Abstract
Bacillus anthracis elaborates a homopolymeric capsule composed of γ-d-glutamic acid residues. Mice were immunized with formalin-fixed encapsulated B. anthracis bacilli, and the serum antibody response to a γ-d-glutamyl capsular epitope was measured. Antiglutamyl antibodies were elicited in athymic BALB/c Nu/Nu, BALB/c Nu/+, and CBA/J mice but not in CBA/N xid mice. These response patterns define the capsule of B. anthracis as a thymus-independent type 2 antigen.
Bacillus anthracis is an encapsulated, endospore-forming, gram-positive rod and is the causative agent of anthrax (1, 8). Pathogenicity of B. anthracis depends upon elaboration of both toxins and capsule. The capsule, a homopolymer of d-glutamic acid residues linked via the γ-carboxyl (2), is thought to function as a virulence factor by inhibiting phagocytosis. The B. anthracis capsule consists of a repeating epitope structure, is of high molecular weight, and is likely resistant to degradation. These properties are characteristic of antigens classified as thymus-independent type 2 (TI-2) (9). TI-2 antigens are able to directly activate B lymphocytes in the absence of T cells, presumably by their ability to extensively cross-link membrane immunoglobulin. Activation of B lymphocytes by TI-2 antigens requires functional expression of Bruton's tyrosine kinase (Btk), a molecule involved in the surface immunoglobulin receptor-mediated signaling cascade. CBA/N xid mice have an X-linked mutation that results in the failure to express functional Btk, and consequently, they do not respond to TI-2 antigens. Although polysaccharides are the prototypic example of TI-2 antigens, proteins containing repeating antigenic determinants, viruses, and synthetic amino acid polymers also may function as TI-2 antigens (9, 12, 13).
In this study we sought to determine whether the capsule of B. anthracis behaved as a TI-2 antigen. Mice received two intraperitoneal injections of 2 × 108 formalin-fixed B. anthracis bacilli (Ames strain). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. The culture and preparation of the bacilli have been described in a previous report (15). Sera were taken before immunization and 10 days after each injection. Serum anti-γ-d-glutamyl antibody levels were measured in pre- and postvaccination sera by enzyme-linked immunosorbent assay (ELISA), where a synthetic biotinylated nonamer, γ-d-glutamyl peptide (γ-d-Glu)9, was attached to avidin-coated microtiter wells (15). Our previous work has shown that the (γ-D-Glu)9 peptide expresses a B. anthracis capsular epitope (15).
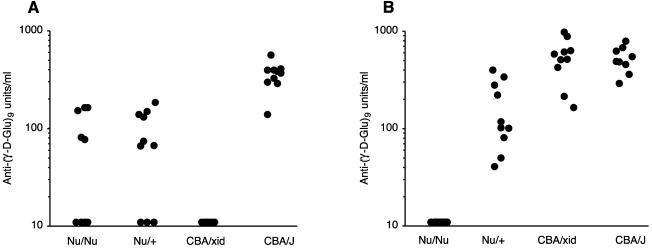
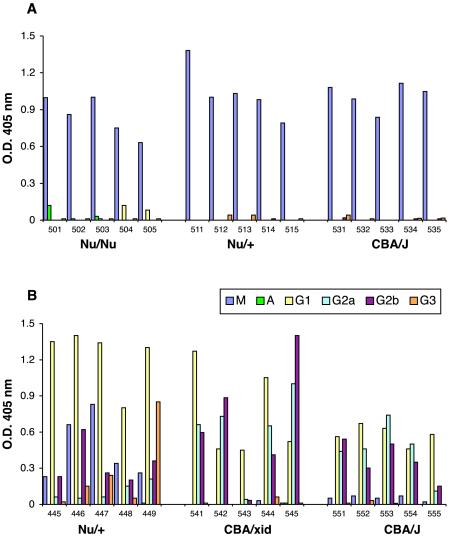
Antiglutamyl antibodies were not detected (<10 units/ml) in preimmunization sera (data not shown). We tested sera after a single immunization with formalin-fixed B. anthracis but found that the seroconversion rate was low, particularly in BALB/c Nu/Nu and Nu/+ mice. Mice were rested for 4 weeks and then challenged again with B. anthracis. Figure 1A shows the serum antiglutamyl antibody responses elicited after the second immunization. Student's t test was used for significance testing of antibody responses. Five of 10 athymic Nu/Nu mice responded with production of antiglutamyl antibodies, and 7 of 10 control Nu/+ mice produced antiglutamyl antibodies. There was no significant difference between the antibody levels in these two strains (P = 0.67). The response to the glutamyl epitope required expression of Btk, as shown by the complete lack of response in CBA/N xid mice. The antiglutamyl responses of the Nu/Nu mice were significantly different from the responses of CBA/N xid mice (P = 0.02). CBA/J control mice responded more uniformly and produced significantly higher levels of antiglutamyl antibodies than did either BALB/c Nu/Nu or BALB/c Nu/+ mice (P < 0.001), a result suggesting that capsule immunogenicity is strain dependent. The presence of a response in athymic mice and the lack of response in CBA/N xid mice thus formally define the capsule of B. anthracis as a TI-2 antigen.
