Abstract
A critical first line of defense against infection is constituted by the binding of natural antibodies to microbial surfaces, activating the complement system via the classical complement activation pathway. In this function, the classical activation pathway is supported and amplified by two antibody-independent complement activation routes, i.e., the lectin pathway and the alternative pathway. We studied the contribution of the different complement activation pathways in the host defense against experimental polymicrobial peritonitis induced by cecal ligation and puncture by using mice deficient in either C1q or factors B and C2. The C1q-deficient mice lack the classical complement activation pathway. While infection-induced mortality of wild-type mice was 27%, mortality of C1q-deficient mice was increased to 60%. Mice with a deficiency of both factors B and C2 lack complement activation via the classical, the alternative, and the lectin pathways and exhibit a mortality of 92%, indicating a significant contribution of the lectin and alternative pathways of complement activation to survival. For 14 days after infection, mannan-binding lectin (MBL)-dependent activation of C4 was compromised. Serum MBL-A and MBL-C levels were significantly reduced for 1 week, possibly due to consumption. mRNA expression profiles did not lend support for either of the two MBL genes to respond as typical acute-phase genes. Our results demonstrate a long-lasting depletion of MBL-A and MBL-C from serum during microbial infection and underline the importance of both the lectin and the alternative pathways for antimicrobial immune defense.
The discovery of specific pattern recognition components which direct and coordinate responses to microbial invasion has reformed the perception of how the innate immune defense system works. Pattern recognition molecules direct specific antimicrobial responses to a wide range of microbial structures. A large number of cell surface-associated pattern recognition receptors has recently been identified (52). Many of these specifically bind to the carbohydrate moieties present on the surfaces of pathogens and induce cellular responses such as the enhancement of phagocytosis and the release of immune mediators, anaphylatoxins, and bactericidal compounds from intracellular stores. Likewise, the humoral part of the innate immune system is capable of sensing and immediately reacting to pathogenic stimuli on microorganisms. The plasma proteins mannan-binding lectin (MBL) (25), L-ficolin (p35) (30), and H-ficolin (Hakata antigen) (31) have recently been described as serum defense molecules with different carbohydrate binding specificities (20, 23, 26) that serve as recognition molecules of the lectin pathway of complement activation. Each of the three different carbohydrate recognition molecules may form multimeric lectin pathway activation complexes with the three MBL-associated serine proteases (MASPs), which, upon binding to specific microbial carbohydrates, will initiate complement activation by cleaving C4 and C4b-bound C2 (29, 50).
MBL, a member of the collectin family, has been described in many species (19). While only one form of MBL is present in humans, due to conversion of a gene into a pseudogene between monkey and ape (19), two forms of MBL are found in mouse serum, MBL-A and MBL-C, which differ in their carbohydrate binding specificity (17, 18). The concentration in serum of mouse MBL-C is about sixfold higher than that of MBL-A, and a mild increase in the levels of MBL-A was observed after injection of the acute-phase inducers (24, 37). The liver is the main source of MBL biosynthesis, but expression was also shown in other tissues (46, 51). Opsonization (22) and activation of the complement system via the lectin pathway (18, 21) seem to be the main biological MBL functions. In humans, low MBL levels in serum are linked to a common opsonic defect that predisposes individuals to recurrent infections (27, 40). A recent study of patients admitted to hospital with systemic inflammatory response syndrome underlines the importance of MBL during sepsis, showing that patients carrying MBL variant alleles had a higher risk of developing severe sepsis and septic shock and were more likely to have positive cultures for microbial species (14).
Although the classical and the lectin pathways of complement activation show a high degree of similarity and lead to the generation of identical C3 and C5 convertases, they differ significantly in their composition and specificity (1, 45, 50).
The classical complement activation pathway is initiated by the binding of its recognition subcomponent C1q to Fc regions of antigen-bound immunoglobulins. This leads to the conversion of the C1q-associated serine protease C1r into its enzymatically active form. C1r then cleaves its substrate C1s, which leads to the cleavage of C4 and C4b-bound C2 to generate the classical C3 convertase, C4b2b, which converts native C3 to C3b. The deposition of multiple C3b molecules in close proximity of the C3 convertase complex changes its substrate specificity, forming the C5 convertase complex, C4b2b-(C3b)n, which converts native C5 to C5b and C5a. These enzymatic reactions are accompanied by the production of potent anaphylatoxins (C5a, C3a, and C4a). The alternative activation pathway is now considered a powerful amplification loop of complement activation, which is required for stabilization of (C3b)n to form the alternative pathway C5 convertase (38).
