Abstract
Two recombinant Taenia solium oncosphere antigens, designated TSOL18 and TSOL45-1A, were investigated as vaccines to prevent transmission of the zoonotic disease cysticercosis through pigs. Both antigens were effective in inducing very high levels of protection (up to 100%) in three independent vaccine trials in pigs against experimental challenge infection with T. solium eggs, which were undertaken in Mexico and Cameroon. This is the highest level of protection that has been achieved against T. solium infection in pigs by vaccination with a defined antigen. TSOL18 and TSOL45-1A provide the basis for development of a highly effective practical vaccine that could assist in the control and, potentially, the eradication of human neurocysticercosis.
Cysticercosis in humans is caused by infection with the larval stage of the tapeworm parasite Taenia solium. The infection has a propensity to occur in the brain and other nervous tissue, and the resultant disease, neurocysticercosis, is an important cause of human morbidity and mortality (11). The disease is most prevalent in many Latin American, African, and Asian countries.
Human cysticercosis results from ingestion of T. solium eggs derived from the feces of a person infected with the adult tapeworm parasite. The normal life cycle of the parasite involves transmission through pigs acting as intermediate hosts. Pigs develop cysticerci in their muscle tissues following ingestion of tapeworm eggs from the feces of a human tapeworm carrier. The life cycle is completed when incompletely cooked pig tissues containing cysticerci are eaten by a human, leading to the growth of the adult tapeworm in the small intestine. However, the tapeworm eggs not only are infective for pigs but also can infect humans, leading to the disease neurocysticercosis. Control of transmission of this disease can be achieved by improvements in public sanitation, particularly the provision and use of latrines (6, 7). Also, it is possible to remove the adult tapeworm by use of highly effective drugs, for example, praziquantel (30). Despite the availability of these methods for control of cysticercosis, the disease continues to be prevalent in many parts of the world. A potential additional method for control of this disease would be vaccination. Substantial progress has been made with the development of highly effective, practical vaccines against closely related cestode parasites (19). Use of an effective vaccine in the natural animal intermediate hosts of T. solium would remove the source of tapeworm infection in humans, breaking the parasite's life cycle and indirectly eliminating the causative agent of human neurocysticercosis.
A number of approaches are being used by different groups towards the development of a vaccine against T. solium infection (2, 13, 32, 37). The approach that has been most successful in development of vaccines against other taeniid cestode parasites has been the use of recombinant oncosphere antigens (21). Three independently host-protective oncosphere antigens have been identified from Taenia ovis (12, 14), and homologues of these proteins have been found to be host protective against infection with Taenia saginata in cattle (25).
Gauci and colleagues (8, 9) identified and cloned cDNAs encoding T. solium oncosphere proteins TSOL18 and TSOL45-1A, which are homologues of host-protective antigens in T. ovis (To18 and To45W) (12, 14) and T. saginata (TSA18 and TSA9) (25). Here we describe investigations into the use of recombinant T. solium oncosphere antigens as vaccines against challenge infection with T. solium in pigs. In order to utilize these proteins in vaccine trials, TSOL18 and TSOL45-1A cDNAs were subcloned into expression vectors and fusion proteins were expressed in Escherichia coli.
MATERIALS AND METHODS
Subcloning and expression of antigen-expressing cDNA.
The region of the TSOL18 cDNA (8) comprising the 5′ untranslated region was removed by amplification under standard PCR conditions with the primers 5′CCGGAATTCATGGTGTGTCGGTTTGCTC3′ and 5′CCGCTCGAGGCATTGCCTGCTCCGCGC3′. These primers included DNA sequence designed to create 5′ EcoRI and 3′ XhoI restriction sites which enabled directional cloning of the PCR products. The PCR products were separated by agarose gel electrophoresis, excised from the gel, purified by using Geneclean (Bio101), and cloned into pGEM-T (Promega). Following plasmid purification, TSOL18 cDNA was excised by restriction digestion with EcoRI and XhoI, gel purified as described above, and subcloned into pGEX-1T (33) that was modified to accept directional EcoRI-XhoI insert sequences (pGEX-1TEX). The plasmid containing the TSOL18 cDNA was then transformed into E. coli JM109.
