Abstract
The temperature-sensitive dam mutant strain of Salmonella enterica serovar Enteritidis SD1 is highly attenuated and induces innate and protective immunity in mice. SD1 activates NF-κB and induces gamma interferon secretion. Early interaction of the SD1 mutant with intestinal epithelial cells was associated with ruffling of enterocytes. Invading bacteria were found inside Peyer's patches after inoculation.
Salmonella species dam mutants are highly attenuated for virulence and have been proposed as live vaccines (10, 11). The safety of dam mutants of Salmonella spp. is enhanced by the inability of the microorganisms to invade enterocytes or to be toxic to M cells of ileal Peyer's patches (7). These features, however, together with the fact that dam mutants present a defective induction of inducible nitric oxide (NO) synthase and gamma interferon (IFN-γ) (22), would limit their use as bacterial carriers or delivery systems.
In the last few decades, Salmonella enterica serovar Enteritidis has emerged as a major cause of food-borne illness worldwide; in Argentina, for instance, the proportion of salmonellosis cases attributed to this pathogen showed a 275-fold increase in that time (12, 15, 18). In contrast, few studies using Salmonella serovar Enteritidis dam mutants as vaccine strains have been published. Earlier, we obtained a dam insertion mutant of Salmonella serovar Enteritidis named SD1. The insertion dam-231::Tn10dTet rendered in the SD1 mutant a functional (but defective) Dam that was 10 amino acids shorter than the native protein (3; M. N. Giacomodonato, S. H. Sarnacki, F. Sisti, R. Caccuri, and M. C. Cerquetti, Am. Soc. Microbiol. Conf. Salmonella: pathogenesis, epidemiology, and vaccine development, abstr. 106(A) p. 69, 2003). Some differences were found between the null dam mutant TT11694 of Salmonella serovar Typhimurium and the SD1 strain (Table 1). Filamentation and sensitivity to 2-aminopurine were observed in the SD1 mutant only at 37°C, whereas the TT11694 strain filamented and was sensitive to 2-aminopurine regardless of the incubation temperature. Here, we investigated the ability of the temperature-sensitive dam mutant of Salmonella serovar Enteritidis SD1 to interact, in vivo, with the intestinal mucosa and to induce protective immunity in mice.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| Salmonella serovar Enteritidis wild-type strain 5694 | Wild type | M. C. Cerquetti et al. (4) |
| SD1 | dam-231::Tn10dTet in wild-type strain 5694 | This study |
| SentΔdam | damΔ231 in wild-type strain 5694 | This study |
| Salmonella serovar Typhimurium TT11694 | LT2 dam-10d::MudJ | Salmonella Genetic Stock Centre |
The SD1 mutant induces early host responses in the murine model.
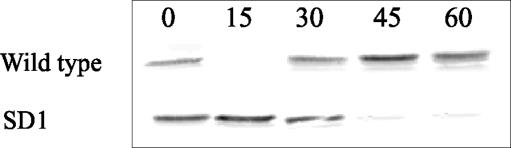
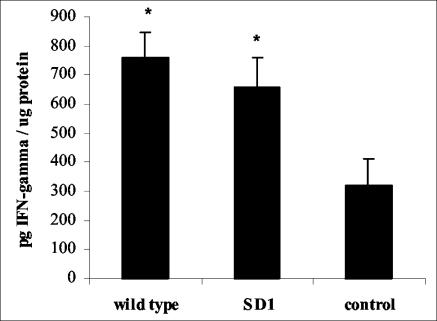
To determine whether the SD1 mutant was able to induce early responses in murine intestines, short-term experiments using an ileal loop (13) were performed. Infection with the wild-type strain of Salmonella serovar Enteritidis resulted in the rapid but transient degradation of IκB-α. Similarly, SD1 inoculation resulted in IκB-α degradation, although it was slower degradation than that seen with the wild-type strain (Fig. 1). The cytokines secreted 60 min after bacterial inoculation were determined by enzyme-linked immunosorbent assay. The SD1 mutant was able to induce significantly higher levels (P < 0.05) of IFN-γ in the gut early after inoculation (660 ± 101 pg/μg of protein) than levels induced in control mice (mean ± standard deviation, 320 ± 91 pg/μg of protein). No significant differences were found between the amounts of IFN-γ induced by the wild-type strain (762 ± 87 pg/μg of protein) and those induced by the SD1 mutant (Fig. 2).
