Abstract
Five exposures of baboons to the attenuated schistosome vaccine gave greater protection than three exposures, but this attenuation was not sustained when challenge was delayed. Within the scope of the data collected, fecal egg counts and circulating antigen levels did not accurately predict the observed worm burdens. Levels of immunoglobulin G at challenge correlated best with protection, but there was little evidence of a recall response.
The olive baboon (Papio anubis) provides a robust primate model for the study of schistosomiasis (7, 10) and has already been the subject of limited experiments with the radiation-attenuated (RA) vaccine (2). Given the efficacy of this vaccine in rodents, it is important to demonstrate that it represents a realistic paradigm for a human vaccine incorporating defined antigens. In one study, >50% protection was achieved after three vaccinations (14), and in another study, >80% protection was achieved after four vaccinations with a shorter interval between the last vaccination and challenge (9). However, while three exposures of vervet monkeys elicited 48% protection, increasing the number to five exposures resulted in only 39% protection (15), suggesting that immunoregulatory mechanisms impose a ceiling on protection. In this study, we sought to determine whether the protective immunity induced in the baboon by the RA vaccine could be boosted to a high level and then sustained.
Juvenile olive baboons ranging in weight from 4.6 to 4.9 kg were used, and on the basis of final weight (<9 kg), none reached sexual maturity before perfusion. They were housed according to international standards and guidelines for primates, and all experimental procedures were approved by independent scientific and ethical committees at the Institute of Primate Research, Nairobi, Kenya. All exposures to irradiated and normal cercariae were as described in previous work (14), using a different skin site on the abdomen, groin, or axilla on each occasion to avoid interference from any potential local inflammation. The degree of vaccine-induced protection was determined by the number of adult worms recovered from baboons at 6 weeks postchallenge by portal perfusion (11, 14). As an indirect measure of worm burden, the mean number of eggs per gram of feces was determined on three 50-mg fresh fecal samples at weeks 4, 5, and 6 postchallenge by using the Kato/Katz smear technique (12). Samples of either 5 ml or 20 ml of blood were obtained from test animals, at intervals throughout the experiments, for serum or recovery of peripheral blood mononuclear cells (PBMC), respectively (14); sampling of controls began at the time of challenge. For assays of antibody and cellular responses, the following parasite antigens were used: material released during cercarial transformation into schistosomula from hours 0 to 3 (RAP); soluble proteins from lung schistosomula (SLAP); and soluble proteins from eggs (SEA) (1, 6). Concanavalin A (Sigma, Dorset, United Kingdom) was used in cell assays as a positive control at a concentration of 10 μl/ml. Lymphocyte proliferation was measured after 72 h (14), and cytokine production was measured after 96 h of culture. Interleukin-5 (IL-5) and gamma interferon (IFN-γ) were measured by capture enzyme-linked immunosorbent assay (ELISA), using anti-human reagents (5). Antigen-specific immunoglobulin M (IgM) and IgG antibodies were measured by ELISA (7, 14). Plates were coated overnight with RAP (0.25 μg/ml for IgM; 1 μg/ml for IgG), SLAP (2 μg/ml), or SEA (5 μg/ml); after blocking with 3% bovine serum albumin in phosphate-buffered saline-Tween 20 (0.05%), sera were diluted to the following concentrations: for RAP, 1/3,200 for IgM and 1/6,400 for IgG; for SLAP, 1/5,000; and for SEA, 1/12,000. The plates were subsequently probed with horseradish peroxidase-labeled rabbit anti-monkey IgM or IgG (Sigma) at a 1:2,000 dilution, and the reactions were developed with tetramethylbenzidine substrate (Kirkegaard and Perry Labs, Gaithersburg, Md.). As a second indirect estimate of worm burden, schistosome circulating anodic antigen (CAA), which originates in the gut of blood-feeding stages in the portal system (4), was measured by antigen capture ELISA using a pair of specific monoclonal antibodies (3, 8). Data from all assays were analyzed by a two-tailed Student's t test; differences between groups were considered significant at a P value of <0.05.