In T. solium oncospheres, the TSOL45 proteins are encoded by a family of related genes that produce alternatively spliced mRNA transcripts (9). Since at least eight TSOL45-related proteins were identified in T. solium oncospheres, the transcript selected initially for expression and testing of the associated protein in vaccine trials was that which encoded a protein (referred to by Gauci and Lightowlers [9] as TSO45-1A) having the closest homology to the To45W protective antigen from T. ovis. The TSOL45-1A cDNA was excised from pGEM-T by EcoRI digestion, gel purified, and cloned into the EcoRI site of pGEX-3TEX. The plasmid was transformed into E. coli JM109.
Recombinant proteins were prepared by growth of the bacterial cultures overnight followed by 10-fold dilution into fresh culture medium (per liter, 20 g of tryptone, 5 g of yeast extract, 0.5 g of NaCl, 0.2 g of KCl, 2 g of MgCl2 [SOB] containing 100 μg of ampicillin per ml), growth in shaker flasks until an optical density at 600 nm of 1.0 was reached, and induction with 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 5 h prior to purification of the glutathione S-transferase (GST) fusion proteins as described by to Smith and Johnson (33). Yields of the TSOL18 and TSOL45-1A fusion proteins, containing the predicted full-length parasite polypeptides, were poor, and copurification of degradation products was also observed in each protein preparation (not shown). Bacterial expression levels and stability of the fusion proteins were improved by deletion of the cDNA sequence encoding the first 16 N-terminal amino acids predicted to be secretory signals in both TSOL18 and TSOL45-1A (9, 20, 22). The secretory signals were removed by PCR amplification of the cDNA encoding amino acids 17 to 130 for TSOL18 and amino acids 17 to 253 for TSOL45-1A by using the following PCR primers: for TSOL18, 5′CCGGAATTCGACCGAACATTCGGCGACG3′ and 5′CCGCTCGAGGCATTGCCTGCTCCGCGC3′; for TSOL45-1A, 5′CCGGAATTCGGAAACCACAAGGCAACATC3′ and 5′CCGCTCGAGGGAAATGGGCATTGACCGAG3′. The PCR-amplified products encoding the truncated proteins were each cloned into pGEX-1TEX and transformed into E. coli JM109. The DNA sequences of cloned PCR amplification products were confirmed on both strands by using BigDye terminator cycle sequencing reactions (Applied Biosystems) and a PRISM automated sequencing system (Applied Biosystems). GST fusion proteins were prepared as described above. The nomenclature TSOL18 and TSOL45-1A was retained for these truncated recombinant proteins, which were used as GST fusion proteins in vaccination trials unless otherwise specified.
For production of maltose binding protein (MBP) fusion proteins, the truncated TSOL18 and TSOL45-1A cDNAs were digested from pGEX with EcoRI and XhoI, purified as described above, subcloned into the EcoRI/SalI sites of pMAL-C2 (New England Biolabs), transformed into E. coli, and expressed and purified according to the manufacturer's recommendations.
Vaccine trials.
Recombinant antigens TSOL18 and TSOL45-1A were used to vaccinate pigs prior to an experimental challenge infection with T. solium eggs. Three independent experiments were performed, two in Mexico and one in Cameroon. Specific details for individual trials are provided below. Immunizations were given intramuscularly in the right hind quarter. Booster immunizations were given into the same site. Subsequently, each pig received a challenge infection with mature viable T. solium eggs obtained following treatment of people harboring intestinal tapeworms. The identity of the tapeworm as T. solium was made on the basis of gravid segment morphology. The eggs were maintained at 4°C in phosphate-buffered saline containing 1,000 U of penicillin G, 1 mg of streptomycin sulfate, and 0.25 μg of amphotericin B/ml until use. Following establishment of cysticerci from the challenge infection, necropsies were performed, carcass musculature was sliced with hand-held knives or scalpels, and the number and viability of cysticerci were determined. Cysticerci were categorized as being viable when a clear-fluid-filled sac containing a detectable scolex was present, while necrotic, fibrosed, or calcified lesions without a recognizable scolex were designated nonviable. Statistical comparisons were made by using the Mann-Whitney U test.
Serological analyses.
Serum or plasma samples were obtained from each pig in vaccine trials at the time of each immunization, at challenge infection, and at postmortem. Specific antibody levels were assessed in an enzyme-linked immunosorbent assay (ELISA) against the T. solium-specific component of TSOL18 or TSOL45-1A by using MBP fusion proteins of these antigens in immunoassays. Routine procedures were used for ELISA (29), with 20 ng of antigen per well. Antibody binding was detected by using anti-porcine immunoglobulin G (IgG)-horseradish peroxidase conjugate (Serotec), and antibody titers were calculated as the highest serum or plasma dilution with an optical density at 450 nm of 1.0. Sera taken at the time of necropsy in trials 1 and 2 were assessed by Western blotting for the presence of anticysticercal antibodies (34).