FIG. 1.
Serum antiglutamyl antibody responses elicited from various mouse strains (10 mice per group) following immunization with either B. anthracis bacilli (A) or (γ-d-Glu)9-KLH conjugate (B). Antiglutamyl antibodies were measured by ELISA as described previously (15) and in the text. Levels of antibody were calculated from a standard curve generated by using a pool of sera obtained from mice immunized with (γ-d-Glu)9-KLH and assigned a value of 1,000 U/ml. Each closed circle represents the response of an individual mouse. Antibody levels indicated as 10 U/ml represented negative responses below the assay threshold of detection.
Early studies on the immunogenicity of synthetic polypeptides demonstrated that metabolizability and a repeating antigenic subunit structure were necessary for thymus independence. Antibody responses to amino acid copolymers composed of d-amino acids were found to be independent of T cells, whereas responses to copolymers of similar composition and size but containing l-amino acids were T cell dependent (12). d-Amino acid polymers, compared to l-amino acid polymers, were metabolized more slowly and were retained in tissues for extended periods of time (3, 12). Thus, it is likely that the capsule of B. anthracis functions as a TI-2 antigen because it consists entirely of d-glutamyl residues and is a homopolymer expressing repetitive epitopes.
We also examined the T cell dependence of the antiglutamyl response elicited by immunization with a conjugate consisting of (γ-d-Glu)9 covalently coupled to keyhole limpet hemocyanin (KLH). Previously, we demonstrated that immunization with this conjugate induces immunoglobulin G (IgG) antiglutamyl antibodies that recognize surface capsular epitopes of B. anthracis and that mediate opsonophagocytosis of the bacilli (15). Mice received two intraperitoneal injections of conjugate spaced 4 weeks apart. A single dose consisted of 20 μg of conjugate in 1.0 mg of aluminum hydroxide. As might be expected, (γ-d-Glu)9-KLH behaved as a thymus-dependent (TD) antigen as shown by the lack of antiglutamyl antibody response in Nu/Nu mice (Fig. 1B). There was no difference between the magnitude of the antibody responses of CBA/N xid and CBA/J mice (P = 0.99), a result showing that glutamyl-specific B cells are present in xid mice but that their activation requires presentation of the epitope in a TD form. As seen with B. anthracis, immunization with (γ-d-Glu)9-KLH induced significantly greater responses in CBA mice than in BALB/c Nu/+ mice (P < 0.001). Thus, the difference in responsiveness between these two strains is apparent with both TI and TD forms of the glutamyl epitope.
Isotype analysis of the antiglutamyl antibodies showed that B. anthracis immunization elicited an IgM response (Fig. 2A), whereas (γ-d-glu)9-KLH elicited an IgG response (Fig. 2B). These isotype patterns are consistent with the conclusion that the capsule of B. anthracis functions as a TI-2 antigen and is unable to promote isotype switching. In contrast, when presented as a hapten-like molecule coupled to KLH, the glutamyl epitope functions as a TD antigen and is able to stimulate extensive switching to a variety of IgG subclasses. IgM and IgG3 typically predominate in murine responses to TI-2 polysaccharide antigens, although there are exceptions (9). In humans, TI-2 antigens, such as the pneumococcal polysaccharides, can elicit IgG responses, but this probably results from activation of preexistent class-switched memory B cells and not from the ability of the polysaccharide to directly induce isotype switching (6).
FIG. 2.
Isotype profile of antiglutamyl antibodies elicited in response to either B. anthracis bacilli (A) or (γ-d-Glu)9-KLH conjugate (B). The analysis was performed by ELISA, using anti-isotype antibodies manufactured by Southern Biotechnology Associates (Birmingham, Ala.).