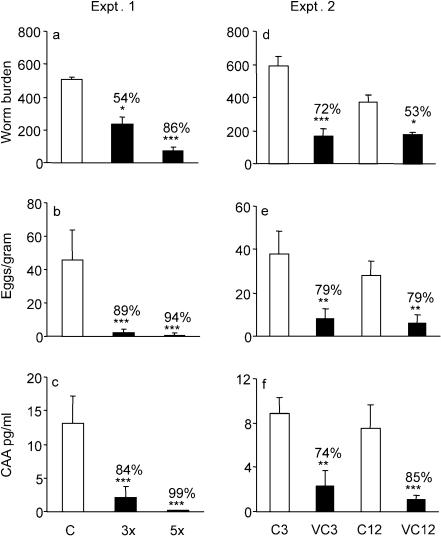
We first wished to discover whether increasing the number of vaccinations would enhance the level of protection. Two groups of five baboons were vaccinated three or five times (designated groups 3x and 5x) with 9,000 RA cercariae at 4-week intervals. The experiment was synchronized so that the first exposure of the 3x group coincided with the third exposure of the 5x group. Thus, 3 weeks after the last vaccination, both test groups together with a group of five control animals were challenged with 1,000 normal cercariae from the same batch. The mean worm burden of the challenge control group (Fig. 1a) was much higher than those of the 3x and 5x vaccinated groups, representing 54 and 86% protection against challenge, respectively. Comparison of fecal egg counts (Fig. 1b) revealed a substantially lower output in the 3x and 5x groups compared to the controls, equating to 89 and 94% protection. Estimation of CAA in the serum immediately prior to perfusion (Fig. 1c) also indicated much lower worm burdens in the 3x and 5x groups than were actually recovered, equating to 84 and 99% protection.
FIG. 1.
Protection against challenge estimated by worm burden (number of worms recovered per animal) (a and d), fecal egg output (number of eggs per gram of feces) (b and e), and levels of CAA (c and f) in experiments 1 (a, b, and c) and 2 (d, e, and f). Percentage protection was calculated using the formula (C − V)/C × 100, where V is the worm burden of the vaccinated group and C is the worm burden of the challenge control group. Significances are given for the absolute values of each parameter in the vaccinated groups relative to their respective controls. ***, P < 0.001; **, P < 0.01; *, P < 0.05. Values depicted are means ± standard errors of the mean (SEM) for results from five animals.
We then sought to determine whether the high level of protection would be sustained for a significant period, a crucial requirement for an effective vaccine. Two groups of five baboons were vaccinated five times at 4-week intervals before they and their respective controls were challenged with 1,000 normal cercariae at either 3 weeks (VC3) or 12 weeks (VC12) after the last vaccination. The difference in mean worm burdens between the 3-week challenge controls and the VC3 group at perfusion amounted to 72% protection, while that between the 12-week challenge controls and the VC12 group represented only 53% protection (Fig. 1d). In contrast, the indirect estimates of worm burden suggested that protection did not wane, with fecal egg counts indicating identical values in both groups (79%; Fig. 1e), and circulating antigen levels indicated a higher protection in the VC12 than in the VC3 group (85% versus 74%; Fig. 1f).