RESULTS
Vaccine trial 1 was carried out in Mexico. Twenty 2-month-old Landrace pigs were obtained from a farm known to be free from T. solium cysticercosis and transported to a laboratory animal house. The pigs were allocated into four groups of five animals each, and pigs in the groups were immunized with either 200 μg of control protein (GST), 200 μg of TSOL18, 200 μg of TSOL45-1A, or 200 μg each of TSOL18 and TSOL45-1A, with 1 mg of Quil A as an adjuvant. A second identical immunization was given 2 weeks after the first injection. Three weeks after the second injection, each pig received a challenge infection with 40,000 T. solium eggs that were obtained from two tapeworm carriers from the state of Yucatan, Mexico, and used within 1 week of collection. The eggs were diluted in phosphate-buffered saline and introduced into a gelatin capsule which was given orally to each pig. Beginning 10 weeks after the challenge infection, pigs were humanely euthanatized; the hind legs, heart, tongue, and diaphragm were sliced at approximately 3-mm intervals; and each slice was inspected for the presence of cysticerci.
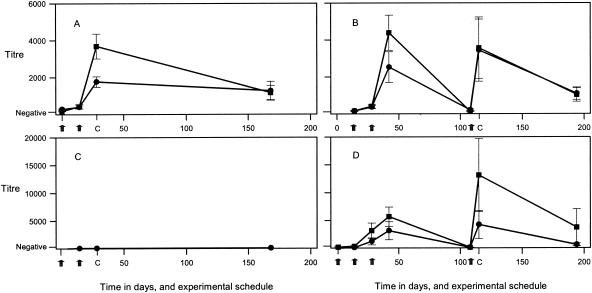
The number of cysticerci detected in each of the experimental pigs is shown in Table 1. T. solium infections in the five control pigs ranged between 167 and 415 cysts. The proportion of viable cysts differed between the individual control pigs, ranging from less than 1% viable in one pig to 100% viable in another (data not shown). No cysticerci were detected in any of the five pigs vaccinated with TSOL18. T. solium cysticerci established in all of the pigs vaccinated with TSOL45-1A, and while the mean number of cysticerci in this group was 47% lower than the mean number in the control group, this difference was not statistically significant. Vaccination with a combination of both TSOL18 and TSOL45-1A induced a 94.7% reduction in the number of cysticerci, with two individual pigs having no cysticerci detected. This was a statistically significant (P < 0.01, Mann-Whitney test) level of protection in comparison with the control group of pigs and did not represent a statistically significant difference from the group vaccinated with TSOL18 alone. Serological analyses of pig sera taken during the vaccine trial indicated that the pigs had responded to both primary and secondary immunizations with TSOL18, since specific IgG antibodies were produced (Fig. 1). However, the pigs vaccinated with TSOL45-1A in trial 1 failed to raise a detectable antibody response to the vaccine protein.
TABLE 1.
Protection against experimental infection with T. solium cysticerci by vaccination with the TSOL18 and/or TSOL45-1A recombinant oncosphere antigena
| Trial and vaccine | Trial location |
T. solium cysticerci in pigs
|
Mean % cysticercus viability (range) at time of necropsy | Mean protection (%)c | |
|---|---|---|---|---|---|
| No. in individual animalsb | Mean | ||||
| 1 | Mexico | ||||
| GST | 167, 206, 234, 262, 415 | 256.8 | 40 (0.4-100) | ||
| TSOL18 | 0, 0, 0, 0, 0 | 0 | 100d | ||
| TSOL45-1A | 28, 51, 151, 193, 258 | 136.2 | 72 (11-99) | 0 | |
| TSOL18+45 | 0, 0, 3, 9, 56 | 13.6 | 30 (22-100) | 94.7d | |
| 2 | Mexico | ||||
| GST or none | 6, 10, 11, 13, 17, 26, 28, 40, 59, 64, 100, 127 | 41.8 | 17 (0-51) | ||
| TSOL18 | 0, 0, 0, 0, 1 | 0.2 | 0 | 99.5d | |
| TSOL45-1A | 0, 0, 0, 0, 6 | 1.2 | 0 | 97.1d | |
| 3 | Cameroon | ||||
| None | 0, 4, 18, 39, 89 | 30.0 | 11 (0-94) | ||
| TSOL18 | 0, 0, 0, 0, 0 | 0 | 0 | 100e | |
Pigs received intramuscular immunizations with the recombinant antigen(s) together with 1 mg of Quil A adjuvant prior to challenge infection with viable eggs of T. solium, and necropies were performed approximately 3 months after the challenge infection.
