein p38 (p-p38) observed in circulating TB-PMN and with the fact that M. tuberculosis is able to induce the expression of p-p38 in PMN in vitro.
MATERIALS AND METHODS
Patients.
A total of 45 hospitalized patients with active TB were studied. Their informed consent for experimentation was obtained according to the Ethics Commission of the Hospital Francisco J. Muñiz. All of the patients had advanced radiographic abnormalities on chest X-ray as well as a sputum smear that was positive for acid-fast bacilli and a confirmed sputum-positive culture for M. tuberculosis. None of them had extrapulmonary tuberculosis, and all of them were seronegative for human immunodeficiency virus and had not used intravenous drugs. Patients with other infectious or underlying disease were not included in the study. Ten patients were without treatment, and 35 patients were treated with a standard regimen of four anti-TB drugs regimen for 1 to 15 days. The mean age was 35 years (range, 23 to 58). A total of 25 healthy volunteer blood donors (controls) were also studied, and their informed consent was received. Their mean age was 30 years (range, 20 to 60). All controls had received BCG vaccination in childhood, and their tuberculin test status was unknown.
Antigens.
Mycobacterial lipoarabinomannan purified from M. tuberculosis H37Rv (ManLAM), whole-cell lysate (WCL), and gamma irradiated M. tuberculosis H37-Rv strain were provided by John Belisle (Colorado State University, Fort Collins). ManLAM and WCL were resuspended in pyrogen-free phosphate-buffered saline (PBS) at 500 and 1,000 μg/ml, respectively, and sonicated M. tuberculosis suspensions were adjusted to an optical density at 600 nm of 1, which corresponds to a bacterial suspension of ∼108 bacteria/ml.
PMN purification and culture.
Human PMN were isolated from heparinized venous blood by Ficoll-Hypaque gradient centrifugation (8) followed by sedimentation in 3% dextran (Sigma Chemical Co, St. Louis, Mo.). The PMN-rich supernatant was then collected, and residual red blood cells were removed by hypotonic lysis. The cells were washed immediately and resuspended at 3 × 106 cells/ml in RPMI 1640 medium (Gibco, Grand Island, N.Y.) supplemented with 1% heat-inactivated fetal calf serum (Gibco) and 50 μg of gentamicin per ml (complete medium). The viability was consistently >95% as determined by trypan blue dye exclusion. The purity of the final PMN preparation was up to 95% as assessed by morphological examination by staining with Wright-Giemsa stain and by fluorescence-activated cell sorting light scatter patterns.
Cultures were performed by incubating, in Falcon 2063 tubes, 1 ml of a PMN suspension (3 × 106 cells) stimulated with M. tuberculosis, ManLAM (1 and 10 μg/ml), or WCL (5, 10, and 15 μg/ml), or not stimulated (control), at 37°C in a humidified 5% CO2 incubator for 3 and 18 h. An M. tuberculosis-to-PMN ratio of 1:3 were used unless otherwise stated.
To modify cellular MAPK activity, compound SB20358, a specific inhibitor for p38 MAPK, or PD98059, which indirectly blocks the activation of p44/42 MAPK, was used (Calbiochem-Behring, La Jolla, Calif.). One milliliter of PMN suspension was preincubated with or without SB20358 or PD98059 (final concentrations, 20 and 50 μM, respectively) at 4°C for 40 min. When specified, endogenous TNF-α was neutralized by using a polyclonal anti-human TNF-α blocking antibody (Preprotech Inc., Rocky Hill, N.J.) at a final concentration of 10 μg/ml. Thereafter, cells were stimulated as indicated above.
Blocking of surface antigens in PMN.
Blocking of CD11b, CD14, and Fcγ receptors (CD16, CD32, and CD64) and TLR2 on PMN with specific antibodies was done for 30 min on ice before to the culture; these antibodies, at a final concentration of 10 μg/ml, were present throughout the subsequent culture period. Purified anti-CD11b (ICRF44; Ancell, Bayport, Minn.), anti-CD14 (IgG2b from hybridoma 3C10; American Type Culture Collection, Manassas, Va.), anti-CD16 (immunoglobulin G1 [IgG1], clone 3G8; Medarex Inc., Annandale, N.J.), anti-CD32 (IgG2b, clone IV3; Medarex), anti-CD64 (IgG1, clone 10.1; Calbiochem, La Jolla, Calif.), FG anti-TLR2 (IgG2a,κ, clone TL2.1; eBioscience, San Diego, Calif.) and their corresponding irrelevant isotypes were employed.
Measurement of PMN apoptosis. (i) AV binding assay.
The percentage of apoptotic PMN was assessed based on the Annexin V (AV)-fluorescein isothiocyanate (FITC) (Sigma) protein binding assay. Briefly, 5 μl of Annexin V-FITC (10 μg/ml) and 5 μl of propidium iodide (PI) (250 mg/ml) (Sigma) were added to 1.2 × 106 cells suspended in 500 μl of binding buffer and incubated for 15 min at room temperature in the dark, as previously described (20). The AV−/PI− cell population was regarded as alive, and the AV+/PI− population was considered an early apoptotic population. The AV+/PI+ population represented late-stage apoptotic or necrotic cells.
(ii) Microscopy assessment of PMN apoptosis.
Cytospin preparations were fixed in methanol, stained with May-Grünwald-Giemsa stain (Merck, Rahway, N.J.), and examined by oil immersion light microscopy at a final magnification of ×1,000. The percentage of apoptotic PMN was determined by counting the number of cells showing features associated with apoptosis. For all samples analyzed, 300 to 400 PMN per slide were counted by two different operators without prior knowledge of the sample.
