Abstract
We compared the immune defense of mice with chronic granulomatous disease (CGD mice) with that of wild-type C57BL/6 mice for their response to Sporothrix schenckii. A subcutaneous injection of 5 × 104 CFU S. schenckii strain IFM41598 into CGD mice resulted in systemic infection and death within 84 days. In contrast, injected C57BL/6 mice did not develop systemic infection and were able to survive through 100 days of observation. Differences in host resistance were analyzed in vitro. Neutrophils and macrophages obtained from CGD mice were found to allow greater growth of this organism than did those obtained from C57BL/6 mice. Moreover, macrophages obtained from immunized CGD mice were able to simply inhibit the growth of this fungus whereas macrophages obtained from immunized C57BL/6 mice killed the fungus within 48 h after phagocytosis. These results suggest that (i) the lack of NADPH oxidase function is a risk factor for lethal S. schenckii infection and (ii) superoxide anion and its reactive oxidative metabolites produced by neutrophils and macrophages are involved in fungistatic and fungicidal activities.
Sporothrix schenckii is a dimorphic fungus that is widely distributed in nature. It causes sporotrichosis, a subcutaneous infection, in humans. The subcutaneous infection usually stays localized in the skin, although it can spread along the lymphatics or become ulcerative. The infection, however, has been reported to sometimes develop into systemic mycosis in humans.
The defense mechanisms of humans are known to be composed of both innate and adaptive immunity. Innate immunity involves phagocytosis and intracellular killing by neutrophils and macrophages that act during the early stages of infection, while adaptive immunity involves T-cell stimulation, macrophage activation, and antibody production (19). Neutrophils and macrophages kill microorganisms by generating microbicidal reactive oxygen species, such as superoxide anions, hydrogen peroxide, hydroxyl anions, and hypochlorous acid (18). In 1998, a severe Sporothrix infection, which turned fatal, was reported among AIDS patients (1), indicating that CD4+ T-cell-mediated immunity is important to protect against S. schenckii infection in humans. In fact, there are animal experiments that support this clinical observation. It has been reported that nude mice are more susceptible to sporotrichosis (5) and that acquired immunity to S. schenckii is expressed mainly by macrophages activated by CD4+ T cells (21, 22).
In contrast, the role(s) of neutrophils in protection against this fungus has not been well studied, and the data are controversial. Cunningham et al. (4) studied phagocytosis and intracellular killing of S. schenckii in human polymorphonuclear cells and concluded that the H2O2-KI-myeloperoxidase system is important for fungicidal activity in vitro. In contrast, Schaffner et al. (20) reported that virulent S. schenckii is resistant to killing by neutrophils and also by H2O2. As a result of this controversy, no clear conclusion can be drawn about the role of reactive oxygen species produced by neutrophils in the host defense against S. schenckii.
Chronic granulomatous disease (CGD) is a rare inherited disorder due to the defective function of the NADPH oxidase complex. As a result of this defect, generation of microbicidal reactive oxidants is impaired; hence phagocytes from CGD patients cannot sufficiently kill H2O2-nonproducing or catalase-producing organisms (13, 23). This deficiency in the key host defense pathway is the reason why CGD patients suffer from recurrent life-threatening bacterial and fungal infections, including those due to Aspergillus, Torulopsis, and Candida species (3). Most CGD patients with fungal infection have fungal pneumonia and/or widely disseminated diseases (3). S. schenckii is also an H2O2-nonproducing and catalase-producing fungus. Hence, we considered that this fungus might also threaten CGD patients. Since this genetic defect effectively eliminates the generation of microbicidal oxidative species, we decided to examine the role(s) of these oxidative species in phagocytes during S. schenckii infection. We used a mouse strain that has been established as a mouse model for an X-linked (gp91-phox−) form of CGD, generated by Pollock et al. (17). In this study, we injected S. schenckii into mice with CGD (CGD mice) and their wild-type C57BL/6 counterparts and compared the susceptibility of the mice, dissemination of the parasites to organs, and antifungal activity of neutrophils and macrophages after in vitro and in vivo activation. Here we report that CGD is a risk factor for fatal dissemination of S. schenckii.
MATERIALS AND METHODS
Fungus.