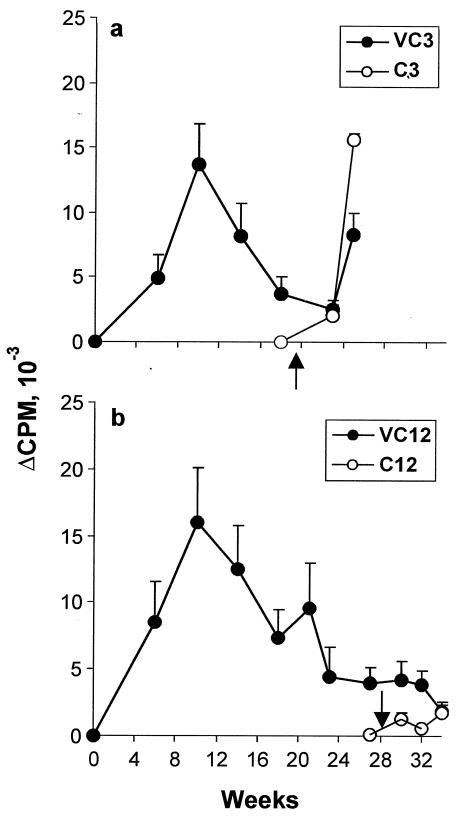
Immunological parameters were monitored throughout both experiments, and as similar profiles were obtained for both groups vaccinated 5 times and challenged at 3 weeks, only those from the second experiment are presented. The nonspecific reactivity of PBMC, determined by concanavalin A stimulation, was in the range of 6 × 104 to 8 × 104 cpm/well throughout (data not shown). Furthermore, there was no upward drift in the baseline [3H]thymidine incorporation by unstimulated cultures with successive exposures to vaccinating parasites, indicating an absence of generalized immune system stimulation. When the proliferation of PBMC in response to SLAP (Fig. 2a and b) and RAP (data not shown) was monitored, [3H]thymidine incorporation revealed only a small, nonsustained response in both the VC3 and VC12 groups, suggesting that even irradiated larvae are poorly immunogenic. Moreover, there was little evidence that normal cercariae provided a significant boost in responsiveness to antigens after challenge. (These results argue against the view that trickle infections of individuals in areas in which the parasite is endemic might continually prime the immune response to incoming larvae.) However, there was a dramatic rise in incorporation at 6 weeks in the VC3 group and its control, undoubtedly the result of egg deposition; this was not seen in the VC12 group or its control. The limited peripheral responses after vaccination were matched by low cytokine production (<400 pg of both IFN-γ and IL-5/ml), making it impossible to delineate distinct Th1 or Th2 patterns (data not shown). The inability to detect a prominent cytokine response was not due to a failure of the anti-human reagents to capture baboon IFN-γ or IL-5, because where egg deposition was evident at 6 weeks postchallenge, SEA-stimulated cultures contained up to 2,590 pg of IFN-γ/ml and 837 pg of IL-5/ml.
FIG. 2.
Cellular responses over the time course of experiment 2. Incorporation of [3H]thymidine by PBMC after stimulation by SLAP, with a 3-week interval (a) or a 12-week interval (b) between the last vaccination and challenge. Closed circles represent values for vaccinated groups; open circles represent values for control groups. Arrows correspond to time of challenge. Values depicted are means ± SEM for results from five animals.
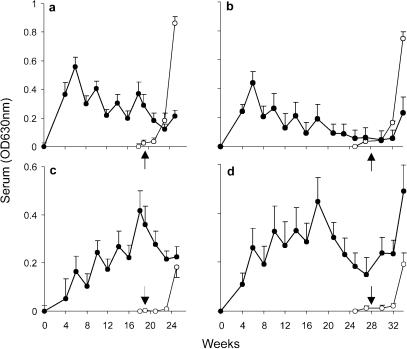
Antibody responses were a more informative indicator of immune status. IgM reactive with larval secretions (RAP) rose rapidly to a peak after two vaccinations before declining in a sawtooth pattern (Fig. 3a and b), indicating that this isotype is not crucial for protection. At 6 weeks postchallenge in both control groups, there was a dramatic rise in IgM responses, mirrored to a much smaller extent in the vaccinated groups, coinciding with the onset of egg deposition. IgG levels, in contrast, rose progressively in a sawtooth fashion with successive vaccinations in both VC3 and VC12 groups (Fig. 3c and d); the former group received its challenge virtually at the peak of the IgG response, whereas levels had declined by two-thirds when the latter group was challenged. The sawtooth antibody profile associated with priming, where the boost provided by vaccination rapidly wanes, is an unusual feature. We can rule out a contribution from CAA because negligible amounts were detected prior to the onset of blood feeding after challenge.