FIG. 1.
Western blotting analysis showing IκB-α degradation induced by the SD1 mutant and the wild-type strain in the ligated ileal loop. Mice were sacrificed 15, 30, 45, or 60 min after bacterial inoculation. Ileal loops inoculated with physiologic solution were used as controls (time zero). Results are representative of three separate experiments.
FIG. 2.
IFN-γ induced by the SD1 mutant. Murine ileal loops were inoculated with 108 CFU of the SD1 mutant or the wild-type strain of Salmonella serovar Enteritidis. The gut loops were removed 60 min after inoculation, and the production of IFN-γ was measured by enzyme-linked immunosorbent assay. Data are means (five mice). Asterisks indicate significant differences (P < 0.05) from results for the control loop. Results are representative of two separate experiments. The Student t test was used to compare mean values.
Two of the host responses that follow wild-type Salmonella sp. infection are the activation of macrophages and the concomitant release of NO. In this work, NO in plasma was quantified by using the Griess reaction (8). We found that mice inoculated intraperitoneally with 104 CFU of the SD1 mutant showed a delayed increase in plasma NO compared to the plasma NO levels of mice inoculated with the wild-type strain (Table 2). Two days after inoculation, the virulent strain induced significantly higher levels (P < 0.01) of NO than levels in either control mice or animals inoculated with SD1. A significant (P < 0.05) elevation of nitrite levels in plasma was found by day 5 postinoculation in mice receiving the SD1 mutant compared with levels in control mice (by this time point, all mice inoculated with the wild-type strain were dead). It is well documented that the release of cytokines, such as IFN-γ, interleukin 12, and tumor necrosis factor alpha, enhances early innate immunity (23) and thereafter creates an inflammatory context that favors the maturation of dendritic cells to have an antigen-presenting function (2). Also, IFN-γ-inducible proteins, like inducible NO synthase and the class II transactivator protein, regulate, respectively, the production of the antimicrobial agent NO and the induction of major histocompatibility complex class II molecules that facilitate the ability to present processed microbial antigens (19, 21). Thus, the benefit of using attenuated Salmonella sp. strains able to induce proinflammatory cytokines as bacterial carriers is that they may function as natural adjuvants (16).
TABLE 2.
NO levels induced in plasma by the SD1 mutanta
| Time of collection of plasma | Nitrite production (μM)
|
||
|---|---|---|---|
| SD1 | Wild type | Control | |
| Day 2 | 8.5 ± 0.20 | 33.4 ± 1.89 | 12.4 ± 0.39 |
| Day 5 | 23.4 ± 0.87 | NSb | NDc |
Mice were inoculated intraperitoneally with physiologic solution (control) or with 104 CFU of SD1 or the wild-type strain of Salmonella serovar Enteritidis. Blood was collected by cardiac puncture at days 2 and 5 after infection, and nitrite production was measured in plasma with Griess reagent. Data are means ± standard deviations of results for five samples.
NS, no survivors.
ND, not determined.
The role of NO in host immunity against Salmonella spp. is controversial; it mediates immunosuppression but at the same time is crucial in protection against even some attenuated Salmonella sp. strains. Studies performed by Eisenstein and colleagues found that NO induced by attenuated mutants of Salmonella spp. correlated with both immunosuppression and protection, although in one case, at least, protection occurred without NO induction (6, 14, 20). It was demonstrated earlier that the ability of attenuated Salmonella sp. strains to induce intestinal NO and apoptosis at the time of immunization correlates with the induction of a protective immune response (4).
Knockout dam mutants of Salmonella serovar Typhimurium show defects in several virulence-related traits, such as the ability to invade the intestinal epithelium and toxicity to M cells (7). Our results indicate that the temperature-sensitive dam mutant SD1 induces innate immunity in the gut.