S. schenckii IFM 41598 isolated from lungs of humans with sporotrichosis were provided by K. Nishimura (Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Japan). This fungus shows dimorphism depending on the culture temperature; that is, the mycelial form occurs at 25°C and the yeast form occurs at 37°C. The organism was cultured for 72h at 37°C on brain heart infusion (Eiken Chemical Co., Ltd., Tokyo, Japan) agar plates containing 1.5% agar and 1% dextrose to obtain the yeast form. The yeasts were washed once with sterile phosphate-buffered saline (PBS) for in vivo infection and RPMI 1640 medium (GIBCO Laboratories, Grand Island, N.Y.) containing 10% (vol/vol) newborn calf serum (NBCS) (GIBCO Laboratories) for in vitro infection. The suspension was examined for viable counts by culture for 4 days at 25°C on BHI agar containing 1% dextrose. The number of yeast-form cells was expressed as CFU.
Mice.
X-linked CGD mice, which were produced by a knockout of gp91-phox from C57BL/6 mice, were donated by M. C. Dinauer (Riley Children's Hospital, Indianapolis, Ind.) and A. Kume (Division of Genetic Therapeutics, Center for Molecular Medicine, Jichi Medical School, Tochigi, Japan), and reproduced in a specific- pathogen-free animal room in the Department of Bacteriology, Faculty of Medical Sciences, Kyushu University, Fukuoka, Japan. Six-week-old X-linked CGD mice were used in the experiment. C57BL/6 mice of the same age, used as controls, were purchased from Japan SLC, Inc., Hamamatsu, Japan. Mice were given food and water ad libitum throughout all the experiments.
Infection experiment.
Cells (5 × 104) of S. schenckii suspended in 0.05 ml of PBS were inoculated subcutaneously into the left hind footpads of mice. The footpad thickness was measured by using a dial thickness gauge caliper (Peacock model G 0.01-10mm; Ozaki Mfg. Co., Ltd.) at intervals after inoculation. The difference between the thickness of the left and right footpads was taken as the footpad swelling. The swelling was noted as an indicator of the degree of inflammation in the footpads. Survival of mice was noted daily until 100 days after infection. To measure the fungal burden after inoculation, the infected feet of mice were cut off at appropriate intervals and homogenized using a homogenizer (model AM type; Nihon Seiki Kaisha Ltd., Japan). Viable counts of fungi in the homogenate were determined as described in the previous section. To assess dissemination of the fungi to internal organs, the liver, left kidney, spleen, and left lung of mice were removed under aseptic conditions, homogenized, and examined for viable counts of fungi. This experiment was reviewed by the Ethics Committee on Animal Experiment in the Faculty of Medical Sciences, Kyushu University, and carried out under the supervision of the Guideline for Animal Experiments in the Faculty of Medical Sciences, Kyushu University, and The Law (no. 105) and Notification (no. 6) of the Government.
Histological examination of footpads.
Mouse footpads were studied for histological examination after 3, 10, and 30 days of infection. The thin sections of formalin-fixed, paraffin-embedded samples were stained with hematoxylin and eosin and periodic acid-Schiff and observed under a light microscope.
Phagocyte monolayer preparation from mice.
Mice were injected intraperitoneally with 2 ml of 8% casein dissolved in PBS to collect neutrophil-rich peritoneal cells or with 2 ml of 4% thioglycolate medium (Difco Laboratory, Detroit, Mich.) to collect macrophages. After 10 h or 4 days, respectively, peritoneal phagocytes were collected by lavaging the peritoneal cavity of C57BL/6 and CGD mice with RPMI 1640 medium containing 10 U of heparin per ml. The cell suspensions obtained from four or five mice were washed with RPMI 1640 medium and pooled. Giemsa staining revealed that the casein-induced or thioglycolate-induced peritoneal cells consisted of more than 70% neutrophils or more than 90% macrophages, respectively. On 96-well flat-bottom tissue culture plates (Falcon no. 353072; Becton Dickinson Labware, Oxnard, Calif.), neutrophil-rich peritoneal cells (henceforth referred to as neutrophils) or macrophages were adjusted to 3 × 105 cells per well while the macrophages were adjusted to 106 cells per well on similar 24-well plates (Falcon no. 353047). RPMI 1640 medium containing 10 Units of heparin per ml was used for cell adjustment. The plates were incubated for 2 h at 37°C in a humid atmosphere containing 5% CO2 in air. After incubation, the wells were washed with sterile PBS to remove nonadherent cells.
Macrophage activation in vitro.
The adherent cells on 24-well tissue culture plates were incubated for 20 h in RPMI 1640 medium containing 1,000 U of recombinant mouse gamma interferon (IFN-γ; 1.1 × 107 U/ml [PBL Biomedical Laboratories, Piscataway, N.J.]) per ml and 10 μg of lipopolysaccharide (LPS; Escherichia coli serotype O111: B4 [Fluka, Buchs, Switzerland]) per ml to activate the adherent macrophages. After the culture media were removed, the cells were washed and used for antifungal assay.