FIG. 3.
Humoral responses over the time course of the duration of the experiment in groups with a 3-week interval (a and c) or a 12-week interval (b and d) between vaccination and challenge. IgM (a and b) and IgG (c and d) reactivities against RAP are shown. Closed circles represent values for vaccinated groups; open circles represent values for control groups. Arrows correspond to time of challenge. Values depicted are means ± SEM for results from five animals.
The best correlation coefficient (0.43) between antibody response and protective immunity was obtained by comparing IgG levels in response to RAP at challenge with the percentage protection of individual animals at perfusion, suggesting that this isotype mediates the effector response. It was noteworthy that once vaccination ceased, specific IgG levels went into a rapid decline, with no boost provided by the challenge before egg deposition began. At the 6-week postchallenge sampling, the VC12 group and both controls showed a significant rise in IgG responses to RAP compared to the 4-week values (P < 0.01). Moreover, both IgM and IgG responses to SEA at 6 weeks were virtually superimposable onto those to larval secretions (data not shown); this result is readily explained by the cross-reactivity of the two antigen preparations.
As the principal purpose of the experiments was to measure protection, biopsies of skin exposure sites were precluded since they would remove unknown numbers of parasites. Furthermore, we sought to avoid generating localized dermal responses by applying cercariae to different sites on each occasion. However, we were able to compare hepatic responses in all animals at 6 weeks postchallenge. Moderate to severe granulomatous inflammation was visible in all challenge controls but was either absent or mild in vaccinated animals. Histology further revealed that controls formed significantly larger granulomas than their vaccinated counterparts (data not shown). Thus, prior exposure to attenuated larvae appears to ameliorate the hepatic response to eggs.
The reduction in worm burden in test animals, relative to controls, is the unequivocal measure of vaccination success. On this basis we conclude that increasing the number of vaccinations boosts protection in baboons to very high levels. If such a level could be achieved with a human vaccine, it would have an impact on both morbidity and transmission. However, in the absence of restimulation, the high level of protection is not sustained. Assuming that protection is antibody-mediated, the reason protection is not sustained is probably the inability to maintain antibody titer. This result suggests that a recombinant antigen replacement will require a vaccine formulation that maintains high antibody titers for an extended period. Such a strategy may be crucial to vaccination success because parasites may only be vulnerable to immune attack for a short period after penetration, leaving insufficient time for a secondary response to be mounted before they have become established.
A major problem that will need to be addressed in trials of human schistosomiasis vaccines is the impossibility of determining worm burden directly as the measure of protection. We have, for the first time, evaluated vaccine efficacy in a primate host by comparing actual worm burdens with the indirect alternatives of number of eggs per gram of feces and CAA levels in serum. These parameters are routinely used for diagnosis of schistosome infection in humans (3, 13), but their ability to predict worm burden cannot be validated. We found that both provided overestimates of protection, relative to worm burden, in three out of the four groups. For egg counts, a possible explanation is that perfusions were performed shortly after the start of oviposition. Thus, if immune responses delayed parasite arrival in the portal tract of vaccinated animals, eggs would appear later in the feces of vaccinated animals than in that of controls. In future experiments, we will use a later perfusion time to resolve this issue. The discrepancies between worm burden and circulating antigen, which originates primarily in the worm gut (4), are more difficult to reconcile, as blood feeding commences upon arrival of parasites in the liver some weeks before perfusion. One potential explanation is that there are differences in the rates of clearance of circulating antigen immune complexes in vaccinated groups and control groups. A second explanation is that female worms in particular may be rendered less fit by immune pressure; this could be reflected both in blood feeding or regurgitation of gut contents and in egg output. Such an antifecundity effect would be a valuable adjunct to successful vaccination, but a larger data set is needed before its existence can be confirmed.
Acknowledgments
This work was supported by INCO-DC contracts IC18CT97-0212 and ICA4-CT99-10006 from the European Commission.
