Abstract
Over the past four decades, there has been a significant increase in allergy and asthma in westernized countries, which correlates with alterations in fecal microbiota (microflora) and widespread use of antibiotics (the “hygiene hypothesis”). Antibiotics also lead to overgrowth of the yeast Candida albicans, which can secrete potent prostaglandin-like immune response modulators. We have developed a mouse model of antibiotic-induced microbiota disruption that includes stable increases in gastrointestinal (GI) enteric bacteria and GI Candida levels with no introduction of microbes into the lungs. Mice are treated for 5 days with cefoperazone in the drinking water, followed by a single oral gavage of C. albicans. This results in alterations of GI bacterial populations and increased yeast numbers in the GI microbiota for at least 2 to 3 weeks and can drive the development of a CD4 T-cell-mediated allergic airway response to subsequent mold spore (Aspergillus fumigatus) exposure in immunocompetent mice without previous systemic antigen priming. The allergic response in the lungs is characterized by increased levels of eosinophils, mast cells, interleukin-5 (IL-5), IL-13, gamma interferon, immunoglobulin E, and mucus-secreting cells. In the absence of antibiotics, mice exposed to Aspergillus spores do not develop an allergic response in the airways. This study provides the first experimental evidence to support a role for antibiotics and fungal microbiota in promoting the development of allergic airway disease. In addition, these studies also highlight the concept that events in distal mucosal sites such as the GI tract can play an important role in regulating immune responses in the lungs.
Over the past four decades, there has been an explosive increase in allergy and asthma in westernized countries (67). According to a Centers for Disease Control and Prevention study, asthma rates in the United States increased 75% from 1980 to 1994, with the largest increase (160%) reported for children ages 0 to 4 (36). In the United States, the incidence among 13- to 14-year-old children is 22%, while in Canada, the United Kingdom, Ireland, New Zealand, and Australia, the incidence is 28 to 32% (22). While host genetics can influence allergy and asthma development, this marked increase is almost certainly due to changes in environmental factors and has lead to the “hygiene hypothesis” of allergies and asthma (62, 67). One of the factors in the hygiene hypothesis that correlates with this increased incidence is the rise in antibiotic use in industrialized countries (1, 12, 39, 65, 68). Epidemiologic studies in humans have also demonstrated a positive correlation between altered fecal microbiota (microflora) and atopic disease (4, 6, 23, 29). Since germfree animals also display numerous defects in the regulation of immune responses (10, 35, 58, 70), these observations raise the question of whether antibiotic-induced changes in the gastrointestinal (GI) microbiota are a predisposing factor for developing allergic responses in the airways. To date, this facet of the hygiene hypothesis has never been tested in an experimental model.
Increased fungal microbiota growth is a common side effect of antibiotic therapy. The yeast Candida albicans is a normal part of the human microbiota and resides in the mouth, vagina, and GI tract in low numbers (reviewed in reference 7). The factors affecting C. albicans numbers on mucosal surfaces are multifaceted and include the normal microbiota, hormones, stress, innate immunity, and adaptive immunity. Antibiotic-treated and germfree mice are dramatically more susceptible to C. albicans colonization and infection. Thus, control of C. albicans by the normal microbiota is very important. We recently reported that C. albicans (and many other fungi) secrete prostaglandin-like oxylipin molecules de novo or via conversion of exogenous arachidonic acid (42, 43). Prostaglandins such as prostaglandin E2 (PGE2) and prostaglandin D2 (PGD2) can inhibit Th1-type immune responses (3, 37, 57), promote Th2-type responses (11, 37, 54), and play a role in overall immune regulation (positive and negative) (44, 45). Thus, growth of fungal microbiota in a mucosal site could potentiate or alter immune responses on the mucosa via the production of prostaglandin-like oxylipins.
The objective of our present studies was to test one aspect of the hygiene hypothesis and investigate whether antibiotic therapy and alterations in microbiota populations, including growth of C. albicans, can dysregulate immune response generation in the airways, leading to allergic (Th2) responses to inhaled fungal elements. To test this hypothesis, we developed a murine model and focused primarily on the generation of allergic responses to mold spores from the ubiquitous fungus Aspergillus fumigatus, one of the most common indoor allergens affecting humans (30).
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (18 g ± 2 g) were purchased from Harlan Laboratories (Indianapolis, Ind.) and housed in specific pathogen-free conditions in enclosed filter-top cages. Food and sterile water were given ad libitum. The mice were maintained by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor, Mich.), and protocols were approved by an animal institutional review board.
Antibiotic treatment.
Cefoperazone (0.5 mg/ml; Sigma-Aldrich, St. Louis, Mo.) was administered orally to mice ad libitum in drinking water. Antibiotic treatment was continued for 5 days to allow for C. albicans colonization. After 5 days, drinking water was replaced with sterile water. Fecal cultures were analyzed at the termination of antibiotic therapy to confirm the efficacy of the antibiotic in decreasing GI microbiota populations. To insure consistent microbiota recolonization, an untreated mouse was always housed with the antibiotic-treated mice.
A. fumigatus intranasal inoculation.
A. fumigatus (ATCC 13073) was grown on Sabouraud dextrose agar (SDA; Difco, Detroit, Mich.) for 14 days. Conidia were harvested by washing plates with sterile 0.1% Tween 80. The resulting fungal suspension was then filtered through two layers of sterile gauze to remove hyphae. For infection, the conidia were washed in nonpyrogenic saline (Abbott Laboratories, Chicago, Ill.), counted with a hemocytometer, and diluted to 109 conidia/ml in sterile nonpyrogenic saline to administer 107 conidia/mouse in a 10-μl volume. Prior to intranasal inoculation, mice were anesthetized by intraperitoneal injection with a ketamine-xylazine solution (2.5 mg of ketamine/mouse [Fort Dodge Animal Health, Fort Dodge, Iowa] plus 0.1 g of xylazine/mouse [Lloyd Laboratories, Shenandoah, Iowa]). Mice were given a 5-μl bolus of A. fumigatus in each nostril followed by a 10-μl flush with sterile saline in each nostril.
C. albicans GI inoculation.
C. albicans strain CHN1 was grown to stationary phase (72 h) at 37°C in Sabouraud dextrose broth (1% neopeptone, 2% dextrose; Difco) with shaking. For infection, the cultures were washed in sterile nonpyrogenic saline, counted with a hemocytometer, and diluted to 2 × 108 CFU/ml in sterile nonpyrogenic saline. Mice were inoculated with C. albicans (107 CFU in 50 μl) by oral administration with a 24-gauge feeding needle attached to a 1-ml syringe. The syringe containing C. albicans was mounted onto a Stepper repetitive pipette (Tridak, Brookfield, Conn.) to deliver an equal amount of inoculum to each mouse. Aliquots of the inoculum were analyzed for CFU to monitor the amount delivered.