Assay of antifungal activity after in vitro phagocytosis.
For in vitro phagocytosis, 105 S. schenckii cells per well suspended in RPMI 1640 medium containing 10% NBCS were inoculated onto phagocyte monolayers, and the plates were centrifuged at 2,000 × g for 10 min at 4°C to bring the fungi into contact with the surface of the phagocytes. After centrifugation, the plates were incubated for 10 min at 37°C in a water bath. Each well was then washed with sterile cold PBS to remove nonphagocytosed fungi and incubated at 37°C in RPMI 1640 medium containing 10% NBCS in a CO2 incubator. After an appropriate period of incubation, the culture media, which contained fungal cells released from lysed phagocytes, were collected. Fungi inside phagocytes were released by osmotic lysis of phagocytes in sterile distilled water. The two fractions were pooled, and the total numbers of S. schenckii CFU were determined as described above.
Colony inhibition assay.
S. schenckii was suspended in PBS and serially diluted twofold. Aliquots of 0.1 ml of each diluted suspension were layered on neutrophils or macrophages on 96-well plates, prepared as described above. After 4 days of incubation at 37°C in a CO2 incubator, the culture plates were transferred to 25°C and incubated for 4 days. The change of culture temperature was made in order to make colony counting easy by causing the differentiation from the yeast form to the mycelial form. The outgrowth of S. schenckii cells in each well was determined by counting the colonies in each well under an inverted phase-contrast microscope. Cell viability was assayed by the trypan blue dye exclusion test.
Assay of antifungal activity in macrophage of immune mice.
Mice were immunized by subcutaneous inoculation of 5 × 104 S. schenckii cells suspended in 0.05 ml of PBS into the hind footpad. The mice were used as immune mice 30 days after the inoculation. Peritoneal macrophages of C57BL/6 mice and CGD mice were induced by intraperitoneal injection with 2 ml of 4% thioglycolate medium. For in vivo phagocytosis, 3 × 106 cells of S. schenckii suspended in 0.5 ml of PBS were inoculated into the peritoneal cavity of mice after 4 days. After 30 min, peritoneal cells phagocytosing the fungi were harvested, centrifuged (180 × g for 10 min), and resuspended in RPMI 1640 medium supplemented with 10% NBCS. The cell suspension was adjusted to 6 × 104 cells per well on 96-well plates, and the plates were incubated for 48 h at 37°C in an atmosphere of 5% CO2. Fungal CFU were measured as described in “Assay of antifungal activity after in vitro phagocytosis” (above).
Statistical analysis.
Values are reported as mean ± standard deviation. Student's t test was used to compare the differences between groups. A P value of <0.05 was considered statistically significant.
RESULTS
Susceptibility of CGD mice to S. schenckii IFM 41598.
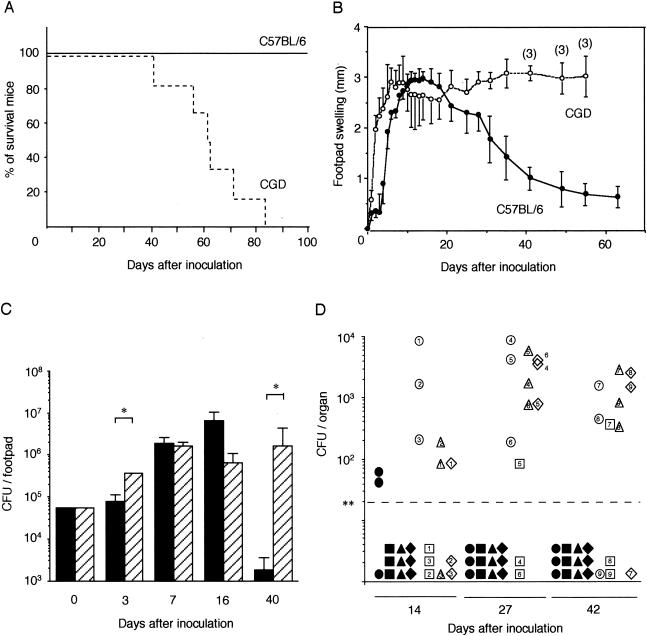
To examine the influence of a defect in NADPH oxidase function on the susceptibility of mice to S. schenckii, we studied the differences in death rate, footpad swelling, histological observation of footpads, and fungal burden in organs between C57BL/6 and CGD mice after subcutaneous inoculation of the footpads. Inoculation of 5 × 104 CFU of the IFM41598 strain resulted in the death of all CGD mice within 84 days. In contrast, all C57BL/6 mice survived for over 100 days of observation (Fig. 1A).
