Abstract
Lipid rafts are highly ordered, cholesterol-rich, and detergent-resistant microdomains found in the plasma membrane of many eukaryotic cells. These domains play important roles in endocytosis, secretion, and adhesion in a variety of cell types. The parasitic protozoan Entamoeba histolytica, the causative agent of amoebic dysentery, was determined to have raft-like plasma membrane domains by use of fluorescent lipid analogs that specifically partition into raft and nonraft regions of the membrane. Disruption of raft-like membrane domains in Entamoeba with the cholesterol-binding agents filipin and methyl-β-cyclodextrin resulted in the inhibition of several important virulence functions, fluid-phase pinocytosis, and adhesion to host cell monolayers. However, disruption of raft-like domains did not inhibit constitutive secretion of cysteine proteases, another important virulence function of Entamoeba. Flotation of the cold Triton X-100-insoluble portion of membranes on sucrose gradients revealed that the heavy, intermediate, and light subunits of the galactose-N-acetylgalactosamine-inhibitible lectin, an important cell surface adhesion molecule of Entamoeba, were enriched in cholesterol-rich (raft-like) fractions, whereas EhCP5, another cell surface molecule, was not enriched in these fractions. The subunits of the lectin were also observed in high-density, actin-rich fractions of the sucrose gradient. Together, these data suggest that pinocytosis and adhesion are raft-dependent functions in this pathogen. This is the first report describing the existence and physiological relevance of raft-like membrane domains in E. histolytica.
Recent evidence suggests that plasma membrane lipids are nonhomogeneously distributed and that microdomains with specialized functions exist in the membrane. One such domain, a lipid raft, is a highly ordered, less-fluid, and tightly packaged membrane domain enriched in cholesterol (or other sterols), glycosphingolipids, and phospholipids with a higher degree of saturated fatty acyl chains than those of the rest of the membrane (reviewed in references 37 and 54). These domains are also detergent insoluble and are thus often referred to as detergent-resistant membranes (DRMs). The presence of microdomains in membranes allows for the inclusion or exclusion of membrane proteins based on their attachment to the membrane via lipid anchors or specific protein-lipid interactions. For example, proteins that are modified with a hydrophobic attachment, such as a glycosylphosphatidylinositol (GPI) anchor, or double acylation or transmembrane proteins with the capacity to interact with cholesterol are often found in lipid microdomains.
The physiological role of lipid rafts has been the subject of numerous recent studies, and it has recently become clear that these membrane regions play an important role in a variety of cellular functions, including polarization, signal transduction, endocytosis, secretion, and cell-cell and cell-pathogen adhesion (17, 18, 21, 34, 36, 44). A range of cell surface receptors mediating signal transduction pathways through lipid rafts have been described, including the FcɛRI, T-cell, B-cell, epidermal growth factor, and Hedgehog receptors, as well as integrins (reviewed in reference 54). Typically, these receptors stably associate with the raft only after ligand binding. Once in the microdomain, the receptor cluster either recruits or encounters other signaling proteins triggering the signaling cascade (54).
Endocytic mechanisms may also rely on lipid rafts. For example, depletion of cholesterol from the membrane results in the inhibition of pinocytosis (23, 24), particularly caveolin- and clathrin-coated pit internalization (59, 67). More recently, a requirement for cholesterol has been identified for macropinocytosis in A431 epidermoid carcinoma cells (17). Interestingly, phagocytosis does not appear to be dependent on intact rafts (46).
Several studies also suggest a role for lipid rafts in secretion. Wang et al. (70) determined that cholesterol depletion in AtT-20 tumor cells blocks constitutive and regulated secretory vesicle formation while Martin-Belmonte et al. (36) demonstrated that the depletion of cholesterol results in the failure of Madin-Darby canine kidney (MDCK) cells to traffic exogenously expressed thyroglobulin, a principal secretory protein of thyroid epithelial cells. Accumulation of secretory proteins in the trans-Golgi network after cholesterol depletion suggests a loss in the ability to properly form secretory vesicles in depleted cells. By deduction, delivery of plasma membrane-resident proteins may also be inhibited by cholesterol depletion. In support of this, yeast mutants that were incapable of synthesizing sphingolipids and ergosterol were deficient in trafficking the plasma membrane-associated proteins Gas1p and Pma1p (3).
Lipid rafts are also important in regulating and maintaining cell-cell and cell-matrix adhesions. For example, the slime mold Dictyostelium discoideum adhesion molecule gp80 associates with raft-like microdomains (21, 22). In addition, in activated T cells, lipid rafts have been shown to segregate proteins, such as cell adhesion molecules, including the β2 integrin LFA-1 (33). These data suggest that microdomains may also regulate integrin activity.
Entamoeba histolytica is a protozoan parasite that is the causative agent of 50 million cases of invasive amebiasis (reviewed in reference 57). The parasite, initially ingested as an environmentally stable cyst via contaminated food or water, passes through the stomach and excysts in the small intestine, releasing multiple amoeboid trophozoites. The amoebae move to the large intestine, where they rely on the endocytic pathway to obtain nutrients. Fluid-phase pinocytosis (1), phagocytosis (42), and receptor-mediated endocytosis (4, 47) have been described for this organism. Although little is known about the lipids or proteins that participate in endocytosis in Entamoeba, it has been demonstrated that a Rab7-like GTPase, EhRab7, associates with early pinosomes (71). Constitutive secretion of cysteine proteases from the pathogen, which also occurs during infection, participates in host cell destruction. Twenty cysteine protease genes have been isolated from Entamoeba (6), and it has been demonstrated that EhRab7 colocalized in compartments that harbor a well-characterized 27-kDa cysteine protease (71). Like for endocytosis, the lipids or proteins that participate in secretion of hydrolases from Entamoeba are not well characterized.
Virulence also relies on host cell contact, which triggers the regulated secretion of pore-forming peptides known as amoebapores (32). Adhesion to intestinal cells is mediated by a multisubunit lectin with specific affinity for galactose (Gal) or N-acetyl-d-galactosamine (GalNAc) (35). The adhesion lectin is comprised of a transmembrane heavy subunit (Hgl; 170 kDa) disulfide linked to a GPI-anchored light subunit (Lgl; 31 to 35 kDa) (14, 35). This heterodimer associates noncovalently with a GPI-anchored intermediate subunit (Igl; 150 kDa) (9, 43). Hgl has been shown to contain a carbohydrate recognition domain specific for Gal/GalNAc and, more recently, a cytoplasmic tail with sequence homology to those of β2 and β7 integrins (14, 68). A study involving a mutagenized heavy subunit of the Gal/GalNAc lectin has demonstrated the importance of the cytoplasmic tail of this subunit for inside out signaling (68). Integrins are known to bind to actin through several actin-binding proteins, and actin may also play a role in the adhesion of Entamoeba to host cells (7). For example, actin is localized to pathogen-host contact sites (66). Moreover, it is postulated that the Gal/GalNAc adherence lectin interacts with actin and stimulates pathways that induce actin polymerization (15).