Tissue CFU assay.
Murine tissues were excised in a laminar flow hood, placed in tubes containing 10 ml of sterile water, weighed, and homogenized mechanically by using a Tissue-tearor (Biospec Products, Bartlesville, Okla.). For the oral cavity, the tongue and both cheek pouches were removed. For all the other organs, the entire organ was removed and homogenized. Aliquots of the homogenates were plated out in 10-fold dilutions. Several types of media were used to select for bacteria or yeast (SDA [Difco] for yeast, violet red bile agar [VRBA; Difco] for facultative anaerobic coliform bacteria, and Trypticase soy agar with 5% sheep blood [TSA II; BD Biosciences, Franklin Lake, N.J.] for total bacteria). SDA and VRBA plates were incubated aerobically at 37°C, and TSA II plates were incubated under anaerobic conditions (85% N2, 10% CO2, 5% H2) at 37°C. Colonies were counted at 24 h for the bacterial plates and 72 h for the fungal plates. The data are presented as the CFU per tissue for the cross-tissue comparisons of Candida deposition and CFU per gram of cecum for the bacterial counts.
Lung leukocyte culture and cytokine ELISA.
Mice were euthanized by CO2. Lungs were excised, minced, and enzymatically digested as described previously. Cell concentrations were determined by counting cells diluted in trypan blue with a hemocytometer. Isolated leukocytes from individual mice were standardized to 5 × 106 cells/ml and cultured in complete medium without additional stimulation at 37°C and 5% CO2. Supernatants were harvested at 24 h and assayed for cytokine production by enzyme-linked immunosorbent assay (ELISA) (OptEIA; Pharmingen, San Diego, Calif.).
Cell staining.
Leukocyte differentials (neutrophils, eosinophils, macrophages, and moncytes or lymphocytes) were visually counted after Wright-Giemsa staining of lung leukocyte samples cytospun onto glass slides (Shandon Cytospin, Pittsburgh, Pa.). The percentage of a leukocyte subset was multiplied by the total number of leukocytes to yield the absolute number of that leukocyte subset.
CD4+-T-cell depletion in mice.
Anti-CD4 antibody (GK1.5) was diluted in sterile saline to deliver 300 μg of antibody/mouse in a 250-μl volume. Mice were injected intraperitoneally with the diluted antibody 2 days prior to the initial A. fumigatus exposure (day 0 of the experimental timeline). A second injection of anti-CD4 antibody was given at day 7 postinoculation with C. albicans at a concentration of 100 μg/mouse in a 250-μl volume. Administration of this antibody resulted in a 99.95% depletion of CD4+ T cells as determined by fluorescence-activated cell sorter analysis. For fluorescence-activated cell sorter analysis of CD4 T-cell populations, an antibody (RM4-4) with specificity for an epitope of CD4 different from that of the depleting antibody (GK1.5) was used.
Statistical analysis.
The Student's t test (two-tailed, unequal variance) was used to analyze the significance of differences between experimental groups. For multiple comparisons, Bonferroni's correction was applied. Data with a P value of ≤0.05 for a single comparison and ≤0.015 for three comparisons were considered to be significant.
RESULTS
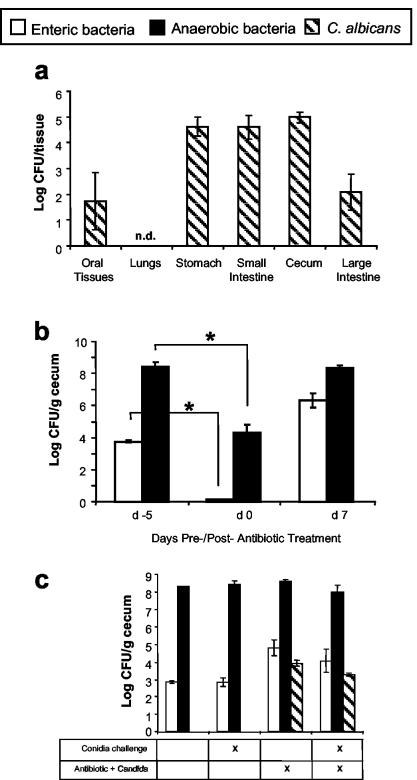
To establish a model of antibiotic therapy that included reproducible yeast persistence in the microbiota, C57BL/6 mice were first treated with a short course of a broad-spectrum antibiotic (cefoperazone for 5 days) in the drinking water to decrease total bacterial microbiota followed by a single oral gavage dose of C. albicans to establish a reproducible, low-level elevation of yeast in the microbiota (outlined in the Fig. 1 legend). Greater than 99.95% of the C. albicans colonies cultured at day 1 after gavage were found in the GI tract, with <100 CFU in the oral tissue and no detectable yeast in the lungs (Fig. 2a). C. albicans could not be cultured from the lungs at any time point examined in these studies (1 h, 12 days, and 19 days; data not shown), nor could we culture any bacteria from the lungs. This protocol for antibiotic treatment and gavage reduced culturable anaerobic and enteric bacteria levels in the gut by 99.99% at day 4 of antibiotic treatment and resulted in elevated C. albicans levels in the GI tract (Fig. 2b and c). When antibiotic treatment was discontinued (day 0), the numbers of both anaerobic and enteric bacteria increased. However, the recolonization by the enteric bacteria resulted in 10- to 100-fold-higher enteric bacteria levels at days 7 and 12 post-antibiotic treatment (referred to hereafter as posttreatment) compared to untreated mice (Fig. 2b and c). The levels of anaerobic bacteria in the cecum in our studies is consistent with that reported previously (27). The levels of C. albicans in the GI tract decreased during the regrowth of the bacterial microbiota but remained elevated at day 12 posttreatment (Fig. 2c) and could be found in the feces (data not shown). C. albicans did not cause overt disease symptoms and did not disseminate from the GI tract (data not shown). In summary, there was no evidence that this procedure introduced any microbes into the lungs, the GI microbiota of these mice were still disrupted 12 days after the completion of antibiotic treatment, and the GI microbiota was characterized by an elevated enteric bacteria-to-anaerobe ratio combined with low-level persistence of C. albicans.