Measurement of p38 MAPK activation. (i) Cell staining.
p-p38 was measured in permeabilized cells by using a Fix and Perm kit (Caltag, Burlingame, Calif.). PMN (3 × 106) were incubated with M. tuberculosis (1 × 106 bacteria) for 2 h in the presence or absence of the specific p-38 MAPK inhibitor SB203580 (20 μM). Thereafter, cells were washed with PBS and resuspended in 100 μl of solution A (fixation) for 15 min at room temperature. After washing with PBS containing 1% azide and 5% fetal calf serum, cells were resuspended in solution B (permeabilization) and the mouse anti-human p-p38 IgM anti-p-p38 FITC-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). After a 20-min incubation on ice in the dark, cells were washed, resuspended in FACSflow (Bectin-Dickinson) and analyzed by using a FACScan (Becton-Dickinson). An isotype-matched control was used to determine autofluorescence and nonspecific staining. Results were expressed as mean fluorescence intensity (MFI).
(ii) Western blot analysis.
To detect the activation of p38 MAPK, 200 μl of PBS containing 20 μl of a protease inhibitor cocktail from Sigma (P-8340) and 2 μM phenylmethylsulfonyl fluoride was added to the cells. The cells were then placed on ice and disrupted by ultrasonic irradiation. A 200-μl volume of 2× Laemmli buffer (4% [wt/vol] sodium dodecyl sulfate [SDS], 20% [vol/vol] glycerol, 10% [vol/vol] 2-mercaptoethanol, 0.004% [wt/vol] bromphenol blue, and 0.125 M Tris-HCl [pH 6.8]) was added and thoroughly mixed (22a). Samples were immersed in a boiling water bath for 5 min and then immediately settled on ice. Proteins were resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE) (10% acrylamide-bisacrylamide for the resolving gel and 4.3% acrylamide-bisacrylamide for the stacking gel) in a Mini Protein II Cell (Bio-Rad, Hercules, Calif.). After SDS-PAGE, gels were equilibrated in transfer buffer for 10 min and electrotransferred at 100 V for 60 min onto polyvinylidene difluoride membranes (Hybond-P; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) by using a Mini Trans-Blot Cell (Bio-Rad). The membranes were probed with a commercial kit (Phosphoplus p38 MAPK [Thr180/Tyr182] antibody kit; New England Biolabs, Inc., Beverley, Mass.) that allows specific recognition of both nonphosphorylated and serine-phosphorylated p38 MAPK. The intensities of the autoradiographic bands were estimated by densitometric scanning with NIH Image (Scion Corporation) software.
TLR2 expression on PMN.
The expression of TLR2 in TB-PMN and N-PMN was evaluated by using an FITC-conjugated anti-TLR2 antibody (IgG2a,κ, clone TL2.1; eBioscience) and analyzed by using a FACScan (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.); 10,000 events were collected in linear mode for forward scatter, side scatter, and log amplification for FL-1 and FL-2. Analysis was performed with the CellQuest software (Becton-Dickinson), and isotype-matched controls were used to determine autofluorescence and nonspecific staining. Results were expressed as the relative MFI (specific MFI/isotype MFI).
Statistical analysis.
The Mann-Whitney U test was used for comparison between groups, and the Wilcoxon test was used to compare different treatments of same sample. Statistical significance was assumed at a P value of <0.05.
RESULTS
M. tuberculosis induces apoptosis in a dose-dependent manner.
Although apoptosis of PMN is known to be induced during phagocytosis of opsonized M. tuberculosis (6), we have observed that nonopsonized M. tuberculosis is able to induce PMN apoptosis at a low ratio of M. tuberculosis to PMN (1:3) (3). Thus, in order to compare the apoptotic effects of nonopsonized M. tuberculosis at low or high number of bacteria, N- and TB-PMN were stimulated with M. tuberculosis at different M. tuberculosis/PMN ratios (1:3, 1:1, 10:1, and 20:1) for 18 h. Thereafter, morphological examination was performed and cell surface exposure of phosphatidylserine by FITC-conjugated AV was determined.
Flow cytometric analysis showed that the level of AV+ cells increased in M. tuberculosis-stimulated PMN from either controls or TB patients (Fig. 1) and that the increase in the percentage of apoptotic PMN critically depends on the ratio of bacteria to PMN in the culture. Moreover, the lower the ratio, the larger the differences in PMN apoptosis between controls and TB patients. These results demonstrate that nonopsonized M. tuberculosis-induced apoptosis is dose dependent and that the low ratio of M. tuberculosis to PMN (1:3) used in our experiments is sufficient to induce apoptosis in TB-PMN.
FIG. 1.
M. tuberculosis-induced apoptosis in PMN depends on M. tuberculosis/PMN ratio. N-PMN (▴) and TB-PMN (▪) (3 × 106/ml) were cultured in medium alone (control) or stimulated with M. tuberculosis (Mtb) at different M. tuberculosis/PMN ratios (1:3, 1:1, 10:1, and 20:1) for 18 h. Thereafter, apoptosis was evaluated on the basis of AV-FITC binding. Results are expressed as means (± standard errors of the means) of the percentage of AV+ cells (n = 10). Statistical differences for control versus M. tuberculosis: *, P < 0.0001; ϕ, P < 0.0005; #, P < 0.01. Statistical differences for N-PMN versus TB-PMN: Ψ, P < 0.02, Φ, P < 0.005.
LAM or WCL does not induce PMN apoptosis.
We wondered whether the whole bacteria or one of the components of M. tuberculosis could be exerting the proapoptotic effect. We therefore stimulated PMN with different concentrations of a WCL of M. tuberculosis which includes cellular wall, cytosol, and membranes and with the major mycobacterial cell wall glycolipid (LAM), which is a representative component of the bacteria generating early immune responses (12). Apoptosis was evaluated in 18-h cultured PMN, since the proapoptotic effect of M. tuberculosis had been previously observed in TB-PMN at this time point (3). Thereafter, the percentage of apoptotic PMN was determined with AV-FITC and compared to M. tuberculosis-induced apoptosis. In accordance with our previous results (3), no differences in apoptosis between treated and untreated patients were observed (data not shown), so they were taken as one group. As can be observed in Table 1 and in accordance to our previous results (3), a significant increase in apoptosis by whole M. tuberculosis was observed in TB-PMN, and although ManLAM did not induce apoptosis in N- and TB-PMN, the WCL tended to increase the apoptosis in TB-PMN in a dose-dependent manner, suggesting that antiapoptotic components might be present in the WCL. The results obtained with LAM were also independent of the dose used (10 μg/ml) (data not shown).