The SD1 mutant is capable of invading the intestinal mucosa.
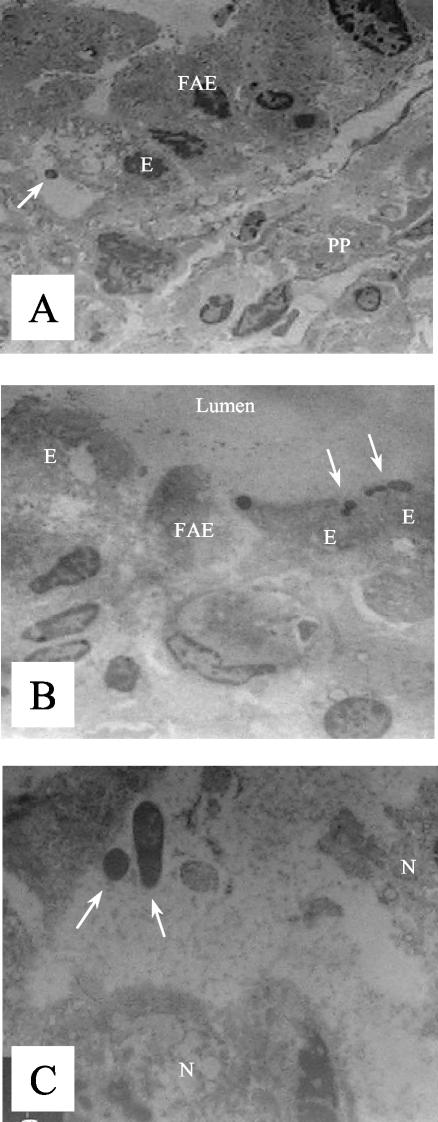
Electron microscopy revealed that the SD1 mutant induces cytotoxicity in the intestinal epithelium soon after inoculation into the ileal loop. Bacterial attachment was often associated with ruffling of the apical cell surface (Fig. 3A). Moreover, the mutant was found both at the apical side of the epithelial cells (Fig. 3B) and inside the Peyer's patches (Fig. 3C) within 75 min following inoculation. Cytoplasmic rarefaction was observed in many enterocytes (Fig. 3B), and signs of necrobiosis appeared in Peyer's patches (Fig. 3C). Almost all bacteria observed within the Peyer's patches had an extracellular location. These features are essential for bacterial carriers. In this regard, Darji et al. (5) have demonstrated that increasing the invasiveness of an attenuated Salmonella serovar Typhimurium resulted in a stronger immune response.
FIG. 3.
Transmission electron micrographs taken 75 min postinfection with the SD1 mutant. (A) Bacteria (arrow) in contact with an enterocyte with ruffling formation. Magnification, ×3,000. (B) Invading bacteria (arrows) within the apical side of enterocytes. Magnification, ×3,000. (C) Bacteria (arrows) inside a Peyer's patch. Magnification, ×16,000. E, enterocyte; FAE, follicle-associated epithelium; PP, Peyer's patches; N, nucleus.
The SD1 mutant is highly attenuated for virulence in mice.
Salmonella sp. dam mutants are highly attenuated and have been proposed as live vaccines (11). To examine whether the temperature-sensitive dam mutant SD1 was attenuated for virulence, intraperitoneal and intragastric 50% lethal doses (LD50s) were calculated by the method of Reed and Muench (17). Mice were inoculated intragastrically or intraperitoneally with different doses of the SD1 mutant or the wild-type strain of Salmonella serovar Enteritidis. Results showed that in the SD1 mutant, the lack of the last 10 amino acids of the Dam protein decreases in 4 log units the LD50s of the wild-type strain of Salmonella serovar Enteritidis. The LD50s for intraperitoneal inoculation were >105 CFU and <10 CFU for SD1 and the wild type, respectively. For intragastric inoculation, the LD50s were >109 CFU for SD1 and 1.7 × 104 CFU for the wild type. Similar attenuation was found with the deletion mutant SentΔdam.