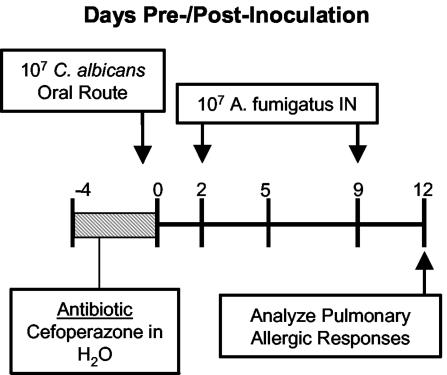
FIG. 1.
Experiment timeline for the induction of allergic airway disease following antibiotic therapy and fungal microbiota increase. C57BL/6 mice were given oral cefoperazone (0.5 mg/ml) in their drinking water for 5 days (days −4 through 0). At day 0, C. albicans (107 CFU) was administered orally, and mice were challenged intranasally (days 2 and 9) with A. fumigatus conidia (107 conidia/mouse). Mice were harvested at day 12 posttreatment.
FIG. 2.
Effect of antibiotic treatment on microbiota populations. At various times, murine tissues were harvested, homogenized in sterile water, and plated with the following media to enumerate cecal bacterial populations: VRBA for enteric bacteria, TSA II blood agar in an anaerobic chamber for total anaerobic bacteria, and SDA for yeast. (a) C. albicans tissue deposition at day 1 postinfection. (b) Cecal bacterial populations before and after antibiotic treatment. (c) Cecal bacterial populations at day 12 postinoculation with C. albicans. n = 7 to 8 mice per time point pooled from two separate experiments. Asterisk, P < 0.01; n.d., not detected.
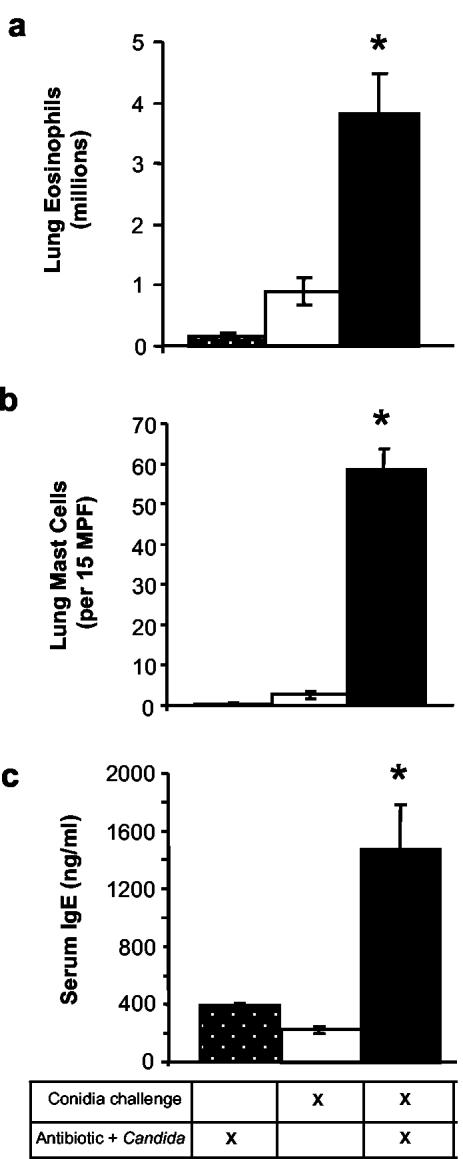
We exposed mice intranasally to mold spores (A. fumigatus conidia) at days 2 and 9 posttreatment to determine whether the combination of antibiotic therapy and concomitant increase in the fungal microbiota could promote the development of a hypersensitivity response to pulmonary allergen exposure. Intranasal Aspergillus conidia exposure did not lead to detectable Aspergillus infection in any of the treated or untreated groups at the time points examined in these studies (data not shown). The pulmonary immune response parameters that were measured included well-documented features of airway allergic responses to Aspergillus: eosinophilia, increased mast cell numbers, induction of serum immunoglobulin E (IgE), goblet cell metaplasia, and lung leukocyte production of interleukin-5 (IL-5) and IL-13 (5, 26, 52, 53).
The treatment of mice with antibiotics followed by oral gavage of C. albicans (Anb/Ca group) did not induce any changes in the pulmonary allergic and inflammatory response parameters measured in these studies if the mice were not exposed to conidia (Fig. 3 and 4). Exposure of mice to conidia in the absence of antibiotic therapy and C. albicans growth resulted in the production of gamma interferon (IFN-γ) by lung leukocytes (Fig. 4c) and an increased number of neutrophils in the lungs (data not shown), but otherwise, the response in the airways consisted of few eosinophils and mast cells, low-level production of IL-5, IL-13, and serum IgE, and minimal goblet cell metaplasia (Fig. 3 to 5).
FIG. 3.
Effect of antibiotic therapy and fungal microbiota increase on the host response to intranasal conidia exposure. Mice were treated as described in the legend to Fig. 1 and analyzed at day 12 posttreatment. (a) Leukocytes were isolated from lungs by enzymatic digestion and mechanical dispersion. Lung eosinophils were phenotyped by Wright-Giemsa staining of cytospun samples. Results are expressed as the increase in the mean number of lung eosinophils per mouse ± the standard error of the mean compared to unchallenged, untreated mice (<105 eosinophils/lung). (b) Lung mast cells were phenotyped by toluidine blue staining of histological sections. Results are expressed as mean number of leukocytes per 15 medium-power fields (200×); unchallenged, untreated mice showed <1 mast cell/medium-power field. (c) Total concentrations of IgE in serum were measured by using ELISA. Unchallenged, untreated mice produced <150 ng of IgE/ml. n = 7 to 9 mice per time point pooled from two separate experiments. Asterisks, P < 0.01 for group 3 compared to groups 1 and 2.