For trials 2 and 3, the entire body musculature was assessed; for trial 1, the numbers of cysticerci in the right hind leg, heart, tongue, were and diaphragm assessed.
Protection was calculated as the percent reduction in the mean number of cysticerci in each group in comparison with the mean number of cysticerci in the control group of the same experiment and is shown only for those groups for which the difference was statistically significant.
P < 0.01 versus control group.
P < 0.05 versus control group.
FIG. 1.
Specific antibody titers, measured by ELISA, in pigs vaccinated with TSOL18 (A and B) or TSOL45-1A (C and D) recombinant T. solium oncosphere antigen in separate vaccine trials 1 (A and C) and 2 (B and D). Squares, IgG1; circles, IgG2; arrows, times at which pigs received immunizations; C, day on which pigs were challenged with T. solium eggs.
Vaccine trial 2 was also undertaken in Mexico. The aims of this trial were to confirm the protective efficacy of TSOL18 in to a vaccination protocol similar to that used in trial 1 and to reassess TSOL45-1A following three immunizations. Difficulties with obtaining viable T. solium eggs for the challenge infection led to a modification of the experimental plan for trial 2 such that each group of pigs received an additional booster immunization prior to the challenge infection. Four groups of pigs, of similar breed and age to those used in trial 1, were used. Two groups of six pigs each acted as controls. One group was immunized with 200 μg of GST plus 1 mg of Quil A on day 0 and at 2, 4, and 20 weeks after the primary injection. The second control group was not immunized with either vaccine protein or adjuvant. Two groups of five pigs each were vaccinated with T. solium recombinant antigens. One group was immunized with 200 μg of TSOL18 plus 1 mg of Quil A on day 0 and at 2 and 20 weeks after the primary injection, and another group was vaccinated with 200 μg of TSOL45-1A plus 1 mg of Quil A on day 0 and at 2, 4, and 20 weeks after the primary injection, with the final immunization undertaken with TSOL45-1A as an MBP fusion protein. The pigs were challenged 1 week after the final immunization with 9,000 viable T. solium eggs obtained from one tapeworm carrier from the state of Yucatan, Mexico, and several proglottids from another carrier from Lima, Peru. Necropsies began 14 weeks after the challenge infection. All body musculature was sliced for assessment of the level of infection in each animal.
All animals in the two control groups were found to be infected with cysticerci at necropsy, and there was no statistically significant difference between the numbers of cysticerci in the two groups; therefore, the control groups were combined for comparison with the experimental groups (Table 1). The numbers of cysticerci in controls ranged between 6 and 127, with the majority of lesions detected being nonviable; two control animals were found to harbor viable cysticerci (data not shown). Only a single nonviable lesion was detected in one of the pigs vaccinated with TSOL18. Four of the five pigs vaccinated with TSOL45-1A had no cysticerci, while one pig had six nonviable cysts. Serological analyses of the antibody responses in the pigs in trial 2 indicated that all pigs vaccinated with TSOL18 and TSOL45-1A had specific antibody against the corresponding vaccine protein at the time of the challenge infection (Fig. 1). Pigs vaccinated with TSOL45-1A responded particularly well to the final (fourth) immunization, with specific IgG1 titers ranging between 3,500 and 34,000 in individual animals. In both trials 1 and 2, all control pigs showed clear positive serological reactions at the time of necropsy for anticysticercal antibodies by Western blotting (data not shown); however, all of the animals in groups that were protected from infection following vaccination with TSOL18 or TSOL45-1A were negative for anticysticercal antibodies.
Vaccine trial 3 was undertaken in Cameroon. Pigs were Duroc and Duroc cross-bred with local pigs of uncertain breed. Five pigs in one group were untreated and acted as controls, while a second group of five pigs received three immunizations with 200 μg of TSOL18 plus 1 mg of Quil A, with the first two immunizations being given 2 weeks apart and the third immunization being given approximately 6 months later. Six days after the third immunization, the pigs received a challenge infection orally with 5,000 viable T. solium eggs obtained from taeniasis patients in Ecuador. Necropsies were undertaken to determine the level of infection between weeks 12 and 22 after the challenge infection. All body musculature and brain were sliced to determine the number of cysticerci in each pig (Table 1). Four of the five control pigs were found to harbor cysticerci at the time of the necropsies. There was a wide variability in the proportion of viable cysticerci between individual animals. While three pigs had only nonviable cysticerci, one animal had almost entirely (17 of 18) viable cysts. No cysticerci were detected in any of the pigs vaccinated with TSOL18.