The lectin pathway of complement activation can be initiated by the recognition of oligosaccharide moieties on the surface of pathogens by the serum lectins and MASP complexes (30, 45). The MASP-2 complex has been shown to sequentially cleave C4 and C2 to initiate further downstream activation of complement in a comparable way to the classical pathway activation complex (29, 50). Experiments indicate that, as expected, the lectin pathway is also amplified by the alternative pathway (39). Thus, both the classical and the lectin pathways, supported by the alternative pathway, lead to the final, nonenzymatic assembly of the bactericidal membrane attack complex.
To study the pathophysiological consequences of selective complement deficiency, mouse strains made deficient in the expression of the complement components C1q (2) and factors B and C2 (42) by gene-specific targeting were used. C1qa−/− mice are selectively deficient in the antibody-mediated classical pathway of complement activation (2), while the lectin and alternative pathways are functionally active. H2-Bf/C2−/− mice are deficient in both the classical and alternative routes of complement activation (42). Recently, it has been demonstrated that these mice also lack the lectin pathway of complement activation (4). Therefore, they exhibit complete deficiency of all three complement activation pathways, which makes them exquisitely sensitive to bacterial infection.
The cecal ligation and puncture (CLP) model is considered a clinically relevant model of septic peritonitis with bacteremia (47). Mice can be saved by antibiotics from CLP-induced mortality, demonstrating that the animals die as a consequence of the bacterial infection (12). For survival after CLP, tumor necrosis factor (TNF) stored in mast cells has been shown to be important (10) to initiate the cellular innate immune response by recruiting neutrophils and for containment of the septic focus by fibrous adhesions (12). From an exploration of the role of complement activation in this more clinically related model of septic peritonitis, the importance of the lectin pathway of complement activation for survival again became obvious. In addition, depletion of the serum lectins MBL-A and MBL-C was found to last for more than 1 week after CLP. MBL-A and MBL-C expression was not downregulated during the course of CLP; the proteins, rather, seemed to be consumed beyond the time expected for bacterial clearance in this model.
MATERIALS AND METHODS
Animals.
The study was performed in accordance with German federal regulations. C1qa−/− (2) and H2-Bf/C2−/− (42) breeding pairs were obtained from Marina Botto (Imperial College School of Medicine, London, United Kingdom). Both strains were on a 129/SV genetic background. These mice as well as control 129/SV mice were reared, bred, and kept under conventional conditions in the animal facilities of the University of Regensburg. All genetically modified mice used in the experiments were genotyped. Female NMRI mice were purchased from Charles River (Sulzfeld, Germany). All groups were age matched.
CLP.
CLP was performed as described earlier (8). Briefly, mice were anaesthetized and the cecum was exteriorized. The distal 30% of the cecum was ligated and punctured once with a needle with a 0.4-mm-diameter opening. The cecum was then replaced, and the skin was closed with clamps.
Bacterial counts.
Mice were sacrificed 24 h after CLP, and livers and spleens were removed. The organs were homogenized in an Ultra-Turrax (IKA-Labortechnik, Staufen, Germany) at 8,000 rpm in 2 ml of Standard I nutrient broth (VWR, Darmstadt, Germany) for 5 s. Homogenates were diluted serially in 0.85% NaCl and incubated on Mueller-Hinton plates at 36°C for 24 h in aerobic atmosphere. The resulting bacterial colonies were counted and expressed as bacteria per organ.
C4 cleavage assay.
A C4 cleavage assay was performed as described earlier (35). Microtiter wells (Maxisorp; Nunc, Kamstrup, Denmark) were coated overnight at room temperature with 1 μg of mannan (Sigma, Schnelldorf, Germany) in 100 μl of 15 mM Na2CO3, 35 mM NaHCO3 (pH 9.6) (coating buffer). Residual protein binding sites were blocked with 0.1% (wt/vol) human serum albumin (Statens Serum Institute, Copenhagen, Denmark) in 10 mM Tris-HCl, 140 mM NaCl, pH 7.4 (TBS), for 1 h. After washing with TBS plus 0.05% Tween 20 (Fluka, Buchs, Switzerland) and 5 mM CaCl2 (TBS/Tw/Ca2+), wells were filled with 100 μl of serum dilutions in 20 mM Tris-HCl, 10 mM CaCl2, 1 M NaCl, 0.05% (vol/vol) Triton X-100 (Serva Feinbiochemica, Heidelberg, Germany), 0.1% (wt/vol) human serum albumin (pH 7.4) (MBL binding buffer). Plates were incubated overnight at 4°C and washed three times with TBS/Tw/Ca2+. Wells then received 1 μg of human C4 (prepared as described previously) (7) in 100 μl of 8.9 mM KH2PO4, 27.8 mM NaHPO4, 111 mM NaCl, 0.15 mM CaCl2, 0.5 mM MgCl (pH 7.4). After incubation for 90 min at 37°C and another wash in TBS/Tw/Ca2+, deposited C4b was detected by adding alkaline phosphatase-conjugated chicken anti-human C4 antibody (100 μl of a 1:1,000 dilution in TBS/Tw/Ca2+) (Immunsystem AB, Uppsala, Sweden). Alkaline phosphatase activity was determined by adding p-nitrophenyl substrate solution (100 μl; Sigma). Plates were read at 405 nm. The optical density induced by mouse serum samples was determined in duplicate and compared to an internal standard (human serum) assigned the arbitrary activity value of 1 U/ml.