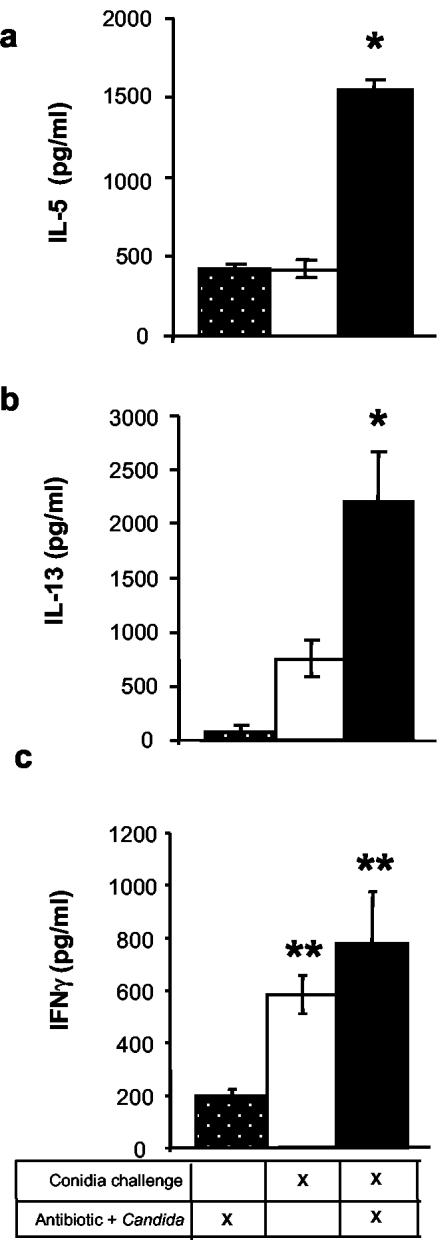
FIG. 4.
Effect of antibiotic therapy and fungal microbiota increase on lung leukocyte cytokine production in response to intranasal conidia exposure. Mice were treated as described in the legend to Fig. 1. At day 12 posttreatment, leukocytes were isolated from whole lungs and cultured (5 × 106 cells/ml) for 24 h without additional stimulation. Supernatants were collected and assayed by ELISA for IL-5 (a), IL-13 (b), and IFN-γ (c). Results are expressed as the increase in the levels of cytokine production compared to equivalent numbers of lung leukocytes from unchallenged, untreated mice (mean ± standard error of the mean). Lung leukocyte cultures from unchallenged, untreated mice produced <50 pg of IFNγ and IL-13/ml and <500 pg of IL-5/ml. n = 7 to 9 mice per time point. The experiments were repeated two times with similar results. Asterisks, P < 0.015 for group 3 compared to groups 1 and 2; double asterisks, P < 0.015 compared to group 1 only.
FIG. 5.
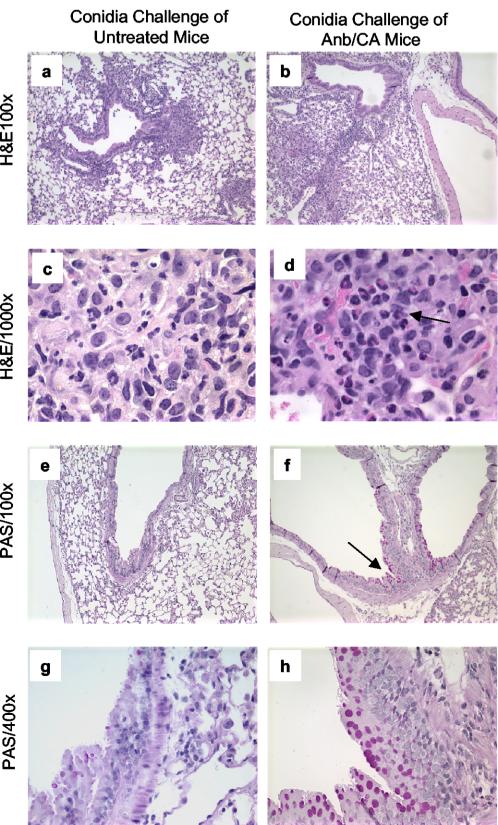
Effect of antibiotic therapy and fungal microbiota increase on lung inflammation and goblet cell metaplasia in response to intranasal conidia exposure. Mice were treated as described in the legend to Fig. 1. At day 12 posttreatment, lungs were harvested, fixed, sectioned, and stained with hematoxylin and eosin (H&E) (a to d) or PAS (e to h). In panel d, the arrow highlights the presence of numerous eosinophils in mice treated with antibiotics and colonized with GI C. albicans. In panel f, PAS stains mucus pink, indicating goblet cell metaplasia in the airway epithelium (arrow). Magnifications and groups are indicated on the photomicrographs.
In sharp contrast, conidia exposure produced a significant allergic response in the lungs of Anb/Ca mice (Fig. 3 to 5). The number of eosinophils and mast cells in the lungs increased (Fig. 3a and b). Serum IgE levels were significantly higher (Fig. 3c). Production of IL-5 and IL-13 by lung leukocytes was elevated (Fig. 4). Neutrophil influx and IFN-γ levels were elevated compared to those of unexposed Anb/Ca mice but similar to those seen in the conidia only group (Fig. 4c and data not shown). The eosinophilic nature of the inflammatory response was evident in a histological analysis of the lungs (Fig. 5). In addition, the high IL-13 levels were accompanied by widespread goblet cell metaplasia, as indicated by the increased number of periodic acid-Schiff (PAS) staining cells in the airways (Fig. 5). High-level production of IL-13 is both necessary and sufficient to induce the features of allergic disease including goblet cell metaplasia (60, 66). While we were unable to demonstrate that the lung leukocytes produced IL-4, the increase in serum IgE levels in Anb/Ca mice following conidia exposure indirectly indicated that IL-4 was induced in these mice (Fig. 2c). Thus, conidia exposure produced a significant allergic airway response in Anb/Ca mice but not in untreated mice.
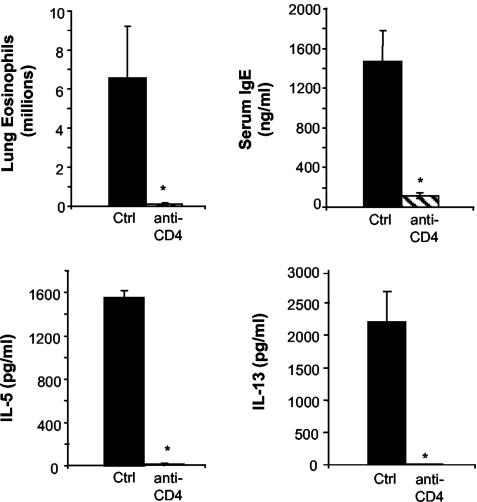
Our next objective was to determine whether the allergic response in the airways of Anb/Ca mice was a CD4 T-cell-mediated response (Th2). Anb/Ca mice that were exposed to conidia were treated with a CD4 T-cell-depleting monoclonal antibody prior to mold spore exposure. This well-established procedure reliably renders the mice CD4 T-cell deficient and resulted in a 99.95% reduction in CD4 T cells in these experiments (data not shown). In CD4 T-cell-deficient Anb/Ca mice, pulmonary eosinophil numbers, lung IL-5 and IL-13 production, and serum IgE levels did not increase following conidia exposure (Fig. 6). In contrast, all of these allergic response indices were elevated in CD4 T-cell-sufficient Anb/Ca mice (Fig. 6). The conclusion from these studies is that the response was CD4 T-cell mediated, and the implication is that alterations in the microbiota populations can have a significant impact on the development of a CD4 Th2 response in the lungs.