FIG. 1.
Influence of a defect in NADPH oxidase function on the susceptibility of mice to S. schenckii. Survival curves (A), footpad swelling (B), time course of fungal infection in footpads (C), and the appearance of the fungi in several organs (D) of C57BL/6 and CGD mice were examined after subcutaneous infection with 5 × 104 CFU of S. schenckii. (A) The solid line represents the survival curve of C57BL/6 mice (n = 6), and the dotted line represents that of CGD mice (n = 6). (B) Solid circles show the course of footpad swelling of C57BL/6 mice, and open circles show the course of footpad swelling of CGD mice. The data are the means and standard deviations (SD) for five C57BL/6 mice and four CGD mice, except that three CGD mice were used for the measurement after 40 days of infection. (C) The number of fungi are expressed as the mean CFU and SD for three mice. Brackets indicate statistical comparisons. *, P < 0.05. (D) Fungal numbers were determined 14, 27, and 42 days after inoculation. Symbols show the CFU of the fungi in the liver (•, ○), left kidney (▪, □), spleen (▴, Δ) and left lung (⋄, ⋄) of C57BL/6 mice (solid symbols) and CGD mice (open symbols). Symbols with the same numbers mean that the organs were derived from the same mouse. **, detection limit of 20 CFU.
We also measured local footpad swelling, which we took as a rough indicator of inflammation, after the subcutaneous inoculation. Footpad swelling of CGD mice increased at an earlier stage than that of C57BL/6 mice, and the swelling continued until their death (Fig. 1B). Footpad swelling of C57BL/6 mice, on the other hand, reached a peak at 12 to 15 days of infection but decreased thereafter.
To examine the relationship between footpad swelling and the size of the S. schenckii population in the footpads of C57BL/6 and CGD mice, we determined the number of fungi in the footpads at appropriate intervals after subcutaneous infection (Fig. 1C). In the footpads of C57BL/6 mice, the number of fungi increased for 16 days but decreased and, at 40 days after infection, had become about 30-fold smaller than the number inoculated, showing an apparent clearance. In contrast, the number of fungi in the footpads of CGD mice at 40 days after infection was about 30-fold larger than the number inoculated. These results correlate well with the footpad swelling data.
Footpads infected with S. schenckii were used for histological examination (data not shown). At 3 days of infection, infiltration of inflammatory cells was more prominent in CGD mice than in C57BL/6 mice. At 10 days of infection in C57BL/6 mice, the yeast forms of S. schenckii were observed along with many neutrophils and mononuclear cells, although the pus-like inflammatory focus was limited. In contrast, small pus lesions accompanied by many neutrophils and S. schenckii cells were observed in CGD mouse footpads. At 30 days, cell infiltrations, composed mainly of mononuclear cells, were observed in wild-type mice, but S. schenckii cells were not found. In CGD mouse footpads, many pus lesions measuring 200 to 300 μm in diameter and containing many neutrophils and S. schenckii cells were observed in the connective tissue. Diffuse infiltrations of inflammatory cells were observed in the muscle tissue. These histopathological findings correlated well with the footpad swelling (Fig. 1B) and fungal burden in the footpads (Fig. 1C).
The dissemination of S. schenckii into internal organs was examined next. For this, we determined the number of fungi at 14, 27, and 42 days after subcutaneous infection (Fig. 1D). At 27 and 42 days, we detected the fungi disseminating in the liver, left kidney, spleen, and left lung of CGD mice but did not detect them in these organs of C57BL/6 mice.
Hence, an NADPH oxidase defect in mice clearly resulted in an inability to clear S. schenckii from the site of infection despite an apparent inflammation-like response (swelling). The defect also resulted in greater dissemination of the fungi to deeper organs and eventual death.
Assays for antifungal activity of neutrophils. (i) In vitro killing assay.
Since a defect in NADPH oxidase function impairs certain aspects of neutrophil activities, we next examined the ability of neutrophils to suppress fungal growth. Neutrophils from C57BL/6 and CGD mice were collected, and intracellular growth of S. schenckii was examined at 1, 2, 4, and 6 h after in vitro infection of these neutrophils with the fungi. The number of fungi in the cells from CGD mice at 4 h after infection was consistently about 1.5-fold larger than the initial number of phagocytosed fungi, in comparison to only 1.1-fold higher in the cells from C57BL/6 mice (data not shown). However, statistical analysis indicated that this difference was not significant.
(ii) Colony inhibition assay.