Since Entamoeba membranes contain cholesterol (64), it is conceivable that raft-like domains exist in the plasma membrane of this organism. Therefore, we have conducted a study to identify and characterize raft-like domains in this pathogen. Treatment of Entamoeba cells with raft-disrupting agents demonstrated the importance of cholesterol in pinocytosis and adhesion of the parasite to a host-cell monolayer. Moreover, the isolation of DRMs revealed that the lectin heavy, intermediate, and light subunits were partially enriched in these microdomains. These results are the first to demonstrate the existence of raft-like microdomains in Entamoeba and illustrate the role of these microdomains in the virulence functions of this pathogen.
MATERIALS AND METHODS
Strains and culture conditions.
E. histolytica trophozoites, strain HM-1:IMSS, were cultured axenically in TYI-S-33 in screw-cap glass tubes at 37°C (13). Chinese hamster ovary (CHO) cells were cultured at 37°C in 25-cm2 angle-necked cell culture flasks in F-12K nutrient medium (Gibco, Carlsbad, Calif.) supplemented with fetal bovine serum (10% vol/vol), 7.5% sodium bicarbonate (2% vol/vol), and penicillin-streptomycin (1% vol/vol).
Lipid microdomain disruption.
Raft-like microdomains were chemically disrupted by depleting cholesterol with methyl-β-cyclodextrin (MBCD) (Sigma, St. Louis, Mo.) or by sequestering cholesterol with filipin (Fluka, Seelze, Germany). In all cases, MBCD was dissolved in TYI-33 medium (TYI-S-33 medium without serum) at the appropriate concentration; filipin was stored as a stock solution in ethanol (5 mg/ml) and diluted appropriately in medium as required (see below). Cells were treated for 30 min at 37°C with either MBCD (7.5 or 15 mM) or filipin (3.8 μM). For all experiments, mock-disrupted controls were utilized.
Fluorescent lipid analog staining.
To stain raft and nonraft regions of the membrane, Entamoeba cells were allowed to adhere to a two-well coverslip slide (Lab-Tek, Christchurch, New Zealand) for 2 h in serum-free medium at 37°C. In some trials, rafts were disrupted by also treating cells with MBCD (7.5 mM) or filipin (3.8 μM) during the last 30 min of incubation. The medium was removed, and the cells were incubated with dialkyindocarbocyanine (DiIC16), 1.1 μM; (Molecular Probes, Eugene, Oreg.) or 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarboxyanine (FAST-DiI, 0.9 μM; Molecular Probes) for 2 min at room temperature. The cells were then fixed with 4% (vol/vol) paraformaldehyde (10 min at room temperature). The slides were then rinsed twice with phosphate-buffered saline (PBS), mounted in SlowFade antifade reagent in PBS (Molecular Probes), and viewed on a Zeiss LSM 510 confocal microscope.
Measurement of fluid-phase pinocytosis.
Log-phase Entamoeba cells were iced for 10 min to remove them from the glass, pelleted by centrifugation (500 × g for 5 min), and resuspended in TYI-33 medium prewarmed to 37°C. Cells were then dispensed into 4-ml glass vials and allowed to recover at 37°C for 3 h. To carry out cholesterol depletion, additional prewarmed TYI-33 medium supplemented with MBCD (7.5 mM) or prewarmed TYI-33 medium alone (control) was added to the cells during the last 30 min of recovery. After the 30-min treatment, concentrated fluorescein isothiocyanate dextran (Sigma), diluted in prewarmed TYI-33 medium, was added to the cells at a final concentration of 2 mg/ml. At each time point (0 or 60 min), the cells were iced as described above, collected by centrifugation (500 × g, 1 min), and washed twice in ice-cold PBS. The cells were stored on ice as pellets. Ice-cold PBS was then added to each pellet, 10% of each sample was removed, and total protein was measured by using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, Ill.) according to the manufacturer's instructions. The remaining cells were lysed in 10% (vol/vol) Triton X-100 (Sigma) in PBS. Intracellular fluorescence was measured by using an FLx800 microplate fluorescence reader (Bio-Tek, Winooski, Vt.) with excitation and emission wavelengths of 485 and 528 nm. Fluorescence was corrected for autofluorescence by using the 0-min sample. Pinocytosis is reported as the fluorescence per milligram of protein.
Measurement of cysteine protease secretion.
Secretion of cysteine protease was measured according to the method of Leippe et al. (32). Briefly, confluent Entamoeba cultures were iced to remove the cells from the glass, centrifuged (500 × g, 5 min), and resuspended in 1 ml of TYI-33 in which the phosphates had been replaced with 10 mM HEPES and supplemented with 0.15 mM CaCl2 and 0.5 mM MgCl2. To establish values for maximum release of protease, 0.4 ml of the suspension was withdrawn and subjected to three freeze-thaw cycles. The lysates were cleared by centrifugation (12,000 × g, 5 min) and stored at −80°C until the enzyme activity was measured. The remaining cell suspensions were placed in a 37°C water bath. For raft disruption prior to measurement of secretion, cells were allowed to recover for 30 min prior to the addition of MBCD, filipin, or ethanol (diluent control for filipin) as described above. At 0, 30, 60, 90, 120, and 180 min, samples of each suspension were collected and cell viability was estimated by trypan blue exclusion (0.5 mg/ml). Cells and supernatants were separated by centrifugation (500 × g, 5 min). Supernatants were removed and placed on ice until used for the activity assay. The peptide substrate, benzyloxycarbonyl-l-arginyl-l-arginine-p-nitroaniline, was diluted from a stock solution (10 mM in 90% dimethyl sulfoxide) to 0.1 mM in 0.1 M KH2PO4 and 2 mM EDTA (pH 7.0 with KOH). A 1:200 ratio of sample to peptide was added to the wells of a 24-well plate and incubated for 10 min at 37°C. Accumulation of free p-nitroaniline (yellow) was measured by using a μQuant plate reader (Bio-Tek Instruments) at 405 nm. The results were reported as percentages of the maximum release.
Adhesion of E. histolytica to CHO cell monolayer.
Entamoeba adhesion was measured as described by Padilla-Vaca et al. (42). Briefly, CHO cells were plated and grown to confluence in a 24-well culture plate. The CHO cells were fixed in 4% paraformaldehyde to prevent cytolysis, washed twice with PBS, incubated in 200 mM glycine, and washed twice more in PBS. Control or raft-disrupted (15 mM MBCD, 3.8 μM filipin, or diluent control [0.0475% ethanol]) Entamoeba (in TYI-33) cells (104) were added to each well containing CHO cells and incubated for 30 min at 37°C. At the end of the incubation, the wells were gently washed twice with prewarmed medium to remove nonadherent trophozoites. The number of adherent Entamoeba cells from 90 fields of view were counted at a magnification of ×40 on an Olympus CK2 inverted microscope.
Purification of detergent-resistant membranes and sucrose density centrifugation.