FIG. 6.
Effect of CD4 T-cell depletion on the development of pulmonary hypersensitivity responses to conidia exposure in antibiotic-treated mice or mice with altered microbiota (Anb/Ca). All mice were treated with cefoperazone followed by C. albicans gavage and intranasal conidia challenge as described in the legend to Fig. 1. For CD4+-T-cell depletion, mice were injected with anti-CD4 monoclonal antibody (GK1.5) at days 0 and 7. n = 4 mice per group. Asterisks, P < 0.01 compared to undepleted challenged Anb/Ca mice (group 1).
DISCUSSION
Our studies present a system “perturbation” that is caused by an interrelated, multifactoral process. We have presented a model of a clinically feasible, common scenario that occurs for a number of humans: antibiotic treatment followed by a non-life-threatening low-grade increase in fungal microbiota accompanied by an increase in the enteric microbiota. These are interrelated, dependent processes. In our studies, we have viewed this as one “change,” a perturbation in the system. Our data demonstrate that the perturbation itself does not elicit an allergic response. Airway antigen exposure alone also does not elicit an allergic response. However, if the system is perturbed and then antigen exposure occurs, a vigorous airway allergic response develops. This is a cause-and-effect equation with two variables, (i) antibiotic-induced change with fungal microbiota increase and (ii) airway exposure to antigen. The conclusion of these studies is that inhalation of antigen induces an allergic response only when the physiologic perturbation has occurred. This study may raise more questions than it answers. However, it is important to realize that this study definitively points out one very important point: we have demonstrated in an animal model a type of system perturbation that allows airway allergic responses to be generated where they would not normally occur. This perturbation is a clinically relevant model. We do not know which aspect of the perturbation is most important; however, the perturbation is the cause of the immune deviation.
These studies address a major concept of the hygiene hypothesis and experimentally test in mice whether the correlation between antibiotics or microbiota changes and allergies in humans could be a cause-and-effect relationship. Repeated Aspergillus spore exposure of untreated mice did not produce any indices of an allergic response. In sharp contrast, repeated Aspergillus spore exposure of Anb/Ca (microbiota-disrupted) mice produced a strong CD4 T-cell-mediated allergic response as indicated by increased levels of eosinophils, mast cells, IL-5, IL-13, IFN-γ, IgE, and mucus-secreting cells in the airways. The presence of IFN-γ along with Th2 cytokines in the lungs is consistent with an allergic airway response (19).
Is this response restricted to mold spores and C57BL/6 mice? We have performed additional studies similar to those described in this paper using BALB/c mice for mold spore challenge. An allergic response also developed in the lungs of Anb/Ca but not non-antibiotic-treated BALB/c mice (our unpublished data). We also used a similar multiple intranasal challenge protocol with ovalbumin (OVA) in Anb/Ca and non-antibiotic-treated BALB/c mice (which did not include any systemic priming to OVA). The allergic response in the airways of untreated mice exposed intranasally to OVA was low, while Anb/Ca mice produced a significant allergic response in the airways (our unpublished data). Thus, our additional studies indicate that the pulmonary allergic response in mice with altered microbiota can occur in other inbred genetic backgrounds of mice and in response to nonfungal antigens.
In this study, we have demonstrated that the physiologic perturbation of cefoperazone and increased yeast microbiota can allow or promote the development of an allergic airway response upon subsequent exposure to Aspergillus spores. This is a complex physiologic perturbation but one that is clinically common. While our studies demonstrate a cause-and-effect relationship between this perturbation and the subsequent response, at this point we cannot identify which factors are most important. Cephalosporins and other antibiotics have been shown to have direct effects on leukocytes (for examples, see references 34, 48, 51, 61, and 63). This is not likely the major mechanism of immune deviation in our mice because exposure of mice treated with antibiotics but not inoculated with C. albicans to Aspergillus conidia did not induce the fulminant allergic response seen in Anb/Ca mice (data not shown), indicating that both antibiotic-induced and fungal microbiota changes appear to be necessary for promoting an allergic response in the airways. At this point, we cannot rule out the possibility that the growth of the fungal microbiota alone is all that is necessary. However, it must be kept in mind that growth of the fungal microbiota is largely influenced by the bacterial microbiota, which in turn will be significantly influenced by antibiotics. Our data demonstrate that this complex physiologic perturbation can alter immune regulation in the lungs, leading to allergic airway disease upon mold spore exposure.
The studies presented here indicate that alterations in the microbiota can significantly modify a T-cell-mediated immune response in the lungs. It has been proposed that the lung microenvironment is generally predisposed to Th2 responses (9). However, repeated intranasal antigen exposure in the lungs leads to decreasing reactivity, a form of tolerance (18, 59). Emerging models of T-regulatory responses suggest that both Th1 and Th2 responses can be down-modulated by regulatory T cells (Treg cells) and/or Th3 responses (38). Oral tolerance is an example of a Th3 response that has been studied in models of experimental allergic encephalitis (40, 64). Oral tolerance can suppress the Th1 response in the central nervous system during experimental allergic encephalitis (40, 64) and has also been reported to modulate an airway Th2 response in a model of OVA hypersensitivity (8). The GI microbiota likely plays an important role in tolerance since oral tolerance cannot be generated in germfree mice (35). The mechanism underlying this phenomenon remains to be determined. A number of studies have demonstrated that fluids, particles, and microbes introduced into the nasal cavity are largely found in the GI tract shortly thereafter (13, 46, 55). Even volumes as small as 2.5 μl introduced intranasally into mice will largely end up being swallowed due to the mucociliary anatomy of the nasopharyngeal cavity (46). Thus, the GI tract will be exposed to any antigens to which the respiratory tract is also exposed. Since ingestion of antigens can induce tolerance to that antigen (“oral tolerance” [71]), the GI tract may act as a “sensor” for the development of tolerance to inhaled antigens. Oral tolerance is believed to be mediated by regulatory T-cell (Treg/Th3) responses and these Treg/Th3 cells may down-modulate Th2 responses in the airways to the same antigens (49, 50, 69, 71). Furthermore, oral tolerance is defective in germfree mice (58), indicating a role for the microbiota in the development of the response. Thus, our data are consistent with the hypothesis that alterations of the GI bacterial and fungal microbiota may prevent the development of Treg/Th3 responses that control overexuberant mucosal Th2 responses to inhaled antigens such as mold spores.