Intact B. anthracis bacilli, as opposed to purified capsule, were used as an immunogen in this study in order to simulate the antigenic stimulus encountered by the host during natural infection. High-dose intraperitoneal injection of bacilli, as used in this study, and the high concentration of bacteria present in the bloodstream during the later stage of inhalational anthrax infection are conditions that favor activation of B1 and marginal-zone B cells. Both populations have been implicated in responses to TI-2 antigens (7). Encapsulated bacteria are complex immunogens comprised not only of surface antigens but also of other molecules having adjuvant and immunomodulatory properties that activate both the adaptive and innate arms of the immune system (5, 9, 14). While the capsule of B. anthracis behaves as a TI-2 antigen in accordance with the classical criteria, the response to B. anthracis bacilli likely involves multiple immunoregulatory pathways. T cells, NK cells, complement, and cytokines are known to play a role in responses to TI antigens (9, 14), and antibody responses to TI antigens have TD components as well (4, 5).
Based upon previous studies of humoral responses to encapsulated bacterial pathogens (14), we think that the host response to B. anthracis infection will include a substantive TI-2 component. Anticapsular marginal-zone B lymphocytes would be rapidly mobilized by the septicemia which develops during the fulminant stage of inhalational anthrax infection (7). Although this TI-2 response may provide a rapid and early defense mechanism, it may not be sufficiently robust to clear an infection where bacterial levels in the blood can reach 108 organisms per ml. We and others have suggested that conversion of the capsular antigen into a TD form by coupling it to immunogenic carrier proteins may be a worthwhile approach towards the development of an anthrax vaccine component that elicits an immune response directed against encapsulated vegetative cells (5a, 6, 10, 11). The benefit of having a TD capsular component in an anthrax vaccine would be the generation of an expanded, isotype-switched and possibly affinity-matured memory-B-cell population capable of secreting IgG antibodies that would opsonize and promote clearance of vegetative cells during systemic anthrax infection.
Acknowledgments
We thank Patricia F. Fellows, Southern Research Institute, Frederick, Md., for provision of B. anthracis.
This work was supported by Public Health Service grant AI25008 from the National Institute of Allergy and Infectious Diseases and by a grant from Children's Hospital Oakland Research Institute.
Editor: A. D. O'Brien
REFERENCES
- 1.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 2.Hanby, W. E., and H. N. Rydon. 1946. The capsule substance of Bacillus anthracis. Biochem. J. 40:297-309. [PubMed] [Google Scholar]
- 3.Janeway, C. A., and J. H. Humphrey. 1968. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology 14:225-234. [PMC free article] [PubMed] [Google Scholar]
- 4.Jeurissen, A., M. Wuyts, A. Kasran, S. Ramdien-Murli, L. Boon, J. L. Ceuppens, and X. Bossuyt. 2002. Essential role for CD40 ligand interactions in T lymphocyte-mediated modulation of the murine immune response to pneumococcal capsular polysaccharides. J. Immunol. 168:2773-2781. [DOI] [PubMed] [Google Scholar]
- 5.Khan, A. Q., A. Lees, and C. M. Snapper. 2004. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J. Immunol. 172:532-539. [DOI] [PubMed] [Google Scholar]
- 5a.Kozel, T. R., W. J. Murphy, S. Brandt, B. R. Blazar, J. A. Lovchik, P. Thorkildson, A. Percival, and C. R. Lyons. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 101:5042-5047. [DOI] [PMC free article] [PubMed]
- 6.Lucas, A. H., K. D. Moulton, V. R. Tang, and D. C. Reason. 2001. Combinatorial library cloning of the human antibody repertoire to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect. Immun. 69:853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 8.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 9.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 10.Rhie, G.-E., M. H. Roehrl, M. Mourez, R. J. Collier, J. J. Mekalanos, and J. Y. Wang. 2003. A dually active anthrax vaccine that confers protection against both bacilli and toxins. Proc. Natl. Acad. Sci. USA 100:10925-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneerson, R., J. Kubler-Kielb, T.-Y. Liu, Z.-D. Dai, S. H. Leppla, A. Yergey, P. Backlund, J. Shiloach, F. Majadly, and J. B. Robbins. 2003. Poly(γ-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA 100:8945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sela, M., E. Mozes, and G. M. Shearer. 1972. Thymus-independence of slowly metabolized immunogens. Proc. Natl. Acad. Sci. USA 69:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szomolanyi-Tsuda, E., and R. M. Welsh. 1998. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 10:431-435. [DOI] [PubMed] [Google Scholar]
- 14.Vos, Q., A. Lees, Z.-Q. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154-170. [DOI] [PubMed] [Google Scholar]
- 15.Wang, T. T., P. F. Fellows, T. J. Leighton, and A. H. Lucas. 2004. Induction of opsonic antibodies to the γ-D-glutamic acid capsule of Bacillus anthracis by immunization with a synthetic peptide-carrier protein conjugate. FEMS Immunol. Med. Microbiol. 40:231-237. [DOI] [PubMed] [Google Scholar]