Typically, 4 × 106 Entamoeba cells were centrifuged (500 × g for 5 min), resuspended in ice cold buffer 1 with protease inhibitors (40 mM sodium pyrophosphate, 0.4 mM dithiothreitol, 0.1 mg of phenylmethylsulfonyl fluoride/ml, 2 mM EDTA, 1 mM EGTA, 3 mM sodium azide, 10 mM Tris-HCl [pH 7.6]) containing 0.5% Triton X-100 at 4°C for 30 min and then centrifuged (14,000 × g for 5 min) at 4°C. The Triton-soluble supernatant (TSS) was removed, and the Triton-insoluble pellet (TIP) was resuspended in 80% (wt/wt) sucrose in buffer 1. A noncontinuous sucrose gradient was generated by using equal volumes of 80 (containing the TIP), 50, 30, and 10% (wt/wt) sucrose solutions in buffer 1. Samples were then centrifuged in a Beckman TL-100 ultracentrifuge (125,000 × g for 16 h) at 4°C. After centrifugation, the gradient was fractionated into 20 equal volumes (140 μl/fraction). A sample was immediately removed from each fraction, and the proteins were precipitated by the addition of trichloroacetic acid as described elsewhere (72). The precipitated proteins were resuspended in double-distilled H2O and mixed with 4× LDS buffer (Invitrogen, Carlsbad, Calif.) and 2-mercaptoethanol (10% [vol/vol] final concentration). Samples were stored at −20°C and used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as described below. The remainder of the sample was used to quantify cholesterol, sucrose, and protein levels from fractions as described below.
SDS-PAGE and Western blot analysis.
SDS-PAGE and Western blot analyses were performed as described previously by Welter et al. (71). Protein samples from each fraction and the TSS were prepared as described above, loaded onto the wells of a 4 to 12 or 12% polyacrylamide gel (Invitrogen), electrophoresed at 200 V for 1 h, and transferred to a polyvinylidene difluoride membrane (Invitrogen) in Towbin buffer (62) for 1 h at 100 V. Blotted membranes were decorated with primary antibodies specific for the 170-kDa heavy subunit (polyclonal, 1:5,000 dilution), the 150-kDa intermediate subunit (polyclonal, 1:2,000 dilution), the 31- to 35-kDa light subunit (monoclonal, 1:4,000 dilution) (generous gifts of W. Petri, University of Virginia, Charlottesville), the membrane-bound cysteine protease EhCP5 (polyclonal, 1:1,333 dilution) (generous gift of Matthius Leippe, Research Center for Infectious Disease, Würzburg, Germany) (9, 25), or a commercially available pan-actin primary antibody (monoclonal, 1:800 dilution) (Novus Biologicals, Littleton, Colo.). Immunoblots were visualized by using the appropriate peroxidase-conjugated secondary antibody (1:5,000 dilution for goat anti-rabbit; 1:2,000 dilution for goat anti-mouse) (Cappel; ICN Pharmaceuticals, Costa Mesa, Calif.) and the enhanced chemiluminescence Western blotting detection system (Amersham Biosciences, Piscataway, N.J.) according to the manufacturer's instructions. Alternately, samples from each fraction were electrophoresed and silver stained according to the manufacturer's instructions (Pierce).
Sucrose gradient fraction characterization.
Each fraction from the sucrose gradient was analyzed to determine the relative level of cholesterol and protein and the percentage of sucrose. Cholesterol quantification was performed by using the Amplex Red cholesterol assay kit (Molecular Probes) according to the manufacturer's instructions. Levels of cholesterol were reported as fluorescent units (fl) of cholesterol per microgram of protein. Protein was measured by using the bicinchoninic acid protein assay kit (Pierce) according to the manufacturer's instructions. Sucrose levels were determined by refractometry by using an ADP220 polarimeter (Bellingham and Stanley, Inc., Lawrenceville, Ga.) according to the manufacturer's instructions.
Statistical analysis.
Unpaired t tests were performed with the computer program GraphPAD Instat (version 3.05; IBM). All values are represented as the means of the results from at least three trials (± standard deviations [SD]).
RESULTS
The plasma membrane of E. histolytica contains both raft and nonraft domains.
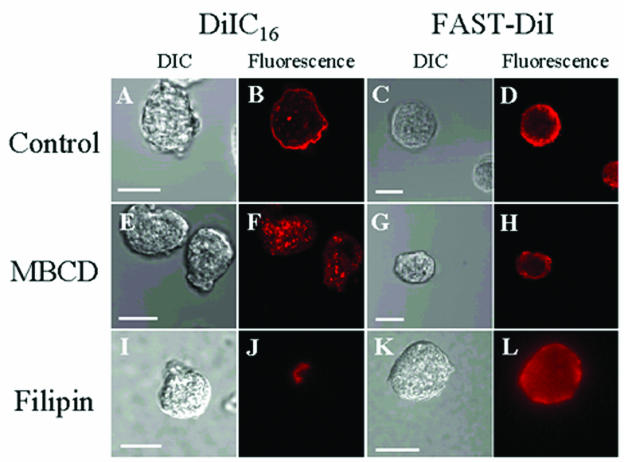
To determine whether Entamoeba cells possess highly ordered raft-like microdomains and to distinguish them from more-fluid-phase membrane regions, we stained cells with either the order-preferring lipid analog DiIC16 or the non-order-preferring lipid analog FAST-DiI with or without chemical disruption of rafts (Fig. 1). Although DiIC16 can diffuse into nonraft regions (56), it is commonly used as a marker for raft-like membranes because of its ability to partition into ordered domains due to its saturated acyl chains (16-carbon chain with no double bonds) (11, 19, 40, 53, 60). On the other hand, FAST-DiI, which possesses unsaturated fatty acyl chains (18-carbon chain with two double bonds), resides exclusively in the fluid domain of the membrane (11, 40). These lipid analogs are appropriate for studies in this system, as their fatty acid chain lengths and levels of saturation are comparable to major components of the nascent fatty acids found in Entamoeba cells (12). Both lipid analogs were localized to the plasma membrane and some intracellular structures in Entamoeba cells after 2 min of staining (Fig. 1B and D). The similar staining pattern observed for the two dyes may be the result of a uniform distribution of both domain types on the plasma membrane. This is consistent with the notion that a large proportion of the plasma membrane is in fact DRM-like in nature (19, 40). Alternatively, this pattern may simply be the result of association of both fluorophores with fluid domains and/or the limitations in the resolution of light microscopy. Several other studies have reported similar staining patterns with comparable fluorescent lipid analogs (19, 20, 53). To test this, cells were treated with a known raft-disrupting agent, MBCD or filipin. MBCD has been widely used to remove cholesterol selectively from the membranes of cells, thereby disrupting membrane rafts. The concentration of MBCD utilized was similar to that used elsewhere (19, 33, 44, 65). Filipin, a cholesterol-sequestering agent, also disrupts membrane rafts by coalescing cholesterol in the plasma membrane. Since lipid microdomains are known to require cholesterol, depletion or sequestration of cholesterol is commonly interpreted to result in the disruption or destabilization of such domains (19). Treatment with MBCD abolished staining in the plasma membrane by DiIC16 (Fig. 1F). Alternately, treatment with filipin resulted in alteration in the DiIC16 staining pattern such that fluorescence was observed in a single region after treatment. Both staining patterns are consistent with the proposed actions of these agents (Fig. 1J). As expected, the raft-disrupting agents did not affect FAST-DiI staining (Fig. 1H and L). Together, these data authenticate the colocalization of DiIC16 with cholesterol-rich membrane regions and suggest the existence of raft-like domains in Entamoeba cells. The significant reduction in visible staining of the plasma membrane with DiIC16 after MBCD treatment suggests that the majority of DiIC16 resided in raft-like microdomains. The increased intensity of staining of intracellular structures observed in Fig. 1F (compared to Fig. 1B) is due to the increased microscope laser power utilized to ensure that plasma membrane staining was absent. However, filipin was able to disrupt intracellular staining by DiIC16 (Fig. 1J), indicating that putative raft-like domains may also be present on the intracellular structures. This is consistent with several previous reports that have demonstrated the existence of raft-like domains on intracellular structures, such as the Golgi apparatus, and secretory vesicles in other cells (36, 55).