MBL time-resolved immunofluorometric assay.
Plates were coated and blocked as described for the C4 cleavage assay. Samples and standard serum were diluted in MBL-binding buffer. After incubation overnight at 4°C, plates were washed, and biotinylated anti-mouse MBL-A or MBL-C antibody (24) diluted in TBS/Tw/Ca2+ was added. Anti-mouse MBL-A was used at a dilution of 1:3,000, and anti-mouse MBL-C was used at a dilution of 1:5,000 (from stock solutions at 1 mg/ml). Plates were incubated for 2 h at room temperature and washed, followed by 1 h of incubation with Eu3+-conjugated streptavidin (Wallac, Turku, Finland) at a dilution of 1:1,000 in TBS plus 0.05% Tween and 25 μM EDTA. After another wash, enhancement solution (Wallac) was added. The amount of fluorescent chelate was estimated by time-resolved immunofluorometry with a DELFIA fluorometer (Wallac).
Northern blotting.
Total RNA was extracted from mouse liver according to standard protocols (5). Approximately 15 mg of total RNA per lane was separated on a denaturing 0.8% (wt/vol) agarose gel and transferred to Hybond N membrane (Amersham Pharmacia, Little Chalfont, United Kingdom). The blots were hybridized according to standard protocols (36) with random-primed [32P]dCTP-labeled (Random Primed Labeling Kit; Boehringer Mannheim, Mannheim, Germany) cDNA probes specific for the 3′ untranslated regions of MBL-A and MBL-C, as previously described (51). RNA loading and quality were normalized by using a probe specific for human β-actin (16). Blots were scanned and analyzed by Image Quant software (Amersham Pharmacia).
Statistical evaluation.
All experiments were performed at least three times with similar results, and the results of one representative experiment are shown. Descriptive statistics were expressed as the means ± standard deviations. Statistical significance was determined by using Student's t test for lectin pathway activity and the Mann-Whitney U test for bacterial counts. Time courses of survival were analyzed by Kaplan-Meier survival curve calculations and the log-rank test. P values of <0.05 were defined as significant for discrimination. Data were analyzed by using SPSS software.
RESULTS
Survival of complement-deficient mice after CLP.
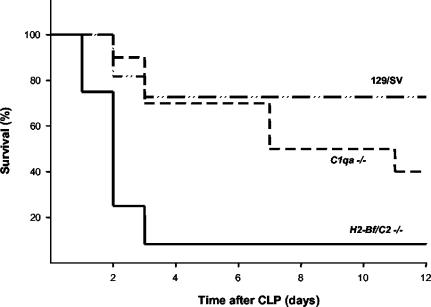
The mortality of wild-type and complement-deficient mice was assessed in the experimental model of polymicrobial septic infection induced by CLP. Survival of wild-type mice, C1q-deficient mice, and factor B- and C2-deficient mice was monitored for 12 days after CLP (Fig. 1). While 3 of 11 (27%) wild-type mice died, twice as many (6 of 10, or 60%) mice lacking the classical activation pathway of complement succumbed to the infection. The survival of mice lacking all three pathways of complement activation was severely impaired. Only one of 12 factors B- and C2-deficient mice survived the infection (92% mortality).
FIG. 1.
Groups of wild-type mice (129/SV, n = 11), C1q-deficient mice (C1qa−/−, n = 10), and factors B- and C2-deficient mice (H2-Bf/C2−/−, n = 12) were subjected to CLP, and cumulative survival was monitored over 12 days. Compared to wild-type mice, the increase in mortality of C1qa−/−mice was statistically insignificant (P = 0.2189), whereas the mortality of H2-Bf/C2−/− mice was significantly higher (P = 0.001).