The attenuation of SD1 may be due in part to the increased sensitivity of the mutant to some components of the innate immunity. Virulent Salmonella spp. are highly resistant to bile (9). Like other dam mutants, SD1 showed increased sensitivities to deoxycholate and ox bile extracts compared with those of the wild-type strain (Table 3). Regarding the MIC of hydrogen peroxide (1), we found that the SD1 mutant is more labile than the wild-type strain (27 mM versus 55 mM).
TABLE 3.
The SD1 mutant is sensitive to detergents and hydrogen peroxide at 37°Ca
| Bacterial strain | % of growth inhibition on:
|
MIC of H2O2 (mM)d | |
|---|---|---|---|
| 0.5% Deoxycholateb | 0.85% Ox bilec | ||
| SD1 | 86 | 98 | 27 |
| Wild type | 0 | 0 | 55 |
No significant differences in sensitivity were found when experiments were performed at 28°C.
Salmonella serovar Enteritidis strains were grown to log phase in Trypticase soy broth (TSB), centrifuged, and suspended in physiologic solution. Appropriated dilutions were then placed onto Trypticase soy agar with different concentrations of deoxycholate.
Salmonella serovar Enteritidis strains were grown in TSB and plated on different concentrations of ox bile extracts.
Salmonella serovar Enteritidis strains were grown to log phase in TSB and incubated with hydrogen peroxide for 18 h at 37°C.
Immunization with the SD1 mutant induces protection against the wild-type strain.
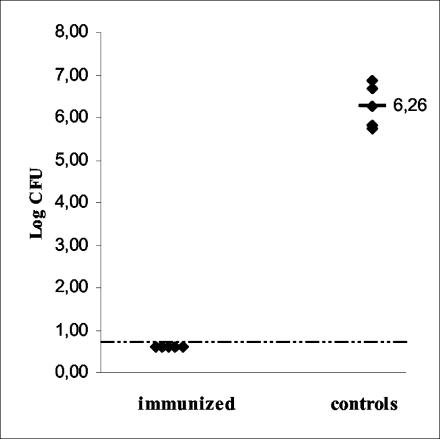
The capacity of the SD1 mutant to generate protective immunity was assessed in a murine model. Mice were immunized with two oral doses (a week apart) of 6 × 109 CFU of the SD1 mutant per animal. Nonimmunized animals were included as the control group. Twenty-one days later, mice were challenged orally with 3 × 105 CFU of the wild-type strain of Salmonella serovar Enteritidis per animal. Five days after the challenge, the numbers of virulent bacteria remaining in the spleens were determined. The results showed that immunization with the SD1 mutant dramatically improves the clearance of the wild-type strain from the spleen (Fig. 4). The numbers of bacteria recovered from immunized mice were under the level of detection (<5 CFU/organ). The median log number of CFU per organ calculated for control animals was 6.26 (range, 5.72 to 6.87 CFU). This finding indicates that the temperature-sensitive dam strain SD1 confers protection against homologous challenge.
FIG. 4.
Clearance. Groups of five mice were immunized with two oral doses (a week apart) of 6 × 109 CFU of the SD1 mutant per animal. Twenty-one days later, mice were challenged orally with 3 × 105 CFU of the wild-type strain of Salmonella serovar Enteritidis per animal. Five days after the challenge, mice were sacrificed and their spleens were removed. Appropriate dilutions were plated on Trypticase soy agar for determination of colony counts. The dotted line represents the limit of detection (<5 CFU/organ). Median values were compared by using the Mann-Whitney unpaired test for nonparametric samples. The difference between results for immunized mice and for the control group was significant (P = 0.0159).
In summary, the deletion of the last 10 amino acids produced a Dam protein with a temperature-sensitive phenotype in Salmonella serovar Enteritidis. The SD1 dam mutant is capable of interacting with the intestinal mucosa and of inducing innate immunity in mice. Although SD1 invades Peyer's patches early after infection, the mutant is highly attenuated; moreover, SD1 induces protective immunity in the murine model. This temperature-sensitive dam strain appears to be a promising bacterial carrier and deserves further investigation.