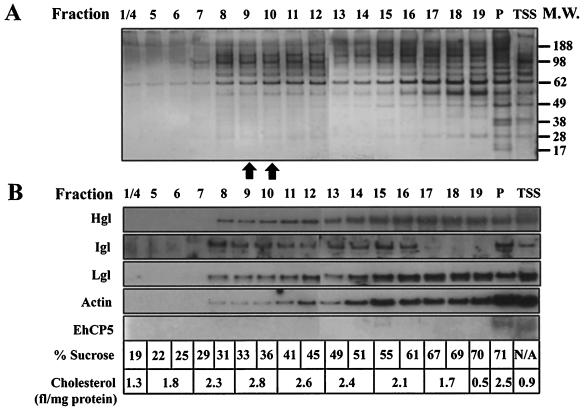
FIG. 1.
Fluorescence microscopy of Entamoeba stained with the fluorescent lipid analogues DiIC16 (raft) and FAST-DiI (nonraft). Both stains were detected in the plasma membrane in untreated control cells and in intracellular structures (B and D). Treatment of cells with the cholesterol-depleting agent MBCD or the cholesterol-sequestering agent filipin resulted in an altered staining pattern for DiIC16 (F and J) but not for FAST-DiI (H and L). Panels A, C, E, G, I, and K represent differential interference contrast (DIC) images. Scale bars, 20 μm.
Role of lipid microdomains in pinocytosis and secretion.
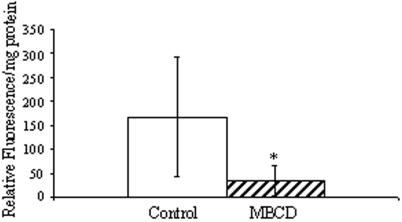
Raft-like microdomains are known to play an important role in pinocytosis and secretion. We sought to determine whether raft-like microdomains also play an important role in these processes in the pathogen. To examine fluid-phase pinocytosis, cells were pretreated with MBCD for 30 min and then exposed to a fluid-phase marker, fluorescein isothiocyanate-conjugated dextran. At 0 and 60 min, cells were collected and intracellular fluorescence was determined as a measure of pinocytosis. It was observed that pinocytosis was significantly inhibited in cells treated with MBCD, suggesting that, in Entamoeba cells, this process is dependent on the presence of cholesterol (Fig. 2). Pinocytosis was not analyzed for cells pretreated with filipin, as the diluent itself (ethanol) was found to inhibit fluid-phase pinocytosis (data not shown).
FIG.2.
Effect of lipid microdomain disruption on fluid-phase pinocytosis. Fluid-phase uptake was measured for cells that were not treated (control) or that were treated with 7.5 mM MBCD. The uptake of fluid-phase cells was significantly inhibited by treatment with MBCD. Means ± SD are shown (P < 0.05; n = 6 for MBCD treatment).
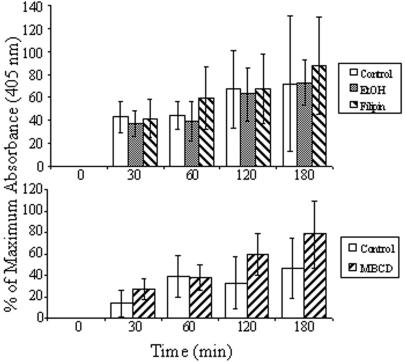
The role of raft-like domains in the constitutive secretion of cysteine proteases was also investigated with MBCD and filipin. Cells were treated with these agents, and the release of cysteine protease into conditioned medium was measured (Fig. 3). Neither cholesterol-binding agent significantly inhibited the ability of the cells to secrete cysteine proteases, suggesting that constitutive secretion of these proteases from Entamoeba cells is not a raft-dependent cellular function. For both treated and untreated cells, approximately 80% of cells remained viable during the experiments, as determined by trypan blue exclusion (data not shown). This suggests that any change in cellular function observed after treatment was a result of the physiological effects of cholesterol depletion from the plasma membrane and not cell death.
FIG. 3.
Effect of lipid microdomain disruption on secretion of cysteine proteases. The release of cysteine proteases was measured for cells that were not treated (white bars) and for cells treated with 7.5 mM MBCD (striped bars in bottom panel), 3.8 μM filipin (striped bars in top panel), or ethanol (0.0475% vol/vol; diluent control for filipin) (gray bars). The release of cysteine proteases was not inhibited by treatment with agents that disrupt lipid rafts. Means ± SD are shown (n = 4 for filipin and ethanol; n = 3 for MBCD).
Role of lipid microdomains in Entamoeba adhesion to epithelial cells.
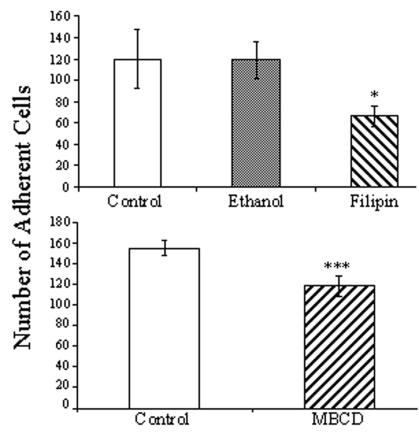
A critical event in Entamoeba pathogenesis is adhesion to host epithelial cells (15). In other systems, several studies demonstrate that interactions between lipid microdomains and adhesion complexes are necessary for proper attachment and signal transduction (2, 18, 58). Therefore, we examined the possible role of raft-like microdomains in the adhesion of Entamoeba trophozoites to a mammalian cell monolayer after raft disruption by MBCD and filipin. Adhesion of Entamoeba trophozoites to CHO monolayers was significantly inhibited by treatment with MBCD or filipin, suggesting that intact microdomains are important to adhesion (Fig. 4). Moreover, ethanol, the filipin diluent, had no effect on adhesion, suggesting that the reduction in adhesion in the presence of filipin was specific.
FIG. 4.
Effect of disruption of lipid rafts on Entamoeba adhesion to host cells. Adhesion to a CHO monolayer was measured for cells that were not treated (control) and cells that were treated with 15 mM MBCD, 3.8 μM filipin, or ethanol (0.0475% vol/vol; diluent control for filipin). Adhesion was significantly inhibited by treatment with agents that disrupt lipid rafts. Means ± SD are shown (P < 0.05, n = 3 for filipin; P < 0.001, n = 4 for MBCD).