TABLE 1.
Induction of apoptosisa
| Treatment | Mean AV+ cells (n = 15) ± SEM
|
|
|---|---|---|
| N-PMN | TB-PMN | |
| Control | 46.3 ± 7.8 | 47.0 ± 7.8 |
| M. tuberculosis (1 × 106/ml) | 54.0 ± 6.2 | 66.1 ± 8.4b |
| ManLAM (1 μg/ml) | 45.3 ± 3.5 | 42.2 ± 5.2 |
| WCL | ||
| 5 μg/ml | 52.8 ± 2.2 | 51.0 ± 2.6 |
| 10 μg/ml | 53.0 ± 2.0 | 53.5 ± 2.8 |
| 15 μg/ml | 54.3 ± 6.0 | 55.5 ± 2.6 |
PMN (3 × 106) were incubated for 18 h, stimulated with M. tuberculosis (106 ml), LAM (1μg/ml), WCL (5, 10, or 15 μg/ml), or medium alone (control). Thereafter, the percentage of apoptotic cells was evaluated by A V-FITC binding assay.
P < 0.002 (control versus M. tuberculosis).
Neutralization of secreted TNF-α does not inhibit M. tuberculosis-induced apoptosis in TB-PMN.
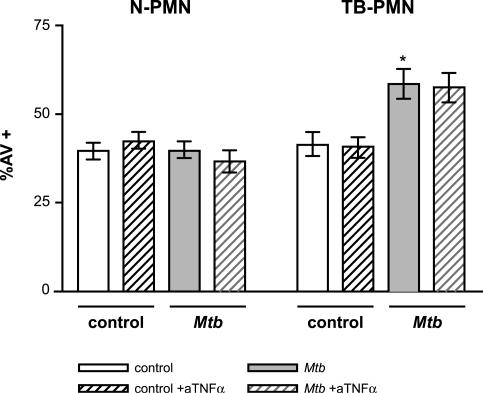
It has been shown that PMN stimulated with both M. tuberculosis and TNF-α undergo rapid apoptosis (20, 26, 30). Considering that M. tuberculosis induces the secretion of TNF-α in TB-PMN cultured overnight (3), we evaluated whether the M. tuberculosis-induced apoptosis observed in TB-PMN after an 18-h culture was due to an autocrine or paracrine effect of TNF-α secretion. As can be observed in Fig. 2, M. tuberculosis-induced apoptosis was not abolished when endogenous TNF-α was neutralized with a specific antibody. Furthermore, the concentration of antibody used in these experiments was sufficient to block the proapoptotic effect of TNF-α (data not shown). Therefore, M. tuberculosis-induced apoptosis in TB-PMN is not mediated by the production of TNF-α.
FIG. 2.
Endogenous TNF-α is not involved in the M. tuberculosis-induced apoptosis of TB-PMN. PMN (3 × 106/ml) cells were treated with anti-TNF-α (aTNFα) or an irrelevant isotype antibody following incubation with or without M. tuberculosis (Mtb) (106 /ml) for 18 h. The percentage of apoptotic cells was evaluated and expressed as means (± standard errors of the means) of the percentage of AV+ cells (n = 10). *, P < 0.001 (TB-PMN control versus M. tuberculosis).
CD11b, CD14, and Fcγ receptors are not involved in M. tuberculosis-induced apoptosis.
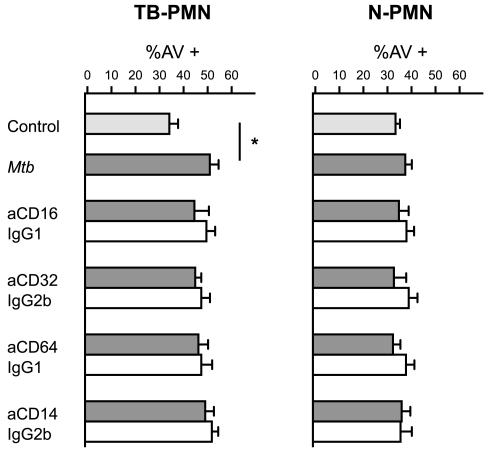
Taking into account that many surface receptors are involved in M. tuberculosis recognition, we evaluated which of them could be involved in the triggering of apoptosis. The CD11b/CD18 receptor, which mediates the uptake of opsonized and nonopsonized mycobacteria, has been implicated in the modulation of the PMN apoptotic process (16), and its expression is markedly induced by M. tuberculosis in cultured TB-PMN (2). Although we did not employ opsonized mycobacteria, we evaluated Fcγ (CD16, CD32, and CD64) receptors, since ligation of different receptors in macrophages could alter TLR-induced inflammatory signaling (31). Also, the expression of CD16 and CD64 is up-regulated in circulating TB-PMN (2).
To this purpose, PMN were treated with specific blocking antibodies or isotype-matched control antibodies and then stimulated with M. tuberculosis for 18 h. As shown in Fig. 3, the blockade of the CD11b, CD14, CD16, CD32, and CD64 receptors had no effect on M. tuberculosis-induced apoptosis in either N- or TB-PMN.
FIG. 3.
CD11b, CD14, and Fcγ receptors are not involved in M. tuberculosis-induced apoptosis. N-PMN and TB-PMN (3 × 106/ml) were treated with specific antibodies against CD11b (aCD11b), CD14, CD16, CD32, and CD64 (solid bars) or their corresponding isotype controls (open bars) following incubation without (control) or with M. tuberculosis (Mtb) (106/ml) for 18 h. Apoptotic cells were evaluated by AV-FITC binding assay as described in Materials and Methods. Results are expressed as means (± standard errors of the means) of the percentage of AV+ cells (n = 8). IgG, immunoglobulin G.