DISCUSSION
In each of three vaccine trials, immunization of pigs with the TSOL18 recombinant antigen alone induced complete, or near-complete (99.5%), protection against the development of cysticerci following experimental challenge infection. This is the highest level of protection that has been achieved by vaccination against T. solium infection in pigs with a defined antigen (2, 13, 37), and it equates to the level of protection that has been achieved in pigs by using native T. solium oncosphere antigens (15, 27, 28, 35). While such high levels of protection are unusual for an antiparasite vaccine (4, 18), there are precedents for similar levels of protection having been achieved in vaccination against other taeniid cestode species by using recombinant oncosphere antigens (12, 14, 23-26). The TSOL18 and TSOL45-1A antigen vaccines were effective in preventing infections with T. solium eggs derived from Mexico, Peru, and Ecuador, indicating that these antigens are protective against isolates of T. solium occurring in different geographical locations.
The TSOL45-1A antigen induced a high level of protection (97%) against the challenge infection with T. solium in trial 2, in which serological analyses revealed a specific immune response to the vaccine protein in the immunized animals. It is not clear why the pigs in trial 1 failed to respond to immunization with the TSOL45-1A protein. Indeed, inclusion of TSOL45-1A together with TSOL18 appeared to detract from the level of protection afforded by TSOL18 in trial 1. The absence of any detectable specific antibody response against TSOL45-1A in this experiment (Fig. 1C) suggests that a technical error may have been made with preparation, storage, or application of the vaccine used. In trial 2, as well as in other experiments where pigs have been immunized with TSOL45-1A (data not shown), a prominent specific antibody response to the antigen has been detected in all animals after their second immunization. Additional vaccine trials will be required to determine the reliability of TSOL45-1A as an effective immunogen and protective antigen in pigs.
In sheep immunized with recombinant oncosphere antigens fused to GST, much of the antibody response has been found to be raised against the highly immunogenic GST fusion partner (29, 39), and similar responses were found in pigs vaccinated with the TSOL18 and TSOL45-1A fusion proteins (data not shown). Expression of taeniid oncosphere antigens in E. coli has been often found to be relatively toxic for the host bacteria (5, 22, 26), and their expression as fusions, involving a protein seemingly more compatible with the bacterial hosts, leads to enhanced and more stable expression. It is for this reason, and because of the simplicity with which GST fusions can be purified, that the TSOL18 and TSOL45 recombinant proteins were expressed as fusions with GST. In trial 2, the final immunization for the TSOL45-1A group was undertaken with an MBP fusion, with the aim of using a fusion partner other than GST being to boost the antibody specificities to the TSOL45-1A-encoded component of the vaccine and not GST. The TSOL45-1A-specific antibody titer increased dramatically after this immunization (anti-MBP titers were not raised significantly following immunization); however, the absence of data from animals boosted similarly with the GST fusion protein prevents any interpretation of the particular merits of alternating fusion partners in the immunization protocol. Nevertheless, the TSOL45-1A protein was found to be highly protective in trial 2, correlating with the enhanced immune response that was achieved against this antigen in comparison with trial 1.
Pigs in trials 1 and 2 were assessed at necropsy for anticysticercal antibodies, and while all control animals were positive in the assay, none of the pigs in groups protected by either TSOL18 or TSOL45-1A was positive. It is not known whether TSOL18 or TSOL45-1A is restricted in its expression to the oncosphere, as is the case for some of the host-protective oncosphere antigens from other taeniid species (3, 10, 38). However, under the conditions used in these experiments, immunization with TSOL18 or TSOL45-1A does not lead to seropositivity in assays used to detect infection with cysticerci. This serological discrimination between vaccinated and infected pigs may be important in future epidemiological assessment of T. solium infection in pigs, in association with practical use of a vaccine based on TSOL18 and/or TSOL45-1A.
The viability of cysticerci in the control pigs in each experiment varied greatly between individual animals. While no viable cysticerci were detected in any of the pigs in the groups where immunization with TSOL18 or TSOL45-1A induced a protective response, this cannot be interpreted to suggest that the viability of the few parasites that may establish in vaccinated animals will be affected by the vaccine. In the case of the other cestode vaccines, immunization with oncosphere antigens greatly reduces the proportion of parasites that are able to establish following a challenge infection but may not affect the longer-term viability of the metacestodes that do successfully establish in vaccinated animals (1, 23, 24).