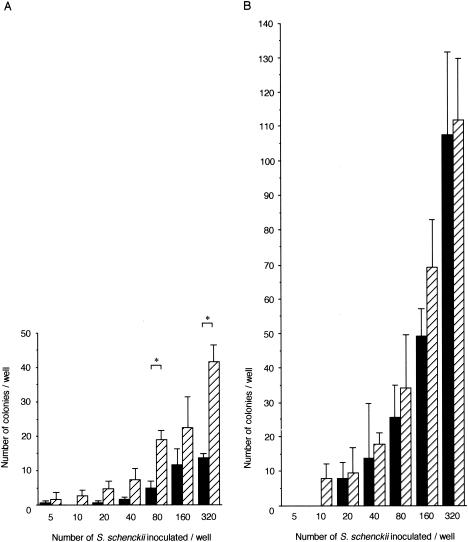
As a result of the outcome of the above experiment, we decided to directly observe the effect of neutrophils on fungal growth in order to compare more closely the difference between neutrophils from CGD and wild-type mice. For this, we modified an assay for the microbicidal activity of macrophages by Nozawa et al. (15) to use with neutrophils. We further modified the quantification procedure so that we could directly count the number of colonies of S. schenckii formed on neutrophil monolayers, 8 days after inoculation, using an inverted phase-contrast microscope. This assay shows that the these fungi survived for 4 days before the plates were transferred to a 25°C incubator and formed colonies thereafter. The viability of these neutrophils was 78 to 89% during the first 4 days after the peritoneal neutrophils were collected (data not shown). When 80 and 320 fungal cells were added in the wells, neutrophils from CGD mice had significantly more colonies than those from C57BL/6 mice after 8 days (Fig. 2A). We also tested isolated peritoneal macrophages by the same methods to examine the contribution of contaminating macrophages to fungal growth in neutrophil-rich peritoneal cells (Fig. 2B). The colony inhibition activity of thioglycolate-induced peritoneal macrophages from either mouse strain was weaker than that of casein-induced peritoneal neutrophils from either strain, and we did not find a significant difference between macrophages from C57BL/6 and CGD mice. Hence, there was an observable decrease in the antifungal activity of casein-induced neutrophils when they were defective in NADPH oxidase function.
FIG. 2.
Inhibition of S. schenckii colony formation by neutrophils (A) and macrophages (B) from C57BL/6 (black bars) and CGD (hatched bars) mice. Data shown are the means and SD obtained from triplicate samples. Brackets indicate statistical comparisons. *, P < 0.05
Assays for antifungal activity of macrophages.
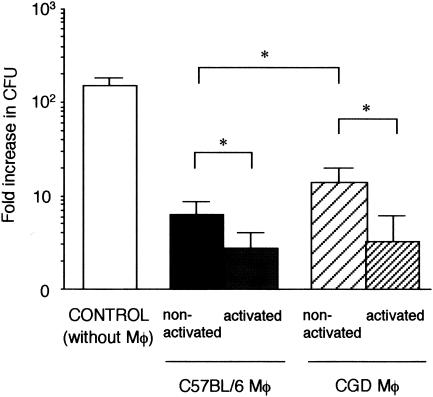
We next analyzed the effect of NADPH oxidase impairment on the antifungal activity of immune-activated macrophages, another aspect that is likely to be affected by this genetic defect. We chose to look at macrophage activation and its effect on intracellular fungal growth. First, in vitro activation of macrophages was attempted. Peritoneal macrophages from C57BL/6 and CGD mice were activated in vitro by LPS and IFN-γ and then infected with S. schenckii (Fig. 3). Intracellular growth of S. schenckii in macrophages was determined after 48 h of infection with the fungi. In all cases, the presence of macrophages resulted in decreased fungal growth compared to that in the control without macrophages. Looking at the nonactivated macrophages, we observed that those from CGD mice allowed the fungi to proliferate more than did those from C57BL/6 mice. Activated macrophages from both C57BL/6 and CGD mice reduced fungal CFU significantly more than nonactivated macrophages did.
FIG. 3.