The inhibition of adhesion by treatment with raft-disrupting agents suggests that Entamoeba adhesion molecules, such as the Gal/GalNAc lectin, may localize to rafts. Therefore, we isolated rafts by sucrose gradient flotation and examined the enrichment of the subunits of the Gal/GalNAc lectin by Western blot analysis. After extraction with cold Triton X-100, the TIP of cell lysates was subjected to ultracentrifugation on sucrose gradients; proteins associated with rafts float to the low-density regions of the gradient, whereas soluble or cytoskeleton-associated proteins distribute to higher-density regions (5). To characterize each of the fractions from the sucrose gradient, the relative amount of cholesterol, the percentage of sucrose, and the quantity of protein were determined. Cholesterol enrichment, a criterion used to identify lipid rafts, was highest in fractions 9 and 10 of the sucrose gradient (Fig. 5B). Upon analysis of the percentage of sucrose in each fraction, it was determined that these same fractions contained approximately 35% sucrose, a region of the gradient that is consistent with the known flotation properties of lipid rafts (16, 69).
FIG. 5.
Silver stain and Western blot analysis of a fractioned sucrose gradient (fractions 1 through 4 combined [1/4] to 19 and P) and the TSS. (A) A silver-stained SDS-PAGE gel of sucrose gradient fractions (fractions 1/4 to 19 and P) and the TSS. (B) Western blots of a sucrose gradient-fractionated TIP and TSS membrane fractions with antibodies to the heavy (Hgl), intermediate (Igl), and light (Lgl) subunits of the Gal/GalNAc lectin as well as a blot for actin and a membrane-bound protein not involved in adhesion, EhCP5. The percent sucrose (wt/wt) and amount of cholesterol (relative fluorescence [fl]/milligram of protein) for each fraction or fraction combination are given below the blot. Hgl, Igl, and Lgl are enriched in the low-density, cholesterol-rich region of the gradient (fractions 9 and 10) as well as in medium- and high-density sucrose fractions (fractions 11 to 19). Actin is enriched in the high-density region of the sucrose gradient (fractions 15 to 19). The membrane-bound protein EhCP5, which is not involved in adhesion, is found exclusively in the pellet-containing fraction P and the TSS. N/A, not defined.
Consistent with the proposed role of lipid rafts in adhesion, Western blot analysis with antibodies specific to Hgl, Igl, or Lgl demonstrated that a portion of these proteins localized to the raft-like region of the gradient (fractions 9 to 10), suggesting interaction with lipid microdomains (Fig. 5B). A proportion of Hgl, Igl, and Lgl was also found in higher-density sucrose fractions (fractions 15 to 19) and the pellet that formed at the base of the gradient (fraction P). It has been suggested that actin-associated proteins cannot float to low-density regions because of their actin cytoskeletal anchorage. These proteins typically float in high-density fractions (41, 53). To test whether the presence of Hgl and Lgl in the high-density fraction may be due to association with actin, we performed Western blot analysis on the gradient fractions by using a commercially available pan-actin antibody that has been shown to specifically react with actin from a variety of organisms including several mammals, chickens, and the slime molds Physarum polycephalum and D. discoideum. This antibody strongly recognized a protein band of approximately 42 kDa in Entamoeba cell lysates consistent with the known molecular mass of this protein (data not shown). Decoration of the gradient fractions with this antibody indicated that actin was indeed enriched in the higher-density fractions. Given the evidence that the lectin interacts with actin (15, 66), it is possible that the localization of the subunits in these fractions may be due to cytoskeletal anchorage. Given the covalent association of Hgl and Lgl, it is not surprising that these two subunits exhibit a similar distribution in the gradient.
The pellet (fraction P) may represent incompletely solubilized lysates which may harbor unbroken cells and/or unbroken intracellular transport vesicles. Indeed, this would explain the presence of Hgl, Igl, Lgl, and actin in this fraction. Alternatively, the pellet may represent very large and stable protein complexes that include the lectin. Given the proposed role of the lectin in signaling (14, 68), it would not be surprising to find this adhesion molecule interacting with a variety of proteins (see Discussion). Further experimentation is necessary to distinguish between these possibilities. The lack of Igl staining in high-density, actin-rich fractions may indicate that Igl association with actin is weaker than that of the Hgl-Lgl heterodimer and/or that the subunit is excluded from some of the protein complexes containing Hgl and Lgl. The latter may have important regulatory consequences.
Finally, a membrane protein not involved in adhesion, EhCP5, was determined to localize partially to the TIP (fraction P) but did not float in the gradient. These results support the specificity of the flotation observed for the lectin. In all cases, the proteins described above were also observed in the TSS fraction, suggesting the ability of these proteins to move between these two membrane domains. A silver stain of the sucrose gradient fractions and TSS revealed a marked increase in protein staining beginning at ∼31% sucrose (fraction 8) over less-dense fractions, and differential protein band patterns can be observed among the fractions (Fig. 5A).
DISCUSSION
In this study, we have identified raft-like microdomains in the plasma membrane of E. histolytica by staining with two different fluorescent lipid analogs, FAST-DiI and DiIC16. We have also shown that pinocytosis and adhesion to host cell monolayers are raft-dependent cellular events in this pathogen. Interestingly, constitutive secretion of cysteine proteases was not inhibited by raft disruption. Finally, we determined that the heavy, intermediate, and light subunits of the Gal/GalNAc adherence lectin partially purify with the detergent-insoluble portion of the membrane and are enriched in cholesterol-containing regions of the membrane.
In other systems, it is established that cholesterol-rich membrane domains play a role in endocytosis. Reduction of cholesterol by inhibiting its synthesis with oxygenated cholesterol derivatives was shown to decrease pinocytic rates in L cells (23, 24). More recently, the uptake of an apoptotic agent alkyl-lysophospholipid or the transferrin receptor was blocked by treatment with MBCD or filipin (63). In another example, depletion of cholesterol was coupled with an increased residency of clathrin in the plasma membrane and a decrease in the proportion of deeply invaginated clathrin-coated pits, suggesting that raft-like domains participate in the early formation of endocytic vesicles (59). While clathrin has been identified in Entamoeba cells (61), its role in endocytosis in this organism has not been established. Here, we demonstrate the importance of intact raft-like domains to fluid-phase pinocytosis in Entamoeba cells, suggesting that molecular mechanisms, similar to those in higher eukaryotes, may govern this process in this pathogen.
Secretion is a vital component to the virulence of Entamoeba cells, as several released toxic molecules, such as cysteine proteases and the pore-forming peptide amoebapore, participate in the destruction of the colonic epithelium, gut-resident bacteria, and erythrocytes. Lipid rafts have been implicated in the secretory capabilities of several cell lines. For example, the neuroendocrine tumor cell line, AtT-20, was defective in the formation of both constitutive and regulated secretory vesicles from the trans-Golgi network upon cholesterol depletion (70). Notably, after cholesterol depletion of rat pancreatic cells, amylase was secreted constitutively rather than in a regulated fashion (50). These results suggest that, at least for rat pancreatic cells, regulated secretory events are lipid raft dependent, whereas constitutive secretion is lipid raft independent. Similarly, the release of cysteine protease from Entamoeba cells, which is a constitutive secretory event (32), was not affected by the disruption of raft-like domains. However, we cannot rule out the possibility that regulated secretory events, such as the release of the amoebapore, are sensitive to raft disruption in Entamoeba cells.