Human cysticercosis caused by infection with T. solium has been identified as a potentially eradicable disease (17, 31). Emphasis has been placed on the possibility of parasite control by using anthelmintics to treat tapeworm carriers. However, reliance on this strategy has been of limited effectiveness in control campaigns to prevent transmission of other taeniid cestodes, because the presence of a reservoir population of infected animal intermediate hosts provides a source for new infections in definitive hosts, resulting in new episodes of parasite transmission (16, 36). Incorporation of an effective vaccine for prevention of infection in pigs would be likely to increase the effectiveness of T. solium disease control efforts and reduce the impact of reintroduction of disease transmission by immigration of Taenia carriers into disease control areas. The TSOL18 and TSOL45-1A antigens have been demonstrated to induce very high levels of protection against T. solium infection in pigs in the trials described here and provide a solid basis on which to develop a practical vaccine to assist with control of, and potentially the eradication of, human neurocysticercosis.
Acknowledgments
Funding from the Australian National Health and Medical Research Council and the Belgian Directorate General for Development Cooperation (DGDC, Brussels, and Framework Agreement with the Institute of Tropical Medicine, Antwerp) is acknowledged.
We acknowledge Hector Garcia (Lima, Peru) and W. Benitez-Ortiz (Quito, Ecuador) for the supply of some of the T. solium eggs used to infect pigs. The following colleagues provided invaluable participation in the assessment of parasite burdens: Francisco Cen, Rosa María Gonzalez, Idalia Hernandez, Giovanni Hernadnez, Diego Jimenez, Margarita Leyva, Cintia Lojero, Karina Marquez, Fela Mendlovic, Juan Perez, Mirza Romero, Nancy Teran, Violeta Quintero, Elizabeth Toral, Denisse Vazquez, Jessica Villanueva, and Nguekam. Sara Parraguirre performed histologic analysis on samples from nonviable lesions in vaccine trial 2 to confirm their parasitic origin. We thank Martin Pinon, Ramon Garcia, Luis Enrique Fernandez, and Jorge Campos for their outstanding efforts in pig handling and maintenance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bogh, H. O., M. D. Rickard, and M. W. Lightowlers. 1988. Studies on stage-specific immunity against Taenia taeniaeformis metacestodes in mice. Parasite Immunol. 10:255-264. [DOI] [PubMed] [Google Scholar]
- 2.Cai, X., Z. Chai, Z. Jing, P. Wang, X. Luo, J. Chen, Y. Dou, S. Feng, C. Su, and J. Jin. 2001. Studies on the development of DNA vaccine against Cysticercus cellulosae infection and its efficacy. Southeast Asian J. Trop. Med. Public Health 32(Suppl. 2):105-110. [PubMed] [Google Scholar]
- 3.Chow, C., C. G. Gauci, A. F. Cowman, and M. W. Lightowlers. 2004. Echinococcus granulosus: oncosphere-specific transcription of genes encoding a host-protective antigen. Exp. Parasitol. 6:183-186. [DOI] [PubMed] [Google Scholar]
- 4.Dalton, J. P., and G. Mulcahy. 2001. Parasite vaccines—a reality? Vet. Parasitol. 98:149-167. [DOI] [PubMed] [Google Scholar]
- 5.Dempster, R. P., C. M. Robinson, and G. B. Harrison. 1996. Parasite vaccine development: large-scale recovery of immunogenic Taenia ovis fusion protein GST-45W(B/X) from Escherichia coli inclusion bodies. Parasitol. Res. 82:291-296. [DOI] [PubMed] [Google Scholar]
- 6.Flisser, A. 1998. Larval cestodes, p. 539-560. In L. Collier, A. Balows, and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed., vol. 5. Arnold, London, United Kingdom. [Google Scholar]
- 7.Garcia, H. H., A. E. Gonzalez, and R. H. Gilman. 2003. Diagnosis, treatment and control of Taenia solium cysticercosis. Curr. Opin. Infect. Dis. 16:411-419. [DOI] [PubMed] [Google Scholar]
- 8.Gauci, C. G., A. Flisser, and M. W. Lightowlers. 1998. A Taenia solium oncosphere protein homologous to host-protective Taenia ovis and Taenia saginata 18 kDa antigens. Int. J. Parasitol. 28:757-760. [DOI] [PubMed] [Google Scholar]
- 9.Gauci, C. G., and M. W. Lightowlers. 2001. Alternative splicing and sequence diversity of transcripts from the oncosphere stage of Taenia solium with homology to the 45W antigen of Taenia ovis. Mol. Biochem. Parasitol. 112:173-181. [DOI] [PubMed] [Google Scholar]
- 10.Gauci, C. G., and M. W. Lightowlers. 1995. Developmental regulation of Taenia ovis 45W gene expression. Mol. Biochem. Parasitol. 73:263-266. [DOI] [PubMed] [Google Scholar]
- 11.Gemmell, M. A., Z. Matyas, Z. Pawlowski, and E. J. L. Soulsby. 1983. Guidelines for surveillance, prevention and control of taeniasis/cysticercosis. World Health Organization, Geneva, Switzerland.