This is also the first animal model of fungal airway allergy in which immunocompetent mice develop an allergic response to mold spores without prior systemic immunization with the fungus or fungal antigens. It has been previously demonstrated that some pure Aspergillus proteins alone can induce an airway allergic response (17, 28, 33); however, intranasal challenge with Aspergillus spores, culture filtrate, or mycelial extracts cannot induce an allergic response without prior systemic sensitization (16, 17, 21, 31, 32). Systemic immunization and airway challenge models have been extremely useful for the study of the manifestation and pathological process of the allergic response. However, these models cannot address potential afferent mechanisms of allergic responses in humans because humans are exposed to mold spores via inhalation, not systemic immunization. Our studies present a new model to study the afferent phase of allergic airway responses.
These studies also indicate that increased numbers of yeast cells in the microbiota can be a contributing factor in upregulating Th2 responses to antigen exposure in the lungs. In these studies, C. albicans was never isolated from the lungs, even immediately following gavage, and >99.9% of the yeast cells were found in the GI tract when analyzed 1 day posttreatment. If oral C. albicans was not included in the system perturbation (i.e., mice treated with antibiotic only), then a fulminant allergic response did not occur (data not shown), indicating a requirement for an alteration in the fungal microbiota in this system. C. albicans is a normal constituent of the human microbiota (14), and one potential mechanism for the immunomodulatory activity of C. albicans is via the production of prostaglandin-like oxylipins (41). C. albicans (and many other fungi) secrete PGE2- and PGD2-like molecules de novo or via conversion of exogenous arachidonic acid (42, 43). A PGE2-cross-reactive compound can be purified from C. albicans and other fungi that are biologically active on mammalian cells with activity comparable to that of purified PGE2 (42). Prostaglandins such as PGE2 and PGD2 are potent immunomodulatory molecules (3, 37, 54), and microbe-derived PGD2 can alter dendritic cell migration and biology (2, 20). Fungal cell wall glucans are also powerful inflammatory stimulants in tissues (15) and may also play a role in the immunomodulatory activity of yeast in the GI tract. Thus, increased levels of fungal microbiota, such as often occurs during antibiotic therapy, may diminish the ability to generate Treg/Th3 responses to swallowed antigens, possibly by interfering with tolerance-inducing antigen presentation via fungal oxylipins and glucans.
The studies presented here are the first to examine whether antibiotics and fungal microbiota changes can promote the development of an airway Th2 response. The potential link between antibiotic use and allergies is one of the major observations that led to the hygiene hypothesis of allergic diseases (56, 67). We propose that the link between antibiotic use and dysregulated pulmonary immunity is through antibiotic-induced long-term alterations in the bacterial and fungal GI microbiota, which we predict disrupts the regulatory T-cell response. We have also demonstrated that enteric bacteria numbers can remain elevated for 2 weeks after the end of antibiotic therapy, and we have additional data that these numbers remain stably elevated for at least 3 weeks (data not shown). Numerous studies have shown a correlation between altered fecal bacterial microbiota counts (including high levels of enteric bacteria) and the development of allergies (4, 6, 23, 29). A number of other studies have suggested a correlation between antibiotic use and allergies in humans (1, 12, 39). Furthermore, other factors that may affect the incidence of allergies, such as diet and probiotic therapy (24, 25, 47), also affect the GI microbiota composition. So, while it remains to be tested whether human allergies result from altered microbiota, we have demonstrated in an animal model that antibiotic use leading to altered bacterial and fungal microbiota can allow the development of an airway allergic response to subsequent allergen exposure via the nose. These studies also provide a conceptual mechanistic framework for investigating whether probiotic and prebiotic regimens may be useful in preventing or reducing allergies in infants and even adults.
Acknowledgments
We thank Rod McDonald and Nicole Falkowski for technical support.
This work was supported by a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs-Wellcome Fund (G.B.H.). M.C.N. was supported by NIH-NHLBI training grant 2T32HL007749-11. Additional support was provided by the following grants from the National Institutes of Health: RO1-HL65912 (G.B.H.) and RO1-AI059201 (G.B.H.).
Editor: T. R. Kozel
REFERENCES
- 1.Alm, J. S., J. Swartz, G. Lilja, A. Scheynius, and G. Pershagen. 1999. Atopy in children of families with an anthroposophic lifestyle. Lancet 353:1485-1488. [DOI] [PubMed] [Google Scholar]
- 2.Angeli, V., C. Faveeuw, O. Roye, J. Fontaine, E. Teissier, A. Capron, I. Wolowczuk, M. Capron, and F. Trottein. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 4.Bjorksten, B., E. Sepp, K. Julge, T. Voor, and M. Mikelsaar. 2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108:516-520. [DOI] [PubMed] [Google Scholar]
- 5.Blease, K., C. Jakubzick, J. Westwick, N. Lukacs, S. L. Kunkel, and C. M. Hogaboam. 2001. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 166:5219-5224. [DOI] [PubMed] [Google Scholar]
- 6.Bottcher, M. F., E. K. Nordin, A. Sandin, T. Midtvedt, and B. Bjorksten. 2000. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 30:1590-1596. [DOI] [PubMed] [Google Scholar]
- 7.2001. Calderone, R. A. (ed.) 2001. Candida and candidiasis. ASM Press, Washington, D.C.