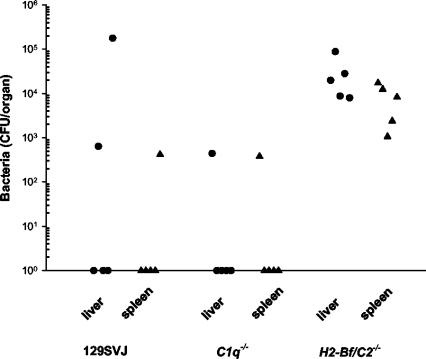
In accordance with their increased susceptibility to infection, mice lacking the classical, the alternative, and the lectin pathways of complement activation were also impaired in their ability to clear bacteria from liver and spleen. Bacteria were detected in livers (3.1 × 104± 3.3 × 104 CFU/organ) and spleens (8.3 × 103± 6.8 × 103 CFU/organ) from all tested mice deficient for factors B and C2 1 day after CLP (Fig. 2), while bacteria were found only in very few control or C1q-deficient mice.
FIG. 2.
Bacterial counts were determined in spleen or liver cultures of groups (n = 5) of control 129/SV mice, C1qa−/− mice, and H2-Bf/C2−/− mice 24 h after CLP. Bacterial counts are given as CFU per organ. A statistically significant enhancement in bacterial counts from liver (P = 0.007) and spleen (P = 0.007) of H2-Bf/C2−/− mice compared to C1qa−/− was determined.
Lectin pathway activity.
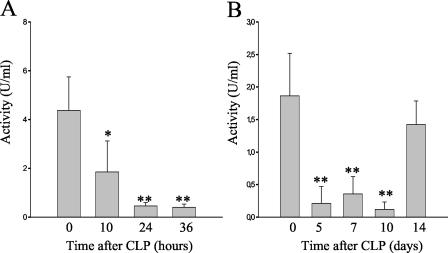
To further investigate whether the lectin pathway is involved in host defense in the CLP model of peritonitis, a C4 cleavage assay was performed. Plasma samples of a complement-sufficient strain (NMRI) were assayed in a mannan-dependent C4 cleavage test by trapping plasma MBL-MASP complexes on mannan-coated plates and measuring MASP-2-mediated C4 cleavage. This test specifically measures lectin pathway activation via MBL without interference from the classical pathway. By 10 h after CLP, the MBL-dependent C4 cleavage capacity of the mouse sera was significantly diminished compared to the capacity of untreated control serum (Fig. 3A). Whereas the serum of untreated mice showed an activity of 4.37 ± 1.38 U/ml, sera taken 10 h after CLP displayed an activity of 1.85 ± 1.28 U/ml (P = 0.017). This decrease was even more pronounced after 24 h (0.46 ± 0.14 U/ml, P = 0.003) and 36 h (0.40 ± 0.14 U/ml, P = 0.003) (Fig. 3A). Mice stayed compromised for more than 10 days, with a C4 activation capacity below 0.5 U/ml. (P < 0.005). A recovery could only be observed 14 days later (Fig. 3B). Experiments performed in C1q-deficient and factors B- and C2-deficient mice provided similar results (data not shown).
FIG. 3.
MBL-dependent C4 cleavage activity of mouse serum samples (NMRI; n = 5) at different time points after CLP and from untreated control mice was compared to an internal standard with the arbitrary activity of 1 U/ml. (A) Statistically significant reduced C4 activation in sera was determined at 10, 24, and 36 h after CLP. *, P < 0.05; **, P < 0.005. (B) MBL-dependent C4 cleavage activity 5 (n = 4), 7 (n = 4), 10 (n = 3), and 14 (n = 3) days after CLP. **, P < 0.005.
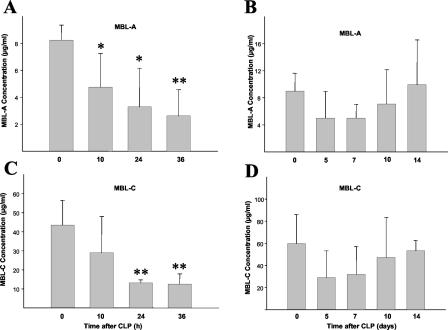
Serum MBL-A and MBL-C levels were determined to directly test whether the observed decrease in lectin pathway-dependent C4 activation was due to a lack of MBL or consumption of MASP-2. By 10 h after CLP, the serum MBL-A levels dropped in mice that had undergone CLP (4.76 ± 2.49 μg/ml) compared to serum MBL-A levels of untreated mice (8.26 ± 1.15 μg/ml) (P = 0.021) (Fig. 4A). Depletion of MBL-A from the sera was even more pronounced after 24 h (3.29 ± 2.87 μg/ml, P = 0.015) and 36 h (2.62 ± 1.96 μg/ml, P = 0.001), with two mice below the detection range of 2.5 ng/ml after 24 and 36 h, respectively. Serum MBL-A levels rose gradually over the next week, reaching normal levels again after 14 days (Fig. 4B). Due to the high variation of MBL titers in individual mice, the difference of serum MBL levels after CLP compared to serum levels before CLP did not reach statistical significance.