Antifungal activity of macrophages (Mφ) from C57BL/6 and CGD mice. To activate macrophages, LPS and IFN-γ were added to the macrophage culture. Growth of fungi in the absence of macrophages was also examined as a control. The number of viable fungi in the wells was determined after incubation for 48 h. Data are expressed as means and SD of the fold increase in CFU of six nonactivated macrophages and five activated macrophages. The fold increase in CFU is calculated as (fungal CFU at 48 h after phagocytosis)/(fungal CFU at 0 h after phagocytosis). Brackets indicate statistical comparisons. *, P < 0.05
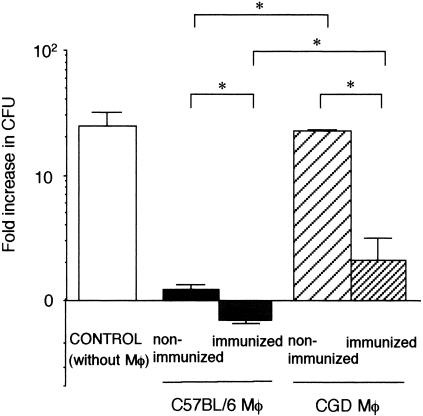
Activation of macrophages during an actual infection is influenced by many interrelated factors in the host biological system, which may not be represented in vitro. Hence, we also examined the antifungal activity of macrophages activated in vivo (Fig. 4). Mice were first immunized with a dose of S. schenckii. Immunized C57BL/6 and CGD mice both have antibodies to this fungus 35 days after subcutaneous inoculation (unpublished observation), and these antibodies may act as opsonizing antibodies for intraperitoneal phagocytosis by macrophages. For the experiment, phagocytosis of the fungi by macrophages was tested by using the peritoneal cavities of nonimmunized or immunized mice. The intracellular growth of phagocytosed fungi in peritoneal macrophages was then evaluated after transferring the macrophages to an in vitro system. Macrophages from nonimmunized CGD mice clearly allowed greater proliferation of this fungus than did those from nonimmunized C57BL/6 mice. Immunization of both C57BL/6 and CGD mice resulted in a significant reduction of CFU. Notably, macrophages from immunized C57BL/6 mice not only inhibited the growth of this fungus but also killed the organism, while those from immunized CGD mice could only inhibit growth. Therefore, under in vivo conditions, the NADPH oxidase defect impairs the fungicidal activity of macrophages in a more pronounced manner than in vitro.
FIG. 4.
Antifungal activity of macrophages (Mφ) from nonimmunized and immunized mice. Mice were immunized by subcutaneous inoculation of S. schenckii 30 days before the challenge. Growth of fungi in the absence of macrophages was also examined as a control. The number of viable fungi in the wells was determined after incubation for 48 h. Data are expressed as means and SD of the fold increase in the CFU in three mice. Fold increase in CFU is calculated as (fungal CFU at 48 h after in vitro culture)/(fungal CFU at 0 h after in vitro culture). Brackets indicate statistical comparisons. *, P < 0.05
DISCUSSION
C57BL/6 mice infected subcutaneously with S. schenckii succeeded in eliminating this fungus at 40 days after infection. In contrast, CGD mice not only were unable to clear the fungi from the site of inoculation, but also permitted it to spread to deeper organs, resulting in fatal systemic mycoses. These results suggest that a defect in NADPH oxidase function causes lethal systemic sporotrichosis, and hence superoxide anion and its metabolites produced by phagocytes play an important role in host protection against this fungus.
To examine the role of microbicidal oxygen species produced by neutrophils in S. schenckii infection, neutrophils of both C57BL/6 and CGD mice were infected with this fungus in vitro. They could not kill the organism within 6 h after phagocytosis, although the activity of the neutrophils from CGD mice was lower than that of neutrophils from C57BL/6 mice (data not shown). These data are similar to the results of Schaffner et al. (20), which showed only fungistatic activity during that time. In our colony inhibition assay of neutrophil-rich monolayers, neutrophils of CGD mice also showed lower inhibitory activity than those of C57BL/6 mice (Fig. 2A), and the inhibitory activity of neutrophils from C57BL/6 mice was not caused by contaminating macrophages (Fig. 2B). The observation that the fungal burden in the footpads of CGD mice was greater than that in the footpads of C57BL/6 mice 3 days after subcutaneous infection (Fig. 1C) is consistent with the observation that the antifungal activity of neutrophils from CGD mice is lower than that of neutrophils from C57BL/6 mice.
Immune activation of macrophages induces the production of microbicidal reactive oxygen and nitrogen intermediates such as nitric oxide (NO) (14). In the experiment to examine the effect of the activation of macrophages on fungal growth, the activated macrophages from CGD mice had a lower fungistatic activity than did those from C57BL/6 mice (Fig. 3). Therefore, reactive oxygen species seem to be required for a wild-type level of activity. However, it should be noted that activated macrophages from CGD mice were not entirely deficient in inhibiting fungal growth. We have confirmed that NO production by macrophages from both CGD mice and C57BL/6 mice is similar (unpublished observation). Hence, we postulate that inhibition of the growth of this fungus shown by in vitro activated macrophages from CGD mice was due to the production of NO. Fernandes et al. (6) have also demonstrated that stimulation of murine macrophages with IFN-γ and LPS induced the inhibition of S. schenckii growth predominantly through NO production. Therefore, NO produced by activated macrophages plays a role in fungal growth inhibition and, along with oxygen radicals, participates in the defense against this fungus.