M. tuberculosis-induced apoptosis in TB-PMN is dependent on TLR2.
Soluble and cell wall-associated factors are able to mediate cellular activation through TLR2 (30), and it has been demonstrated that TLR2 mediates apoptotic signals (4) as well as the activation of p38 in response to gram-positive bacteria (42). Therefore, we evaluated whether TLR2 was involved in M. tuberculosis-induced apoptosis.
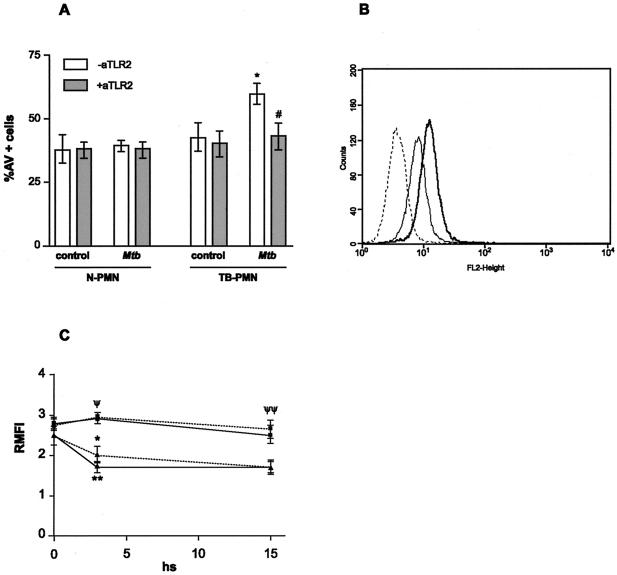
To this purpose, PMN were treated with either specific blocking or isotype-matched control antibodies and then stimulated with M. tuberculosis for 18 h. A significant inhibition of M. tuberculosis-induced apoptosis in TB-PMN as a result of the blockade of TLR2 was observed (percentages of AV+ cells: control, 40 ± 6; M. tuberculosis, 59.5 ± 4 [P < 0.001]; M. tuberculosis plus anti-TLR2, 43 ± 5 [P < 0.04] [n = 8]) (Fig. 4A). Consistent with this, an increase of 20% in the MIF of TLR2 expression was observed in recently isolated TB-PMN (P < 0.03) compared with N-PMN, as shown in an example of 13 experiments in Fig. 4B. Moreover, TLR2 expression in TB-PMN remained unaltered during the culture period even in the presence of M. tuberculosis, whereas N-PMN decreased TLR2 expression in culture (n = 8; P < 0.05) (Fig. 4C). Therefore, activation in TB-PMN may be mediated by a ligand(s) that recognizes TLR2 and, in the presence of M. tuberculosis, induces apoptosis in TB-PMN.
FIG. 4.
TLR2 triggers apoptosis in TB-PMN. (A) Blocking of TLR2 abolishes M. tuberculosis-induced apoptosis in TB-PMN. PMN (3 × 106/ml) from eight controls and eight TB patients were treated with specific antibody against TLR2 (aTLR2) or its corresponding isotype following incubation with or without M. tuberculosis (Mtb) (106 /ml) for 18 h. Apoptotic cells were evaluated by AV-FITC binding assay as described in Materials and Methods). *, P < 0.001 (M. tuberculosis versus control); #, P < 0.04 (M. tuberculosis versus M. tuberculosis plus anti-TLR2. (B) TLR2 expression in freshly isolated PMN. Expression of TLR2 in TB-PMN () and N-PMN (—) assessed by using an FITC-anti-TLR2 antibody and FACScan. Isotype-control antibody-stained cells are shown as a dotted line histogram. An example from 13 experiments done in each group are shown. (C) TLR expression in N-PMN (▴) and TB-PMN (▪) cultured with () or without (—) M. tuberculosis at 0, 3, and 18 h. Expression of TLR2 was evaluated and expressed as relative MFI as detailed in Materials and Methods. *, P < 0.04; **, P < 0.002 (control versus M. tuberculosis N-PMN) (n = 8). ψ, P < 0.02; ψψ, P < 0.0004 (TB-PMN vs. N-PMN) (n = 8).
M. tuberculosis-induced apoptosis involves MAPK p38 activation.
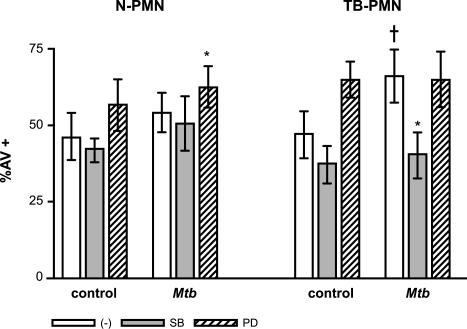
MAPK activation represents an important signaling pathway in PMN. It has been demonstrated that MAPK activation participates in that of PMN stimulated with M. tuberculosis as well as in apoptosis induced by phagocytosis of M. tuberculosis (29, 30). On the other hand, TNF-α induces activation of p38 MAPK, while IL-8 induces activation of ERK (27). Considering that the secretion of these cytokines is induced by M. tuberculosis in TB-PMN in culture (2, 3, 32) we used the specific kinase inhibitors SB203580 and PD98059 in order to evaluate the roles of p38 and ERK in spontaneous and M. tuberculosis-induced apoptosis at the low M. tuberculosis/PMN ratio (1:3). As shown in Fig. 5, neither the spontaneous apoptosis of PMN from controls and TB patients nor M. tuberculosis-induced apoptosis in N-PMN was modified by inhibition of p38, but M. tuberculosis-induced apoptosis was inhibited in TB-PMN (P < 0.03). On the other hand, a significant increase in M. tuberculosis-induced apoptosis in N-PMN was observed when ERK was inhibited (P < 0.02), whereas in TB-PMN spontaneous apoptosis tended to be enhanced. Taken together, these results indicate that M. tuberculosis-induced apoptosis is dependent on the p38 pathway.