FIG. 4.
Serum levels of MBL-A and MBL-C were determined in mouse serum samples (NMRI) at different time points after CLP and compared to the serum levels of untreated control mice (n = 5). (A) MBL-A levels in untreated mice and 10 and 36 h after CLP. *, P < 0.05; **, P < 0.005. (B) MBL-A levels 5, 7, 10, and 14 days after CLP. (C) MBL-C levels in untreated mice and 10, 24, and 36 h after CLP. **, P < 0.005. (D) MBL-C levels 5, 7, 10, and 14 days after CLP.
Depletion of MBL-C in serum after CLP was also clearly shown by the drop in the concentrations of MBL-C in serum of untreated mice (43.52 ± 12.92 μg/ml) to 28.96 ± 19.36 μg/ml (P = 0.208) after 10 h, 13.12 ± 1.63 μg/ml (P = 0.006) after 24 h, and 12.46 ± 1 μg/ml (P = 0.001) after 36 h (Fig. 4C). A slow recovery in MBL-C levels could also be observed over the next 14 days (Fig. 4D).
To establish whether the decreased serum MBL levels were due to consumption or decreased protein expression, MBL-A and MBL-C mRNA levels in livers of mice that had undergone CLP were determined by Northern blot analysis. Groups of three mice each were sacrificed at 5 and 10 h as well as 5 and 10 days after CLP. Bands were normalized to β-actin. The results demonstrated that transcription of both MBL-A and MBL-C was not changed by CLP over the whole period of 10 days after CLP (data not shown).
DISCUSSION
Peritoneal bacterial infection and sepsis induced by CLP can be considered a relevant animal model to study the importance of different mechanisms of host innate and adaptive immune responses to combat bacterial infections. CLP serves as a clinically relevant model for severe abdominal infection because an early hyperinflammatory phase is followed by a subsequent hypoinflammatory phase during which the animals are extremely sensitive to bacterial superinfections (11, 53). This parallels the clinical observation of immunoparalysis that develops in patients during the course of sepsis (49).
The results from this study clearly demonstrate the importance of an intact complement system for survival after CLP. As already shown recently in a different model of experimentally induced polymicrobial peritonitis, both the classical pathway and the lectin pathway of complement activation are important in combating bacterial infection (4). A dominant role of the classical pathway in the innate immune response to bacterial pathogens by binding of natural antibodies to microbial antigens has previously been indicated (3). The recognition of microorganism-specific carbohydrate patterns by serum defense molecules such as MBL may constitute another important mechanism by which the innate immune system is activated, thus providing critical residual protection for C1q-deficient mice, as also supported by the results of the present study. Mouse MBL-A, under physiological conditions associated with MASP-2, after binding to specific oligosaccharides, initiates the lectin pathway of complement activation (28, 44, 50). Mouse MBL-C has been detected in sera of different mouse strains in about a sixfold higher concentration than MBL-A and was also shown to activate the lectin pathway via MASP-2 (24). MBL-A and MBL-C have different avidities for carbohydrate structures, which may broaden the spectrum of microorganisms they recognize (17). The two molecules might also have different effector functions besides complement activation. Both MBL and C1q have been shown to bind to CR1/CD35 (15) and calreticulin and CD91 (34, 48), thereby facilitating phagocytosis. However, this mechanism alone does not seem to be able to protect mice from bacterial infection, as H2-Bf/C2−/− mice, which still possess both molecules but lack all complement activation downstream of C4, were significantly more susceptible to CLP than wild-type or C1q-deficient mice. Also, these mice showed an impaired bacterial clearance from organs, indicating a clear need for further complement activation for the successful defense against infections. Interestingly, MBL-C is expressed in epithelial cells of the mouse small intestine, possibly indicating a role in mucosal immunity (46). However, MBL-A and MBL-C expression was also found in other tissues (51).
A comparison of the human system to the mouse model has to take into account that humans express only one form of MBL, which is more closely related to the rodent MBL-C form (13, 19). However, there is good evidence that MBL also plays an important role in the human system in protection from bacterial infection (14, 27, 40).