- 8.Chung, Y., J. Cho, Y. S. Chang, S. H. Cho, and C. Y. Kang. 2002. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology 206:408-423. [DOI] [PubMed] [Google Scholar]
- 9.Constant, S. L., K. S. Lee, and K. Bottomly. 2000. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur. J. Immunol. 30:840-847. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, M. D., P. W. Kincade, D. E. Bockman, and A. R. Lawton. 1974. Origin, distribution and differentiation of IgA-producing cells. Adv. Exp. Med. Biol. 45:13-22. [DOI] [PubMed] [Google Scholar]
- 11.Demeure, C. E., L.-P. Yang, C. Desjardins, P. Raynauld, and G. Delespesse. 1997. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 27:3526-3531. [DOI] [PubMed] [Google Scholar]
- 12.Droste, J. H., M. H. Wieringa, J. J. Weyler, V. J. Nelen, P. A. Vermeire, and H. P. Van Bever. 2000. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 30:1547-1553. [DOI] [PubMed] [Google Scholar]
- 13.Eyles, J. E., I. D. Spiers, E. D. Williamson, and H. O. Alpar. 2001. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J. Pharm. Pharmacol. 53:601-607. [DOI] [PubMed] [Google Scholar]
- 14.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogelmark, B., J. Thorn, and R. Rylander. 2001. Inhalation of (1→3)-beta-D-glucan causes airway eosinophilia. Mediat. Inflamm. 10:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunig, G., D. B. Corry, M. W. Leach, B. W. Seymour, V. P. Kurup, and D. M. Rennick. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunig, G., and V. P. Kurup. 2003. Animal models of allergic bronchopulmonary aspergillosis. Front. Biosci. 8:e157-e171. [DOI] [PubMed] [Google Scholar]
- 18.Hall, G., C. G. Houghton, J. U. Rahbek, J. R. Lamb, and E. R. Jarman. 2003. Suppression of allergen reactive Th2 mediated responses and pulmonary eosinophilia by intranasal administration of an immunodominant peptide is linked to IL-10 production. Vaccine 21:549-561. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, G., G. Berry, R. H. DeKruyff, and D. T. Umetsu. 1999. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Investig. 103:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herve, M., V. Angeli, E. Pinzar, R. Wintjens, C. Faveeuw, S. Narumiya, A. Capron, Y. Urade, M. Capron, G. Riveau, and F. Trottein. 2003. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur. J. Immunol. 33:2764-2772. [DOI] [PubMed] [Google Scholar]
- 21.Hogaboam, C. M., K. Blease, B. Mehrad, M. L. Steinhauser, T. J. Standiford, S. L. Kunkel, and N. W. Lukacs. 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 156:723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Study of Asthma and Allergies in Childhood Steering Committee. 1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 351:1225-1232. [PubMed] [Google Scholar]
- 23.Kalliomaki, M., P. Kirjavainen, E. Eerola, P. Kero, S. Salminen, and E. Isolauri. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107:129-134. [DOI] [PubMed] [Google Scholar]
- 24.Kalliomaki, M., S. Salminen, H. Arvilommi, P. Kero, P. Koskinen, and E. Isolauri. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076-1079. [DOI] [PubMed] [Google Scholar]
- 25.Kankaanpaa, P., K. Nurmela, A. Erkkila, M. Kalliomaki, D. Holmberg-Marttila, S. Salminen, and E. Isolauri. 2001. Polyunsaturated fatty acids in maternal diet, breast milk, and serum lipid fatty acids of infants in relation to atopy. Allergy 56:633-638. [DOI] [PubMed] [Google Scholar]
- 26.Kauffman, H. F. 2003. Immunopathogenesis of allergic bronchopulmonary aspergillosis and airway remodeling. Front. Biosci. 8:e190-e196. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, M. J., and P. A. Volz. 1985. Effect of various antibiotics on gastrointestinal colonization and dissemination by C. albicans. Sabouraudia 23:265-273. [DOI] [PubMed] [Google Scholar]
- 28.Kheradmand, F., A. Kiss, J. Xu, S. H. Lee, P. E. Kolattukudy, and D. B. Corry. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169:5904-5911. [DOI] [PubMed] [Google Scholar]
- 29.Kirjavainen, P. V., T. Arvola, S. J. Salminen, and E. Isolauri. 2002. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut 51:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurup, V., H. Shen, and B. Banerjee. 2000. Respiratory fungal allergy. Microbes Infect. 2:1101-1110. [DOI] [PubMed] [Google Scholar]
- 31.Kurup, V. P., H. Choi, A. Resnick, J. Kalbfleisch, and J. N. Fink. 1990. Immunopathological response of C57BL/6 and C3H/HeN mice to Aspergillus fumigatus antigens. Int. Arch. Allergy Appl. Immunol. 91:145-154. [DOI] [PubMed] [Google Scholar]
- 32.Kurup, V. P., S. Mauze, H. Choi, B. W. Seymour, and R. L. Coffman. 1992. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J. Immunol. 148:3783-3788. [PubMed] [Google Scholar]
- 33.Kurup, V. P., J. Q. Xia, R. Crameri, D. A. Rickaby, H. Y. Choi, S. Fluckiger, K. Blaser, C. A. Dawson, and K. J. Kelly. 2001. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin. Immunol. 98:327-336. [DOI] [PubMed] [Google Scholar]
- 34.Larson, S. E., G. J. DaMert, C. Collins-Lech, and P. G. Sohnle. 1980. Direct stimulation of lymphokine production by cephalothin. J Infect. Dis. 142:265-272. [DOI] [PubMed] [Google Scholar]
- 35.Maeda, Y., S. Noda, K. Tanaka, S. Sawamura, Y. Aiba, H. Ishikawa, H. Hasegawa, N. Kawabe, M. Miyasaka, and Y. Koga. 2001. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer's patches under germfree conditions. Immunobiology 204:442-457. [DOI] [PubMed] [Google Scholar]
- 36.Mannino, D. M., D. M. Homa, C. A. Pertowski, A. Ashizawa, L. L. Nixon, C. A. Johnson, L. B. Ball, E. Jack, and D. S. Kang. 1998. Surveillance for asthma—United States, 1960-1995. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 47:1-27. [PubMed] [Google Scholar]
- 37.Matsuoka, T., M. Hirata, H. Tanaka, Y. Takahashi, T. Murata, K. Kabashima, Y. Sugimoto, T. Kobayashi, F. Ushikubi, Y. Aze, N. Eguchi, Y. Urade, N. Yoshida, K. Kimura, A. Mizoguchi, Y. Honda, H. Nagai, and S. Narumiya. 2000. Prostaglandin D2 as a mediator of allergic asthma. Science 287:2013-2017. [DOI] [PubMed] [Google Scholar]