FIG. 5.
p38 MAPK is required for M. tuberculosis-induced apoptosis in TB-PMN. PMN (3 × 106/ml) were pretreated with 25 μM SB203580 or 50 μM PD98059 and incubated with medium alone (control) or M. tuberculosis (Mtb) (106 /ml) for 18 h. Apoptotic cells were evaluated by AV-FITC binding assay as described in Materials Methods. Results are expressed as means (± standard errors of the means) of the percentage of AV+ cells (n = 15). *, P < 0.05 (M. tuberculosis versus control); †, P < 0.03; ‡, P < 0.05 (inhibitor versus untreated PMN).
PMN stimulation with M. tuberculosis induces p38 expression.
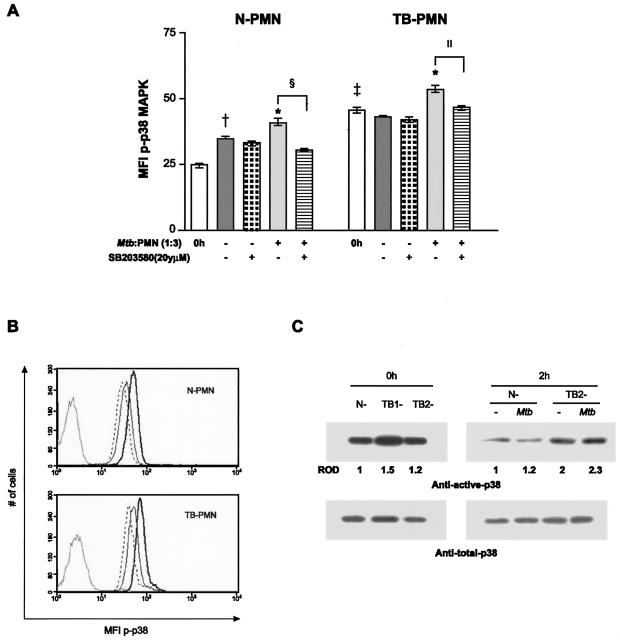
Taking into account that activation of p38 participates in M. tuberculosis-induced apoptosis and in PMN activation, we wondered whether the bacteria were capable of inducing the activation of this kinase. Therefore, we evaluated the capacity of M. tuberculosis to induce the cytoplasmic expression of p-p38 in TB-PMN and N-PMN in a 2-h culture, the time at which p-p38 expression reached the maximum value, according to previous experiments (data not shown). As shown in Fig. 6A, higher p-p38 expression was observed in circulating TB-PMN than in N-PMN (P < 0.0008). Moreover, M. tuberculosis-induced p-p38 expression in both N- and TB-PMN (P < 0.03) was inhibited by SB203580, as shown in Fig. 6B. These results were confirmed by Western blot analysis, in which larger amounts of p-p38 were observed in circulating TB-PMN and p-p38 was induced by M. tuberculosis (Fig. 6C). Therefore, the activation state in TB-PMN could be due to the in vivo activation of this kinase.
FIG. 6.
p38 MAPK activation in circulating PMN and in M. tuberculosis-stimulated PMN. (A) Expression of p-p38 in recently isolated or cultured PMN from N- and TB-PMN was evaluated. As detailed in Materials and Methods, 3 × 106 cells/ml were pretreated with 25 μM SB203580 and incubated with medium alone (control) or M. tuberculosis (Mtb) (106/ml) for 2 h, and the p-p38 expression was evaluated by flow cytometry. Results are expressed as the means (± standard errors of the means) of the MFI (n = 10). Statistical differences: *, P < 0.03 (M. tuberculosis versus control); †, P < 0.03 (2 h versus 0 h); ‡, P < 0.001 (TB-PMN versus N-PMN); §, P < 0.02; ‖, P < 0.05 (M. tuberculosis versus M. tuberculosis-SB). (B) Representative histograms of p-p38 expression in control (—), M. tuberculosis-stimulated (—), and M. tuberculosis-SB-treated (- - -) PMN in a 2-h culture. (C) Equal amounts of cellular proteins were separated by SDS-PAGE and blotted with anti-active p38 MAPK (upper panel). To ascertain that each lane received similar amounts of p38 MAPK, the blots were reprobed with anti-total-p38 MAPK antibody (lower panel). Results are for freshly isolated PMN from one control (N) and two patients (TB1 and TB2) (0 h) or from a control and one TB patient and cultured with or without M. tuberculosis (2 h). The intensities of the autoradiographic bands were estimated by densitometric scanning, and the relative densities (ROD) of the p-p38 bands were referred to N-PMN.
DISCUSSION
Although tuberculous lesions are characterized by a predominant migration of monocytes/macrophages to the site of infection, the earliest response to mycobacteria is primarily an influx of PMN (5), which play a significant role in the acute phase of the disease (14). However, as the disease evolves, an increased number of PMN are observed in the bronchoalveolar lavage fluid of the affected lung (28). This mobilization and subsequent activation are important for microbicidal function (35), even though the powerful mediators helping PMN to be effective killers, such as oxidants and elastase, can also injure endothelial cells and produce structural damage (7). Therefore, the lack of timely removal of these cells from the inflamed sites may result in tissue damage or progression to chronic inflammation (15). We have previously observed that PMN exposed to M. tuberculosis or TNF-α up-regulate CD11b expression in TB-PMN and N-PMN, demonstrating an in vitro PMN activation. Moreover, M. tuberculosis-induced activation leads to the acceleration of apoptosis of PMN (3). In this study we wanted to identify the signaling pathways triggered in PMN by M. tuberculosis-PMN interaction which might be involved in the pathology of TB.