Amoebic infection is dependent on the ability of the trophozoite to adhere to the colonic epithelium. Here, adhesion of Entamoeba cells to a CHO monolayer was blocked upon treatment with raft-disrupting agents MBCD and filipin (Fig. 4). Our results are consistent with those of other reports that have described the necessity of microdomains for cell-cell adhesion. In D. discoideum, cell-cell adhesion was also shown to be sensitive to raft-disrupting agents, such as filipin or digitonin (21). Furthermore, the Dictyostelium cell adhesion molecule gp80 was isolated in the low-density, raft-like fractions of a sucrose gradient (21, 22).
Adhesion complexes in higher eukaryotes have also been shown to be raft associated. In RBL-2H3 mast cells, the immunoglobulin E receptor, FcɛRI, was evenly distributed in the plasma membrane but was observed to colocalize with the lipid raft marker, fluorescent cholera toxin B subunit, upon ligand binding (reviewed in reference 28). Connexin 43, an adhesion molecule important in gap junctions, has been determined to reside in raft-like microdomains at the junctional membrane regions of NIH 3T3 fibroblasts and human embryonic kidney 293T cells (51). In leukocytes, lipid rafts are critical in adhesion as well as in the cellular response to a presented antigen (30, 33). For example, binding of the T-cell receptor to antigen initiates its association with a number of proteins, including cytoplasmic protein tyrosine kinases, membrane-associated Src-family kinases, and several receptor-associated proteins in raft-like microdomains (27). Once this activation and signaling machinery assembles in the raft, downstream signaling events mediated by the small GTPase, Rap1A, focal adhesion kinase, proline-rich tyrosine kinase-2, and mitogen-activated protein kinase and actin reorganization occur (27, 38, 48, 52). Disruption of microdomains in these cells results in an inability to propagate this antigen-dependent signal (27).
The precise molecular mechanism by which Entamoeba adheres to mammalian cells has not been discerned. However, it is known that the Gal/GalNAc-inhibitible lectin is necessary for both adhesion to host cells and virulence (14, 31, 42, 45, 49). To date, the heavy and light subunits have always been detected jointly in amoebae or by Western blots of native proteins (35). Likewise, in this study, the heavy and light subunits exhibit similar distribution in the sucrose gradient. Studies in which the heavy subunit was mutagenized identified β2 and β7 integrin-like sequences in the cytoplasmic tail of the heavy subunit (68). Interestingly, the integrin family of adhesion proteins, which consists of allosteric signaling molecules that mediate intracellular (inside out) or extracellular (outside in) signals, are often associated with raft-like microdomains and with the cytoskeleton through several actin-binding proteins (29, 30). The results of this study, which demonstrate the enrichment of the Gal/GalNAc lectin heavy subunit in both the cholesterol- and actin-rich domains supports the notion (68) that this adhesion molecule may be functionally similar to integrins.
Several reports demonstrate a direct interaction between lipid rafts and the actin cytoskeleton, which may account for the dual localization of the Gal/GalNAc heavy (and consequently, light) subunit. First, upon cholesterol depletion of hippocampal cells with MBCD, dendritic spines, typical membrane outgrowths of this cell type, were observed to immediately collapse due to F-actin redistribution from the spines to dendritic shafts (26). Second, actin depolymerization was observed in microvilli and lamellipodia of fibroblasts upon cholesterol depletion (39). Third, proteomic analysis of the detergent-resistant membrane from neutrophils revealed the association of an F-actin-binding protein, supervillin (41). Interestingly, the β2-integrin, LFA-1, is excluded from microdomains in unstimulated T cells due to cytoskeletal constraints. Upon activation, the protein moves into the more-ordered domain to presumably mediate a signal either from internal or external sources (33). Although it is not clear why the subunits of Entamoeba Gal/GalNAc lectin partition into both raft-like sucrose gradient fractions and actin-rich fractions of higher density, the data support the previously reported notion that mechanisms similar to those for LFA-1 may regulate the Gal/GalNAc lectin (68). In other words, the Gal/GalNAc heterodimer (which consists of Hgl and Lgl) may be excluded from microdomains through its interaction with the actin cytoskeleton. Upon binding to Gal- or GalNAc-containing ligands, the proteins may be released from the cytoskeleton and become incorporated into raft-like regions of the membrane. This may initiate a signaling cascade that would trigger virulence functions, including the release of the pore-forming peptide amoebapore.
Hgl and Lgl were also found in the pellet that formed at the bottom of the gradient. The pellet (fraction P) may represent incompletely solubilized low-density fractions or large, stable protein complexes that include the lectin. Given the proposed role of the lectin in signaling (68), it would not be surprising to find this adhesion molecule forming complexes with other proteins. In support of this, it has been shown that integrins form large complexes with transmembrane 4 superfamily proteins that are >20 million Da and remain intact after treatment with Triton (10).
The precise function and role of Igl in adhesion and virulence has not been described; however, it is known that amoebic adhesion to CHO cells is blocked upon incubation of Entamoeba with a monoclonal antibody to the protein (8). Previously, Igl was shown to have a GPI anchor sequence motif on its carboxy terminus, Gal/GalNAc binding affinity independent of that of Hgl, and a noncovalent association with the Hgl/Lgl heterodimer, and it has recently been postulated to function as a coreceptor for the heterodimer (35, 43). Our work demonstrates that Igl is associated with lipid raft fractions and actin-rich fractions; however, unlike Hgl and Lgl, it is not found in the densest fractions of the sucrose gradient. This indicates that Igl does not associate with actin as strongly as the Hgl/Lgl heterodimer and/or may not be included in some of the protein complexes containing the other two subunits. In the second scenario, Igl may serve as a regulator for adhesion and/or downstream signaling events associated with virulence.
This analysis is the first to describe raft-like lipid microdomains in Entamoeba cells and to illustrate their potential physiological significance for this human intestinal parasite. Lipidomic and proteomic analyses of the detergent-insoluble regions of the Entamoeba plasma membrane, currently under way, will greatly advance the knowledge of host-pathogen interactions. These future studies will provide greater insight into the role of lipid rafts and the mechanisms governing the virulence of this human pathogen.
Acknowledgments
The work was supported by a grant from the National Institutes of Health (R01 AI046414) awarded to L.A.T.
We thank William Petri, University of Virginia, for antibodies to the Gal/GalNAc lectin, Matthius Leippe, Research Center for Infectious Disease, for antibodies to EhCP5, John Kaup and Andy Mount, Clemson University, for assistance in quantifying sucrose, Karl Franek, Clemson University, for critical review of the manuscript, and Gary Powell, Clemson University, for helpful discussion.