- 12.Harrison, G. B., D. D. Heath, R. P. Dempster, C. Gauci, S. E. Newton, W. G. Cameron, C. M. Robinson, S. B. Lawrence, M. W. Lightowlers, and M. D. Rickard. 1996. Identification and cDNA cloning of two novel low molecular weight host-protective antigens from Taenia ovis oncospheres. Int. J. Parasitol. 26:195-204. [DOI] [PubMed] [Google Scholar]
- 13.Huerta, M., A. S. de Aluja, G. Fragoso, A. Toledo, N. Villalobos, M. Hernandez, G. Gevorkian, G. Acero, A. Diaz, I. Alvarez, R. Avila, C. Beltran, G. Garcia, J. J. Martinez, C. Larralde, and E. Sciutto. 2001. Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine 20:262-266. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, K. S., G. B. Harrison, M. W. Lightowlers, K. L. O'Hoy, W. G. Cougle, R. P. Dempster, S. B. Lawrence, J. G. Vinton, D. D. Heath, and M. D. Rickard. 1989. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature 338:585-587. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, V., S. N. S. Gaur, and K. M. L. Pathak. 1993. Immunization of pigs against the cysticercus of Taenia solium using fractionated first and second peaks of Cysticercus cellulosae scolex antigen. Indian J. Anim. Sci. 57:932-935. [Google Scholar]
- 16.Lawson, J. R., and M. A. Gemmell. 1983. Hydatidosis and cysticercosis: the dynamics of transmission. Adv. Parasitol. 22:261-308. [DOI] [PubMed] [Google Scholar]
- 17.Lightowlers, M. W. 1999. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int. J. Parasitol. 29:811-817. [DOI] [PubMed] [Google Scholar]
- 18.Lightowlers, M. W. 1994. Vaccination against animal parasites. Vet. Parasitol. 54:177-204. [DOI] [PubMed] [Google Scholar]
- 19.Lightowlers, M. W., A. L. Colebrook, C. G. Gauci, S. M. Gauci, C. T. Kyngdon, J. L. Monkhouse, C. Vallejo Rodriquez, A. J. Read, R. A. Rolfe, and C. Sato. 2003. Vaccination against cestode parasites: anti-helminth vaccines that work and why. Vet. Parasitol. 115:83-123. [DOI] [PubMed] [Google Scholar]
- 20.Lightowlers, M. W., A. Flisser, C. G. Gauci, D. D. Heath, O. Jensen, and R. Rolfe. 2000. Vaccination against cysticercosis and hydatid disease. Parasitol. Today 16:191-196. [DOI] [PubMed] [Google Scholar]
- 21.Lightowlers, M. W., and C. G. Gauci. 2001. Vaccines against cysticercosis and hydatidosis. Vet. Parasitol. 101:337-352. [DOI] [PubMed] [Google Scholar]
- 22.Lightowlers, M. W., C. G. Gauci, C. Chow, D. R. Drew, S. M. Gauci, D. D. Heath, D. C. Jackson, D. L. Dadley-Moore, and A. J. Read. 2003. Molecular and genetic characterisation of the host-protective oncosphere antigens of taeniid cestode parasites. Int. J. Parasitol. 33:1207-1217. [DOI] [PubMed] [Google Scholar]
- 23.Lightowlers, M. W., O. Jensen, E. Fernandez, J. A. Iriarte, D. J. Woollard, C. G. Gauci, D. J. Jenkins, and D. D. Heath. 1999. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int. J. Parasitol. 29:531-534. [DOI] [PubMed] [Google Scholar]
- 24.Lightowlers, M. W., S. B. Lawrence, C. G. Gauci, J. Young, M. J. Ralston, D. Maas, and D. D. Health. 1996. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 18:457-462. [DOI] [PubMed] [Google Scholar]
- 25.Lightowlers, M. W., R. Rolfe, and C. G. Gauci. 1996. Taenia saginata: vaccination against cysticercosis in cattle with recombinant oncosphere antigens. Exp. Parasitol. 84:330-338. [DOI] [PubMed] [Google Scholar]