Acknowledgments
We thank María Isabel Bernal for excellent technical assistance.
This work was supported in part by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina; the Universidad de Buenos Aires, Buenos Aires, Argentina (UBACyT M037); and the Fundación Antorchas, Buenos Aires, Argentina.
Editor: F. C. Fang
REFERENCES
- 1.Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Brawer, R., B. F. D. Burrone, D. O. Sordelli, and M. C. Cerquetti. 1998. A temperature-sensitive DNA methyltransferase of Salmonella typhimurium. Arch. Microbiol. 169:530-533. [DOI] [PubMed] [Google Scholar]
- 4.Cerquetti, M. C., N. B. Goren, A. J. Ropolo, D. Grasso, M. N. Giacomodonato, and M. I. Vaccaro. 2002. Nitric oxide and apoptosis induced in Peyer's patches by attenuated strains of Salmonella enterica serovar Enteritidis. Infect. Immun. 70:964-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darji, A., S. zur Lage, A. I. Garbe, T. Chakraborty, and S. Weiss. 2000. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol. Med. Microbiol. 27:341-349. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein, T. K., J. J. Meissler, Jr., S. I. Miller, and B. A. D. Stocker. 1998. Immunosuppression and nitric oxide production induced by parenteral live Salmonella vaccines do not correlate with protective capacity: a phoP::Tn10 mutant does not suppress but does protect. Vaccine 16:24-32. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:11578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granger, D. L., J. B. Hidds, J. R. Perfect, and D. T. Durack. 1990. Metabolic fate of l-arginine inhalation to microbiostatic capability of murine macrophages. J. Clin. Investig. 85:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn, J. S. 2000. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2:907-913. [DOI] [PubMed] [Google Scholar]
- 10.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 12.Hogue, A., P. White, J. Guard-Petter, W. Schlosser, R. Gast, E. Ebel, J. Farrar, T. Gomez, J. Madden, M. Madison, A. M. McNamara, R. Morales, D. Parham, P. Sparling, W. Sutherlin, and D. Swerdlow. 1997. Epidemiology and control of Salmonella enteritidis in the United States of America. Rev. Sci. Tech. Off. Int. Epizoot. 16:542-553. [DOI] [PubMed] [Google Scholar]
- 13.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacFarlane, A. S., M. G. Schwacha, and T. K. Eisenstein. 1999. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophages and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 67:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales, R. A., and R. M. McDowell. 1999. Economic consequences of Salmonella enterica serovar Enteritidis infection in humans and the U.S. egg industry, p. 271-290. In A. M. Saeed, R. K. Gast, M. E. Potter, and P. G. Wall (ed.), Salmonella enterica serovar Enteritidis in humans and animals. Iowa State University Press, Ames.
- 16.Paglia, P., E. Medina, I. Arioli, C. A. Guzman, and M. P. Colombo. 1998. Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella serovar Typhimurium, results in protective immunity against a murine fibrosarcoma. Blood 92:3172-3176. [PubMed] [Google Scholar]
- 17.Reed, L. J., and H. Muench. 1935. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 18.Rodrigue, D. C., R. V. Tauxe, and B. Rowe. 1990. International increase in Salmonella enteritidis: a new pandemic? Epidemiol. Infect. 105:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwacha, M. G., J. J. Meissler, Jr., and T. K. Eisenstein. 1998. Salmonella typhimurium infection in mice induces nitric oxide-mediated immunosuppression through a natural killer cell-dependent pathway. Infect. Immun. 66:5862-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shtrichman, R., and C. E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 22.Shtrichman, R., D. M. Heithoff, M. J. Mahan, and C. E. Samuel. 2002. Tissue selectivity of interferon-stimulated gene expression in mice infected with Dam+ versus Dam− Salmonella enterica serovar Typhimurium strains. Infect. Immun. 70:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson, M., R. Seymour, and B. Henderson. 1998. Bacterial perturbation of cytokine networks. Infect. Immun. 66:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]