We acknowledge the support of the Biomedical Sciences and Engineering Institute of Clemson University.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aley, S. B., Z. A. Cohn, and W. A. Scott. 1984. Endocytosis in Entamoeba histolytica. Evidence for a unique non-acidified compartment. J. Exp. Med. 160:724-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, M. A., and J. Millan. 2001. The role of lipid rafts in signaling and membrane trafficking in T lymphocytes. J. Cell Sci. 114:3957-3965. [DOI] [PubMed] [Google Scholar]
- 3.Bagnat, M., S. Keranen, A. Shevchekno, A. Shevchekno, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batista, E., L. de Menezes Feitosa, and W. de Souza. 2000. The endocytic pathway in Entamoeba histolytica. Parasitol. Res. 86:881-890. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 6.Bruchhaus, I., T. Roeder, H. Lotter, M. Schwerdtfeger, and E. Tannich. 2002. Differential gene expression in Entamoeba histolytica isolated from amoebic liver abscess. Mol. Microbiol. 44:1063-1072. [DOI] [PubMed] [Google Scholar]
- 7.Burchard, G. D., and R. Bilke. 1992. Adherence of pathogenic and non-pathogenic Entamoeba histolytica strains to neutrophils. Parasitol. Res. 78:146-153. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, X. J., H. Tsukamoto, Y. Kaneda, and T. Tachibana. 1998. Identification of the 150-kDa surface antigen of Entamoeba histolytica as a galactose- and N-acetyl-d-galactoseamine-inhibitable lectin. Parasitol. Res. 84:632-639. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, X. J., M. A. Hughes, C. D. Huston, B. Loftus, C. A. Gilchrist, L. A. Lockhart, S. Ghosh, V. Miller-Sims, B. J. Mann, W. A. Petri, Jr., and H. Tachibana. 2001. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect. Immun. 69:5892-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claas, C., S. S. Stipp, and M. E. Hemler. 2001. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J. Biol. Chem. 276:7974-7984. [DOI] [PubMed] [Google Scholar]
- 11.Colarusso, P., and K. R. Spring. 2002. Reticulated lipid probe fluorescence reveals MDCK cell apical membrane topography. Biophys. J. 82:752-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, S., T. Stevens, C. Castillo, A. Villasenor, H. Arredondo, and K. Reddy. 2002. Lipid metabolism in mucous-dwelling amitochondriate protozoa. Int. J. Parasitol. 32:655-675. [DOI] [PubMed] [Google Scholar]
- 13.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic culture of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 14.Dodson, J. M., P. W. Lenkowski, Jr., A. C. Eubanks, T. F. Jackson, J. Napodano, D. M. Lyerly, L. A. Lockhart, B. J. Mann, and W. A. Petri, Jr. 1999. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J. Infect. Dis. 179:460-466. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa-Cantellano, M., and A. Martinez-Palomo. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin. Microbiol. Rev. 13:318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedler, K., T. Kobayashi, T. V. Kurzchalia, and K. Simons. 1993. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry 32:6365-6373. [DOI] [PubMed] [Google Scholar]
- 17.Grimmer, S., B. van Deurs, and K. Sandvig. 2002. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J. Cell Sci. 115:2953-2962. [DOI] [PubMed] [Google Scholar]
- 18.Ha, H., H. B. Kwak, S. K. Lee, D. S. Na, C. E. Rudd, Z. H. Lee, and H. H. Kim. 2003. Membrane rafts play a crucial role in receptor activator of nuclear factor kappaB signaling and osteoclast function. J. Biol. Chem. 278:18573-18580. [DOI] [PubMed] [Google Scholar]
- 19.Hao, M., S. Mukherjee, and F. R. Maxfield. 2001. Cholesterol depletion induces large-scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. USA 98:13072-13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao, M., S. Mukherjee, Y. Sun, and F. R. Maxfield. 2004. Effects of cholesterol depletion and increased lipid unsaturation on the properties of endocytic membranes. J. Biol. Chem. 279:14171-14178. [DOI] [PubMed] [Google Scholar]
- 21.Harris, T. J. C., D. E. Awrey, B. J. Cox, A. Ravandi, A. Tsang, and C. H. Siu. 2001. Involvment of a triton-insoluble floating fraction in Dictyostelium cell-cell adhesion. J. Biol. Chem. 276:18640-18648. [DOI] [PubMed] [Google Scholar]
- 22.Harris, T. J. C., A. Ravandi, and C. H. Siu. 2001. Assembly of glycoprotein-80 adhesion complexes in Dictyostelium. J. Biol. Chem. 276:48764-48774. [DOI] [PubMed] [Google Scholar]
- 23.Heiniger, H. J., A. A. Kandutsch, and H. W. Chen. 1976. Depletion of L-cell sterol depresses endocytosis. Nature 263:515-517. [DOI] [PubMed] [Google Scholar]
- 24.Heiniger, H. J., and J. D. Marshall. 1979. Pinocytosis in L cells: its dependence on membrane sterol and the cytoskeleton. Cell Biol. Int. Rep. 3:409-420. [DOI] [PubMed] [Google Scholar]
- 25.Hellberg, A., N. Nowak, M. Leippe, E. Tannich, and I. Bruchhaus. 2002. Recombinant expression and purification of an enzymatically active cysteine proteinase of the protozoan parasite Entamoeba histolytica. Protein Exp. Purif. 24:131-137. [DOI] [PubMed] [Google Scholar]
- 26.Hering, H., C. C. Lin, and M. Sheng. 2003. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 23:3262-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg, N., R. Henderson, B. Leitinger, J. Porter, and P. Stanley. 2002. Mechanisms contributing to the activity of integrins on leukocytes. Immunol. Rev. 186:164-171. [DOI] [PubMed] [Google Scholar]
- 28.Holowka, D., and B. Baird. 2001. Fc(epsilon)RI as a paradigm for a lipid raft-dependent receptor in hematopoietic cells. Semin. Immunol. 13:99-105. [DOI] [PubMed] [Google Scholar]
- 29.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signing machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 30.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz, U., S. Ankri, T. Stolarsky, Y. Nuchamowitz, and D. Mirelman. 2002. Entamoeba histolytica expressing a dominant negative N-truncated light subunit of its Gal-lectin are less virulent. Mol. Biol. Cell 13:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leippe, M., H. J. Sievertsen, E. Tannich, and H. D. Horstmann. 1995. Spontaneous release of cysteine proteinases but not of pore-forming peptides by viable Entamoeba histolytica. Parasitology 111:569-574. [DOI] [PubMed] [Google Scholar]
- 33.Leitinger, B., and N. Hogg. 2001. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115:963-972. [DOI] [PubMed] [Google Scholar]
- 34.Manes, S., E. Mira, C. Gomez-Mouton, R. Lacalle, P. Keller, J. Labrador, and C. Martinez-A. 1999. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18:6211-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann, B. 2002. Structure and function of the Entamoeba histolytica Gal/GalNAc lectin. Int. Rev. Cytol. 216:59-80. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Belmonte, F., M. A. Alonso, X. Zhang, and P. Arvan. 2000. Thyroglobulin is selected as luminal protein cargo for apical transport via detergent-resistant membranes in epithelial cells. J. Biol. Chem. 275:41074-41081. [DOI] [PubMed] [Google Scholar]