- 26.Lightowlers, M. W., J. G. Waterkeyn, J. S. Rothel, C. G. Gauci, and G. B. Harrison. 1996. Host-protective fragments and antibody binding epitopes of the Taenia ovis 45W recombinant antigen. Parasite Immunol. 18:507-513. [DOI] [PubMed] [Google Scholar]
- 27.Pathak, K. M., and S. N. Gaur. 1990. Immunization of pigs with culture antigens of Taenia solium. Vet. Parasitol. 34:353-356. [DOI] [PubMed] [Google Scholar]
- 28.Plancarte, A., A. Flisser, C. G. Gauci, and M. W. Lightowlers. 1999. Vaccination against Taenia solium cysticercosis in pigs using native and recombinant oncosphere antigens. Int. J. Parasitol. 29:643-647. [DOI] [PubMed] [Google Scholar]
- 29.Rothel, J. S., M. W. Lightowlers, H. F. Seow, P. R. Wood, L. J. Rothel, D. D. Heath, and G. B. Harrison. 1996. Immune responses associated with protection in sheep vaccinated with a recombinant antigen from Taenia ovis. Parasite Immunol. 18:201-208. [DOI] [PubMed] [Google Scholar]
- 30.Sarti, E., P. M. Schantz, G. Avila, J. Ambrosio, R. Medina-Santillan, and A. Flisser. 2000. Mass treatment against human taeniasis for the control of cysticercosis: a population-based intervention study. Trans. R. Soc. Trop. Med. Hyg. 94:85-89. [DOI] [PubMed] [Google Scholar]
- 31.Schantz, P. M., M. Cruz, E. Sarti, and Z. Pawlowski. 1993. Potential eradicability of taeniasis and cysticercosis. Bull. Pan Am. Health Organ. 27:397-403. [PubMed] [Google Scholar]
- 32.Sciutto, E., G. Fragoso, K. Manoutcharian, G. Gevorkian, G. Rosas-Salgado, M. Hernandez-Gonzalez, L. Herrera-Estrella, J. Cabrera-Ponce, F. Lopez-Casillas, C. Gonzalez-Bonilla, A. Santiago-Machuca, F. Ruiz-Perez, J. Sanchez, F. Goldbaum, A. Aluja, and C. Larralde. 2002. New approaches to improve a peptide vaccine against porcine Taenia solium cysticercosis. Arch. Med. Res. 33:371-378. [DOI] [PubMed] [Google Scholar]
- 33.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 34.Tsang, V. C., J. A. Brand, and A. E. Boyer. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J. Infect. Dis. 159:50-59. [DOI] [PubMed] [Google Scholar]
- 35.Verastegui, M., R. H. Gilman, A. Gonzales, H. H. Garcia, C. Gavidia, N. Falcon, T. Bernal, Y. Arana, and V. C. Tsang. 2002. Taenia solium oncosphere antigens induce immunity in pigs against experimental cysticercosis. Vet. Parasitol. 108:49-62. [DOI] [PubMed] [Google Scholar]
- 36.Vidal Oqueta, S. 1995. The hydatidosis control program: the Chilean model, p. 172-216. In A. Ruiz, P. Schantz, and P. Arambulo (ed.), Proceedings of the scientific working group on the advances in the prevention, control and treatment of hydatidosis. Pan American Health Organization, Washington, D.C.
- 37.Wang, Q. M., S. H. Sun, Z. L. Hu, D. Wu, and Z. C. Wang. 2003. Immune response and protection elicited by DNA immunisation against Taenia cysticercosis. Vaccine 21:1672-1680. [DOI] [PubMed] [Google Scholar]
- 38.Waterkeyn, J., C. Gauci, A. Cowman, and M. Lightowlers. 1997. Sequence analysis of a gene family encoding Taenia ovis vaccine antigens expressed during embryogenesis of eggs. Mol. Biochem. Parasitol. 86:75-84. [DOI] [PubMed] [Google Scholar]
- 39.Woollard, D. J., C. G. Gauci, D. D. Heath, and M. W. Lightowlers. 1998. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immunol. 20:535-540. [DOI] [PubMed] [Google Scholar]