In this study we show for the first time that the lectin pathway is actively engaged in combating bacterial infection to such an extent that a rapid depletion of MBL in serum occurs. Most interestingly, this depletion lasted for more than 14 days, even though transcription of both MBL-A and MBL-C was still going on. A possible reason for this might be that MBL not only helps fend off acute bacterial invasions but also may be necessary for removal of bacteria in later stages after CLP. Although mice seem to have overcome the infection and give a healthy impression after about 1 week, the resolution of peritoneal adhesions and abscesses that are formed after CLP and which are crucial for survival (12) takes up to 4 weeks (unpublished observation). Continuous consumption of MBL during this process could explain the long-lasting depletion. Complement activation via the lectin pathway after tissue injury was also suggested by a report demonstrating the binding of MBL to endothelial cells after oxidative stress (6). The identification of the structures and molecules involved in the process leading to long-lasting MBL consumption after CLP is ongoing.
We could not detect a rise in MBL-A levels after CLP, even though MBL-A has been reported to behave like an acute- phase protein as elevated levels were induced by the injection of azocasein, thioglycolate medium, or lipopolysaccharide (24, 37). A modest increase in MBL levels was also reported in patients undergoing major surgery (43). However, promoter studies have cast doubt on the notion that MBL is an acute- phase protein, as transcriptional activity was not increased upon stimulation with proinflammatory cytokines. Dexamethasone even downregulated MBL expression (33). Our observation that MBL-A and MBL-C mRNA levels were not increased at any measured time point after CLP also casts doubt on the idea of MBL as an acute-phase protein.
A recent report shows that MBL-A deficiency enhanced survival in the same mouse peritonitis model of CLP; however, mice with the C57BL/6 genetic background were used for this study (41). As a possible explanation for the unexpected finding, a significant decrease in the TNF response in MBL-A-deficient mice 48 h after CLP was noted. However, other reports showed that TNF neutralization up to 8 h after CLP increased lethality (8). Also, TNF-deficient mice as well as mice deficient in the TNF receptor type 1 were reported to be significantly more susceptible to CLP than wild-type mice (9, 32). Thus, the relative importance of either MBL-A or MBL-C for survival of CLP-induced septic peritonitis is far from being clear at the moment. It will be interesting to see how mouse strains deficient in both MBL-A and MBL-C will perform in infection models and especially in the CLP model. However, a recent study of patients admitted to hospital with systemic inflammatory response syndrome underlines the importance of MBL during sepsis, showing that patients carrying MBL variant alleles had a higher risk of developing severe sepsis and septic shock and were more likely to have positive cultures for microbial species (14).
In conclusion, our results show that the lectin pathway of complement activation plays an important role in combating bacterial peritonitis. CLP leads to a long-lasting depletion of MBL from serum but not to an upregulation of MBL expression.
Acknowledgments
We thank Marina Botto for providing the C1qa−/− and H2-Bf/C2−/− mouse lines and Petra Lindner for assistance with C4 preparation.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft Ma 760/10-1.
Editor: F. C. Fang
REFERENCES
- 1.Ambrus, G., P. Gal, M. Kojima, K. Szilagyi, J. Balczer, J. Antal, L. Graf, A. Laich, B. E. Moffatt, W. Schwaeble, R. B. Sim, and P. Zavodszky. 2003. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J. Immunol. 170:1374-1382. [DOI] [PubMed] [Google Scholar]
- 2.Botto, M., C. Dell'Agnola, A. E. Bygrave, E. M. Thompson, H. T. Cook, F. Petry, M. Loos, P. P. Pandolfi, and M. J Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56-59. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celik, I., C. Stover, M. Botto, S. Thiel, S. Tzima, D. Kunkel, M. Walport, W. Lorenz, and W. Schwaeble. 2001. Role of the classical pathway of complement activation in experimentally induced polymicrobial peritonitis. Infect. Immun. 69:7304-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirgwin, J., A. Przybyla, R. MacDonald, and W. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonucleases. Biochemistry 18:5294. [DOI] [PubMed] [Google Scholar]