We are grateful to Simon Kiarie and Sammy Kisara for providing help with parasitological assays and baboon perfusions, Fred Nyundo and Kiio Kithome for excellent technical support in immunological assays, and the veterinarians and animal caretakers at the Institute of Primate Research.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ashton, P. D., R. S. Curwen, and R. A. Wilson. 2001. Linking proteome and genome: how to identify parasite proteins. Trends Parasitol. 17:198-202. [DOI] [PubMed] [Google Scholar]
- 2.Coulson, P. S. 1997. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv. Parasitol. 39:271-336. [DOI] [PubMed] [Google Scholar]
- 3.Deelder, A. M., N. De Jonge, O. C. Boerman, Y. E. Fillie, G. W. Hilberath, J. P. Rotmans, M. J. Gerritse, and D. W. Schut. 1989. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am. J. Trop. Med. Hyg. 40:268-272. [DOI] [PubMed] [Google Scholar]
- 4.de Water, R., J. A. Fransen, and A. M. Deelder. 1986. Ultrastructural localization of the circulating anodic antigen in the digestive tract of Schistosoma mansoni using monoclonal antibodies in an immunogold labeling procedure. Am. J. Trop. Med. Hyg. 35:549-558. [DOI] [PubMed] [Google Scholar]
- 5.Mola, P. W., I. O. Farah, T. M. Kariuki, M. Nyindo, R. E. Blanton, and C. L. King. 1999. Cytokine control of the granulomatous response in Schistosoma mansoni-infected baboons: role of exposure and treatment. Infect. Immun. 67:6565-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mountford, A. P., R. Harrop, and R. A. Wilson. 1995. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with attenuated larvae. Infect. Immun. 63:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyindo, M., and I. O. Farah. 1999. The baboon as a non-human primate model of human schistosome infection. Parasitol. Today 15:478-482. [DOI] [PubMed] [Google Scholar]
- 8.Polman, K., M. M. Diakhate, D. Engels, S. Nahimana, G. J. Van Dam, S. T. Falcao Ferreira, A. M. Deelder, and B. Gryseels. 2000. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop. Med. Int. Health 5:534-537. [DOI] [PubMed] [Google Scholar]
- 9.Soisson, L. A., G. D. Reid, I. O. Farah, M. Nyindo, and M. Strand. 1993. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J. Immunol. 151:4782-4789. [PubMed] [Google Scholar]
- 10.Sturrock, R. F. 1986. A review of the use of primates in studying human schistosomiasis. J. Med. Primatol. 15:267-279. [PubMed] [Google Scholar]
- 11.Sturrock, R. F., A. E. Butterworth, and V. Houba. 1976. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology 73:239-252. [DOI] [PubMed] [Google Scholar]
- 12.Sturrock, R. F., A. E. Butterworth, V. Houba, S. D. Karamsadkar, and R. Kimani. 1978. Schistosoma mansoni in the Kenyan baboon (Papio anubis): the development and predictability of resistance to homologous challenge. Trans. R. Soc. Trop. Med. Hyg. 72:251-261. [DOI] [PubMed] [Google Scholar]
- 13.Sturrock, R. F., J. H. Ouma, H. C. Kariuki, F. W. Thiongo, D. K. Koech, and A. E. Butterworth. 1997. Quality control of Kato slide counts for Schistosoma mansoni: a review of 12 years' experience in Kenya. Bull. W. H. O. 75:469-475. [PMC free article] [PubMed] [Google Scholar]
- 14.Yole, D. S., R. Pemberton, G. D. Reid, and R. A. Wilson. 1996. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology 112:37-46. [DOI] [PubMed] [Google Scholar]
- 15.Yole, D. S., G. D. Reid, and R. A. Wilson.. 1996. Protection against Schistosoma mansoni and associated immune responses induced in the vervet monkey Cercopithecus aethiops by the irradiated cercaria vaccine. Am. J. Trop. Med. Hyg. 54:265-270. [DOI] [PubMed] [Google Scholar]