We found that even with nonopsonized bacteria, the rate of apoptosis is critically dependent on the ratio of bacteria to PMN, as previously demonstrated with opsonized M. tuberculosis (30). In our system, in contrast to N-PMN, apoptosis of TB-PMN is also induced at a low ratio of M. tuberculosis to PMN (1:3), suggesting that two mechanisms underlie induction of apoptosis in TB-PMN, the first being independent of phagocytosis and the second being dependent on phagocytosis. The first mechanism occur at low ratio of bacteria to PMN and would be important during the early phase of M. tuberculosis infection when serum opsonins and M. tuberculosis are limited. The second mechanism would take place in sites with high bacterial burdens, such as the lung in patients with TB, where M. tuberculosis phagocytosis could account for the enhanced apoptosis. Furthermore, apoptosis of PMN either not mediated or mediated by phagocytosis might prevent tissue injury of the lung. Phagocytosis was excluded at this low bacteria/PMN ratio, since the presence of cytochalasin B, an inhibitor of cytoskeleton, did not modify the rate of apoptosis in TB-PMN (data not shown). In addition, electron microscopic assays have demonstrated that about 90% of PMN were unable to phagocytose nonopsonized Mycobacterium bovis BCG (13). It has been shown that some pathogens, such as Escherichia coli (24), Streptococcus pneumoniae (47), and Candida albicans (33), reduce the life span of PMN. However, some intracellular parasites inhibit the PMN apoptosis: in Leishmania major (1) and Chlamydia pneumoniae (41), which survive inside PMN, the delay of apoptosis is mediated by phagocytosis, while in M. bovis BCG, it is not (39). These differences could be attributed to the antigen employed (live, heat-killed, or gamma irradiated), the presence of lipopolysaccharide, and the preactivated state of PMN, which, as we have demonstrated, contribute to the accelerated apoptosis in TB-PMN (3). In addition, the interaction between the antigenic components recognized at the pathogen surface and the receptors involved in the recognition can trigger different signaling pathways. Therefore, activation and apoptosis of PMN do not directly depend on phagocytosis but might be the result of stimulation through soluble factors released by activated PMN and/or immune recognition of M. tuberculosis cell wall components capable of triggering signals that induce activation and/or apoptosis. In fact, the observation that apoptosis of PMN is induced at low ratio of M. tuberculosis to PMN would explain why PMN are not the predominant cell population at the site of TB infection.
In order to discern whether the apoptotic effect observed in PMN could be mediated by M. tuberculosis components, we tested a WCL and LAM. Anti-LAM antibodies have been found in sera of TB patients, suggesting the presence of this molecule outside the macrophages (35). Moreover, AraLAM induces the secretion of IL-8 and Gro-α in cultured PMN (5). Although PMN do not express mannose receptors, we evaluated the effect of ManLAM because it is isolated from H37Rv and could act through CD14 (7) in association with TLR2 (39). However, our results show that ManLAM is not capable of inducing apoptosis in PMN. Therefore, we thought that another mycobacterial component(s) could be exerting the apoptotic effect. As shown in Table 1, the WCL of M. tuberculosis tended to induce apoptosis in PMN, suggesting that an interaction with whole M. tuberculosis would be required to induce apoptosis or that there may be other components in the preparation which exert an antiapoptotic effect. Further studies will be necessary to confirm which ligand triggers the apoptotic process in PMN.
Considering that TNF-α induces activation and apoptosis in PMN (3, 20, 26) and that it also leads to the activation of the p38 pathway (27), we evaluated whether the M. tuberculosis-induced apoptosis observed in TB-PMN was due to enhanced TNF-α secretion induced by M. tuberculosis (30). However, our results indicate that triggering of M. tuberculosis-induced apoptosis is not an autocrine effect of TNF-α release, since inhibition of endogenous TNF-α had no effect on M. tuberculosis-induced apoptosis (Fig. 2).
In the present work we demonstrated that TLR2 is involved in apoptosis induced by M. tuberculosis in PMN from TB patients. It is well known that TLR2-dependent signals participate in the innate inflammatory response to mycobacterial infection, and it is known that M. tuberculosis as well as AraLAM, but not ManLAM, stimulates TNF-α production by macrophages, involving TLR2 (40). As TLRs generate intracellular signals that elicit or modulate inflammatory responses triggered in association with other receptors, we evaluated whether other receptors were also involved in TB-PMN apoptosis. Although CD11b/CD18 has been implicated in the modulation of the PMN apoptotic process (16) and its expression is induced by M. tuberculosis (3), we observed that it is not involved in M. tuberculosis-induced apoptosis. Like CD11b/CD18, neither CD14 nor Fcγ receptors were involved at low ratios of M. tuberculosis to PMN. Therefore, TLR2 is involved in TB-PMN apoptosis, and although CD11b, CD14, and Fcγ receptors are not, we cannot exclude the involvement of other receptors associated with TLR2.
It has been demonstrated that TLR2 mediates apoptotic signals (4) as well as the activation of p38 in response to gram-positive bacteria (42). In addition, M. tuberculosis-induced activation and M. tuberculosis-induced apoptosis by phagocytosis of opsonized mycobacteria involve the MAPK pathways in PMN (29, 30). Therefore, we wanted to evaluate whether this pathway is also involved in our system, where phagocytosis is not involved. The present data show that SB203580 inhibits M. tuberculosis-induced apoptosis in TB-PMN while the specific inhibitor of ERK 1/2 has no effect, indicating that M. tuberculosis-induced apoptosis is dependent on the p38 pathway. It has been demonstrated that PMN undergo apoptosis spontaneously when cultured in vitro or by Fas receptor cross-linking but that these pathways are independent of p38 (44). Thus, the accelerated spontaneous apoptosis of TB-PMN observed in vitro could be due either to the high expression of Fas receptor (3) or to a preactivated state involving the p38 pathway. It is well known that IL-8 delays apoptosis mediated by Fas and TNF-α (21) and generates survival signals that override the p38 death signals (18). Accordingly, we also observed a significant increase of M. tuberculosis-induced apoptosis in N-PMN and of spontaneous apoptosis in TB-PMN when ERK was inhibited (Fig. 4), suggesting that IL-8 generated in culture attenuates the M. tuberculosis-induced apoptosis (3). In this context, the tendency to enhance spontaneous apoptosis observed in TB-PMN would be explained by the preactivated state of PMN in TB patients.