- 6.Collard, C. D., A. Vakeva, M. A. Morrissey, A. Agah, S. A. Rollins, W. R. Reenstra, J. A. Buras, S. Meri, and G. L. Stahl. 2000. Complement activation after oxidative stress: role of the lectin complement pathway. Am. J. Pathol. 156:1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodds, A. W. 1993. Small-scale preparation of complement components C3 and C4. Methods Enzymol. 223:46-61. [DOI] [PubMed] [Google Scholar]
- 8.Echtenacher, B., W. Falk, D. N. Mannel, and P. H. Krammer. 1990. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145:3762-3766. [PubMed] [Google Scholar]
- 9.Echtenacher, B., and D. N. Männel. 2002. Requirement of TNF and TNF receptor type 2 for LPS-induced protection from lethal septic peritonitis. J. Endotoxin Res. 8:365-369. [DOI] [PubMed] [Google Scholar]
- 10.Echtenacher, B., D. N. Männel, and L. Hültner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381:75-77. [DOI] [PubMed] [Google Scholar]
- 11.Echtenacher, B., R. Urbaschek, K. Weigl, M. A. Freudenberg, and D. N. Männel. 2003. Treatment of experimental sepsis-induced immunoparalysis with TNF. Immunobiology 208:381-389. [DOI] [PubMed] [Google Scholar]
- 12.Echtenacher, B., K. Weigl, N. Lehn, and D. N. Männel. 2001. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69:3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezekowitz, R. A. B., L. E. Day, and G. A. Herman. 1988. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J. Exp. Med. 167:1034-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garred, P., J. J. Strom, L. Quist, E. Taaning, and H. O. Madsen. 2003. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J. Infect. Dis. 188:1394-1403. [DOI] [PubMed] [Google Scholar]
- 15.Ghiran, I., S. F. Barbashov, L. B. Klickstein, S. W. Tas, J. C. Jensenius, and A. Nicholson-Weller. 2000. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 192:1797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunning, P., P. Ponte, H. Okayama, J. Engel, H. Blau, and L. Kedes. 1983. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol. Cell. Biol. 3:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen, S., and U. Holmskov. 1998. Structural aspects of collectins and receptors for collectins. Immunobiology 199:165-189. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, S., S. Thiel, A. Willis, U. Holmskov, and J. C. Jensenius. 2000. Purification and characterization of two mannan-binding lectins from mouse serum. J. Immunol. 164:2610-2618. [DOI] [PubMed] [Google Scholar]
- 19.Holmskov, U., S. Thiel, and J. C. Jensenius. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547-578. [DOI] [PubMed] [Google Scholar]
- 20.Holmskov, U. L. 2000. Collectins and collectin receptors in innate immunity. APMIS Suppl. 100:1-59. [PubMed] [Google Scholar]
- 21.Ikeda, K., T. Sannoh, N. Kawasaki, T. Kawasaki, and I. Yamashina. 1987. Serum lectin with known structure activates complement through the classical pathway. J. Biol. Chem. 262:7451-7454. [PubMed] [Google Scholar]
- 22.Kuhlman, M., K. Joiner, and R. A. Ezekowitz. 1989. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le, Y., S. H. Lee, O. L. Kon, and J. Lu. 1998. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 425:367-370. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H., L. Jensen, S. Hansen, S. V. Petersen, K. Takahashi, A. B. Ezekowitz, F. D. Hansen, J. C. Jensenius, and S. Thiel. 2001. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand. J. Immunol. 53:489-497. [DOI] [PubMed] [Google Scholar]
- 25.Lu, J. H., S. Thiel, H. Wiedemann, R. Timpl, and K. B. Reid. 1990. Binding of the pentamer/hexamer forms of mannan-binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J. Immunol. 144:2287-2294. [PubMed] [Google Scholar]
- 26.Lynch, N. J., S. Roscher, T. Hartung, S. Morath, M. Matsushita, D. N. Männel, M. Kuraya, T. Fujita, and W. J. Schwaeble. 2004. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 172:1198-1202. [DOI] [PubMed] [Google Scholar]
- 27.Madsen, H. O., P. Garred, S. Thiel, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013-3020. [PubMed] [Google Scholar]
- 28.Matsushita, M., and T. Fujita. 1992. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176:1497-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita, M., and H. Okada. 1986. Alternative complement pathway activation by C4b deposited during classical pathway activation. J. Immunol. 136:2994-2998. [PubMed] [Google Scholar]
- 30.Matsushita, M., Y. Endo, and T. Fujita. 2000. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 164:2281-2284. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita, M., M. Kuraya, N. Hamasaki, M. Tsujimura, H. Shiraki, and T. Fujita. 2002. Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J. Immunol. 168:3502-3506. [DOI] [PubMed] [Google Scholar]