In experiments performed to elucidate the most effective phagocytes to eliminate this fungus (Fig. 2 to 4), only macrophages from immunized C57BL/6 mice showed both fungicidal and fungistatic activity after in vivo phagocytosis (Fig. 4). It is likely that because of this intense fungicidal activity, C57BL/6 mice eliminated the organism and survived the in vivo infection experiment (Fig. 1A). In contrast, macrophages obtained from immunized CGD mice were not fungicidal (Fig. 4), and infected CGD mice died within 84 days in the in vivo experiment (Fig. 1A). Hence, oxygen radicals employed by immune macrophages are likely to be critical effector molecules for fungistatic and fungicidal activities.
Schaffner et al. (20) demonstrated that in vitro susceptibility of fungi to killing by neutrophils can discriminate between primary and opportunistic pathogens. In this respect, the S. schenckii IFM 41598 strain used in this study could be considered a primary pathogen of humans and mice.
It has been reported that CGD patients are susceptible to fungal infections such as those by Aspergillus, Torulopsis, and Candida and that most of the patients had fungal pneumonia and/or widely disseminated diseases. For C. albicans infection, Aratani et al. (2) reported that intraperitoneal inoculation of 2.3 × 105 CFU of the fungi into CGD mice resulted in the detection of much larger numbers of fungi in all of their organs at 6 days after infection compared with the numbers in wild-type mice and also resulted in 100% mortality. Our present study showed that sporotrichosis is also a threat to CGD patients. We cannot find any case report of sporotrichosis in CGD patients because of the low chance of infection: the incidence of CGD patients is estimated at 1 in 220,000 births in Japan (9). Sporotrichosis is also not common. According to the reports from the dermatology clinics of university hospitals in Japan, the proportion of patients with sporotrichosis relative to the total number of outpatients was 0.13% at Kurume University (11) and 0.15% at Chiba University (10). However, we emphasize that CGD is a risk factor for developing severe sporotrichosis.
Resistance of S. schenckii to killing by phagocytes and subsequent persistence in the tissues suggest the importance of antifungal therapy of this infection, especially in CGD patients. Combined treatment by thermotherapy, iodide, and antifungal agents is likely to be effective against this fungus. Saturated solutions of potassium iodide and itraconazole are recommended, while fluconazole is known to be less effective in treating this infection (8, 16). Nevertheless, a treatment protocol for sporotrichosis needs to be established for patients at risk.
The pathogenesis factors of S. schenckii are not fully understood, and little is known about the differences in virulence among S. schenckii strains isolated from specimens from patients and the environment. Kwon-Chung (12) reported that the ability to grow at 37°C in vitro is a critical factor in the pathogenesis of S. schenckii strains which can grow not only in the skin but also in the internal organs. Fernandes et al. (7) reported that S. schenckii conidia cultured for 4 days are more virulent than conidia cultured for 12 days and that the difference lies in cell wall composition. As mentioned in the introduction to this paper, S. schenckii is reported to be a dimorphic fungus which has a saprophilic mycelial form on plant debris and soil, but the traumatic inoculation of this fungus causes differentiation into a yeast form within the infected tissue. It is reported that the yeast form of S. schenckii is more resistant to H2O2 and neutrophil killing than is the mycelial form (6). The association between dimorphism and virulence of this fungus, however, is not well understood. Since knowledge about various aspects of the pathogenesis of this fungus is limited, work is under way in our laboratory to further explore these points.
Acknowledgments
We thank Tamaki Chou and Osamu Shimokawa for scientific discussion; Kazuko Ohga, Takahiko Ishikawa, Ken-ichiro Iida, Masanori Seki, and Zhenyu Piao for technical assistance; Yuka Eguchi for preparing the histological specimens; and Shigeki Nabeshima for the advice on NO production assay. We also thank Chun Chau Sze and Sharon Villanueva for their critical advice on the manuscript.