- 38.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 39.McKeever, T. M., S. A. Lewis, C. Smith, J. Collins, H. Heatlie, M. Frischer, and R. Hubbard. 2002. Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands General Practice Research Database. J. Allergy Clin. Immunol. 109:43-50. [DOI] [PubMed] [Google Scholar]
- 40.Moreau, M. C., M. Coste, V. Gaboriau, and C. Dubuquoy. 1995. Oral tolerance to ovalbumin in mice: effect of some parameters on the induction and persistence of the suppression of systemic IgE and IgG antibody responses. Adv. Exp. Med. Biol. 371B:1229-1234. [PubMed] [Google Scholar]
- 41.Noverr, M. C., J. R. Erb-Downward, and G. B. Huffnagle. 2003. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 16:517-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noverr, M. C., D. D. Williams, G. B. Toews, and G. B. Huffnagle. 2002. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 70:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters-Golden, M. 2002. ‘Good’ lipids for asthma. Nat. Med. 8:931-932. [DOI] [PubMed] [Google Scholar]
- 45.Peters-Golden, M. 2002. Open mind, open airways: broadening the paradigm of prostaglandins and allergic airway inflammation. Am. J. Respir. Crit. Care Med. 165:1035-1036. [DOI] [PubMed] [Google Scholar]
- 46.Pickett, T. E., M. F. Pasetti, J. E. Galen, M. B. Sztein, and M. M. Levine. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect. Immun. 68:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenfeldt, V., E. Benfeldt, S. D. Nielsen, K. F. Michaelsen, D. L. Jeppesen, N. H. Valerius, and A. Paerregaard. 2003. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 111:389-395. [DOI] [PubMed] [Google Scholar]
- 48.Rouveix, B., F. Groult, and M. Levacher. 1987. Beta-lactam antibiotics and human lymphocyte function: the in vitro effect on blastogenesis, lymphokine production and suppressor cell functions. Int. J. Immunopharmacol. 9:567-575. [DOI] [PubMed] [Google Scholar]
- 49.Russo, M., S. Jancar, A. L. Pereira de Siqueira, J. Mengel, E. Gomes, S. M. Ficker, and A. M. Caetano de Faria. 1998. Prevention of lung eosinophilic inflammation by oral tolerance. Immunol. Lett. 61:15-23. [DOI] [PubMed] [Google Scholar]
- 50.Russo, M., M. A. Nahori, J. Lefort, E. Gomes, A. de Castro Keller, D. Rodriguez, O. G. Ribeiro, S. Adriouch, V. Gallois, A. M. de Faria, and B. B. Vargaftig. 2001. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am. J. Respir. Cell Mol. Biol. 24:518-526. [DOI] [PubMed] [Google Scholar]
- 51.Scheffer, J., and W. Konig. 1993. Cephalosporins and inflammatory host reactions. Respiration 60(Suppl. 1):25-31. [DOI] [PubMed] [Google Scholar]
- 52.Schuh, J. M., K. Blease, and C. M. Hogaboam. 2002. CXCR2 is necessary for the development and persistence of chronic fungal asthma in mice. J. Immunol. 168:1447-1456. [DOI] [PubMed] [Google Scholar]
- 53.Schuh, J. M., C. A. Power, A. E. Proudfoot, S. L. Kunkel, N. W. Lukacs, and C. M. Hogaboam. 2002. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 16:1313-1315. [DOI] [PubMed] [Google Scholar]
- 54.Snijdewint, F. G., P. Kalinski, E. A. Wierenga, J. D. Bos, and M. L. Kapsenberg. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 150:5321-5329. [PubMed] [Google Scholar]
- 55.Southam, D. S., M. Dolovich, P. M. O'Byrne, and M. D. Inman. 2002. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L833-L839. [DOI] [PubMed] [Google Scholar]
- 56.Strachan, D. P. 1989. Hay fever, hygiene, and household size. BMJ 299:1259-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strassmann, G., V. Patil-Koota, F. Finkelman, M. Fong, and T. Kambayashi. 1994. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 180:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sudo, N., S. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 59.Takabayashi, K., L. Libet, D. Chisholm, J. Zubeldia, and A. A. Horner. 2003. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in Th2-sensitized mice. J. Immunol. 170:3898-3905. [DOI] [PubMed] [Google Scholar]
- 60.Taube, C., C. Duez, Z. H. Cui, K. Takeda, Y. H. Rha, J. W. Park, A. Balhorn, D. D. Donaldson, A. Dakhama, and E. W. Gelfand. 2002. The role of IL-13 in established allergic airway disease. J. Immunol. 169:6482-6489. [DOI] [PubMed] [Google Scholar]
- 61.Ueta, E., K. Yoneda, T. Yamamoto, and T. Osaki. 1992. Upregulatory effects of cefpimizole natrium on human leukocytes. Int. J. Immunopharmacol. 14:877-885. [DOI] [PubMed] [Google Scholar]
- 62.Umetsu, D. T., J. J. McIntire, O. Akbari, C. Macaubas, and R. H. DeKruyff. 2002. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 3:715-720. [DOI] [PubMed] [Google Scholar]
- 63.Villa, M. L., S. Armelloni, E. Ferrario, F. Ottaviani, and M. Clerici. 1991. Interference of cephalosporins with immune response: effects of cefonicid on human T-helper cells. Int. J. Immunopharmacol. 13:1099-1107. [DOI] [PubMed] [Google Scholar]
- 64.Whitacre, C. C., I. E. Gienapp, A. Meyer, K. L. Cox, and N. Javed. 1996. Oral tolerance in experimental autoimmune encephalomyelitis. Ann. N. Y. Acad. Sci. 778:217-227. [DOI] [PubMed] [Google Scholar]
- 65.Wickens, K., N. Pearce, J. Crane, and R. Beasley. 1999. Antibiotic use in early childhood and the development of asthma. Clin. Exp. Allergy 29:766-771. [DOI] [PubMed] [Google Scholar]
- 66.Wills-Karp, M., J. Luyimbazi, X. Xu, B. Schofield, T. Y. Neben, C. L. Karp, and D. D. Donaldson. 1998. Interleukin-13: central mediator of allergic asthma. Science 282:2258-2261. [DOI] [PubMed] [Google Scholar]
- 67.Wills-Karp, M., J. Santeliz, and C. L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69-75. [DOI] [PubMed] [Google Scholar]
- 68.Wjst, M., B. Hoelscher, C. Frye, H. E. Wichmann, S. Dold, and J. Heinrich. 2001. Early antibiotic treatment and later asthma. Eur. J. Med. Res. 6:263-271. [PubMed] [Google Scholar]
- 69.Wu, X. M., M. Nakashima, and T. Watanabe. 1998. Selective suppression of antigen-specific Th2 cells by continuous micro-dose oral tolerance. Eur. J. Immunol. 28:134-142. [DOI] [PubMed] [Google Scholar]
- 70.Yamanaka, T., L. Helgeland, I. N. Farstad, H. Fukushima, T. Midtvedt, and P. Brandtzaeg. 2003. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J. Immunol. 170:816-822. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, X., L. Izikson, L. Liu, and H. L. Weiner. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245-4253. [DOI] [PubMed] [Google Scholar]