- 32.Maurer, M., B. Echtenacher, L. Hultner, G. Kollias, D. N. Mannel, K. E. Langley, and S. J. Galli. 1998. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 188:2343-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito, H., A. Ikeda, K. Hasegawa, S. Oka, K. Uemura, N. Kawasaki, and T. Kawasaki. 1999. Characterization of human serum mannan-binding protein promoter. J. Biochem. 126:1004-1012. [DOI] [PubMed] [Google Scholar]
- 34.Ogden, C. A., A. deCathelineau, P. R. Hoffmann, D. Bratton, B. Ghebrehiwet, V. A. Fadok, and P. M. Henson. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen, S. V., S. Thiel, L. Jensen, R. Steffensen, and J. C. Jensenius. 2001. An assay for the mannan-binding lectin pathway of complement activation. J. Immunol. Methods 257:107-116. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Sastry, K., K. Zahedi, J. M. Lelias, A. S. Whitehead, and R. A. Ezekowitz. 1991. Molecular characterization of the mouse mannose-binding proteins. The mannose-binding protein A but not C is an acute phase reactant. J. Immunol. 147:692-697. [PubMed] [Google Scholar]
- 38.Schwaeble, W. J., and K. B. Reid. 1999. Does properdin crosslink the cellular and the humoral immune response? Immunol. Today 20:17-21. [DOI] [PubMed] [Google Scholar]
- 39.Suankratay, C., X. H. Zhang, Y. Zhang, T. F. Lint, and H. Gewurz. 1998. Requirement for the alternative pathway as well as C4 and C2 in complement-dependent hemolysis via the lectin pathway. J. Immunol. 160:3006-3013. [PubMed] [Google Scholar]
- 40.Super, M., S. Thiel, J. Lu, R. J. Levinsky, and M. W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet ii:1236-1239. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, K., J. Gordon, H. Liu, K. N. Sastry, J. E. Epstein, M. Motwani, I. Laursen, S. Thiel, J. C. Jensenius, M. Carroll, and R. A. Ezekowitz. 2002. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 4:773-784. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, P. R., J. T. Nash, E. Theodoridis, A. E. Bygrave, M. J. Walport, and M. Botto. 1998. A targeted disruption of the murine complement factor B gene resulting in loss of expression of three genes in close proximity, factor B, C2, and D17H6S45. J. Biol. Chem. 273:1699-1704. [DOI] [PubMed] [Google Scholar]
- 43.Thiel, S., U. Holmskov, L. Hviid, S. B. Laursen, and J. C. Jensenius. 1992. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin. Exp. Immunol. 90:31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 45.Thiel, S., S. V. Petersen, T. Vorup-Jensen, M. Matsushita, T. Fujita, C. M. Stover, W. J. Schwaeble, and J. C. Jensenius. 2000. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J. Immunol. 165:878-887. [DOI] [PubMed] [Google Scholar]
- 46.Uemura, K., M. Saka, T. Nakagawa, N. Kawasaki, S. Thiel, J. C. Jensenius, and T. Kawasaki. 2002. L-MBP is expressed in epithelial cells of mouse small intestine. J. Immunol. 169:6945-6950. [DOI] [PubMed] [Google Scholar]
- 47.Urbaschek, B., B. Ditter, K. P. Becker, and R. Urbaschek. 1984. Protective effects and role of endotoxin in experimental septicemia. Circ. Shock 14:209-222. [PubMed] [Google Scholar]
- 48.Vandivier, R. W., C. A. Ogden, V. A. Fadok, P. R. Hoffmann, K. K. Brown, M. Botto, M. J. Walport, J. H. Fisher, P. M. Henson, and K. E. Greene. 2002. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169:3978-3986. [DOI] [PubMed] [Google Scholar]
- 49.Volk, H. D., P. Reinke, D. Krausch, H. Zuckermann, K. Asadullah, J. M. Muller, W. D. Docke, and W. J. Kox. 1996. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 22(Suppl. 4):S474-S481. [DOI] [PubMed] [Google Scholar]
- 50.Vorup-Jensen, T., S. V. Petersen, A. G. Hansen, K. Poulsen, W. Schwaeble, R. B. Sim, K. B. M. Reid, S. J. Davis, S. Thiel, and J. C. Jensenius. 2000. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J. Immunol. 165:2093-2100. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, S., N. J. Lynch, W. Walter, W. J. Schwaeble, and M. Loos. 2003. Differential expression of the murine mannose-binding lectins A and C in lymphoid and nonlymphoid organs and tissues. J. Immunol. 170:1462-1465. [DOI] [PubMed] [Google Scholar]
- 52.Werling, D., and T. W. Jungi. 2003. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 91:1-12. [DOI] [PubMed] [Google Scholar]
- 53.Wichterman, K. A., A. E. Baue, and I. H. Chaudry. 1980. Sepsis and septic shock-a review of laboratory models and a proposal. J. Surg. Res. 29:189-201. [DOI] [PubMed] [Google Scholar]