- 37.Maxfield, F. R. 2002. Plasma membrane microdomains. Curr. Opin. Cell Biol. 14:483-487. [DOI] [PubMed] [Google Scholar]
- 38.McDowall, A., D. Inwald, B. Leitinger, A. Jones, R. Liesner, N. Klein, and N. Hogg. 2003. A novel form of integrin dysfunction involving β1, β2, and β3 integrins. J. Clin. Investig. 111:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meivar-Levy, I., H. Sabanay, A. D. Bershadsky, and A. H. Futerman. 1997. The role of sphingolipids in the maintenance of fibroblast morphology. The inhibition of protrusional activity, cell spreading, and cytokinesis induced by fumonisin B1 can be reversed by ganglioside GM3. J. Biol. Chem. 272:1558-1564. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee, S., T. T. Soe, and F. R. Maxfield. 1999. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 144:1271-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nebl, T., K. N. Pestonjamasp, J. D. Leszyk, J. L. Crowley, S. W. Oh, and E. J. Luna. 2002. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 277:43399-43409. [DOI] [PubMed] [Google Scholar]
- 42.Padilla-Vaca, F., S. Ankri, R. Bracha, L. A. Koole, and D. Mirelman. 1999. Down regulation of Entamoeba histolytica virulence by monoxenic cultivation with Escherichia coli O55 is related to a decrease in expression of the light (35-kilodalton) subunit of the Gal/GalNAc lectin. Infect. Immun. 67:2096-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petri, W. A., Jr., R. Haque, and B. J. Mann. 2002. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 56:39-64. [DOI] [PubMed] [Google Scholar]
- 44.Pierini, L. M., R. J. Eddy, M. Fuortes, S. Seveau, C. Casulo, and F. R. Maxfield. 2003. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 278:10831-10841. [DOI] [PubMed] [Google Scholar]
- 45.Ramakrishnan, G., S. Lee, B. J. Mann, and W. A. Petri, Jr. 2000. Entamoeba histolytica: deletion of the GPI anchor sequence on the Gal/GalNAc lectin light subunit prevents its assembly into the lectin heterodimer. Exp. Parasitol. 96:57-60. [DOI] [PubMed] [Google Scholar]
- 46.Rao, M., K. Peachman, C. Alving, and S. Rothwell. 2003. Depletion of cellular cholesterol interferes with trafficking of liposome-encapsulated ovalbumin. Immunol. Cell Biol. 81:415-423. [DOI] [PubMed] [Google Scholar]
- 47.Reyes-Lopez, M., J. Serrano-Luna, E. Negrete-Abascal, N. Leon-Sicairos, A. Guerrero-Barrera, and M. de la Garza. 2001. Entamoeba histolytica: transferrin binding proteins. Exp. Parasitol. 99:132-140. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Fernandez, J. L., M. Gomez, A. Luque, N. Hogg, F. Sanchez-Madrid, and C. Cabanas. 1999. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol. Biol. Cell 10:1891-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saffer, L. D., and W. A. Petri, Jr. 1991. Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. Infect. Immun. 59:4681-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, K., M. Schrader, H. F. Kern, and R. Kleene. 2001. Regulated apical secretion of zymogens in rat pancreas. Involvement of the glycosylphosphatidylinositol-anchored glycoprotein GP-2, the lectin ZG16p, and cholesterol-glycosphingolipid-enriched microdomains. J. Biol. Chem. 276:14315-14323. [DOI] [PubMed] [Google Scholar]
- 51.Schubert, A. L., W. Schubert, D. C. Spray, and M. P. Lisanti. 2002. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41:5754-5764. [DOI] [PubMed] [Google Scholar]
- 52.Sebzda, E., M. Bracke, T. Tugal, N. Hogg, and D. A. Cantrell. 2002. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 3:251-258. [DOI] [PubMed] [Google Scholar]
- 53.Seveau, S., R. J. Eddy, F. R. Maxfield, and L. M. Pierini. 2001. Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol. Biol. Cell 12:3550-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. 1:31-41. [DOI] [PubMed] [Google Scholar]
- 55.Slimane, T. A., G. Trugnan, S. C. Van IJzendoorn, and D. Hoekstra. 2003. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol. Biol. Cell 14:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spink, C. H., M. D. Yeager, and G. W. Feirgenson. 1990. Partitioning behavior of indocarbocyanides probes between coexisting gel and fluid phases in model membranes. Biochim. Biophys. Acta 1023:25-33. [DOI] [PubMed] [Google Scholar]
- 57.Stanley, S. L., Jr. 2003. Amoebiasis. Lancet 361:1025-1034. [DOI] [PubMed] [Google Scholar]
- 58.Stehr, M., R. M. Adam, J. Khoury, L. Zhuang, K. R. Solomon, C. A. Peters, and M. R. Freeman. 2003. Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J. Urol. 169:1165-1170. [DOI] [PubMed] [Google Scholar]
- 59.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, J. L., D. Holowka, B. Baird, W. W. Webb. 1994. Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J. Cell Biol. 125:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tovar, R., M. L. Murguia-Lopez, and M. de Lourdes Munoz. 2000. Immunolocalization of clathrin during electron-dense granule secretion in Entamoeba histolytica. Arch. Med. Res. 31:S143-144. [DOI] [PubMed] [Google Scholar]
- 62.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24:145-149. [PubMed] [Google Scholar]
- 63.van der Luit, A. H., M. Budde, P. Ruurs, M. Verheij, and W. J. van Blitterswijk. 2002. Alkyl-lysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J. Biol. Chem. 277:39541-39547. [DOI] [PubMed] [Google Scholar]
- 64.van Vliet, H. H., F. Spies, W. A. Linnemans, A. Klepke, J. A. Op den Kamp, and L. L. van Deenen. 1976. Isolation and characterization of subcellular membranes of Entamoeba invadens. J. Cell Biol. 71:357-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394:798-801. [DOI] [PubMed] [Google Scholar]
- 66.Vasquez, J., E. Franco, G. Reyes, and I. Meza. 1995. Characterization of adhesion plates by the interaction of Entamoeba histolytica trophozoites with fibronectin. Cell Motil. Cytoskeleton 32:37-45. [DOI] [PubMed] [Google Scholar]
- 67.Verkade, P., T. Harder, F. Lafont, and K. Simons. 2000. Induction of caveolae in the apical plasma membrane of Madin-Darby canine kidney cells. J. Cell Biol. 148:727-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vines, R. R., G. Ramakrishnan, J. B. Rogers, L. A. Lockhart, B. J. Mann, and W. A. Petri, Jr. 1998. Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a β2 integrin motif. Mol. Biol. Cell 9:2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Haller, P. D., S. Donohoe, D. R. Goodlett, R. Aebersold, and J. D. Watts. 2001. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics 1:1010-1021. [DOI] [PubMed] [Google Scholar]
- 70.Wang, Y., C. Thiele, and W. B. Huttner. 2000. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic 1:952-962. [DOI] [PubMed] [Google Scholar]
- 71.Welter, B. H., R. C. Laughlin, and L. A. Temesvari. 2002. Characterization of a Rab7-like GTPase, EhRab7: a marker for the early stages of endocytosis in Entamoeba histolytica. Mol. Biochem. Parasitol. 121:254-264. [DOI] [PubMed] [Google Scholar]
- 72.Wu, F. S., and M. Y. Wang. 1984. Extraction of proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissue. Anal. Biochem. 139:100-103. [DOI] [PubMed] [Google Scholar]