Finally, our results demonstrating that TLR2 triggers apoptosis of TB-PMN correlate with M. tuberculosis-induced expression of p-p38 in PMN and with the increased expression of activated p38 found in circulating TB-PMN. Since in a murine model of pulmonary inflammation the activation of p38 has been implicated in the recruitment of PMN to the infected lung (27), our findings would explain the high influx of PMN to the site of infection during the early stages of TB. Hence, the activation state and the accelerated apoptosis observed in TB-PMN could be due to the in vivo activation of this kinase. In addition, cell activation and apoptosis appear to be two distinct outcomes associated with p38 activation of PMN in response to M. tuberculosis, because once arrived at the site of infection, the presence of M. tuberculosis on activated PMN could accelerate PMN apoptosis. In line with this, enhanced parameters of activation and increased apoptosis were observed in PMN from tuberculous pleural effusions compared to effusions with other causes (unpublished results). This suggests that the microenvironment in which p38 is activated determines its ultimate biological response. Although we did not directly address which M. tuberculosis antigens acting via TLR2 induce p38 activation in PMN, peptidoglycan or lipoproteins would be the most suitable antigens, since they signal through TLR2 (4, 37). Therefore, in our system, human PMN would be detecting whole M. tuberculosis via TLR2, leading to the activation of the p38 MAPK pathway, which in turn could induce PMN activation and apoptosis.
Our results show that apoptosis of TB-PMN accelerated by M. tuberculosis could be acting at the site of infection to prevent tissue damage, because PMN apoptosis is associated with a down-regulation of their proinflammatory capacity (22). In this context, the mechanism described here may be important at low bacterial burdens in the infected lung, where the recognition of M. tuberculosis by activated PMN through TLR2 can trigger apoptosis, preventing the release of their harmful contents and allowing phagocytosis of apoptotic PMN by professional phagocytes. In addition, it has been demonstrated that phagocytosis of apoptotic PMN by human macrophages contributes to the resolution of pulmonary inflammation and increases the production of transforming growth factor-β1 (17), which is also involved in the modulation of cytokine production induced by TLR ligands (23). Furthermore, apoptotic cells can be engulfed and degraded by immature dendritic cells, but the outcome with regard to the immune response may vary depending on the type of cell death and whether maturation signals are received (36). Therefore, it remains to be established whether phagocytosis of apoptotic PMN could affect subsequent maturation of dendritic cells, contributing to the adaptive immune response in human TB.
Acknowledgments
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (grant 05-04816) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 0711/98).
We thank the medical staff of División de Tisioneumonología of Hospital F. J. Muñiz for their great help in providing clinical samples from patients with TB. We are also grateful to Silvina Meroni and Fernanda Riera from Hospital de Niños R. Gutierrez for assistance with the Western blot assay.
Editor: D. L. Burns
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. Van Zandvergen, H. Laufs, B. Hansen, K. Müller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Alemán, M., M. Beigier-Bompadre, C. Borghetti, S. de la Barrera, E. Abbate, M. Isturiz, and M. C. Sasiain. 2001. Activation of peripheral blood neutrophils from patients with active advanced tuberculosis. Clin. Immunol. 100:87-95. [DOI] [PubMed] [Google Scholar]
- 3.Alemán, M., A. García, M. Saab, S. de la Barrera, M. Finiazs, E. Abbate, and M. C. Sasiain. 2002. Mycobacterium tuberculosis-induced activation accelerates apoptosis in peripheral blood neutrophils from patients with active tuberculosis. Am. J. Respir. Cell Mol. Biol. 27:583-592. [DOI] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowsky, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor 2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Appelberg, R. 1992. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J. Leukoc. Biol. 57:472-477. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong, J. A., and P. D. Hart. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo, J., A. M. Billingslea, R. L. Blumenthal, K. F. Seeto, E. R. Simons, and M. J. Fenton. 1998. Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor. Infect. Immun. 66:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Investig. 97:77-89. [PubMed] [Google Scholar]
- 9.Brown, A. E., T. J. Holzer, and B. R., Andersen. 1987. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156:985-989. [DOI] [PubMed] [Google Scholar]
- 10.Brumell, J. H., A. L. Burkhardt, J. B. Bolen, and S. Grinstein. 1996. Endogenous reactive oxygen intermediates activate tyrosine kinases in human neutrophils. J. Biol. Chem. 271:1455-1461. [DOI] [PubMed] [Google Scholar]
- 11.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-26. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee, D., A. D. Roberts, K. Lowell, P. J. Brennan, and I. M. Orme. 1992. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 60:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow, J. C., D. W. Young, D. T. Golembock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 14.Condos, R., W. N. Rom, Y. M. Liu, and N. W. Schluger. 1998. Local immune responses correlate with presentation and outcome in tuberculosis. Am. J. Respir. Crit. Care Med. 157:729-735. [DOI] [PubMed] [Google Scholar]
- 15.Cox, G., J. Crossley, and Z. Xing. 1995. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am. J. Respir. Cell Mol. Biol. 12:232-237. [DOI] [PubMed] [Google Scholar]
- 16.Coxon, A., P. Rieu, F. J. Barkalow, S. Askari, A. H. Sharpe, U. H. von Andrian, M. A. Amin-Arnaout, and T. M. Mayadas. 1996. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5:653-666. [DOI] [PubMed] [Google Scholar]