This work was supported by Grants-in-Aid for Scientific Reseach (B) (2) 14370094 from the Ministry of Education, Science, Culture and Sports of Japan.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.al-Tawfiq, J. A., and K. K. Wools. 1998. Disseminated sporotrichosis and Sporothrix schenckii fungemia as the initial presentation of human immunodeficiency virus infection. Clin. Infect. Dis. 26:1403-1406. [DOI] [PubMed] [Google Scholar]
- 2.Aratani, Y., F. Kura, H. Watanabe, H. Akagawa, Y. Takano, K. Suzuki, M. C. Dinauer, N. Maeda, and H. Koyama. 2002. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J. Infect. Dis. 185:1833-1887. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, M. S., R. E. Isturiz, H. L. Malech, R. K. Root, C. M. Wilfert, L. Gutman, and R. H. Buckley. 1981. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am. J. Med. 71:59-66. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham, K. M., G. S. Bulmer, and E. R. Rhoades. 1979. Phagocytosis and intracellular fate of Sporothrix schenckii. J. Infect. Dis. 140:815-817. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson, C. L., R. L. Taylor, and D. J. Drutz. 1983. Susceptibility of congenitally athymic (nude) mice to sporotrichosis. Infect. Immun. 40:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes, K. S., A. L. Coelho, L. M. Lopes Bezerra, and C. Barja-Fidalgo. 2000. Virulence of Sporothrix schenckii conidia and yeast cells, and their susceptibility to nitric oxide. Immunology 101:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes, K. S., H. L. Mathews, and L. M. Lopes Bezerra. 1999. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J. Med. Microbiol. 48:195-203. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, D. N., J. Robert C. Moellering, and M. A. Sande (ed.). 2001. The Sanford guide to antimicrobial therapy, 31st ed. Antimicrobial Therapy, Inc., Hyde Park, Vt.
- 9.Ishibashi, F., H. Nunoi, F. Endo, I. Matsuda, and S. Kanegasaki. 2000. Statistical and mutational analysis of chronic granulomatous disease in Japan with special reference to gp91-phox and p22-phox deficiency. Hum. Genet. 106:473-481. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, M., S. Okamoto, and H. Kariya. 1986. Survey of 200 cases of sporotrichosis. Dermatologica 172:209-213. [DOI] [PubMed] [Google Scholar]
- 11.Kondo, C., and H. Kaji. 1982. Statistical survay on 100 cases of sporotrichosis. Nishinihon J. Dermatol. 44:983-987. (In Japanese.) [Google Scholar]
- 12.Kwon-Chung, K. J. 1979. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J. Infect. Dis. 139:424-431. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer, R. I. 1975. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J. Clin. Investig. 55:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan, C. F., and J. B. Hibbs, Jr. 1991. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 3:65-70. [DOI] [PubMed] [Google Scholar]
- 15.Nozawa, R. T., R. Sekiguchi, and T. Yokota. 1980. Stimulation by conditioned medium of L-929 fibroblasts, E. coli lipopolysaccharide, and muramyl dipeptide of candidacidal activity of mouse macrophages. Cell. Immunol. 53:116-124. [DOI] [PubMed] [Google Scholar]
- 16.Pickering, L. K., G. S. Peter, C. J. Baker, M. A. Gerber, N. E. MacDonald, W. A. Orenstein, and P. Patriarca. 2000. Sporotrichosis, p. 512-513. In L. K. Pickering, G. S. Peter, C. J. Baker, M. A. Gerber, and N. E. MacDonald (ed.), 2000 red book: report of The Committee on Infectious Diseases, 25th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 17.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 18.Rossi, F., V. Della Bianca, and P. de Togni. 1985. Mechanisms and functions of the oxygen radicals producing respiration of phagocytes. Comp. Immunol. Microbiol. Infect. Dis. 8:187-204. [DOI] [PubMed] [Google Scholar]
- 19.Salyers, A. A., and D. D. Whitt (ed.). 2002. Bacterial pathogenesis: a molecular approach, 2nd ed., p. 53-100. ASM Press, Washington, D.C.
- 20.Schaffner, A., C. E. Davis, T. Schaffner, M. Markert, H. Douglas, and A. I. Braude. 1986. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J. Clin. Investig. 78:511-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraishi, A., K. Nakagaki, and T. Arai. 1992. Role of cell-mediated immunity in the resistance to experimental sporotrichosis in mice. Mycopathologia 120:15-21. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana, T., T. Matsuyama, and M. Mitsuyama. 1999. Involvement of CD4+ T cells and macrophages in acquired protection against infection with Sporothrix schenckii in mice. Med. Mycol. 37:397-404. [DOI] [PubMed] [Google Scholar]
- 23.Washburn, R. G., J. I. Gallin, and J. E. Bennett. 1987. Oxidative killing of Aspergillus fumigatus proceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect. Immun. 55:2088-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]