- 17.Fadok V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y., Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibits proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasch, S., J Nick, V. Fadok, D. Bratton, and G. Worthen. 1998. p38-mitogen-activated protein kinase-dependent and independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J. Biol. Chem. 273:8389-8397. [DOI] [PubMed] [Google Scholar]
- 19.Jones, G. S., H. J. Amirault, and B. R. Andersen. 1990. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J. Infect. Dis. 162:700-704. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara, K., I. Sato, K. Ogura, H. Takeuchi, K. Kobayashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 21.Kettritz, R., M. L. Gaido, H. Haller, F. C. Luft, C. J. Jennette, and R. J. Falk. 1998. Interleukin-8 delays spontaneous and tumor necrosis factor-α apoptosis of human neutrophil. Kidney Int. 53:84-91. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, S. D., J. M. Voyich, K. R. Braughton, and F. R. de Leo. 2003. Down-regulation of proinflammatory capacity during apoptosis in human polymorphonuclear leukocytes. J. Immunol. 170:3357-3368. [DOI] [PubMed] [Google Scholar]
- 22a.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Lucas, M., L. M. Stuart, J. Savill, and A. Lacy-Hulbert. 2003. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J. Immunol. 171:2610-2615. [DOI] [PubMed] [Google Scholar]
- 24.Matzuda, T., H. Saito, T. Inoue. K. Fukatsu, M. T. Lin, I. Han, S. Furukawa, S. Ikeda, and T. Muto. 1999. Ratio of bacteria to polymorphonuclear neutrophils (PMNs) determines PMN fate. Shock 12:365-372. [PubMed] [Google Scholar]
- 25.May, M. E., and P. J. Spagnuolo. 1987. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect. Immun. 55:2304-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, J., J. A. Barbara, and S. A. Dunkley. 1997. Regulation of neutrophil apoptosis by tumor necrosis factor-α: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90:2772-2783. [PubMed] [Google Scholar]
- 27.Nick, J. A., S. K. Young, K. K. Brown, N. J. Avdi, P. G. Arndt, B. T. Suratt, M. S. Janes, P. M. Henson, and G. S. Worthen. 2000. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J. Immunol. 164:2151-2159. [DOI] [PubMed] [Google Scholar]
- 28.Ozaky, T., S. Nakahira, K. Tani, F. Ogushi, S. Yasuoka, and T. Ogura. 1992. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest 2:54-59. [DOI] [PubMed] [Google Scholar]
- 29.Perskvist, N., L. Zheng, and O. Stendahl. 2000. Activation of human neutrophils by Mycobacterium tuberculosis H37Ra involves phospholipase Cγ2, Shc adapter protein, and p38 mitogen-activated protein kinase. J. Immunol. 164:959-965. [DOI] [PubMed] [Google Scholar]
- 30.Perskvist, N., M. Long, O. Stendahl, and L. Zheng. 2002. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/BclxL via an oxygen-dependent pathway. J. Immunol. 168:6358-6365. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer, A., A. Bottcher, E. Orso, M. Kapinsky, P. Nagy, A. Bodnar, I. Spreitzer, G. Liebisch, W. Drobnik, K. Gempel, M. Horn, S. Holmer, T. Hartung, G. Multhoff, G. Schutz, H. Schindler, A. J. Ulmer, H. Heine, F. Stelter, C. Schutt, G. Rothe, J. Szollosi, S. Damjanovich, and G. Schmitz. 2001. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31:3153-3164. [DOI] [PubMed] [Google Scholar]
- 32.Riedel, D. D., and S. H. E. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotstein, D., J. Parodo, R. Taneja, and J. C. Marshall. 2000. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. Shock 14:278-283. [DOI] [PubMed] [Google Scholar]
- 34.Sabroe, I., L. C. Parker, A. G. Wilson, M. K. B Whyte, and S. K. Dower. 2002. Toll-like receptors: their role in allergy and non-allergic inflammatory disease. Clin. Exp. Allergy 32:984-989. [DOI] [PubMed] [Google Scholar]
- 35.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 2:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauter, B., M. L. Albert, L. Francisco, M. Larsson, S. Somersan, and M. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwandner, R., R. Dziarsky, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 38.Seiler, P., P. Aichele, S. Bandermann, A. Hauser, B. Lu, N. P. Gerard, C. Gerard, S. Ehlers, H. Mollenkopf, and S. H. E. Kaufmann. 2003. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 33:2676-2686. [DOI] [PubMed] [Google Scholar]
- 39.Suttmann, H., N. Lehan, A. Böhle, and S. Brandau. 2003. Stimulation of neutrophil granulocytes with Mycobacterium bovis Bacillus Calmette-Guérin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect. Immun. 71:4647-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Zandbergen, G., J. Gieffers, H. Kothe, J. Rupp, A. Bollinger, E. Aga, M. Klinger, H. Brade, K. Dalhoff, M. Maass, W. Solbach, and T. Laskay. 2004. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J. Immunol. 172:1768-1776. [DOI] [PubMed] [Google Scholar]
- 42.Vasselon, T., W. A. Hanlon, S. D. Wright, and P. A. Demeters. 2002. Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. J. Leukoc. Biol. 71:503-510. [PubMed] [Google Scholar]
- 43.Watson, R. W., H. P. Redmond, J. H. Wang, and D. Bouchier-Hayes. 1996. Bacterial ingestion, tumor necrosis factor-alpha, and heat induce programmed cell death in activated neutrophils. Shock 5:47-51. [DOI] [PubMed] [Google Scholar]
- 44.Yousefi, S., D. R. Green, K. Blaser, and H. U. Simon. 1994. Protein-tyrosine phosphorylation regulates apoptosis in human eosinophils and neutrophils. Proc. Natl. Acad. Sci. USA 91:10868-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerli, S., S. Edwards, and J. D. Ernst. 1996. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 15:760-770. [DOI] [PubMed] [Google Scholar]
- 46.Zu, Y.-L., Q. Jiafan, A. Gilchrist, G. A. Fernández, D. Vazquez-Abad, D. L. Kreutzer, C.-K Huang, and R. I. Sha'afi. 1998. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-α or FMLP stimulation. J. Immunol. 160:1982-1989. [PubMed] [Google Scholar]
- 47.Zysk, G., L. Bejo, B. K. Schneider-Wald, R. Nau, and H.-P. Heinz. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin. Exp. Immunol. 122:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]